Abstract
We compared real-world clinical and economic outcomes for insulin glargine treatment administered by disposable pen and traditional vial-and-syringe injections among elderly patients with type 2 diabetes mellitus (T2DM). Using a large database of US retirees, this retrospective longitudinal study examined 1-year follow-up outcomes in patients with T2DM aged 65 years or older who were either insulin naïve and initiated insulin glargine via disposable pen (pen initiators [PI]) or vial (vial initiators [VI]) or were already insulin glargine users but either continued with a vial (vial continuers [VC]) or switched to a disposable pen (pen switchers [PS]). There were 7856 propensity-score-matched patients, including 2930 each in the PI and VI cohorts, and 998 each in the VC and PS cohorts. Compared with vial-and-syringe users, the disposable pen users had significantly greater treatment persistence (P < .0001 for both comparisons), duration of persistence (P < .0001 for both), and adherence (P < .01 for both) and lower insulin daily average consumption (P < .05 for both). Compared with the VI cohort, the PI cohort had significantly fewer hypoglycemia-related events (P = .0164). Total health care costs were comparable for the respective matched cohorts. In elderly patients with T2DM receiving insulin glargine therapy, initiating or switching to a disposable pen was associated with better treatment persistence and adherence than initiating or continuing with vial-and-syringe, without increased total health care costs. Among insulin-naïve patients, initiating insulin glargine by disposable pen was also associated with significantly reduced risk of hypoglycemia compared with vial-and-syringe patients.
Keywords: elderly, insulin glargine, pen, type 2 diabetes
Increasing age is a risk factor for the development of type 2 diabetes mellitus (T2DM).1 Based on 2010 data, an estimated 26.9% of individuals aged 65 years or older living in the United States have diagnosed or undiagnosed diabetes, compared with 8.3% of the total US population.2 This estimate is consistent with the 2004 findings of a cross-sectional survey conducted in a residential care setting, which found that approximately 25% of US nursing home residents aged 65 years or older had diabetes as a primary admission and/or current diagnosis.3 The burden of the disease is significant: in elderly patients aged 65-74 years with diabetes, 30%-40% of all hospitalizations and 30%-60% of all nursing home admissions are related to the disease.4 As the general population continues to age, the prevalence of the disease is expected to increase, making diabetes a significant ongoing health concern for the elderly population.5
Managing the treatment of elderly patients with T2DM presents a number of specific issues. First, in this population, diabetes is associated with increased mortality and serious comorbidities, including cardiovascular disease, pain, and depression.3,4,6,7 Second, treatment options for diabetes may be limited in the elderly, with special care often required in prescribing and monitoring pharmacologic therapy in this population.5 Polypharmacy should also be considered, but the choice of therapies depends on the health status of the patient (defined by the number of comorbidities or impairments of functional status).8 For example, metformin is often contraindicated because of renal insufficiency or significant heart failure, which is especially relevant given that an estimated 36.7% of elderly patients with T2DM in the United States have nephropathy.6 Thiazolidinediones (which can cause fluid retention and are contraindicated in patients with New York Heart Association Class III and IV congestive heart failure [CHF]5) may exacerbate existing heart failure, which affects 17.9% of elderly patients with T2DM;6 extreme caution is required in at-risk patients and those with milder CHF.5 Third, elderly patients with T2DM are at increased risk of hypoglycemia and hypoglycemia-related complications, including confusion and falls. Agents that pose a particular risk for hypoglycemia (such as chlorpropamide and glyburide) should be avoided.9
Due to the progressive nature of diabetes, oral antidiabetic drugs (OADs) may not be sufficient to maintain treatment targets; this necessitates the use of insulin therapy to achieve long-term glycemic control in elderly patients with T2DM.10 Insulin therapy, including the long-acting basal analog insulin glargine, is indicated for the treatment of T2DM when glycemic control is no longer adequately managed using OADs.5 Disposable insulin pens have a number of benefits compared with traditional vial-and-syringe injections, including patient preference, discretion, ease of use, ease of reading the dose, improved accuracy for delivering small doses, and ease of accurate dosing.11-18 Disposable insulin pens may be preferable for individuals with problems with vision or manual dexterity.15,19-21
In addition to these patient-reported benefits, a previous retrospective database study also showed better clinical and economic outcomes when patients with T2DM initiated insulin glargine with a disposable pen compared with vial-and-syringe, although these findings were from a commercially insured population.22 It is currently unclear if the same benefits will be observed in an elderly population. Therefore, the objective of this study was to compare the real-world clinical and economic outcomes of administering insulin glargine via a disposable pen vs the traditional vial-and-syringe method in an elderly population (65 years or older).
Methods
Data Source
This was a retrospective longitudinal cohort analysis of the Thomson Reuters MarketScan® Medicare Supplemental database, covering the period from July 1, 2006, through June 30, 2010. The database represents the health services of approximately 5.6 million retirees in the United States. Members are Medicare beneficiaries with comprehensive employer-sponsored supplemental coverage through private insurance, fee-for-service, point-of-service, or capitated health plans. Both the Medicare-covered payment portion and the portion paid by the former employer are included in this database. All enrollment records and inpatient, outpatient, ancillary, and drug claims were collected. The study consisted of a baseline period of 6 months and a follow-up period that extended 12 months beyond the index date, which was defined according to cohort and is described below.
Study Population
The study sample included elderly patients aged 65 years or older who were diagnosed with T2DM, defined as having at least 1 inpatient or 2 physician visits dated at least 30 days apart with a primary or secondary diagnosis of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 250.x0 or 250.x2. Eligible patients received at least 1 pharmacy prescription for insulin glargine. To facilitate comparison of real-world outcomes of insulin delivery device in this study population across various stages of diabetes treatment, the effect of the device was assessed both in patients initiating insulin treatment for the first time and in patients who were previously treated with insulin. Patients were therefore assigned to the following cohorts: Insulin-naïve patients who initiated insulin treatment with insulin glargine using a vial-and-syringe (cohort 1—vial initiators) or a disposable pen (SoloSTAR®, Sanofi US, Inc, Bridgewater, NJ, USA; cohort 2—pen initiators) between January 1, 2007, and June 30, 2009, with the index date being the date of initiation of insulin glargine treatment. Insulin-naïve patients must have had at least 1 filled prescription for an OAD or glucagon-like peptide-1 analog during the baseline period. Insulin-glargine-experienced patients were continuing users of insulin glargine who either used vial-and-syringe (cohort 3—vial continuers) or switched from vial-and-syringe injections to disposable pen injections (SoloSTAR®; cohort 4—pen switchers). For the latter cohort, the index date was the date of switching from vial-and-syringe to disposable pen. For vial continuers, the index date was randomly selected starting from the third vial prescription fill for insulin glargine. All patients had continuous health plan coverage for at least 6 months before (baseline period) and at least 1 year after the index date (follow-up period).
Follow-up Study Outcomes
Insulin treatment persistence is difficult to measure due to nonfixed dosing. Based on published literature, treatment persistence was defined as the patient remaining on study drugs during the follow-up period, without discontinuation or switching after study drug initiation.22-26 Study medication was considered discontinued if the prescription was not refilled within the expected time of medication coverage, defined as the 90th percentile of the mean time between the initial and second prescription fills, stratified by the metric quantity supplied between first and second fills among patients with at least 1 refill. Patients who restarted their initial medication during follow-up with the time period between 2 refills being greater than the “expected time of medication coverage” were considered nonpersistent patients. Sensitivity analyses were also conducted using 75th and 95th percentiles of the time. Treatment adherence was measured by both the traditional medication possession ratio (MPR) and the adjusted MPR (aMPR), which takes into account the differences in insulin device package size.27 For example, insulin glargine is packaged in either 10 mL vials with a total of 1000 units, or 3 mLF disposable pens in a package of 5 pens with a total of 1500 units. aMPR was calculated by multiplying the traditional MPR (the total days’ supply of all filled study drug prescriptions in the analysis period, divided by the number of days in the analysis period) by the average days between prescription refills divided by the average days’ supply for patients using insulin. Hypoglycemia was defined as a health care encounter (outpatient, inpatient, or emergency department [ED] visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycemia (ICD-9 code 250.8—diabetes with other specified manifestations; 251.0—hypoglycemic coma; 251.1—other specified hypoglycemia; or 251.2—hypoglycemia, unspecified).28 The setting of the hypoglycemic event (outpatient, inpatient, or ED) was used as proxy for severity of the event. Average daily dose was estimated by daily average consumption (DACON), calculated as the total number of units dispensed before the last refill of study drug divided by the total number of days between initiation and last refill during the follow-up period. Health care resource utilization included outpatient visits, ED visits, inpatient admissions, inpatient length of stay (days), and endocrinologist visits. Diabetes-related health care resource utilization included claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx). Health care costs were computed as total paid amounts of adjudicated claims. Diabetes-related health care costs included costs from medical claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx), antidiabetic medications, glucose meters, and test strips. A1C data were not available in this data set.
Statistical Analyses
To remove observed differences in baseline demographic and clinical characteristics, stringent 1:1 propensity score matching (PSM) based on demographic and clinical factors was applied within the cohorts of insulin-naïve patients and insulin-glargine-experienced patients.29 For insulin-naïve patients, vial initiators and pen initiators were matched, and for insulin-glargine-experienced patients, vial continuers and pen switchers were matched. PSM is a statistical technique which attempts to estimate the effect of a treatment by accounting for the covariates that predict receiving the treatment. PSM attempts to reduce the bias due to confounding when simply comparing outcomes among those who received the treatment vs those that did not. In this analysis, the following variables were accounted for: age, gender, initial year (2007, 2008), health plan type, geographic region, copay, baseline diabetes, education index, comorbidities, medication, baseline hypoglycemia rates, baseline all-cause health care utilization, baseline diabetes-related health care utilization, baseline all-cause health care cost, and baseline diabetes-related health care cost.
Among matched patients, baseline characteristics, 1-year clinical outcomes, and economic endpoints were summarized and compared, with P values provided by Student t test or χ2 test as appropriate. Time to discontinuation was analyzed using Kaplan–Meier analysis. A P value of .05 was used to determine the level of statistical significance.
Results
Patient Characteristics
Overall, data from 7856 patients were included for analysis after PSM, including 2930 patients in each of the pen initiator and vial initiator groups (cohorts 1 and 2, respectively) and 998 patients in each of the vial continuer and pen switcher groups (cohorts 3 and 4, respectively). There were no significant differences in clinical characteristics at baseline between the treatment groups in either cohort. In addition, baseline health care resource utilization and costs were similar between groups within the cohorts. The baseline characteristics of propensity-score-matched cohorts are shown in Table 1.
Table 1.
Baseline Patient Demographics and Clinical Characteristics.
| Insulin-naïve patients |
Insulin-glargine-experienced patients |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Vial initiators (n = 2930) | Pen initiators (n = 2930) | P value | Pen switchers (n = 998) | Vial continuers (n = 998) | P value |
| Women, n (%) | 1298 (44.3) | 1281 (43.7) | .6546 | 485 (48.5) | 499 (50.0) | .5308 |
| Age in years, mean (SD) | 74.6 (6.6) | 74.5 (6.5) | .5992 | 73.4 (6.34) | 73.6 (6.35) | .4313 |
| Charlson comorbidity index, mean (SD) | 1.29 (1.71) | 1.29 (1.74) | .9818 | 1.18 (1.61) | 1.18 (1.60) | 1.0000 |
| Comorbidity, n (%) | ||||||
| Hypertension | 1223 (41.7) | 1238 (42.2) | .6914 | 404 (40.4) | 402 (40.2) | .9273 |
| Hyperlipidemia | 513 (17.5) | 548 (18.7) | .2351 | 189 (18.9) | 195 (19.5) | .7333 |
| Congestive heart failure | 535 (18.2) | 516 (17.6) | .5177 | 147 (14.7) | 172 (17.2) | .1267 |
| Peripheral vascular disease | 304 (10.3) | 335 (11.4) | .1939 | 114 (11.4) | 115 (11.5) | .9440 |
| Myocardial infarction | 134 (4.5) | 147 (5.0) | .4267 | 27 (2.7) | 36 (3.6) | .2492 |
| Retinopathy | 239 (8.1) | 245 (8.3) | .7758 | 183 (18.3) | 203 (20.3) | .2570 |
| Neuropathy | 345 (11.7) | 342 (11.6) | .9030 | 168 (16.8) | 170 (17.0) | .9050 |
| Nephropathy | 136 (4.6) | 129 (4.4) | .6599 | 74 (7.4) | 70 (7.0) | .7293 |
| Mental illness | 239 (8.1) | 240 (8.1) | .9620 | 81 (8.1) | 94 (9.4) | .3036 |
| Antidiabetic drug usage | ||||||
| Number of OADs, n (SD) | 2.09 (0.87) | 2.08 (0.85) | .6830 | 0.89 (0.98) | 0.87 (0.94) | .6893 |
| Metformin | 1834 (62.5) | 1827 (62.3) | .8502 | 304 (30.4) | 308 (30.8) | .8460 |
| Sulfonylureas | 2301 (78.5) | 2302 (78.5) | .9746 | 277 (27.7) | 265 (26.5) | .5459 |
| Thiazolidinediones | 1094 (37.3) | 1070 (36.5) | .5159 | 173 (17.3) | 177 (17.7) | .8139 |
| Sitagliptin | 656 (22.3) | 657 (22.4) | .9750 | 81 (8.1) | 73 (7.3) | .5022 |
| GLP-1(exenatide) | 206 (7.0) | 208 (7.0) | .9188 | 46 (4.6) | 45 (4.5) | .9145 |
| Any insulin, n (%) | N/A | N/A | 998 (100) | |||
| Regular insulin | N/A | N/A | 54 (5.4%) | 54 (5.4%) | .1717 | |
| Basal insulin | N/A | N/A | 998 (100.0%) | 998 (100.0%) | ||
| Rapid acting insulin | N/A | N/A | 508 (50.9%) | 513 (51.4%) | .8228 | |
| DACON, U/day (SD) | 43.7 (31.2) | 45.8 (30.3) | .1277 | |||
| Hypoglycemia, n (%) | ||||||
| Any hypoglycemia | 134 (4.5) | 141 (4.8) | .6655 | 101 (10.1) | 100 (10.0) | .9407 |
| Inpatient/ED hypoglycemia | 69 (2.3) | 82 (2.7) | .2838 | 33 (3.3) | 30 (3.0) | .7009 |
| Number of any hypoglycemic events per patient, mean (SD) | 0.08 (0.55) | 0.12 (1.20) | .0922 | 0.22 (0.95) | 0.27 (1.23) | .3492 |
| Number of inpatient/ED hypoglycemic events per patient, mean (SD) | 0.03 (0.18) | 0.03 (0.21) | .2061 | 0.04 (0.21) | 0.05 (0.66) | .4916 |
| Health care utilization | ||||||
| Any hospitalization, n (%) | 994 (33.9) | 1018 (34.7) | .5091 | 190 (19.0) | 193 (19.3) | .8646 |
| Any diabetes-related hospitalization, n (%) | 496 (16.9) | 513 (17.5) | .5564 | 89 (8.9) | 94 (9.4) | .6982 |
| Any ED visit, n (%) | 1001 (34.1) | 1026 (35.0) | .4923 | 295 (29.5) | 167 (26.7) | .1635 |
| Any diabetes-related ED visit, n (%) | 480 (16.3) | 477 (16.2) | .9156 | 129 (12.9) | 119 (11.9) | .4974 |
| Number of hospitalizations, n (SD) | 0.39 (0.59) | 0.39 (0.57) | .9640 | 0.23 (0.51) | 0.23 (0.54) | .9321 |
| Number of diabetes-related hospitalizations, n (SD) | 0.18 (0.43) | 0.18 (0.41) | .9010 | 0.10 (0.36) | 0.10 (0.34) | .8979 |
| Cost, $, mean (SD) | ||||||
| Total cost | 14 289 (27 592) | 13 791 (26 035) | .4769 | 11 051 (15 376) | 11 273 (21 696) | .7924 |
| Total diabetes-related cost | 4636 (15 943) | 4543 (13 321) | .8092 | 3246 (6322) | 3287 (6823) | .8899 |
| Total Rx cost | 2451 (2120) | 2502 (2173) | .3551 | 3572 (2347) | 3494 (2979) | .5184 |
| Total diabetes-related Rx cost | 643 (622) | 646 (613) | .8575 | 1162 (798) | 1156 (828) | .8567 |
| Total diabetes-related Supply cost | 38 (105) | 39 (103) | .5412 | 101 (200) | 96 (205) | .6346 |
Abbreviations: DACON, daily average consumption; ED, emergency department; OADs, oral antidiabetic drugs.
Insulin-naïve Patients (Pen Initiators vs Vial Initiators)
During the 1-year follow-up and compared to vial initiators, pen initiators were significantly more persistent (58.2% vs 50.8%; P < .0001) and adherent (aMPR 0.69 vs 0.64; P < .0001) to insulin glargine treatment (Table 2). Kaplan–Meier analysis showed that pen initiators were persistent for a longer period of time than vial initiators, with a mean duration of persistence of 290.0 days versus 264.6 days, respectively (P < .0001 (Figure 1a). Sensitivity analyses using 75th and 95th percentiles of the mean time between prescription fills yielded similar results. In addition, pen initiators were less likely to have hypoglycemia-related events than vial initiators (8.6% vs 10.4%; P = .0164; Table 2), including fewer hypoglycemia-related events in the first quarter of the follow-up period (3.7% vs 5.0%; P = .0159). Pen initiators also had lower insulin DACON compared with vial initiators (28.6 U/day vs 32.0 U/day; P = .0002) and were less likely to have a claim for hospitalization (all-cause: 33.0% vs 37.5%, P = .0002;diabetes-related: 16.7% vs 18.8%, P = .0374) over the year of follow-up (Table 3). Vial initiators and pen initiators incurred similar total health care costs ($22 265 vs $21 669, respectively; P = .5085), despite significantly higher diabetes drug costs for those initiating treatment with the pen device ($2166 vs $1907; P < .0001; Figure 2a).
Table 2.
Clinical Outcomes Among Insulin-naïve Patients Initiating Treatment With Insulin Glargine by Pen Device or Vial-and-Syringe Injections, 1-Year Follow-up.
| Insulin-naïve patients |
Insulin-glargine-experienced patients |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Pen initiators (n = 2930) | Vial initiators (n = 2930) | P value | Pen switchers (n = 998) | Vial continuers (n = 998) | P value |
| Persistence | ||||||
| Patients persisting with treatment, n (%)a | 1708 (58.2) | 1489 (50.8) | <.0001 | 652 (65.3%) | 567 (56.8%) | <.0001 |
| Number of persistence days, mean (SD)a | 290.0 (94.3) | 264.6 (109.4) | <.0001 | 304 (89) | 282 (105) | <.0001 |
| Adherence | ||||||
| Adjusted MPR, mean (SD) | 0.69 (0.31) | 0.64 (0.35) | <.0001 | 0.75 (0.30) | 0.79 (0.27) | .0052 |
| DACON, U/day, mean (SD) | 28.6 (22.6) | 32.0 (38.5) | .0002 | 42.2 (39.9) | 47.8 (60.2) | .0201 |
| Patients with hypoglycemia | ||||||
| Any hypoglycemic event, n (%) | 253 (8.6) | 307 (10.4) | .0164 | 101 (10.1%) | 100 (10.0%) | .9407 |
| Inpatient hypoglycemic event, n (%) | 11 (0.3) | 10 (0.3) | .8270 | 6 (0.6%) | 6 (0.6%) | 1.0000 |
| Outpatient hypoglycemic event, n (%) | 187 (6.3) | 231 (7.8) | .0255 | 79 (7.9%) | 79 (7.9%) | .8052 |
| ED hypoglycemic event, n (%) | 89 (3.0) | 114 (3.8) | .0741 | 29 (2.9%) | 24 (2.4%) | .4864 |
| Inpatient/ED hypoglycemic event, n (%) | 99 (3.3) | 123 (4.1) | .1006 | 33 (3.3%) | 30 (3.0%) | .7009 |
| Number of hypoglycemic events per patient, mean (SD) | 0.30 (2.47) | 0.29 (1.65) | .9109 | 0.22 (0.95) | 0.27 (1.23) | .3492 |
Abbreviations: DACON, daily average consumption; ED, emergency department; MPR, medication possession ratio.
90% estimation level
Figure 1a.
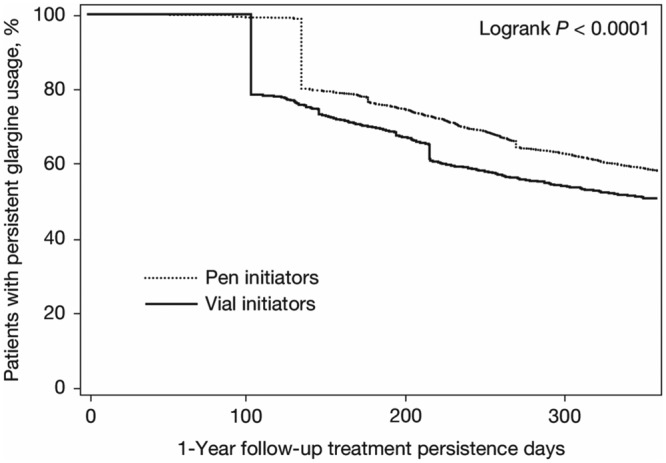
Kaplan–Meier curve for the time to treatment discontinuation for insulin-glargine-naïve patients.
Table 3.
Health Care Resource Utilization.
| Insulin-naïve patients |
Insulin-glargine-experienced patients |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Pen initiators (n = 2930) | Vial initiators (n = 2930) | P value | Pen Switchers (n = 998) | Vial Continuers (n = 998) | P value |
| Hospitalization, n (%) | 967 (33.0) | 1101 (37.5) | .0002 | 320 (32.0) | 344 (34.4) | .2542 |
| Hospitalization, diabetes related, n (%) | 492 (16.7) | 553 (18.8) | .0374 | 174 (17.4) | 181 (18.1) | .682 |
| ED visit, n (%) | 1189 (40.5) | 1247 (42.5) | .1242 | 415 (41.5) | 423 (42.3) | .7167 |
| ED visit, diabetes related, n (%) | 548 (18.7) | 587 (20.0) | .1973 | 195 (19.5) | 197 (19.7) | .9103 |
| Office visit, n (%) | 2900 (98.9) | 2896 (98.8) | .6151 | 990 (99.1) | 990 (99.1) | 1.000 |
| Office visit, diabetes related, n (%) | 2798 (95.4) | 2756 (94.0) | .0137 | 950 (95.1) | 921 (92.2) | .0074 |
| Endocrinologist visit, n (%) | 317 (10.8) | 272 (9.2) | .0506 | 206 (20.6) | 197 (19.7) | .6158 |
| Endocrinologist visit, diabetes related, n (%) | 304 (10.3) | 261 (8.9) | .057 | 199 (19.9) | 190 (19.0) | .6111 |
| Number of hospitalizations, mean (SD) | 0.46 (0.78) | 0.52 (0.81) | .0023 | 0.44 (0.74 ) | 0.52 (0.88) | .0423 |
| Number of hospitalizations, diabetes related, mean (SD) | 0.21 (0.52) | 0.23 (0.53) | .1438 | 0.21 (0.52) | 0.22 (0.55) | .6771 |
| Number of ED visits, mean (SD) | 0.86 (1.52) | 0.90 (1.57) | .3832 | 0.95 (1.81) | 0.98 (1.88) | .7524 |
| Number of ED visits, diabetes related, mean (SD) | 0.28 (0.70) | 0.28 (0.69) | .9404 | 0.30 (0.81) | 0.32 (1.16) | .6387 |
| Number of office visits, mean (SD) | 24.69 (18.31) | 24.63 (19.26) | .8966 | 25.61 (19.64) | 24.24 (20.68) | .1302 |
| Number of office visits, diabetes related, mean (SD) | 6.72 (6.44) | 6.62 (6.92) | .5551 | 6.91 (6.65) | 7.09 (10.85) | .6671 |
| Number of endocrinologist visits, mean (SD) | 0.37 (1.33) | 0.33 (1.48) | .2141 | 0.73 (1.79) | 0.65 (1.68) | .3024 |
| Number of endocrinologist visits, diabetes related, mean (SD) | 0.35 (1.25) | 0.29 (1.15) | .062 | 0.66 (1.63) | 0.61 (1.61) | .5255 |
| Total hospitalization days, mean (SD) | 2.41 (6.90) | 2.78 (7.51) | .0496 | 2.69 (7.14) | 3.55 (9.52) | .0233 |
| Total hospitalization days, diabetes related, mean (SD) | 1.05 (4.15) | 1.08 (3.94) | .7347 | 1.24 (4.00) | 1.64 (5.55) | .0629 |
Abbreviation: ED, emergency department.
Figure 2a.
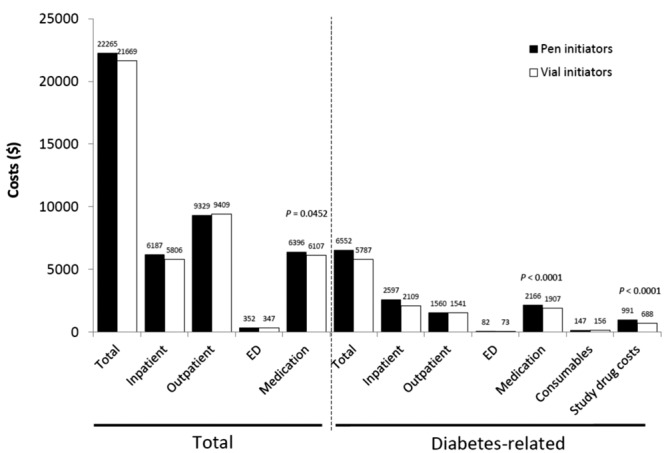
Health care costs associated with initiating treatment with insulin glargine by pen device or vial-and-syringe injections, 1-year follow-up of insulin-glargine-naïve patients. ED, emergency department.
Insulin-glargine-experienced Patients (Pen Switchers vs Vial Continuers)
Similar to the results among insulin-naïve patients, during the 1-year of follow-up, use of the disposable pen was associated with significantly better persistence and adherence than continued vial-and-syringe use (pen switchers vs vial continuers: treatment persistence 65.3% vs 56.8%, P < .0001; aMPR 0.82 vs 0.79, P = .0034; Table 2). Kaplan–Meier analysis showed that pen switchers were persistent for a longer period of time than vial continuers (mean persistence duration 304 days vs 282 days; P < .0001; Figure 1b); sensitivity analyses using 75th and 95th percentiles of the time yielded similar results. Although fewer patients in the pen switcher cohort experienced a hypoglycemic event than those in the vial continuer cohort, the difference in hypoglycemic event rates between the cohorts was not statistically significant (14.4% vs 16.1%; P = .2903; Table 2). However, pen switchers, compared with vial continuers, had significantly lower insulin DACON (42.2 U/day vs 47.8 U/day; P = .0201), significantly fewer all-cause hospitalizations (0.44 vs 0.52; P = .0423), and shorter duration of hospital stay (2.69 vs 3.55 days; P = .0233; Table 3). Total health care costs were similar between pen switchers and vial continuers ($24 211 vs $26 164, P = .2231); however, pen switchers had significantly lower diabetes-related hospitalization costs than vial continuers ($2252 vs $3318; P = .0377), despite higher diabetes medication costs ($3041 vs $2635; P < .0001; Figure 2b).
Figure 1b.
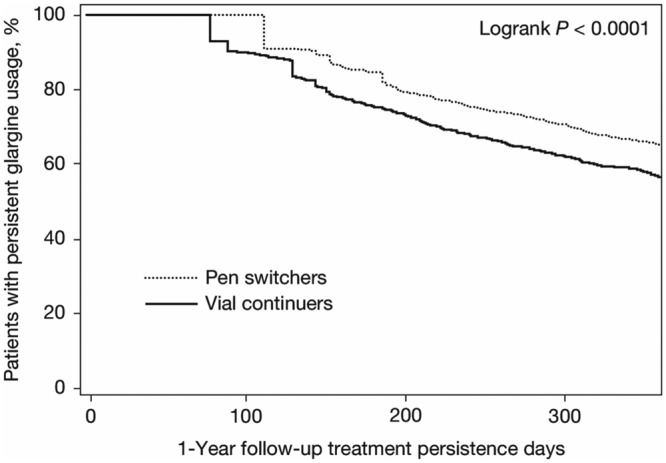
Kaplan–Meier curve for the time to treatment discontinuation for insulin-glargine-experienced patients.
Figure 2b.
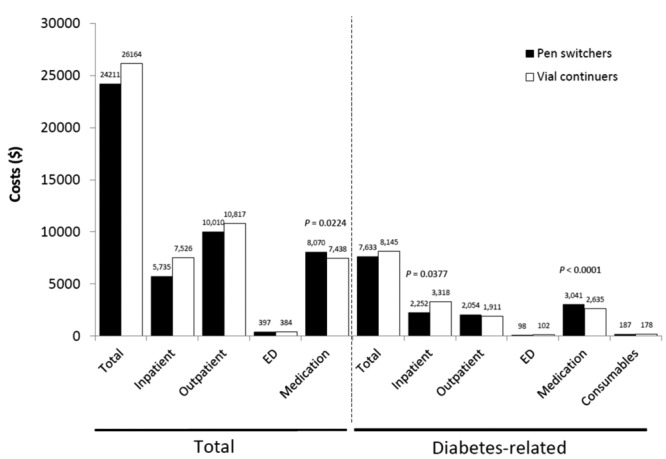
Health care costs associated with initiating treatment with insulin glargine by pen device or vial-and-syringe injections, 1-year follow-up of insulin-glargine-experienced patients. ED, emergency department.
Discussion
The American Diabetes Association (ADA) and American Geriatrics Society (AGS) recently published a consensus report in which it was concluded that most traditional randomized clinical trials excluded older patients—especially the frail elderly. This has resulted in a lack of knowledge of how to treat this group of patients.8 The successful management of T2DM in elderly patients represents a challenge, as this patient group has a high risk of premature death, functional disability, cognitive impairment, injurious falls, and serious comorbid illnesses such as CHF.5 In general, around 50% of elderly patients with T2DM do not achieve adequate glycemic control,6 and when glycemic goals are approached, the risk of hypoglycemia is increased compared to the general diabetes population due to age-related complications, including renal insufficiency, other comorbidities, polypharmacy, drug–drug interactions, irregular dietary habits, and infrequent self-monitoring of blood glucose.9 Frail elderly patients are especially likely to develop complications from hypoglycemia, such as dementia.30 Insulin remains a viable treatment option for many older patients and, as diabetes progresses with age, the majority of patients will likely require insulin to achieve adequate glycemic control. Regimens such as basal insulin glargine, which mimic the body’s natural insulin production, confer a relatively low risk of hypoglycemia.9 Moreover, administration of insulin glargine with a disposable pen offers an array of potential benefits compared with traditional vial-and-syringe methods that have yet to be fully explored in older adults with T2DM.15-22
This real-world study shows that among elderly patients with T2DM treated with insulin glargine, administration with a disposable pen, either as initial insulin treatment or after previous use of vial-and-syringe, offers benefits in terms of persistence and adherence to treatment compared to traditional vial-and-syringe administration. Such improvements may result in improved clinical and economic outcomes, with the present findings showing a reduction in all-cause and diabetes-related hospitalizations among pen initiators, and a reduction in all-cause hospitalizations and length of stay among pen switchers compared with vial-and-syringe initiators and continuers, respectively.
In addition, use of the disposable pen was associated with fewer hypoglycemic events than vial-and-syringe injections among those insulin-naïve patients who initiated insulin glargine. The overall reported hypoglycemia rate, however, was low in both study groups, and relates only to those events involving clinical encounters and captured in claims data. Therefore, one may question the clinical significance of the observed differences in the rates of hypoglycemia between groups. Among insulin-experienced patients, the rates of hypoglycemic events were similarly low in both groups. Other studies have similarly reported lower hypoglycemia rates for pen vs vial-and-syringe users.22,31 This could be due to greater convenience and ease of use of insulin pens, and an associated reduction in dosing errors.32 Schwartz et al. reported that 88% of patients found the insulin pen device to be more reliable in drawing and dispensing insulin, and there was also a significant reduction in administration time (P < .05) compared to vial-and-syringe injections.33 These factors may also explain the lower insulin DACON observed with pen use in the present study.
Consistent with previous published studies conducted in commercial populations,22,34 the use of the disposable pen, compared to vial-and-syringe, did not increase overall treatment costs, despite higher medication costs associated with the disposable pen. Conversely, the use of the disposable pen did not decrease costs despite lower insulin consumption, less hypoglycemia among insulin initiators, and improved treatment persistence and adherence. The combination of increased diabetes drug costs due to the higher acquisition costs of insulin pens compared with vial-and-syringe together with greater persistence and therefore longer duration of pen use may offset any cost benefit associated with pen use. Moreover, another study has shown that hypoglycemia-related costs account for only 0.75% of overall health care costs and 1.5% of diabetes-related health care costs.35 Collectively, these observations potentially explain why there was no significant difference in costs between pen and syringe-and-vial users.
Few studies have reported on the use of disposable insulin pens specifically in the elderly, but the available evidence lends support to the findings of the current study. In a systematic review of insulin therapy in elderly diabetes patients, Tanwani concluded that the ease of use of newer insulin delivery devices may be advantageous, and could facilitate insulin use in a population with a significant burden associated with poor visual function, impaired manual dexterity, poor functioning, and impaired cognition.36 Earlier, Coscelli et al. found that, in elderly patients, acceptability of a disposable pen for insulin delivery was high, and most patients preferred insulin administration by disposable pen compared with administration by conventional vial-and-syringe.11 Unlike the present study, they did not observe a reduction in hypoglycemia with the use of a disposable insulin pen.11 In 2 further US retrospective database studies in the general diabetes population, hypoglycemia risk was shown to be lower for patients using disposable pens than for patients using vial-and-syringe,37,38 but not in a third study.39 Recent pharmacoeconomic analyses of disposable pens also support their cost-effectiveness,40,41 particularly when used to initiate insulin therapy.39
The benefit of tight glycemic control in elderly patients has been questioned, and suitability should be established on a patient-by-patient basis;8 priority should be given to achieving the best quality of life possible.42 Due to its ease-of-use, insulin administration via disposable pen, instead of the traditional vial-and-syringe, may contribute to increased quality of living.
Study Limitations
As a retrospective observational analysis, this study has inherent limitations, such as an inability to establish causality between the treatment and difference in outcomes, as well as the potential for coding errors, and therefore the ability to accurately capture an individual’s claim history.43 In addition, the use of pharmacy claims data to measure adherence and persistence with treatment does not necessarily provide detailed information since a prescription claim does not confirm the drug was taken, or taken as prescribed. A further limitation is the lack of generalizability of the results of the analysis since the population of retirees with Medicare Supplemental insurance may not represent the US elderly population as a whole. Finally, data on A1C levels and weight were not available, and thus we were unable to assess 2 variables typically considered when undertaking any assessment of antidiabetic medication.
Conclusions
This real-world study showed that for elderly patients with T2DM, initiating insulin glargine treatment with a disposable pen or switching from vial-and-syringe to a pen device is associated with overall better treatment persistence and adherence, and among patients who initiated insulin, lower risk of hypoglycemia. In addition, overall health care costs during the first year of use are not increased by use of a disposable pen. These results may help to optimize T2DM management in elderly patients.
Acknowledgments
The authors received writing/editorial support in the preparation of this article from Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Footnotes
Abbreviations: ADA, American Diabetes Association; AGS, American Geriatrics Society; aMPR, adjusted medication possession ratio; CHF, congestive heart failure; DACON, daily average consumption; ED, emergency department; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; MPR, medication possession ratio; OADs, oral antidiabetic drugs; PI, pen initiators; PS, pen switchers; T2DM, type 2 diabetes mellitus; VC, vial continuers; VI, vial initiators.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RM and WW: employees of Sanofi US, Inc. JL: employee at Novosys Health, which received funding to carry out this work from Sanofi US, Inc. LX and OB: employees at STATinMed, which received funding to carry out this work from Sanofi US, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Sanofi US, Inc.
References
- 1. International Diabetes Foundation. Risk factors, 2011. www.idf.org/about-diabetes/risk-factors. Accessed January 6, 2012.
- 2. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed January 6, 2012.
- 3. Resnick HE, Heineman J, Stone R, Shorr RI. Diabetes in U.S. nursing homes, 2004. Diabetes Care. 2008;31(2):287-288. [DOI] [PubMed] [Google Scholar]
- 4. Russell LB, Valiyeva E, Roman SH, Pogach LM, Suh DC, Safford MM. Hospitalizations, nursing home admissions, and deaths attributable to diabetes. Diabetes Care. 2005;28(7):1611-1617. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suh D-C, Kim C-M, Choi I-S, Plauschinat CA. Comorbid conditions and glycemic control in elderly patients with type 2 diabetes mellitus, 1988 to 1994 to 1999 to 2004. J Am Geriatr Soc. 2008;56(3):484-492. [DOI] [PubMed] [Google Scholar]
- 7. Travis SS, Buchanan RJ, Wang S, Kim MS. Analyses of nursing home residents with diabetes at admission. J Am Med Dir Assoc. 2004;5:320-327. [PubMed] [Google Scholar]
- 8. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fravel MA, McDanel DL, Ross MB, Moores KG, Starry MJ. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68(6):500-559. [DOI] [PubMed] [Google Scholar]
- 10. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2008;31(1):173-175. [DOI] [PubMed] [Google Scholar]
- 11. Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173-177. [DOI] [PubMed] [Google Scholar]
- 12. Kadiri A, Chraibi A, Marouan F, et al. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract. 1998;41(1):15-23. [DOI] [PubMed] [Google Scholar]
- 13. Korytkowski M, Bell D, Jacobsen C, Suwannasari R, FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836-2848. [DOI] [PubMed] [Google Scholar]
- 14. Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila). 2004;43(1):69-74. [DOI] [PubMed] [Google Scholar]
- 15. Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27(suppl B):S89-S100. [DOI] [PubMed] [Google Scholar]
- 16. Brunton S. Initiating insulin therapy in type 2 diabetes: benefits of insulin analogs and insulin pens. Diabetes Technol Ther. 2008;10(4):247-256. [DOI] [PubMed] [Google Scholar]
- 17. Carter J, Beilin J, Morton A, De Luise M. Usability, participant acceptance, and safety of a prefilled insulin injection device in a 3-month observational survey in everyday clinical practice in Australia. J Diabetes Sci Technol. 2009;3(6):1425-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hancu N, Czupryniak L, Genestin E, Sourij H. A Pan-European and Canadian prospective survey to evaluate patient satisfaction with the SoloSTAR insulin injection device in type 1 and type 2 diabetes. J Diabetes Sci Technol. 2011;5(5):1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein HH. Pen devices to improve patient adherence with insulin therapy in type 2 diabetes. Postgrad Med. 2008;120(3):172-179. [DOI] [PubMed] [Google Scholar]
- 20. Marcus A. Diabetes care—insulin delivery in a changing world. Medscape J Med. 2008;10(5):120. [PMC free article] [PubMed] [Google Scholar]
- 21. Shaghouly AA, Shah BR. The prescription of insulin pen devices versus syringes for older people with diabetes. Diabetes Technol Ther. 2009;11(7):439-442. [DOI] [PubMed] [Google Scholar]
- 22. Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17(6):845-852. 10.4158/EP10401.OR [DOI] [PubMed] [Google Scholar]
- 23. Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28(11):1000-1011. 10.1007/s12325-011-0074-5 [DOI] [PubMed] [Google Scholar]
- 24. Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14(6):673-680. 10.3111/13696998.2011.605818 [DOI] [PubMed] [Google Scholar]
- 25. Levin P, Wei W, Wang L, Pan C, Douglas D, Baser O. Combination therapy with insulin glargine and exenatide: real-world outcomes in patients with type 2 diabetes. Curr Med Res Opin. 2012;28(3):439-446. 10.1185/03007995.2012.654850 [DOI] [PubMed] [Google Scholar]
- 26. Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15(3):230-236. [DOI] [PubMed] [Google Scholar]
- 27. Baser O, Bouchard J, DeLuzio T, Henk H, Aagren M. Assessment of adherence costs of insulin device (FlexPen®) versus conventional vial/syringe. Adv Ther. 2010;27(2):94-104. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35(5):1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. [Google Scholar]
- 30. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asche CV, Luo W, Aagren M. Differences in rates of hypoglycaemia and health care costs in patients treated with insulin aspart in pens versus vials. Curr Med Res Opin. 2013;29:1287-1296. [DOI] [PubMed] [Google Scholar]
- 32. Molife C, Lee LJ, Shi L, Sawhney M, Lenox SM. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther. 2009;11(8):529-538. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz S, Khutoryansky N, Braceras R. Comparison of resource utilisation, preference and handling of a pre-filled pen and vial-syringe in patients with type 2 diabetes mellitus. J Clin Res. 2007;10:1-10. [Google Scholar]
- 34. Lin J, Wei W, Vlajnic A, et al. Real-world practice pattern and outcomes of patients with type 2 diabetes (T2DM) initiating injectable therapy via insulin glargine disposable pen (GLA-P) or liraglutide (LIRA). Diabetes. 2012;61(suppl 1):A286. [Google Scholar]
- 35. Xie L, Wei W, Pan C, Baser O. Real-world rates, predictors, and associated costs of hypoglycemia among patients with type 2 diabetes mellitus treated with insulin glargine: results of a pooled analysis of six retrospective observational studies. J Med Econ. 2013;16(9):1137-1145. [DOI] [PubMed] [Google Scholar]
- 36. Tanwani LK. Insulin therapy in the elderly patient with diabetes. Am J Geriatr Pharmacother. 2011;9(1):24-36. [DOI] [PubMed] [Google Scholar]
- 37. Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948-962. [DOI] [PubMed] [Google Scholar]
- 38. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712-1725. [DOI] [PubMed] [Google Scholar]
- 39. Lee LJ, Li Q, Reynolds MW, Pawaskar MD, Corrigan SM. Comparison of utilization, cost, adherence, and hypoglycemia in patients with type 2 diabetes initiating rapid-acting insulin analog with prefilled pen versus vial/syringe. J Med Econ. 2011;14(1):75-86. [DOI] [PubMed] [Google Scholar]
- 40. Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12(suppl 1):S101-S108. [DOI] [PubMed] [Google Scholar]
- 41. Niskanen L. A clinical and health economic review of a prefilled insulin pen. Curr Med Res Opin. 2010;26(10):2431-2439. [DOI] [PubMed] [Google Scholar]
- 42. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295(16):1935-1940. [DOI] [PubMed] [Google Scholar]
- 43. Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27(9):1709-1717. [DOI] [PubMed] [Google Scholar]


