Abstract
Background:
Glucagon-like peptide-1 (GLP-1) receptor agonists are a relatively recent addition to the treatment options for type 2 diabetes mellitus (T2DM) and are administered using prefilled pen devices.
Method:
In this open-label task and interview-based pilot study, 3 GLP-1 receptor agonist pen devices—exenatide (Byetta®, Bristol-Myers Squibb/AstraZeneca), liraglutide (Victoza®, Novo Nordisk), and lixisenatide (Lyxumia®, Sanofi-Aventis)—were comparatively assessed in a randomized order in 30 participants with T2DM for ease of use, using a series of key performance measures (time taken to complete a series of tasks, number of user errors [successful performance], and user satisfaction rating). Linear and logistic regression analysis was conducted for the lixisenatide and liraglutide pens versus the exenatide pen. Participants’ mean age was 60 years; 27% and 20% of the participants had visual impairments and reduced manual dexterity, respectively.
Results:
Tasks were completed faster (P < .001) and with higher successful performance (P = .001) with the lixisenatide pen than with the exenatide pen, whereas the liraglutide pen was not statistically significant versus the exenatide pen on these parameters. Overall, user satisfaction was statistically higher for the lixisenatide and liraglutide pens versus the exenatide pen (P < .001 for both).
Conclusions:
Lixisenatide and liraglutide pens are associated with higher user satisfaction compared with the exenatide pen. In addition, the lixisenatide pen is faster and results in fewer errors than its comparator (exenatide). The lixisenatide pen may therefore be a suitable choice for patients with T2DM, including older and pen device-naïve patients, and those with visual impairments and reduced manual dexterity.
Keywords: ease of use, glucagon-like peptide-1 receptor agonists, lixisenatide, prefilled pen devices, type 2 diabetes mellitus
Glucagon-like peptide-1 (GLP-1) receptor agonists are a relatively new therapeutic option for the treatment of type 2 diabetes mellitus (T2DM). These agents are generally well tolerated and provide glycemic control and other clinical benefits.1,2 Aside from their efficacy in reducing glycated hemoglobin via effects on both fasting plasma glucose and postprandial plasma glucose, GLP-1 receptor agonists allow patients to achieve glycemic targets, with beneficial effects on weight being reported.1,3-6 One of the key benefits of GLP-1 receptor agonists is that they stimulate insulin secretion and inhibit glucagon secretion in a glucose-dependent manner, and therefore carry a limited risk of hypoglycemia.3,7 The GLP-1 receptor agonists currently available are exenatide (Byetta®, Bristol-Myers Squibb/AstraZeneca), administered as a twice-daily injection;8 liraglutide (Victoza®, Novo Nordisk), administered as a once-daily injection;9 exenatide (Bydureon®, Bristol-Myers Squibb/AstraZeneca), administered as a once-weekly injection; and lixisenatide (Lyxumia®, Sanofi-Aventis), a once-daily prandial GLP-1 receptor agonist.10 Twice-daily exenatide as well as once-daily liraglutide and once-daily lixisenatide are available as ready-to-use prefilled pen devices for subcutaneous injection. Exenatide is available in 2 different fixed-dose pens: a 5 µg pen and a 10 µg pen, with each prefilled pen designed to deliver a premeasured dose. Exenatide should be initiated at 5 µg per dose administered twice-daily for at least 1 month to improve tolerability; the dose can then be increased to 10 µg twice-daily to further improve glycemic control.11 Liraglutide is a multidose pen that delivers doses of 0.6 mg, 1.2 mg or 1.8 mg. Liraglutide should be initiated at 0.6 mg per day for 1 week; after 1 week the dose can then be increased to 1.2 mg, and if this does not result in acceptable glycemic control, the dose can be increased to 1.8 mg.12 Lixisenatide is available in 2 different fixed-dose pens: a 10 µg pen and a 20 µg pen, with each prefilled pen designed to deliver a premeasured dose. Lixisenatide is initiated at 10 µg once-daily for 14 days, with a fixed maintenance dose of 20 µg once-daily commencing on Day 15.13
Since their introduction in the 1980s, pen devices have been used to administer insulin and are preferred by many patients over traditional administration with a vial and syringe.14-16 Studies demonstrate that pen devices can increase treatment satisfaction and quality of life (QoL) in patients with T2DM,15-18 and provide more accurate and precise delivery of medication doses compared with a vial and syringe, both of which can help improve glycemic control and long-term outcomes.19-21 Ease of use is a particularly important feature of a pen device. In a recent questionnaire-based survey of more than 1000 patients with T2DM, conducted to evaluate which features of a medication injector device were most important for overall user satisfaction, ease of use was found to be the single most important feature.22 Among patients with diabetes, there is a high prevalence of visual impairment due to diabetes-associated retinopathy and maculopathy.23 Reduced manual dexterity, such as limited joint mobility syndrome, is often seen in these patients, and may limit finger or hand strength and the ability to inject medication without assistance.19,24-26 In such patients, the ease with which a pen device can be used without error is of particular importance. In addition, many patients with diabetes are older and may require more time to learn the various functions of a pen device, and ease of use is therefore also important in this population. Research studies on pen devices that include patients with manual and visual impairments, especially with devices that deliver GLP-1 receptor agonists, however, are limited.
Pen device technology is continuously evolving, and such devices are becoming easier to use, more convenient, more flexible, safer, and more socially acceptable.16,19,27,28 Until recently, pen devices had only been used to administer insulin. However, with the introduction of the injectable GLP-1 receptor agonists that are also available in prefilled pen devices, ease of use and satisfaction with device use will become even more important. Pen devices can have varying designs and features, which can impact on dosing accuracy and the ability of patients to easily use the devices. These include the ability to hear and feel clicks (tactile and auditory feedback), easy dialing and delivery of a dose, ease of performing the safety test and cartridge replacement, and overall ease of use. The primary objective of this study was to comparatively assess GLP-1 receptor agonist pen devices—the once-daily lixisenatide pen, with the once-daily liraglutide pen and the twice-daily exenatide pen—for ease of use using 3 important practical aspects.
Methods
Study Design
This was an open-label, task, and interview-based pilot study, conducted in Frankfurt, Germany, and London, United Kingdom. Key performance measurements that were used to assess the 3 pen devices were the time taken to complete a series of tasks, the number of user errors (successful performance), and user satisfaction ratings for each device. This study was not approved by the institutional review board. Following a risk assessment, it was identified that the study involved little or no risk to the patients recruited as the study made use of simulated tasks, and therefore did not require the participant to self-inject, and a moderator was present at all times to observe any potential needle stick injuries.
Participants
A specialist recruitment agency (WorldOne HealthCare Research) recruited participants for this study using a detailed recruitment screener document that clearly specified the participant profile for recruitment into the study and included a series of screening questions (Table 1). Research was conducted at a nonclinical research unit. Overall, 30 participants from Germany (n = 15) and the United Kingdom (n = 15) with T2DM were enrolled using a stratified sampling method, and all enrolled participants completed the study. Participants were GLP-1 receptor agonist-naïve subjects ≥18 years of age with T2DM for >1 year. The study aimed to enroll a total of 60% of participants >60 years old, 25% of participants had to have a visual impairment (self-reported) and 25% a form of manual dexterity impairment (self-reported), and an equal number of males and females. Two-thirds of participants had to be pen device-naïve.
Table 1.
Participant Screening Questionnaire.
| Question | Code | Route |
|---|---|---|
| QX—Firstly, do you or does anyone in your household work in any of the following industries? READ OUT | ||
| Pharmaceutical packaging and manufacture | 1 | |
| Advertising | 2 | |
| Marketing | 3 | |
| Market research | 4 | CLOSE |
| Journalism | 5 | |
| Public relations | 6 | |
| Pharmacy retailing | 7 | |
| Health care (nursing/doctor professions) | 8 | |
| None of these | 0 | QY |
| QY—Have you taken part in any market research in the last 6 months? | ||
| Yes | 1 | CLOSE |
| No | 2 | Q1 |
| Q1—Record sex (do not ask) | ||
| Male | 1 | See quotas |
| Female | 2 | |
| Q2—Could you please tell me which of these bands your age falls into? | ||
| Under 18 | 1 | CLOSE |
| 18-39 | 2 | |
| 40-49 | 3 | See quotas |
| 50-59 | 4 | |
| 60-69 | 5 | |
| Over 70 | 6 | |
| Q3a—Have you ever been diagnosed (by a doctor) with having Diabetes? | ||
| Yes diabetes type 2 | 1 | Q3b |
| Yes diabetes type 1 | 2 | CLOSE |
| No | 3 | CLOSE |
| Q3b—Only if answer was yes to diabetes type 2 | ||
| I am now going to ask you a few questions about the treatment you have been prescribedfor Diabetes. Firstly, what sort of medication have you been prescribed for this condition? | ||
| Injection of insulin | 1 | |
| Oral (eg, tablets) or other | 2 | See quotas |
| Injection of GLP-1 (exenatide or liraglutide) | 3 | CLOSE |
| None of the above | 4 | CLOSE |
| Q3c—Please state the name of the medication you take for this condition: | ||
| Q4—Do you have arthritis in your hands? | ||
| Yes | 1 | See quotas |
| No | 2 | |
| Q5—Do you have a visual impairment (ie, partial blindness due to cataracts, macular degeneration, or glaucoma)? | ||
| Yes | 1 | See quotas |
| No | 2 | |
| Q6 −The research may be filmed or recorded for internal client use only, are you OK with this? | ||
| Yes | 1 | Recruit |
| No | 2 | CLOSE |
Assessments
Assessments were conducted in January 2012 at outpatient clinics by independent moderators from a research agency (DCA Design International, UK; 22030425 and WorldOne, Germany) in line with the study protocol. Each assessment took 90 minutes to complete. To ensure consistency, task assessment was conducted by 1 moderator after study completion using video data and data gathered from study observations (real time via a note taker). The order in which participants tested each device was randomized using a 3 × 3 Latin squares design to avoid bias from user learning. Participants received the manufacturer’s country-specific patient instruction leaflet for each device (prospective leaflet for lixisenatide pen device). Patients were not given formal training on the use of the pen devices. All participants were asked to consecutively demonstrate the use of each of the 3 pens by injecting them into a simulator pad. To measure successful performance, participants carried out a series of tasks with each pen: priming the pen, administering a first dose into a simulator injection pad (without priming), administering a second dose into a simulator injection pad (without priming), determining the remaining dose level in the pen, and identifying when the pen was empty. The number of participants who completed each task without error (successful performance) and the time taken to complete tasks were recorded in real time by direct observation by a researcher (DCA Design International) using a digital stopwatch (Tables 2 and 3).
Table 2.
Summary of User-Based Trial and Time Taken to Complete Tasks.
| Trial Phase | Activity | Specific Activity | Expected Duration (min) |
|---|---|---|---|
| Introduction | Participant registration and briefing | General introduction, consent and confidentiality forms, and overview of trial agenda | 5 |
| Explanation of therapy | Introduction | Overview of therapy | 4 |
| Read device instructions | Participant to read device instructions prior to use-error exercise. | 11 | |
| Use-error exercise with first devicea | Task 1: Attach needle. Prepare device ready for use (no injections). | 2 | |
| Task 2: First dose. Primes device into cup, injects first dose into pad. | 4 | ||
| Task 3: Second dose. Inject second dose into pad. | 2 | ||
| Task 4: Remaining dose level question. State remaining dose level. | 1 | ||
| Task 5: Another dose. Empty cartridge in device. | 2 | ||
| Device ratings | Rate each device (5 min approximately per device) using statements and a 7-point Likert scale. | 15 | |
| Total time to complete exercise with all 3 devices | 90 | ||
Process was repeated with second and third devices.
Table 3.
Use-Error Exercise.
| Expected Behavior | Task Measures (use-errors) | |
|---|---|---|
| Attach needle (2 min) | • Remove the cap • Correctly fit the needle |
• Needle not attached • Needle not attached correctly/securely • User experiences a needle stick injury |
| First dose (4 min) | • Primes the device into the cup (ie, not into the injection pad) • Injects the correct dose into the injection pad |
• Does not correctly set the dose (exenatide and lixisenatide)a
• Does not set the correct dose (liraglutide)b • Primes into the injection pad (instead of into the cup) • Does not fully inject the dose (either priming dose or first dose) • Does not hold down the injection button for the required time (either priming dose or first dose) • Injects the first dose into the cup, instead of injection pad |
| Second dose (2 min) | • Injects the correct dose into the injection pad | • Does not correctly set the dose (exenatide and lixisenatide)a
• Does not set the correct dose (liraglutide)b • Primes the device again • Does not fully inject the dose • Does not hold down the injection button for the required time • Injects the second dose into the cup, instead of into the injection pad |
| Remaining dose level (1 min) | • States correct dose level | • States the incorrect number of doses remaining • Cannot determine the number of doses remaining |
| Another dose (empty cartridge in device) (2 min) | • States that the device is “empty” and that a new device will be needed for the next injection | • Attempts to deliver a dose and incorrectly believes that they have been successful (exenatide and lixisenatide devices)a
• Delivers an incorrect dose (liraglutide device: ie, less than 1.2 mg)b |
Exenatide and lixisenatide are given in a fixed dose.
Liraglutide can be given at a dose of 0.6 mg (starting dose), 1.2 mg, or 1.8 mg.
Participants were asked to provide their own assessment of the overall usability of the 3 pen devices based on a number of questions using a 7-point Likert-type scale (1 = very unsatisfied, 7 = very satisfied).29 User satisfaction measurements were gathered on the following aspects: comfort in using the device, ease of use when priming the device, effectiveness in the feedback when a dose has been delivered, indication of the number of doses remaining, indication that the device is empty/reached the end of its life, and overall experience with the device (Figure 1). Data were recorded and collated in real time by a researcher (DCA Design International).
Figure 1.
Participant assessment of the overall usability of the 3 pen devices based on questions using a 7-point scale.
Statistical Analyses
Statistical analyses were conducted on the 3 main outcome variables: time taken to complete the tasks, successful completion of the tasks, and user satisfaction ratings. The lixisenatide pen and the liraglutide pen were compared with the exenatide pen (reference pen).
Time taken to complete the tasks was derived using random effects linear regression of the total time (seconds) taken to complete all 4 tasks, and represents the difference in time for the comparator pens and the reference pen. For successful performance, a binary variable for overall success was derived, taking an overall score of at least 3 out of 4 successful tasks (administration of first and second doses, determining the remaining dose level in the pen and identifying an empty pen) as overall success. Odds ratios (ORs) were derived using random effects logistical regression. For the user satisfaction rating, random effects linear regression was conducted using the sum of the scores (42 points) for all 6 questions, and refers to the difference in score between the comparator pens and reference pen. The intraclass correlation coefficient and the Akaike information criterion were determined for the random effects logistic regression model.
Results
Patient Characteristics
A total of 19 (63%) participants self-reported that they had never before used an injection device. Nine patients (30%) had experience with injecting insulin. In total, 8 participants (26%) had a visual impairment (partial blindness due to cataracts, macular degeneration, or glaucoma) and 6 participants (20%) had impairment in manual dexterity (arthritis in the hands). Impairments were also self-reported by the participants (Table 4)
Table 4.
Patient Characteristics.
| Patient Characteristic | N = 30a |
|---|---|
| Mean age, years (range) | 60 (31-81) |
| Number of participants with T2DM diagnosis of >1 year, n (%) | 30 (100) |
| Male, n (%) | 15 (50) |
| Diabetes treatment, n (%) | |
| OAD | 19 (63) |
| Insulin | 9 (30) |
| Insulin + OAD | 2 (7) |
| Injection device naïve, n (%) | 19 (63) |
| Visual impairment (%), n (%)b | 8 (27) |
| Impaired manual dexterity, n (%)c | 6 (20) |
OAD, oral antidiabetic drug; T2DM, type 2 diabetes mellitus.
15 patients from Germany and 15 patients from the United Kingdom.
Partial blindness due to cataracts, macular degeneration, or glaucoma.
Arthritis in the hands.
Successful Performance
In administering a first dose, a second dose, and assessing the remaining dose, a high proportion of participants (83%) were able to complete all 3 tasks without error using the lixisenatide pen. Lower proportions of patients were able to complete these tasks without error with the exenatide and liraglutide pens (43%, 77%, 0%, and 60%, 57%, 10%, for the exenatide and liraglutide pens, respectively; Figure 2). No participants were able to assess a remaining dose without error with the exenatide pen, and 3 participants were able to do so with the liraglutide pen. Almost all participants were able to identify an empty device with all the pens (97% of participants for all 3 pens). Regression analysis of successful performance (using successful completion of at least 3 out of 4 tasks) produced an OR of 11.27 for the lixisenatide pen versus the exenatide pen (P = .001). Successful performance with the liraglutide pen versus the exenatide pen was not significant (Table 5). The intraclass correlation coefficient and Akaike information criterion for the random effects logistic regression model were .24 and 114.7, respectively.
Figure 2.
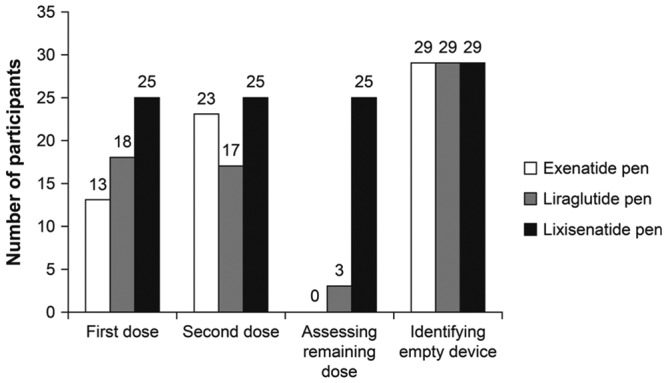
Total number of participants who completed the tasks error free (n = 30 participants).
Table 5.
Regression Analysis for Performance, Task Time, and Overall User Satisfaction of Lixisenatide and Liraglutide Pens Versus the Exenatide Pen.
| Successful Performancea | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Liraglutide pen | 0.76 | 0.23, 2.48 | ns |
| Lixisenatide pen | 11.27 | 2.53, 50.16 | .001 |
| Task Time (sec)b | Time Difference | 95% CI | P Value |
| Liraglutide pen | −55 | −123.8, 14.8 | ns |
| Lixisenatide pen | −177 | −246.3, −107.7 | < .001 |
| Overall User Satisfactionc | Score Difference | 95% CI | P Value |
| Liraglutide pen | 4.6 | 2.1, 7.1 | < .001 |
| Lixisenatide pen | 8.1 | 5.6, 10.6 | < .001 |
CI, confidence interval; ns, not significant.
Successful completion of at least 3 out of 4 tasks.
Difference in time taken to complete all 4 tasks.
Difference in total score (total score = 42) between comparator pen and reference pen.
Task Time
Time taken to complete the first task (administering a first dose) was considerably higher for the exenatide pen than for the other 2 pens (3.39 vs 2.18 minutes and 2.20 minutes for the exenatide, liraglutide, and lixisenatide pens, respectively; Figure 3). Participants also took longer to complete the remaining tasks (administering the second dose, assessing the remaining dose, and identifying an empty device) with the exenatide pen than with the lixisenatide and liraglutide pens. With these tasks, participants took a shorter time with the lixisenatide pen than with the other 2 pens (Figure 3). Time taken to complete all 4 tasks was significantly lower for the lixisenatide pen versus the exenatide pen (time difference: –177 seconds; P < .001), but was not significantly lower for the liraglutide pen versus the exenatide pen (Table 5).
Figure 3.
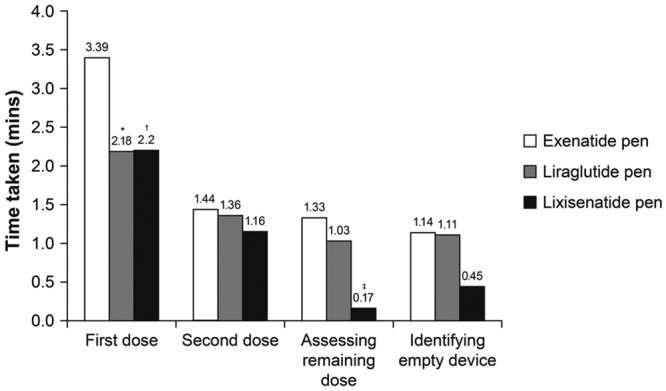
Time taken to complete each task (n = 30 participants). *P < .01 vs exenatide; † P = .001 vs exenatide; ‡ P < .001 vs exenatide.
Overall User Satisfaction
For delivered dose feedback, indication of the remaining dose and indication of an empty device, the lixisenatide pen was given a higher satisfaction rating by the participants compared with the rating given for the liraglutide and exenatide pens (Figure 4). For device comfort and priming the pen, the lixisenatide and liraglutide pens were rated equally high versus exenatide. For overall user experience, participants rated the lixisenatide pen higher than the other 2 pens (scores of 5.8, 4.4, and 5.3 for lixisenatide, exenatide, and liraglutide pens, respectively; Figure 4). Overall user satisfaction score was significantly higher for the lixisenatide pen (score difference 8.1) and the liraglutide pen (score difference 4.6) versus the exenatide pen (P < .001 for both; Table 4).
Figure 4.
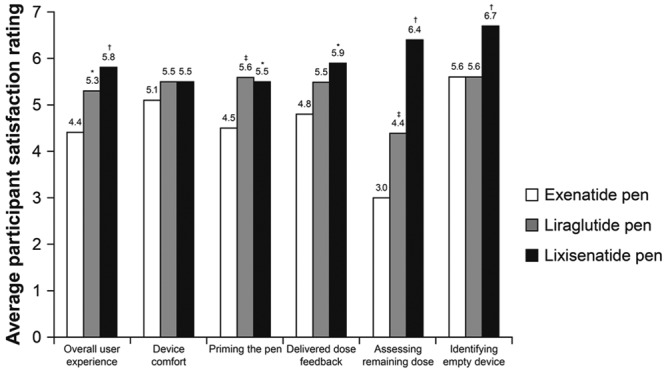
User satisfaction rating of the 3 pen devices (n = 30 participants). *P < .01 vs exenatide; † P < .001 vs exenatide; ‡ P = .001 vs exenatide.
Discussion
Owing to their many advantages over traditional syringes and needles, pen devices have become common for the administration of insulin, and are also being used for administration of the GLP-1 receptor agonists, lixisenatide, exenatide, and liraglutide. Studies of insulin pen devices have shown that accuracy, ease of use, and patient satisfaction are among the most important factors for achieving glycemic control and patient compliance, and thus long-term beneficial outcomes.18,21,22 Real-world studies are a necessary and important aspect of pen device development, and provide assurance to patients and health care professionals regarding the usability of the device in a real-world situation. In this pilot study in participants with T2DM, we comparatively assessed 3 GLP-1 receptor agonist pen devices for ease of use, using time taken to use the device, successful performance, and user satisfaction.
In this study, more than half the study population was pen device-naïve, and none of the participants received any formal training with the use of pen devices. This is an important aspect of the present study, as it demonstrates that accurate use of a pen device can be enhanced through well-designed instructions and simple and intuitive device design that may remove some of the need for costly and time-consuming training by health care providers. However, in this study no comparisons were made between device-naïve and pen experienced patients in terms of overall ease of use.
Participants enrolled in this study had a mean age of 60 years, and 27% and 20% had visual impairments and reduced manual dexterity, respectively. Visual impairments and reduced manual dexterity are frequently found in people with T2DM, especially among the more elderly population, and it is important that pen devices can be accurately and easily used by this population. Studies that include participants with impaired vision and reduced manual dexterity are limited, but recent research with insulin pen devices showed that visual impairments and reduced manual dexterity in patients with T2DM can influence patient preference.24
Pen devices that can be used with accuracy are important tools that contribute to safety in diabetes treatment. In this study, participants made fewer errors with the lixisenatide pen device than with the other 2 pen devices, with significantly higher successful performance versus the exenatide pen (P = .001). Performance difference was especially apparent when assessing remaining dose. It should be noted that, unlike insulin pens, pens for the administration of GLP-1 receptor agonists do not need to be primed before every use, so results for pen priming were therefore not included. The speed and ease of use with which a pen device can administer medication is an important factor that impacts QoL. Efficient administration can help to reduce the stigma of diabetes, and allows more discreet use in public places. In this study, the majority of tasks that influenced ease of use (administering a second dose, assessing the remaining dose level, and identifying an empty device) were completed faster with the lixisenatide pen than with the liraglutide and exenatide pen devices; in addition, overall time (ie, completion of all 4 tasks) was significantly faster with the lixisenatide pen versus the exenatide pen (P < .001). For administering a first dose, participants required >1 minute longer with the exenatide pen than with the liraglutide pen or the lixisenatide pen. Noteworthy differences between the lixisenatide pen and the other pens were seen in assessing the remaining dose level, with a difference of >1 minute between the lixisenatide and exenatide pens. In patients with T2DM, satisfaction with treatment has been demonstrated to enhance compliance, give patients a sense of control over their illness, improve QoL, and give a greater feeling of well-being.30-33 In our study, the lixisenatide pen achieved the highest overall user rating in the participant satisfaction evaluation, consistently achieving high participant scores for all the features evaluated. This provides reassurance that this pen device can provide high satisfaction for patients, including older individuals and those with impaired manual dexterity or vision.
Although intended to be a pilot study, this study has some limitations that should be considered when interpreting the results. The study was open-label, and therefore carries an intrinsic risk of bias. In addition, user feedback with a scale rating may not be the most comprehensive means of evaluation: verbal discussion or comparative rating scales may be more effective. The sample size was small, which may have been a determining factor in the large confidence intervals observed in this trial. In addition, the small sample size did not permit subgroup analyses based on country, insulin-naïve and -experienced users, and visual and dexterity disabilities to be performed. However, this was a pilot study and a large scale study is planned in the future. Although this study included pen device-naïve patients, and those with visual impairments and reduced manual dexterity, no multivariate analyses were performed to assess whether these subgroups had differences in the outcomes assessed and to ascertain which device is the most beneficial for those with these impairments. The fact that visual impairments and reduced manual dexterity were self-reported may also be regarded as a limitation of this study. However, a full study would be designed to screen for such impairments through prestudy assessments. Finally, although not a study limitation, it would be of interest to assess whether twice-daily dosing with exenatide influenced ease of use and to compare the 3 pen devices with exenatide extended-release injection.
Conclusions
Use of the lixisenatide or the liraglutide pen was found to be associated with higher user satisfaction compared with the exenatide pen. In addition, the lixisenatide pen was faster to use and resulted in fewer user errors than its comparator (the exenatide pen), and may be a suitable choice for patients with T2DM, including older and pen device-naïve patients, and those with visual impairments or reduced manual dexterity. Larger-scale, prospective, randomized assessments of these 3 devices involving larger numbers of participants and across a wider range of countries should be conducted.
Acknowledgments
Medical writing assistance was provided by Frances Gambling, Medicus International, London, UK, and was supported by Sanofi-Aventis.
Footnotes
Abbreviations: CI, confidence interval; GLP-1, glucagon-like peptide-1; NS, not significant; OAD, oral antidiabetic drug; OR, odds ratio; QoL, quality of life; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Udo Stauder is an employee of Sanofi-Aventis. Hina Elton is an employee of DCA Design International, which received funding from Sanofi-Aventis. Alfred Penfornis has received fees for consultancy, advisory boards, speaking, travel, or accommodation from Lilly, Novo-Nordisk, and Sanofi-Aventis. Steve Edelman has received consultation fees from Animas Corporation, LifeScan, Inc, DexCom, and Sanofi-Aventis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This pilot study was funded by Sanofi-Aventis (Germany).
References
- 1. Gallwitz B. Preclinical and clinical data on extraglycemic effects of GLP-1 receptor agonists. Rev Diabet Stud. 2009;6(4):247-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lund A, Knop FK, Vilsboll T. Emerging GLP-1 receptor agonists. Expert Opin Emerg Drugs. 2011;16(4):607-618. [DOI] [PubMed] [Google Scholar]
- 3. Unger JR, Parkin CG. Glucagon-like peptide-1 (GLP-1) receptor agonists: differentiating the new medications. Diabetes Ther. 2011;2(1):29-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628-2635. [DOI] [PubMed] [Google Scholar]
- 5. Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, Hanefeld M. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2013. [EPub ahead of print] 10.1111/dme.12328. [DOI] [PubMed] [Google Scholar]
- 6. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47. [DOI] [PubMed] [Google Scholar]
- 7. White J. Efficacy and safety of incretin based therapies: clinical trial data. J Am Pharm Assoc. 2009;49(suppl 1):S30-S40. [DOI] [PubMed] [Google Scholar]
- 8. Bray GM. Exenatide. Am J Health Syst Pharm. 2006;63(5):411-418. [DOI] [PubMed] [Google Scholar]
- 9. Elbrønd B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25(8):1398-1404. [DOI] [PubMed] [Google Scholar]
- 10. Barnett A. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evidence. 2011;6:67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byetta® (exenatide) prescribing information. Available at: http://documents.byetta.com/Byetta_PI.pdf.
- 12. Victoza® (liraglutide) prescribing information. Available at: http://www.novo-pi.com/victoza.pdf.
- 13. Lyxumia® (lixisenatide) summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/27405/SPC/Lyxumia+10+micrograms+solution+for+injection.
- 14. Fischer JS, Edelman SV, Schwartz SL. United States patient preference and usability for the new disposable insulin device Solostar versus other disposable pens. J Diabetes Sci Technol. 2008;2(6):1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498-1505. [DOI] [PubMed] [Google Scholar]
- 16. Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173-177. [DOI] [PubMed] [Google Scholar]
- 17. Hornquist JO, Wikby A, Andersson PO, Dufva AM. Insulin-pen treatment, quality of life and metabolic control: retrospective intra-group evaluations. Diabetes Res Clin Pract. 1990;10(3):221-230. [DOI] [PubMed] [Google Scholar]
- 18. Lee IT, Liu HC, Liau YJ, Lee WJ, Huang CN, Sheu WH. Improvement in health-related quality of life, independent of fasting glucose concentration, via insulin pen device in diabetic patients. J Eval Clin Pract. 2009;15(4):699-703. [DOI] [PubMed] [Google Scholar]
- 19. Penfornis A. Performance of a new reusable insulin pen. Diabetes Technol Ther. 2011;13(3):373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila). 2004;43(1):69-74. [DOI] [PubMed] [Google Scholar]
- 21. Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med. 1999;106(5):57-58, 61,-64, 68. [DOI] [PubMed] [Google Scholar]
- 22. Toscano D, Brice J, Alfaro C. Usage and perceptions of pen injectors for diabetes management: a survey of type 2 diabetes patients in the United States. J Diabetes Sci Technol. 2012;6(3):686-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidson JA, Ciulla TA, McGill JB, Kles KA, Anderson PW. How the diabetic eye loses vision. Endocrine. 2007;32(1):107-116. [DOI] [PubMed] [Google Scholar]
- 24. Pfützner J, Hellhammer J, Musholt P, et al. Evaluation of dexterity in insulin-treated patients with type 1 and type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(1):158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aljahlan M, Lee KC, Toth E. Limited joint mobility in diabetes. Postgrad Med. 1999;105(2):99-101, 105-106. [DOI] [PubMed] [Google Scholar]
- 26. Otto-Buczkowska E, Jarosz-Chobot P. Limited joint mobility syndrome in patients with diabetes. Int J Clin Pract. 2012;66(4):332-333. [DOI] [PubMed] [Google Scholar]
- 27. D’Eliseo P, Blaauw J, Milicevic Z, Wyatt J, Ignaut DA, Malone JK. Patient acceptability of a new 3.0 ml pre-filled insulin pen. Curr Med Res Opin. 2000;16(2):125-133. [PubMed] [Google Scholar]
- 28. Haak T, Edelman S, Walter C, Lecointre B, Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29(4):650-660. [DOI] [PubMed] [Google Scholar]
- 29. Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140(55):5-55. [Google Scholar]
- 30. Saatci E, Tahmiscioglu G, Bozdemir N, Akpinar E, Ozcan S, Kurdak H. The well-being and treatment satisfaction of diabetic patients in primary care. Health Qual Life Outcomes. 2010;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jamous RM, Sweileh WM, Abu-Taha AS, Sawalha AF, Zyoud SH, Morisky DE. Adherence and satisfaction with oral hypoglycemic medications: a pilot study in Palestine. Int J Clin Pharm. 2011;33(6):942-948. [DOI] [PubMed] [Google Scholar]
- 32. Biderman A, Noff E, Harris SB, Friedman N, Levy A. Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract. 2009;26(2):102-108. [DOI] [PubMed] [Google Scholar]
- 33. Nicolucci A, Cucinotta D, Squatrito S, et al. Clinical and socio-economic correlates of quality of life and treatment satisfaction in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2009;19(1):45-53. [DOI] [PubMed] [Google Scholar]