Abstract
To illuminate candidate neural working mechanisms of Mindfulness-Based Cognitive Therapy (MBCT) in the treatment of recurrent depressive disorder, parallel to the potential interplays between modulations in electro-cortical dynamics and depressive symptom severity and self-compassionate experience. Linear and nonlinear α and γ EEG oscillatory dynamics were examined concomitant to an affective Go/NoGo paradigm, pre-to-post MBCT or natural wait-list, in 51 recurrent depressive patients. Specific EEG variables investigated were; (1) induced event-related (de-) synchronisation (ERD/ERS), (2) evoked power, and (3) inter-/intra-hemispheric coherence. Secondary clinical measures included depressive severity and experiences of self-compassion. MBCT significantly downregulated α and γ power, reflecting increased cortical excitability. Enhanced α-desynchronisation/ERD was observed for negative material opposed to attenuated α-ERD towards positively valenced stimuli, suggesting activation of neural networks usually hypoactive in depression, related to positive emotion regulation. MBCT-related increase in left-intra-hemispheric α-coherence of the fronto-parietal circuit aligned with these synchronisation dynamics. Ameliorated depressive severity and increased self-compassionate experience pre-to-post MBCT correlated with α-ERD change. The multi-dimensional neural mechanisms of MBCT pertain to task-specific linear and non-linear neural synchronisation and connectivity network dynamics. We propose MBCT-related modulations in differing cortical oscillatory bands have discrete excitatory (enacting positive emotionality) and inhibitory (disengaging from negative material) effects, where mediation in the α and γ bands relates to the former.
Keywords: Major Depressive Disorder (MDD), Mindfulness-Based Cognitive Therapy (MBCT), ERD/ERS, Oscillatory EEG, α-Band coherence, γ-Band, EEG power
Introduction
Despite accumulating evidence for the efficacy and versatility of Mindfulness-Based Cognitive Therapy (MBCT) for the treatment of current depression (Kenny and Williams 2007; van Aalderen et al. 2012), anxiety-based disorders (Evans et al. 2008; Kim et al. 2009; King et al. 2013), attention-deficit/hyperactivity disorders (Zylowska et al. 2008), borderline personality disorder (Sachse et al. 2010), and reducing risk of depressive relapse (Teasdale et al. 2000; Ma and Teasdale 2004); conclusive neural working mechanisms remain obscure. The MBCT programme entails weekly group training alongside daily individual practise, incorporating cognitive behavioural and mindfulness meditation techniques (e.g. Segal et al. 2012).
Theoretically MBCT; (1) trains attention to enable patients to gain refined insight into their negative cycles and self-defeating thoughts/feelings; (2) trains the cultivation of self-compassion and acceptance towards these distressing ‘negativity networks’ (Feldman and Kuyken 2011; Kuyken et al. 2010). The fine-tuning of attention towards insight, alongside the manifestation of greater empathy (towards self and others), equanimity (relinquishing the need for absolute control and certainty outside oneself), and patience (knowing ‘this too shall pass’, that all experience is transitory), function interdependently facilitating a beneficial and self-directive process of change.
Practically, patients learn to distinguish between “doing” versus “being” modes. The former defining a pattern of experiencing that aims to reduce the discrepancy between how one wants things to be compared to how they actually are (Segal et al. 2012). This processing mode can be selectively useful, albeit when maladaptive, perpetuates and maintains feelings of dissatisfaction and/or avoidance behaviours, contributing to depressive symptoms. Alternatively, during negative experiences, patients can learn flexibility so to disengage from ‘doing mode’ (where thoughts are experienced as facts) into an experiential state of “being”, i.e. within the experience which has no space for cognitive evaluation and judgement—it simply is—thus, putting the brakes on the process that incites distressing experiences to cycle and escalate.
Neurophysiologically, there are plausibly multiple substrates of these action pathways. Attentional control has gained the most empirical examination to date (Grant et al. 2013; Malinowski 2013; Lutz et al. 2008), such as alpha (α) activity and enhanced somatosensory and internalized attention processing (Kerr et al. 2011, 2013; Aftanas and Golocheikine 2001). Likewise, increases in α-power (Travis et al. 2010) and coherence (Travis et al. 2010) have been linked to focused attention meditation which inhibits self-referential processing and mind wandering (Travis and Shear 2010), precursors to rumination in depression (Hamilton et al. 2011). Earlier conceptions emphasise alpha as a measure of ‘cortical idling’ (review; Pfurtscheller et al. 1996), and global arousal (Cantero et al. 1999). However, progress in study design elegance and signal processing techniques mean various aspects of the electro-cortical signal can be extracted to potentially relay discrete neurophysiological dimensions, further revealing the multi-levelled significance of α-band cortical activity. For example, task-related paradigms suggest modulations in alpha (α) correlate with aspects of selective attention regulation (Rihs et al. 2007), and top-down inhibitory control (Klimesch et al. 2007).
Gamma (γ) activity (30–50+ Hz range) connects to the second more subtle, and arguably more complex, component of MBCT; the cultivation of abstract experiences such as compassion and acceptance. For example, elevated fronto-parietal γ-power and coherence during nonreferential compassion meditative states have been reported in long-term (10,000–15,000 h/15–40 years) focused concentration meditators (Lutz et al. 2004), and during altered experiences of dissolution and reconstitution of the ‘self’ (Lehmann et al. 2001). Increased parietal-occipital γ-power (35–45 Hz) has also been reported during Vipassana meditation, i.e. open monitoring mindfulness emphasising non-judgemental awareness, compared to no modulating effects upon theta (θ: 4–8 Hz), α (8–12 Hz) or beta (β: 12–25 Hz) activity (Cahn et al. 2010). Thus, predominant γ-band oscillatory activity appears to be involved in the conscious manifestation of less ego-centric states and experiences in non-clinical healthy populations undergoing both fixed-point/focused and open-monitoring/mindfulness meditation techniques.
Clinically, α and γ activity appear implicated in the emotion processing of negative material in depression. Increased event-related γ-power to negative stimuli (words) has been observed in depressed patients compared to controls (Siegle et al. 2010). Namely, enhanced γ-power localised in frontal distributions for a sustained temporal period (up to 8 s) following negative stimuli presentation, suggesting prolonged and greater elaborative processing to negatively valenced emotive cues. Furthermore, hemispheric hypofrontal α-asymmetry appears a relatively replicated finding (albeit some exceptions; Reid et al. 1998; Carvalho et al. 2011; Segrave et al. 2011) in currently depressed or euthymic mood with former history of depression (Stewart et al. 2010; Allen et al. 2004; Gotlib et al. 1998) suggesting a plausible neurophysiological phenotype of depression, least a mediator of experienced emotion (for a comprehensive review, see Coan and Allen 2004).
Presently, there is minimal EEG research related to mindfulness, and that available, predominantly concentrates on cortical asymmetry/lateralization. Enhanced relative left prefrontal α-activation following mindfulness meditation has been reported in a small group (N = 8) of previously depressed people, more marked in high ruminative brooding scorers (Barnhofer et al. 2010). Alternatively, no change in resting-state α-asymmetry has been reported in a larger remitted depressed sample (N = 78) pre-to-post MBCT (Keune et al. 2011), whereby patients at greater risk of relapse even showed increased right-hemisphere α-activity, i.e. propensity towards negative emotionality, following MBCT exposure. Thus, suggesting severer depression counteracts any potential therapeutic effects of MBCT. Furthermore, resting state frontal EEG α-asymmetry shows no correlation with trait mindfulness scores (Keune et al. 2012). Rather than focusing on EEG asymmetry, examining other EEG parameters in relation to mindfulness may be more illuminating. For example, an event-related potential (ERP) study reported increased late Contingent Negative Variation (CNV) ERP amplitudes in depressed patients pre-to-post MBCT (Bostanov et al. 2012), suggesting patients’ ability to maintain focus on the present moment, which would counteract ruminative attention/processes, ensued.
In sum, enhanced oscillatory activity denotes sustained cognitive effort and elaborative processing. Due to the inherent emotional and attentional biases associated with depression (Foland-Ross et al. 2013; Watters and Williams 2011), this pattern of enhanced cortical activity appears specific to negatively valenced material in such patients.
Aims of study
In light of the nebulous findings above, we examined the potential modulating effects of an 8-week MBCT programme upon event-related EEG variables, depressive severity and self-compassionate experience in MDD. The brain represents a highly open and complex dynamic system. Within this theoretical framework, the brain state space comprises both linear and nonlinear properties, granting multi-dimensional potentiality; the dimensions of which can be confined to self-organising ‘attractor’ states evoked to incoming stimuli (i.e. task-related). Such linear states operate concomitant to task-independent nonlinear states that represent underlying multi-levelled cortical dynamics. Thus, in an attempt to encompass both linear and nonlinear cortical dynamics we examined (1) evoked EEG power, (2) induced event-related (de)-synchronisation (ERD/ERS) dynamics, and (3) EEG coherence, within the α (8–12 Hz) and γ (30–45 Hz) bandwidths. Linear dynamics examining cortical changes associated with task-related functionality during an affective Go/NoGo (valenced words) task were ascertained via power and inter-/intra-hemispheric EEG coherence. We hypothesised MBCT would increase α and γ power and coherence for positively valenced stimuli, with the opposite pattern for negatively valenced stimuli, based on studies outlining increased γ-power to negative stimuli (Siegle et al. 2010), attenuated α-coherence (Suhhova et al. 2009), and general cortical hypofrontality, associated with depression (Galynker et al. 1998). Furthermore, we hypothesised any neural regulatory effects would be associated with decreases in depressive severity and a less negative self-referential perspective, indexed by greater self-compassionate experience.
Nonlinear multi-levelled dynamics of underlying cell synchrony of neuronal networks, i.e. network activity independent from (non-phase-locked) direct Go/NoGo task processing, were ascertained via induced ERD/ERS variables. Event-related de-synchronisation (ERD) represents increased event-related cortical excitability of integrant neuronal assemblies constituting maximal functional readiness, whereas event-related synchronisation (ERS) represents decreased excitability of cortical neurons constituting greater stability and coherent synchrony between neuronal subpopulations. Due to the lack of existing observations of induced ERD/ERS cortical dynamics in mindfulness and/or depression/psychiatric research contexts, further to the premise these theoretically non-linear aspects are largely independent from linear power and coherence cortical measures, no point of axes were available to rationalise a direction in results, presenting an exploratory element of the experiment. Likewise, we were interested whether such cortical synchronisation dynamics would have modulating implications upon depressive severity and aspects of self-compassion.
Experimental procedure
Sample
This report is part II of an overall study examining different cortical dynamics involved in MBCT upon the same depression patient sample. Thus, the sample demographics, clinical, and task performance/behavioural measures, are also outlined in the accompanying article elsewhere, and replicated here so to be of ease for the reader. However, the recruitment procedure is not repeated here, rather can be found in the accompanying article (Schoenberg and Speckens 2014). Patients had suffered 1–3 previous depressive episodes, and were recruited from the Radboud University Medical Centre Nijmegen (UMCN) psychiatry outpatient clinic and associated UMCN Centre for Mindfulness. Primary diagnosis of Major Depressive Disorder (MDD) was ascertained by a consultant psychiatrist using the DSM-IV-TR; current or remitted depression was classified by the Mini-International Neuropsychiatric Interview (MINI: Sheehan et al. 1998). Of the total 51 participating patients, 26 were allocated to an MBCT group where T1/pre was conducted prior to, and T2/post were conducted following, their MBCT intervention; 25 to a wait-list (WL) control group, where T1 and T2 were conducted spaced 8-weeks apart prior to their participation in MBCT. Allocation was quasi-randomised based on MBCT course entry. If patients had applied for their MBCT course with less than 8-weeks to its onset, they were automatically referred to the MBCT group. If not, the waiting period for starting the MBCT course was used as a control condition.
Procedure
MBCT was administered by health care professionals with longstanding clinical and mindfulness experience, meeting the teaching criteria of the Dutch Association of Mindfulness Trainers (www.vmbn.nl). Patients received an 8-week group program of 2.5 h, including one full silent training day, further to independent daily practise lasting .75 h guided by CDs (i.e. Segal et al.’s 2012 standardised program).
Informed written consent to participate in an ethically approved (CMO, Arnhem-Nijmegen) research study was obtained. Patients undertook an affective Go/No-Go task concomitant to EEG recording. The experimental task comprised 12 × 100 stimuli blocks, with rest intervals between each block. Stimuli consisted of Positive, Negative, and Neutral Dutch words, assimilated from two standardised word databases (Arnold et al. 2011; Hermans and De Houwer 1994). Each block consisted of two possible “word valence types” defined as Go or NoGo stimuli [80 × Go − 20 × NoGo (20 % inhibition rate)], constituting 6 possible “block types” [i.e.: (1) Positive (Go)–Negative (NoGo); (2) Positive (Go)–Neutral (NoGo); (3) Negative (Go)–Positive (NoGo); (4) Negative (Go)–Neutral (NoGo); (5) Neutral (Go)–Positive (NoGo); (6) Neutral (Go)–Negative (NoGo)]. Preceding each block onscreen instructions, verbally verified by the experimenter, specified which valence word type to press (Go)/not press (NoGo). Within the overall experiment, 600 different word stimuli were used to reduce stimuli habituation/familiarity, where word stimuli were randomly presented within each block. Stimulus duration was randomly presented between 500 and 1,500 ms, with a random ISI between 800 and 1,750 ms, wherein a button response could be made as soon as stimuli appeared onscreen, i.e. there was no response lag/wait time.
Clinical scales
The following were administered at T1/pre and T2/post; (a) Inventory of Depressive Symptomatology/IDS (Rush et al. 1996), gauging depressive symptom severity; (b) Self-Compassion Scale/SC (Neff 2003), indexing aspects of self-compassion, including a mindfulness component, encompassing: (1) non-judgement and understanding towards oneself; (2) granting a universal perspective to one’s experiential reality, i.e. personal experiences are part of a larger human experience rather than as separating or isolating; (3) keeping painful thoughts/feelings in balanced awareness, opposed to over-identification; (c) Ruminative Response Scale/RRS (Nolen-Hoeksema and Morrow 1991); (d) Five Facet Mindfulness Questionnaire/FFMQ (Baer et al. 2008); (e) State-Trait Anxiety Inventory/STAI (Spielberger et al. 1983); (f) Cambridge Depersonalization Scale/CDS (Sierra and Berrios 2000).
Electrophysiological recording
EEG data were acquired using Brain Vision Recorder 1.03 and QuikAmps 72 hardware (www.BrainProducts.com), recorded from 30 Ag/AgCl active electrode sensors with integrated noise subtraction circuits (actiCAP: Brain Products) located in accordance with the 10-10 electrode system (sites: Fp1, Fp2, AFz, F7, F3, Fz, F4, F8, FC5, FC1, FCz, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, O1, Oz, O2). Average reference was used. Ground electrode was placed on the forehead. Vertical and horizontal ocular activity were calculated by bipolar derivations of electro-oculogram signals recorded using Ag/AgCl cup electrodes above and below the left eye, and 1 cm to the outer canthi of each eye, respectively. Impedance was maintained ≤10 KΩ. Electrical signal was continuously sampled at a digitization rate of 500 Hz, with a band-pass filter of .1–100 Hz.
Signal analysis
Event-related (de)-synchronisation (ERD/ERS)
Oscillatory EEG analysis was conducted using Brain Vision Analyzer 2.0.2. Occular artefacts were removed using Independent Component Analysis/ICA, optimal for event-related time–frequency (T–F) signal processing (Graimann and Pfurtscheller 2006). Data were segmented into 1,400 ms epochs, −500 to 900 ms relative to stimulus onset, for: (1) stimulus-locked NoGo trials (NoGo-T), and (2) stimulus-locked Go trials (Go-T), for each valence type (positive, negative, neutral). Artifact rejection removed electromyographic activity and/or amplifier saturation, where voltages exceeding ±50 µV were discarded. Data were band-pass (Butterworth zero-phase, 24 dB/octave), and Notch (50 Hz), filtered into α (8–12 Hz) and γ (30–45 Hz)-frequency bandwidths.
For phase-locked evoked activity, following bandpass filtering, epoch voltages were squared and subsequently averaged, and expressed as instantaneous power (µV2). Mean power for the early epoch (0–400 ms) and late epoch (400–800 ms) were then calculated, whereby FCz and Pz sites were used in the subsequent statistical analyses. These sites correspond to the fronto-parietal network, implicated in the top-down and bottom-up processing of moment-to-moment control/allocation of specialised functions (Sadaghiani et al. 2012; Sauseng et al. 2005; Wang et al. 2010). Moreover, MDD has shown altered activity within this fronto-parietal loop (Jaworska et al. 2012; Kemp et al. 2010; Ricardo-Garcell et al. 2009; Bauer and Hesselbrock 2002).
Event-related induced activity was extracted via intertrial variance (see Kalcher and Pfurtscheller 1995), by averaging all epochs and subtracting the average power from each individual epoch constituting the average, thus extracting phase-locked activity. ERD/ERS was quantified as percentage change in average inter-trial variance (A) during the time windows (TW): 0–400 ms (early time-window [E-TW]), and 400–800 ms (late time-window [L-TW]), in the nth channel, compared to the average inter-trial variance of the baseline reference (R) = −500 to 0 ms, relative to stimulus-onset, defined as follows:
Mean induced ERD/ERS% during the early temporal epoch (0–400 ms) and late epoch (400–800 ms) were subsequently calculated at each electrode site; FCz and Pz used in the subsequent statistical analyses (as per the previous rationale).
Coherence
Fast Fourier Transform (FFT) converted artefact-rejected and segmented data (as above) from the time series into complex frequency and phase domains using a 10 % Hamming window. The coherence (Coh) between two electrode signals (s1, s2) was then calculated for the α and γ frequency (f) bands [inter-hemispheric electrode pairs: F3–F4, F7–F8, P3–P4, P7–P8; and intra-hemispheric pairs: FCz–Pz, F3–P3, F4–P4, F7–P7, F8–P8], via the cross-spectrum (|CS(s1, s2)(f)|), and normalised with the corresponding autospectrum [| CS(s1, s1)(f) | | CS(s2, s2) (f) |], expressed as:
where CS(s1, s2)(f) = Σ s1, x(f) s2, x(f)*; and x being the totalled number of segments used. Coherence within each frequency band were then expressed as an interval value between 0 and 1; where 0 = s1 + s2 are independent; and 1 = s1 + s2 reflect a linear relationship.
Statistical analyses
Repeated-measures ANOVA (r-ANOVA) examined Time (2 levels: pre/T1, post/T2) × Condition (2 levels: Go, NoGo) × Valence (3 levels: positive, negative, neutral) × Group (2 levels: MBCT, WL), for accuracy and RT behavioural data independently. R-ANOVA examined Time (2 levels: T1, T2) × Epoch (2 levels: early[0–400 ms], late[400–800 ms]) × Condition (2 levels: Go, NoGo) × Valence (3 levels: Positive, Negative, Neutral) × Site (2 levels: FCz, Pz) × Group (2 levels: MBCT, WL) matrices for mean induced ERD/ERS% dynamics, and evoked power, separately. Time × Condition × Valence × Inter-Hemispheric pairs [4 levels: F3–F4, F7–F8, P3–P4, P7–P8] (or Intra-Hemispheric pairs [5 levels: FCz–Pz, F3–P3, F4–P4, F7–P7, F8–P8]) were run for coherence analyses. Greenhouse Geisser corrections were used when assumptions of sphericity were violated. All analyses included Sex as co-variate, as this demographic was not well-matched between groups.
Results
Due to practical restraints, only 38 full questionnaire datasets were collected at both T1 and T2 in the EEG testing sessions.
ERD/ERS
In case of possible baseline differences between the MBCT versus WL groups, one-way ANOVAs showed no significant differences between groups for any baseline (T1/pre) evoked or induced α and γ EEG measure.
Evoked α-power (µV2)
The Condition (2 levels: Go/NoGo) × Epoch (2 levels: early[0–400 ms]/late[400–800 ms]) × Site (2 levels: FCz/Pz) (F(1, 47) = 12.403, p = .001), and Epoch (2 levels: early/late) × Valence (3 levels: positive/negative/neutral) × Group (2 levels: MBCT/WL) (F(2, 94) = 4.396, p = .02) three-way interactions showed increased α-power across all valence conditions for Go-T at FCz and Pz during both epochs. Furthermore, increased power T1-to-T2 for negative stimuli was significant in the WL group (t(24) = 2.581, p = .02 [9.05–12.39 µV2]), compared to marginal increase in the MBCT group (p = .22 [10.85–12.73 µV2]).
For NoGo-T at FCz: the WL group showed increased power across conditions during the early temporal epoch [positive = 8.61–9.37 µV2; negative = 8.95–10.22 µV2; neutral = 8.17–11.23 µV2]; the MBCT group showed minimal incremental increase for positive [7.83–8.71 µV2], and marginal decrease in α-power for negative [8.48–8.123 µV2], and neutral [8.01–7.97 µV2] stimuli. Decrease in α-power became more prominent during the late temporal epoch in the MBCT group for all valence conditions, albeit, to non-significant levels in either group.
For NoGo-T at Pz: the WL group showed increased α-power for all valence types during both epochs, and to a significant extent for neutral stimuli in the early epoch (t(24) = −2.281, p = .03 [9.19–13.15 µV2]). The MBCT group also showed significant increased α-power for positive stimuli during both epochs, significantly so in the early epoch (t(25) = −2.103, p = .05 [10.33–13.22 µV2]).
Valence (3 levels: positive/negative/neutral) × Site (2 levels: FCz/Pz) × Sex (2 levels: female/male) three-way interaction (F(2, 94) = 3.032, p = .05), although no main effect of Sex (p = .57), or Sex interactions pertaining to the MBCT, were observed.
Patient Status (current/remitted) co-variate revealed Time (2 levels: pre/post); Epoch (2 levels: early/late) × Condition (2 levels: go/nogo) × Patient Group (2 levels: current/remitted) (F(1, 47) = 6.685, p = .013), and Epoch × Valence * Patient Group (F(2, 94) = 5.319, p = .006) interactions, although follow-up post hoc analyses were not significant. Patient Status was not found to be a significant main effect, nor had any significant interactions with Time, i.e. treatment effect.
Induced α activity (%)
A Time (2 levels: pre/post) × Epoch (2 levels: early/late) (F(1, 47) = 5.694, p = .02) interaction indicated the late temporal Epoch to NoGo-T comprised ERD in both groups. Moreover, Condition (2 levels: Go/NoGo) × Site (2 levels: Fcz/Pz) (F(1, 47) = 13.535, p = .001), Epoch (2 levels: pre/post) × Site (2 levels: FCz/Pz) (F(1, 47) = 8.385, p = .006), Epoch (2 levels: pre/post) × Valence (3 levels: positive/negative/neutral) × Site (2 levels: FCz/Pz) (F(2, 94) = 3.736, p = .03), Condition × Epoch × Site (F(1, 47) = 17.767, p < .0001), Time × Valence × Group (F(2, 94) = 3.037, p = .05), and Time × Condition × Valence × Group (F(2, 94) = 3.978, p = .02) interactions were found. Disentangling these results revealed desynchronisation increased from the early epoch to late epoch for negative stimuli in the MBCT group at FCz, where ERD increment also significantly increased from T1-to-T2 in the late epoch (t(25) = 2.265, p = .03 [−7.04 to −15.41 %]). Frontocentral (FCz) α-synchronisation shifted from ERS to ERD from the early epoch to late epoch in the WL group for NoGo-T to negative stimuli. For NoGo-T to positive stimuli, increased ERD was observed in the WL from early epoch to late epoch, where incremental change from T1-to-T2 in the late epoch showed a shift from ERS to ERD at FCz (t(24) = 2.700, p = .01 [3.033 to −9.266 %]), and increased ERD at Pz (t(24) = 2.127, p = .05 [−6.74 to −15.15 %]). Contrastingly, the MBCT group showed a decrease in ERD for positive stimuli at both FCz and Pz sites during the early temporal epoch (FCz: p = .72 [−12.52 to −10.97 %]; Pz: p = .14 [−13.49 to −8.42 %]), and late epoch (FCz: p = .80 [−12.85 to −11.32 %]; Pz: p = .98 [−19.78 to −19.67 %]) (Fig. 1a, b).
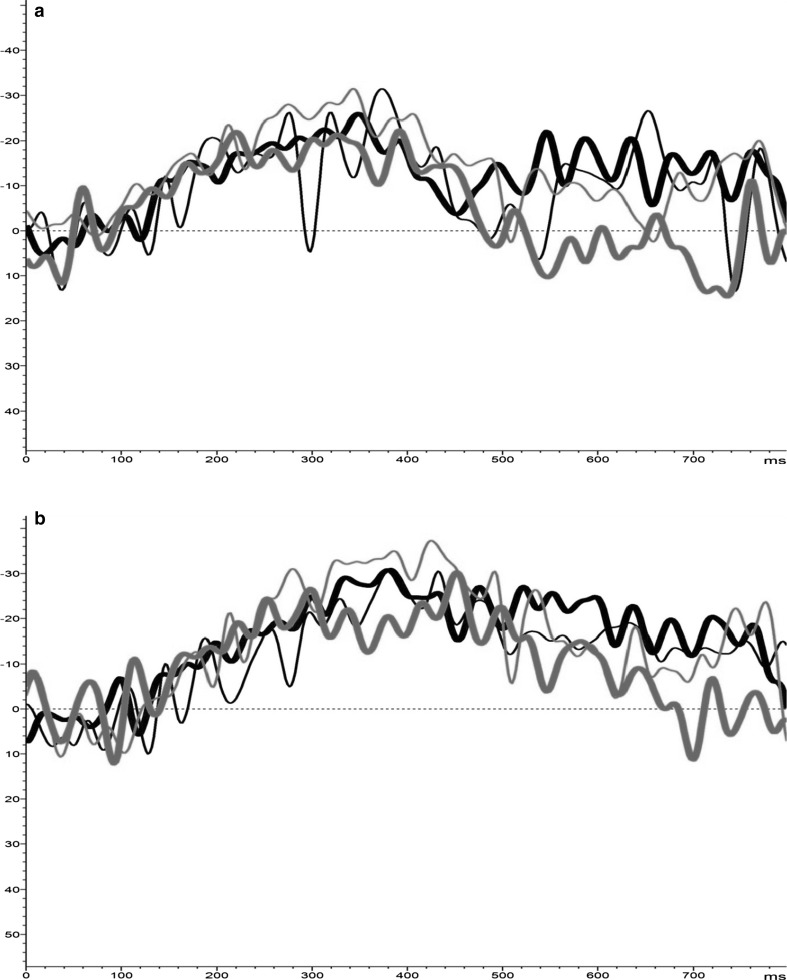
Fig. 1.
a Induced α-synchrony for positive-NoGo (green) trials at FCz [y-axis: +% = ERS; −% = ERD]. b Induced α-synchrony for positive-NoGo (green) trials at Pz [y-axis: +% = ERS; −% = ERD]. (Color figure online)
No significant within-group results were evident for Go-T measures, although a diverging pattern formed between groups during the late epoch; MBCT showed α-ERD across valence conditions, greatest for negative stimuli, compared to the WL group yielding α-ERS. This distinction was more apparent at Pz (Fig. 2a, b).
Fig. 2.
a Induced α-synchrony for negative-Go (red) trials at FCz [y-axis: +% = ERS; −% = ERD]. b Induced α-synchrony for negative-Go (red) trials at Pz [y-axis: +% = ERS; −% = ERD]. (Color figure online)
Taking the co-variate of patient status (current/remitted), Time (pre-to-post/T1-to-T2); Epoch × Condition × Patient Group (F(1, 47) = 5.293, p = 03), and Epoch × Site × Valence × Patient Group (F(2, 94) = 4.211, p = .02) interactions were evident. However, patient status was not found to be a significant main effect, nor had any significant interactions with Time, i.e. treatment effects.
Evoked γ-power (µV2)
A main effect of Condition (2 levels: pre/post) (F(1, 47) = 4.308, p = .04), and Condition (2 levels: Go/NoGo) × Epoch (2 levels: early/late) × Site (2 levels: FCz/Pz) (F(1, 47) = 6.457, p = .01), Time (2 levels: pre/post) × Epoch (2 levels: early/late) × Site (2 levels: FCz/Pz) × Group (2 levels: MBCT/WL) (F(1, 47) = 4.126, p = .05), Time × Epoch × Valence × Group (F(2, 94) = 3.496, p = .03), Condition × Epoch × Valence × Group (F(2, 94) = 3.289, p = .04). No main effects of Group (p = .222) or Sex (p = .95), or Group × Sex interactions were apparent.
Posthoc analyses did not find any significant differences in either group from T1-to-T2. Although the above interactions indicated an overall pattern of increased γ-power in the WL group for Go-T, which was highest during both temporal epochs at Pz for positive [early epoch = 6.15–8.88 µV2; late epoch = 6.33–9.51 µV2] and negative [early epoch = 5.92–9.87 µV2; late epoch = 6.18–9.95 µV2] stimuli. The MBCT group showed marginal decrease in γ-power for positive stimuli during the early epoch [5.72–5.63 µV2] and late epoch [5.73–5.32 µV2] at FCz, compared to marginal increase in power for negative stimuli during early epoch [4.63–5.14 µV2] and late epoch [4.47–5.15 µV2]. Minimal incremental change in evoked γ to Go-T during both epochs were apparent at Pz.
For NoGo-T, at FCz, both groups yielding marginally decreasing power for negative (early epoch: MBCT = 5.33–4.90 µV2; WL = 5.93–5.72 µV2; late epoch: MBCT = 5.78–4.96 µV2; WL = 6.56–5.97 µV2) stimuli at both TWs. Whereas, the MBCT group yielded marginal increase, compared to marginal decrease in the WL group, for positive stimuli (early epoch: MBCT = 4.67–5.14 µV2; WL = 5.65–5.30 µV2; late epoch: MBCT = 4.83–5.00 µV2; WL = 6.40–5.86 µV2). Albeit, these within-group changes were non-significant.
At Pz, increased γ-power across valence types was evident in the WL group during both early epoch [positive = 6.61–7.94 µV2; negative = 6.53–8.80 µV2; neutral = 6.03–11.97 µV2] and late epoch [positive = 7.38–8.67 µV2; negative = 7.28–8.49 µV2; neutral = 6.56–11.79 µV2]. For the MBCT group, increased γ-power was observed for positive stimuli during the early epoch [4.96–5.17 µV2] compared to decreased power in the late epoch [5.14–4.87 µV2]. Whereas, decreased γ-power was observed for negative and neutral stimuli during both early epoch [negative = 5.75–5.06 µV2; neutral = 5.97–5.43 µV2] and late epochs [negative = 6.18–5.33 µV2; neutral = 6.16–5.86 µV2].
Induced γ activity (%)
Main effect of Site (F(1, 47) = 15.938, p < .0001), and Condition × Epoch (F(1, 47) = 4.432, p = .04), Epoch × Site (F(1, 47) = 12.690, p = .001), Condition × Epoch × Site (F(1, 47) = 7.445, p = .009), Time × Condition × Site (F(1, 47) = 4.564, p = .04), and Time × Condition × Epoch × Valence × Site (F(2, 94) = 4.035, p = .02). No main effect of Group (p = .89), Sex (p = .51), or Group × Sex interactions.
These findings illustrated no marked diverging pattern between groups for Go-T at Pz for either temporal epoch, contrary to FCz; where the MBCT group yielded decreased ERD [p = .72: −9.78 to −7.39 %] compared to increased ERD in the WL [p = .41: −9.62 to −13.15 %], for positive stimuli during the E-TW, although not to significant levels. During the late epoch, the MBCT group showed marginal incremental change for positive [p = .87: −11.19 to −10.80 %] and neutral [p = .93: −9.72 to −10.03 %] stimuli, compared to ERS in the WL group. Furthermore, univariate ANOVA examining increment measures, showed Go-T to negative stimuli in the late epoch significantly differed between groups (F(1, 49) = 4.189, p = .05 [MBCT = 2.00 % vs. WL = −4.13 %]), indicating decreased ERD in the MBCT group [p = .34: −12.32 to −10.32 %] compared to increased ERD in the WL [p = .07: −7.07 to −11.20 %].
For NoGo-T, a significant within group finding showed a shift in synchrony from ERD to ERS during the E-TW for positive stimuli was apparent at Pz in the MBCT group (t(25) = −2.498, p = .02 [−8.18 to .47 %]), compared to marginal increase in ERD in the WL [p = .86: −4.98 to −5.66 %].
Inter-/intra-hemispheric coherence
α-Coherence
Examining inter-hemispheric pairs (4 levels: F3–F4, F7–F8, P3–P4, P7–P8); Significant Condition (F(1, 47) = 5.290, p = .026), Inter-hemispheric Sites (F(3, 141) = 27.926, p < .0001), Time × Valence × Inter-hemispheric Sites × Group (F(6, 282) = 2.541, p = .021), effects/interactions revealed increased α-coherence between F3–F4 for neutral NoGo trials (t(24) = −2.138, p = .042 [.20/SD = .12 to .29/SD = .22]) pre-to-post MBCT, and for positive-NoGo trials in the WL (t(24) = −2.081, p = .048: [.19/SD = .13 to .30/SD = .25]). Additionally, between P3–P4 for positive-Go (t(24) = −2.360, p = .026 [.08/SD = .06 to .11/SD = .08]) in the MBCT group.
Examining intra-hemispheric pairs (5 levels: FCz–Pz, F3–P3, F4–P4, F7–P7, F8–P8); Condition (F(1, 47) = 5.823, p = .02), Intra-hemispheric Sites (F(4, 188) = 59.561, p < .0001), Condition × Intra-hemispheric Sites (F(4, 188) = 4.431, p = .003), Valence × Intra-hemispheric Sites × Group (F(8, 376) = 2.280, p = .022), Time × Condition × Valence × Intra-hemispheric Sites × Group (F(8, 376) = 2.808, p = .005) were significant. Follow-up analyses showed significantly increased coherence between F4–P4 for positive-NoGo in WL (t(24) = −2.067, p = .050 [.17/SD = .10 to .27/SD = .24]), not evident for MBCT (p = .27) (Fig. 3). Conversely, coherence increased for the left-hemispheric F3–P3 pair for neutral-Go (t(25) = −2.114, p = .045 [.12/SD = .09 to .17/SD = .11]), negative-NoGo (t(25) = −2.109, p = .045 [.13/SD = .09 to .19/SD = .12], Fig. 4), and neutral-NoGo (t(25) = −2.250, p = .034 [.13/SD = .09 to .21/SD = .19]), in the MBCT group only.
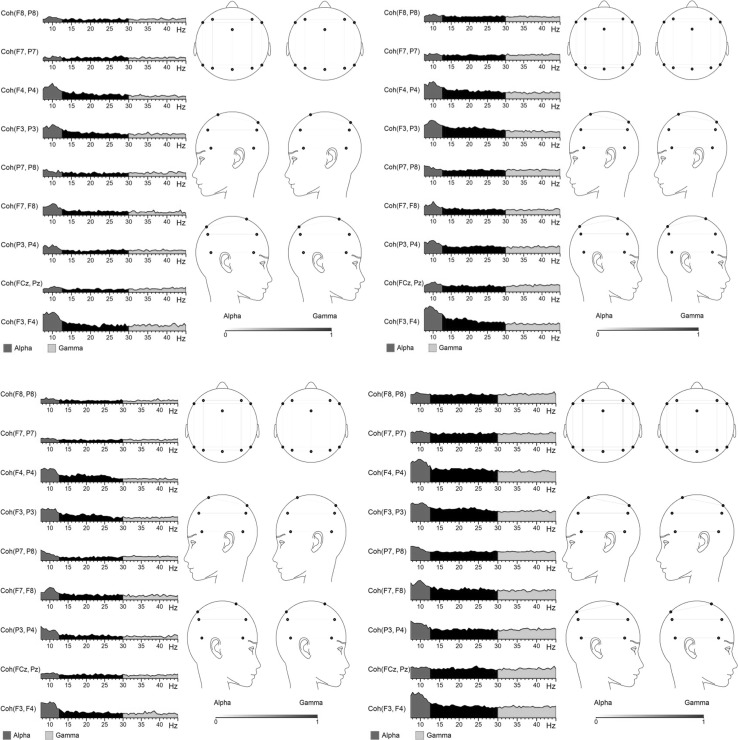
Fig. 3.
Coherence at T1 (left) and T2 (right) in MBCT (above) and WL (below) groups for positive NoGo trials. *Right hemispheric pair F4–P4 significant in WL group only (p = .05)
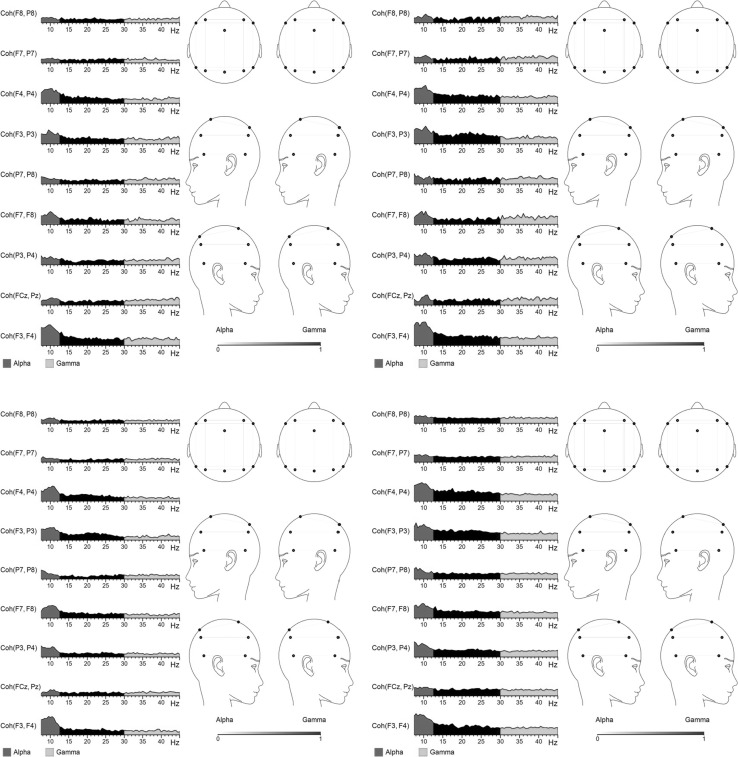
Fig. 4.
Coherence at T1 (left) and T2 (right) in MBCT (above) and WL (below) groups for negative NoGo trials. *Left intra-hemispheric pair F3–P3 significant in MBCT group only (p = .045)
Overall, no main effects of Group (inter-hemispheric pairs = p = .67; intra-hemispheric pairs = p = .77), Sex (inter-hemispheric pairs = p = .29; intra-hemispheric pairs = p = .54), or Sex interactions were found.
γ-Coherence
Condition and Condition × Intra/Inter-hemispheric Sites effect/interaction were found for both inter-hemispheric [Condition: p = .004; Condition × Inter-hemispheric Sites (p = .025), and intra-hemispheric (Condition: p = .008; Condition × Intra-hemispheric Sites (p = .033)] coherence pairs. However, no significant changes in γ-coherence related to the MBCT.
Quantitative scales
Significant changes in clinical scales were exclusive to the MBCT group. See Table 1 for mean/SD values, and pre-to-post statistical reports. Additionally, significant correlations were found in the MBCT group between IDS and self-compassion increment changes (i.e. T2/post–T1/pre) alongside ERD/ERS incremental changes at Pz (Table 2).
Table 1.
Demographic, clinical, and task performance/behavioural, data
| Demographics | MBCT | WL | Between-group comparison |
|---|---|---|---|
| Sex | Female = 20 [76.9 %], Male = 6 [23.1 %] | Female = 12 [48 %], Male = 13 [52 %] | p = .05* |
| Age/years | 47.8 (12.1) | 51.2 (8.5) | p = .27 |
| Medicated | Medicated = 19 [73.1 %], non-med = 7 [26.9 %] | Medicated = 17 [68 %], non-med = 8 [32 %] | p = .76 |
| Primary diagnosis | CD = 11 [42.3 %], RD = 15 [57.7 %] | CD = 7 [28 %], RD = 18 [72 %] | p = .30 |
| Pre | Post | Within-group comparison | Pre | Post | Within-group comparison | [Baseline: T1/Pre] | |
|---|---|---|---|---|---|---|---|
| Clinical variables | |||||||
| IDS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | p = .55 |
| RRS | 60.6 (11.9) | 55.1 (11.0) | p = .03* | 60.2 (13.2) | 59.7 (13.8) | p = .81 | p = .93 |
| FFMQ global | 114.9 (22.8) | 131.3 (17.4) | p = .02* | 119.7 (14.9) | 119.8 (15.7) | p = .96 | p = .43 |
| FFMQ observe | 24.4 (5.9) | 28.6 (4.7) | p = .005** | 25.1 (4.9) | 24.8 (5.2) | p = .51 | p = .70 |
| FFMQ describe | 27.7 (4.5) | 28.6 (4.7) | p = .64 | 28.7 (6.0) | 28.6 (6.1) | p = .77 | p = .56 |
| FFMQ non-judge | 22.3 (8.0) | 26.7 (5.3) | p = .03* | 24.1 (6.8) | 24.2 (6.6) | p = .94 | p = .45 |
| FFMQ non-reaction | 19.0 (5.2) | 22.9 (4.3) | p = .01* | 18.0 (4.7) | 18.0 (4.5) | p = .87 | p = .49 |
| FFMQ awareness | 21.5 (5.8) | 24.7 (6.1) | p = .09 | 23.9 (5.2) | 24.3 (5.5) | p = .55 | p = .20 |
| SC global | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | p = .81 |
| STAI T | 47.0 (3.4) | 46.0 (5.0) | p = .32 | 46.9 (4.6) | 46.4 (4.7) | p = .55 | p = .93 |
| STAI S | 43.8 (5.7) | 43.6 (3.9) | p = .81 | 41.6 (3.8) | 41.3 (3.1) | p = .76 | p = .15 |
| CDS G | 41.4 (26.0) | 36.9 (29.3) | p = .37 | 39.1 (45.8) | 42.5 (43.7) | p = .29 | p = .83 |
| CDS EN | 11.1 (8.2) | 8.4 (7.9) | p = .11 | 11.8 (14.5) | 12.6 (13.1) | p = .55 | p = .82 |
| Behavioural measures | |||||||
| Correct-NoGo ALL | 13.6 (3.0) | 13.9 (2.6) | p = .59 | 13.6 (2.9) | 13.9 (3.3) | p = .39 | p = .94 |
| Correct-Go ALL | 70.0 (8.4) | 71.9 (4.3) | p = .12 | 71.8 (4.6) | 72.3 (4.8) | p = .77 | p = .16 |
| Correct-NoGo POS | 14.2 (3.6) | 14.6 (3.0) | p = .59 | 13.9 (3.1) | 14.3 (3.3) | p = .33 | p = .81 |
| Correct-Go POS | 69.2 (9.8) | 72.2 (4.4) | p = .23 | 72.1 (4.6) | 71.9 (4.9) | p = .60 | p = .20 |
| Correct-NoGo NEG | 13.9 (3.4) | 14.0 (2.8) | p = .84 | 14.0 (3.2) | 14.1 (3.4) | p = .82 | p = .88 |
| Correct-Go NEG | 69.0 (10.0) | 73.0 (6.3) | p = .10 | 73.1 (5.3) | 73.1 (6.6) | p = .94 | p = .08 |
| Correct-NoGo NEU | 12.7 (2.8) | 13.0 (2.8) | p = .56 | 12.9 (3.0) | 13.4 (3.5) | p = .27 | p = .74 |
| Correct-Go NEU | 68.7 (8.1) | 71.0 (5.8) | p = .19 | 70.3 (6.7) | 72.0 (6.3) | p = .27 | p = .48 |
| Correct hits (Go) RT | |||||||
| ALL | 579.7 (75.5) | 587.6 (88.5) | p = .50 | 565.6 (68.4) | 553.8 (60.0) | p = .37 | p = .51 |
| POS | 582.5 (83.2) | 589.9 (84.3) | p = .66 | 562.1 (64.6) | 559.7 (61.5) | p = .79 | p = .36 |
| NEG | 592.1 (85.8) | 601.9 (103.8) | p = .51 | 571.1 (69.1) | 560.8 (54.9) | p = .55 | p = .36 |
| NEU | 564.5 (67.2) | 570.9 (83.4) | p = .52 | 563.5 (79.1) | 541.0 (70.7) | p = .15 | p = .96 |
* CD currently depressed, RD remitted depressed, IDS Inventory of Depressive Symptomatology, RRS Ruminative Response Scale, FFMQ Five facet Mindfulness Questionnaire, SC Self-Compassion Scale; STAI State-Trait Anxiety Inventory [T = Trait/S = State], CDS Cambridge Depersonalization Scale [EN = Emotional Numbing], RT reaction time
▲ = see Table 2
Table 2.
Clinical scale scores for MBCT versus WL groups and correlations between increment changes (T1–T2) in clinical and ERD/ERS evoked and induced measures
| Clinical variable | MBCT (σ) | WL (σ) | Correlations with ERD/ERS activity at Pz in MBCT group▲ [ANOVA between—group effects/interactions] | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Comparison | Pre | Post | Comparison | ||
| IDS | 27.3 (9) | 19.3 (2) | p = .02* | 25.1 (12) | 25.3 (13) | p = .89 | ↓ α-ERD/NG-T/P/E: p = .003* ↓ α-ERD/NG-T/P/L: p = .001** ↓γ-ERD/NG-T/N/L: p = .03* ↑γ-power/Go-T/N/E: p = .004** ↓γ-power/Go-T/n/E: p = .04* ↓γ-power/Go-T/N/L: p = .008** ↑γ-power/NG-T/P/E: p = .04* ↓γ-power/NG-T/P/L: p = .05* [Time x Group: p = .02*] |
| SC Scale☼ | |||||||
| Global score | 83.7 (26) | 107.0 (27) | p < .0001** | 81.7 (29) | 84.0 (23) | p = .45 | ↓α-ERD/NG-T/N/E: p = .05* ↑α-ERD/NG-T/N/L: p = .04* ↓α-ERD/NG-T/P/L: p = .03* [Time: p = .001** Time x Group: p = .001**] |
| SC subscales | |||||||
| Self-Kindness | 13.3 (6) | 17.3 (6) | p = .001** | 13.9 (5) | 13.5 (5) | p = .65 | *No correlations were run for subscales |
| Self-Judgement | 19.3 (6) | 14.9 (5) | p = .001** | 18.1 (5) | 17.8 (5) | p = .61 | |
| Common Humanity | 14.5 (5) | 17.9 (5) | p = .006** | 13.6 (5) | 13.3 (5) | p = .72 | |
| Isolation | 18.8 (6) | 15.1 (5) | p = .005** | 18.5 (5) | 17.1 (6) | p = .07 | [Time: p = .001** Time x Group: p = .001** Subscale: p = .03*] |
| Mindfulness | 16.7 (5) | 19.5 (5) | p = .009** | 14.1 (5) | 15.2 (5) | p = .10 | |
| Over-identification | 18.5 (6) | 13.6 (6) | p = .003** | 18.9 (5) | 19.0 (5) | p = .94 | |
▲, no significant correlations between clinical changes and EEG dynamics were evident in the WL group. Nor any significant correlations at FCz
☼, Self-Compassion Scale (Neff 2003)
Discussion
The effects of MBCT upon evoked event-related α and γ power and coherence, in a sample of MDD patients were observed. This is also the first investigation into MBCT and induced α and γ ERD/ERS dynamics.
MBCT and α-activity
Unlike prevailing meditation training studies, MBCT-related power enhancement was not consistently observed; α-power decreased during the latter 400–800 ms window, particularly for NoGo, pre-to-post MBCT. However, increased parietal α-power for positive stimuli was highlighted for the 400–800 ms window pre-to-post MBCT. These findings grant a potentially multi-faceted interpretation based on the present convoluted scientific understanding of what power values in differing frequency bands precisely embody. Principally, α-power and cortical activity represent an inverse relationship (Bruder et al. 2012), suggesting MBCT enacted excitation within neuronal networks during the post-stimuli-processing (400–800 ms) stage. Elevated α-power has consistently been reported in posterior (Jaworska et al. 2012; Kemp et al. 2010), and anterior (Ricardo-Garcell et al. 2009; Bauer and Hesselbrock 2002) regions in MDD. Furthermore, cortical hypofrontality correlates with negative symptomatology in MDD, whereby a proposed neurobiological mechanism pertains to cerebral hypoperfusion in the dorsolateral and orbitofrontal prefrontal cortex (Galynker et al. 1998). In this light, MBCT elevated blunted levels of cortical excitability (represented by attenuated power) during the later processing stage of 400–800 ms in our patient sample, serving to potentiate greater positive mood. This is further supported by ameliorated depressive symptom severity, and increase in left-hemispheric EEG α-coherence (further discussed later). For example, left frontal cortical excitability has been associated with greater self-reported happiness (Coan and Allen 2004). However, reduced MBCT-related power was also observed in neutral conditions, thus, not emotion-specific. Additionally, behavioural task performance was not mediated by MBCT exposure, suggesting task-related functionality was not targeted in a linear mode. This leads us to consider the nonlinear induced measures towards the better interpretation of these nebulous findings.
A clear pattern emerged regarding induced MBCT effects; of enhanced α-desynchronisation pre-to-post towards negative stimuli, contrary to decreased ERD for positive stimuli, compared to an overall increase in α-desynchronisation in the WL. Combined with the correlational analysis, attenuated α-ERD across the entire 800 ms time window post-positive-NoGo stimuli was significantly associated with amelioration in depressive severity in the MBCT group. No such associations were evident in the WL, suggesting modulatory effects of MBCT on α-desynchronisation are associated with its beneficial role in mood regulation. Furthermore, α-ERD was the sole measure which correlated with increased self-compassion pre-to-post MBCT, whereby reduced ERD during NoGo positive late (400–800 ms) and negative early (0–400 ms) conditions, in addition to elevated α-ERD for NoGo negative late trials, correlated to greater experienced self-compassion. Travis and Shear’s (2010) review categorises differing meditative techniques with discrete neurophysiological signatures, proposing α-activity to be associated with self-transcending meditation processes. In line, an extant hypothesis asserts alterations in self-processing in MDD, particularly inward directed attention causing excessive pathological self-focus, have been attributed to disruptions of a cortical-subcortical midline system (Northoff 2007). It is reasonable to infer from our findings that MBCT possibly targeted this aforementioned system, dampening maladaptive ruminative self-processing networks that serve to impede self-compassionate referential states in depression. Further empirical data is needed to support this hypothesis, although at this juncture, we report elsewhere significant ameliorative effects of the MBCT upon clinical rumination measures (Schoenberg and Speckens 2014).
Whilst it is surmised that α-activity serves an inhibitory role across cortical processing systems; e.g. the ‘inactivation’ of task-irrelevant cortical mechanisms (for a review, see Klimesch et al. 2007); evidence also suggests α-synchrony represents top-down functioning related to attention and consciousness, akin to cortical binding properties within the fronto-parietal ‘global neuronal workspace’ (Palva and Palva 2007), i.e. highly intricate parallel distributed systems of specialised processors constituting neural networks (Dehaene et al. 2011). Furthermore, sub-stratifying α-synchronisation/ERS from α-desynchronisation/ERD, the former has been connected to top-down complex information processing, whilst the latter to bottom-up homeostatic processing (Benedek et al. 2011; Klimesch et al. 2007), and increased cortical excitability (Pfurtscheller and Andrew 1999). As such, α-synchronisation has multi-levelled properties, whereby our findings presented here suggest rather than blunting, or inhibiting, hyper-active negativity networks, MBCT enacted excitatory effects upon intermediate neural properties targeting hypoactive positive emotion processing systems. Increased experiences of positive emotion in patients with a history of depression and residual symptoms after MBCT-exposure (Geschwind et al. 2011) are in line with this theorisation. However, a separate study looking at attention biases in MDD reported a dual-direction outcome following MBCT; i.e. attenuation to negative stimuli, alongside dis-inhibition of positive stimuli (De Raedt et al. 2012). Further empirically rigorous-controlled studies are required to disentangle whether MBCT targets unidirectional or bidirectional clinical action pathways in MDD, tenably regulated by discrete underlying cortical dynamics. For example, we report elsewhere findings of fronto-midline theta (θ) exerting a disengagement mechanism in the processing of, particularly affectively valenced, incoming material (Schoenberg and Speckens 2014).
MBCT and γ-activity
Unlike α-band, no MBCT-related changes were apparent in non-phase-locked induced γ-ERD/ERS. Rather, the two groups diverged regarding γ-power for positive Go trials both frontally and parietally, and negative NoGo conditions for parietal power during the task processing window of 0–400 ms post-stimulus exposure. These measures were characterised by attenuated γ-power post-MBCT, compared to power enhancement in the WL. Furthermore, blunted MBCT-related power versus power increase in the WL was also apparent during the 400–800 ms temporal window for positive Go, positive NoGo, and negative NoGo conditions. Interestingly, of these diverging measures, only attenuated parietal γ-power (reflecting increased cortical activity) during the latter window (400–800 ms) for positive and negative NoGo conditions correlated with amelioration in depressive symptomatology.
From a clinical perspective, enhanced γ-power (decreased neuronal excitability) has been reported in first-episode depression compared to non-depressed controls (Strelets et al. 2007), suggesting that in our sample, the MBCT had a down-regulating effect upon abundant γ-power. Strelets et al.’s study also found considerably reduced γ-coherence, reflecting degraded integration of intra- and inter-hemispheric network activity, unlike that of controls. We found no MBCT-related changes in γ-coherence, implying that MBCT-related clinical improvement was not necessarily facilitated via increased γ-related connectivity between neuronal subpopulations, but increased localised cortical excitability of germane processing regions related to mood regulation. For example, induced γ-synchrony has been assigned an operational function in the activation of globalised cortical networks (Fries 2009), where specifically event-related induced γ represents computational operations within the cerebral cortex for the processing of incoming signals (Müller 2000). Conversely, parietal γ-power has been implicated in emotion processing and the cognitive reappraisal of emotional stimuli (Kang et al. 2012). A primary hypothesised working mechanism of mindfulness is the facilitation of shifting cognitive “sets”, or ‘decentering’, so to promote positive emotion and strategic coping via the positive re-appraisal of potentially stressful/unpleasant input (Garland et al. 2009). Our findings of altered γ-power, particularly to errors towards negatively valenced material alongside attenuation in symptom severity are in line with this conjecture, providing a plausible neurophysiological substrate of this re-appraisal mechanism via adaptive MBCT-regulated γ-power.
Inter- versus intra-hemispheric coherence
Increased left-intra-hemispheric coherence (F3–P3) was specific to the MBCT group, compared to right-hemispheric coherence (F4–P4) in the WL. These findings suggest MBCT enhanced fronto-parietal connectivity within the left-hemisphere, where left frontal hypoactivity is a consistent finding in MDD (Ohta et al. 2008; Rogers et al. 1998 (review); Bajulaiye and Alexopoulos 1994). Moreover, enhanced long-range fronto-parietal coherence was exclusive to the α-band, whereby increased α-coherence has been linked to fluid complex higher-order functions, such as central executive control (Sauseng et al. 2005: also specific to the fronto-parietal network), observational learning (van der Helden et al. 2010), creativity (Orme-Johnson and Haynes 1981), and transcendental experience (Dillbeck and Bronson 1981). Furthermore, α-synchrony across distant fronto-parietal projections regulates the integration of information within the ‘adaptive control network’; a top-down system implemented in the moment-to-moment control and allocation of sensory resources and specialised processing (Sadaghiani et al. 2012). Related findings show enhanced right-intra-hemispheric coherence over fronto-temporal low frequency bands, i.e. delta and theta frequencies have been connected to poorer treatment response to anti-depressant medication (Lee et al. 2011). Therefore, it is reasonable to surmise that enhanced left-hemispheric α-coherence possibly served as an ‘enabling mechanism’ towards the cohesive implementation of conjoining action pathways.
Limitations
First, wait-list control lacks rigour, meaning we cannot rule out the possibility of general psychological intervention effects pertaining to the presented findings. Future investigations may include active controls to consolidate the evidence-base, particularly CBT-only comparison, enabling us to decipher between mindfulness and psychotherapeutic pathways. Second, this was not a fully randomised controlled study. Third, a larger patient sample size would contribute greater empirical strength to this report and the ensuing presented hypotheses. Whilst this may be counter-balanced considering the high sensitivity of EEG measures, it does not extend to the questionnaire data (particularly when factoring the incomplete clinical variables), enforcing us to approach any correlational relationships with great caution. Fourth, the female/male ratio was not equal, although Sex did not show as a significant confound in statistical analyses. Fifth, medication-free patients in both groups throughout the trial would have been optimal, although an unrealistic option considering a substantial part of patients with recurrent depression benefit from pharmacological treatments. Fortunately, medicated and non-medicated patients were equally dispersed between groups, and post hoc analyses did not show medication status as a significant confound. Last but not least, the sample included current and remitted depressed patients, likely engendering differing baseline EEG measures for the two groups. Although, the purpose of the study was to examine relative EEG modulation connected to MBCT. Furthermore, current versus remitted patients were equally dispersed between groups, and Patient Status was not found a significant co-variate, nor had any significant interactions with Time (i.e. pre-to-post/treatment effects).
Summary
The present findings allude to manifold electro-cortical substrates of MBCT, constituting linear and non-linear mechanisms of action. Elevated α-band coherence within the left fronto-parietal network plausibly served as an initial ‘potentiation’ gateway for conjoining emotion related subsystems. In parallel, modulations upon α and γ power, further mediated by α-desynchronisation dynamics, ministered an ‘excitatory’ mechanism upon properties involved in the regulation and execution of positive mood and re-appraisal. Concurrently, a ‘unifying’ mechanism targeted the cortical-subcortical midline system overseeing adaptive self-processing; largely disrupted during current and residual depressive pathology; subsequently promoting subtle and abstract self-represented processing networks towards compassionate and positively-accepting experiential states. Despite our methodological constraints, the present observations provide direction for future lines of enquiry into the discernibly multifarious neural mechanisms of MBCT that seemingly enact via inter-dependent multi-dimensional pathways.
Acknowledgments
This work was supported by the BrainGain SmartMix Programme for the Netherlands Ministry of Economic Affairs and Netherlands Ministry of Education, Culture and Science, funded by the Organisation for Scientific Research (NWO); and the Netherlands Institute for Advanced Study in the Humanities and Social Sciences. Much appreciation and gratitude to all those who participated in the experiments. The authors give many thanks to Addy de Graaf for generous assistance with research co-ordination; Katrin Scheibe and Magdalena Kowlaczuk for assistance with data collection; Sietske Heusinkveld, Joyce Besselink, Danique Smeijers, Irma Veliscek-van Maren, and the Radboud University Medical Centre for Mindfulness team for helpful assistance with patient recruitment.
Conflict of interest
None.
References
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi: 10.1016/S0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Arnold JF, Fitzgerald DA, Fernández G, Rijpkema M, Rinck M, Eling PA, Becker ES, Speckens A, Tendolkar I (2011) Rose or black-coloured glasses? Altered neural processing of positive events during memory formation is a trait marker of depression. J Affect Disord 131:214–223 [DOI] [PubMed]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, Walsh E, Duggan D, Williams MG. Construct validity of the Five Facet Mindfulness Questionnaire in meditating and nonmeditating samples. Assessment. 2008;15:329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- Bajulaiye R, Alexopoulos GS. Pseudodementia in geriatric depression. In: Chiu E, Ames D, editors. Functional psychiatric disorders of the elderly. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Barnhofer T, Chittka T, Nightingale H, Visser C, Crane C. State effects of two forms of meditation on prefrontal EEG asymmetry in previously depressed individuals. Mindfulness. 2010;1:21–27. doi: 10.1007/s12671-010-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM (2002) Lateral asymmetries in the frontal brain: effects of depression and a family history of alcoholism in female adolescents. Alcohol Clin Exp Res 26:1662–1668 [DOI] [PubMed]
- Benedek M, Bergner S, Könen T, Fink A, Neubauer AC. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia. 2011;49:3505–3511. doi: 10.1016/j.neuropsychologia.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanov V, Keune PM, Kotchoubey B, Hautzinger M. Event-related brain potentials reflect increased concentration ability after mindfulness-based cognitive therapy for depression: a randomized clinical trial. Psychiatry Res. 2012;199:174–180. doi: 10.1016/j.psychres.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Bansai R, Tenke CE, Liu J, Hao X, Warner V, Paterson BS, Weissman MM. Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum Brain Mapp. 2012;33:1325–1333. doi: 10.1002/hbm.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Delorme A, Polich J (2010) Occipital gamma activation during vipassana meditation. Cogn Process 11:39–56 [DOI] [PMC free article] [PubMed]
- Cantero JL, Atienza M, Salas RM, Gómez CM. Alpha EEG coherence in different brain states: an electrophysiological index of the arousal level in human subjects. Neurosci Lett. 1999;271:167–170. doi: 10.1016/S0304-3940(99)00565-0. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Moraes H, Silveira H, Ribeiro P, Piedade RAM, Deslandes AC, Laks J, Versiani M (2011) EEG frontal asymmetry in the depressed and remitted elderly: is it related to the trait or to the state of depression? J Affect Disord 129:143–148 [DOI] [PubMed]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Baert S, Demeyer I, Goeleven E, Raes A, Visser A, Wysmans M, Jansen E, Schacht R, Van Aalderen JR, Speckens A. Changes in attentional processing of emotional information following mindfulness-based cognitive therapy in people with a history of depression: towards an open attention for all emotional experiences. Cogn Ther Res. 2012;36:612–620. doi: 10.1007/s10608-011-9411-x. [DOI] [Google Scholar]
- Dehaene S, Changeux J-P, Naccache L. The global neuronal workspace model of conscious access: from neuronal architectures to clinical applications. In: Dehaene S, Christen Y, editors. Characterizing consciousness: from cognition to the clinic? Research perspectives in neurosciences. Berlin: Springer; 2011. pp. 55–84. [Google Scholar]
- Dillbeck MC, Bronson EC (1981) Short-term longitudinal effects of the transcendental meditation technique on EEG power and coherence. Int J Neurosci 14:147–151 [DOI] [PubMed]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D (2008) Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord 22:716–721 [DOI] [PubMed]
- Feldman C, Kuyken W. Compassion in the landscape of suffering. Contemp Buddh. 2011;12:143–155. doi: 10.1080/14639947.2011.564831. [DOI] [Google Scholar]
- Foland-Ross LC, Hamilton PJ, Joorman J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychol Sci. 2013;24:334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Ann Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39:608–612. [PubMed] [Google Scholar]
- Garland E, Gaylord S, Park J. The role of mindfulness in positive reappraisal. Explore. 2009;5:37–44. doi: 10.1016/j.explore.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Drukker M, van Os J, Wichers M (2011) Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: a randomised controlled trial. J Consult Clin Psychol 79:618–628 [DOI] [PubMed]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emot. 1998;12:449–478. doi: 10.1080/026999398379673. [DOI] [Google Scholar]
- Graimann B, Pfurtscheller G. Quantification and visualisation of event-related changes in oscillatory brain activity in the time-frequency domain. In: Neuper C, Klimesch W, editors. Event-related dynamics of brain oscillations. Amsterdam: Elsevier; 2006. pp. 79–97. [DOI] [PubMed] [Google Scholar]
- Grant JA, Duerden EG, Courtemanche J, Cherkasova M, Duncan GH, Rainville P. Cortical thickness, mental absorption and meditative practise: possible implications for disorders of attention. Biol Psychol. 2013;92:275–281. doi: 10.1016/j.biopsycho.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Hamilton HP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, De Houwer J (1994) Affective and subjective familiarity ratings of 740 Dutch words. Psychol Belg 34:115–139
- Jaworska N, Blier P, Fusee W, Knott V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J Psychiatr Res. 2012;46:1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcher J, Pfurtscheller G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroenceph Clin Neurophysiol. 1995;94:381–384. doi: 10.1016/0013-4694(95)00040-6. [DOI] [PubMed] [Google Scholar]
- Kang J-H, Ahn HM, Jeong JW, Hwang I, Kim HT, Kim SH, Kim S-P. The modulation of parietal gamma oscillations in the human electroencephalogram with cognitive reappraisal. NeuroReport. 2012;23:995–999. doi: 10.1097/WNR.0b013e32835a6475. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, Clark CR, Bryant RA. Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biol Psychol. 2010;85:350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kenny M, Williams JMG (2007) Treatment resistant depressed patients show a good response to mindfulness-based cognitive therapy. Beh Res Ther 45:617–625 [DOI] [PMC free article] [PubMed]
- Kerr CE, Jones SR, Wan Q, Pritchett DL, Wasserman RH, Wexler A, Villanueva JJ, Shaw JR, Lazar SW, Kaptchuk TJ, Littenberg R, Hämäläinen MS, Moore CI (2011) Effects of mindfulness meditation training on anticipatory alpha modulation in primary somatosensory cortex. Brain Res Bull 85:96–103 [DOI] [PubMed]
- Kerr C, Sacchet MD, Lazar SW, Moore CI, Jones SR (2013) Mindfulness starts with the body: somatosensory attention and top-down modulations of cortical alpha rhythms in mindfulness meditation. Front Hum Neurosci 7. doi:10.3389/fnhum.2013.00012 [DOI] [PMC free article] [PubMed]
- Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Mindfulness-based cognitive therapy (MBCT), cognitive style, and the temporal dynamics of frontal EEG alpha asymmetry in recurrently depressed patients. Biol Psychol. 2011;88:243–252. doi: 10.1016/j.biopsycho.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Keune PM, Bostanov V, Kotchoubey B, Hautzinger M. Mindfulness versus rumination and behavioural inhibition: a perspective from research on frontal brain asymmetry. Pers Individ Diff. 2012;53:323–328. doi: 10.1016/j.paid.2012.03.034. [DOI] [Google Scholar]
- Kim YW, Lee S-H, Choi TK, Suh SY, Kim B, Kim CM, Cho SJ, Kim MJ, Kook K, Ryu M, Song SK, Yook K-H. Effectiveness of mindfulness-based cognitive therapy as an adjuvant to pharmacotherapy in patients with panic disorder or generalized anxiety disorder. Depress Anxiety. 2009;26:601–606. doi: 10.1002/da.20552. [DOI] [PubMed] [Google Scholar]
- King AP, Erickson TM, Giardino ND, Favorite T, Rauch SAM, Robinson E, Madhur K, Liberzon I. A pilot study of group mindfulness-based cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD) Depress Anxiety. 2013;30:638–645. doi: 10.1002/da.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Watkins E, Holden E, White K, Taylor RS, Byford S, Evans A, Radford S, Teasdale JD, Dalgleish T. How does mindfulness-based cognitive therapy work? Behav Res Ther. 2010;48:1105–1112. doi: 10.1016/j.brat.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Lee T-W, Wu Y-T, Yu YW-Y, Chen M-C, Chen T-J. The implication of functional connectivity strength in predicting treatment response in major depressive disorder: a resting EEG study. Psychiatry Res Neuroimaging. 2011;194:372–377. doi: 10.1016/j.pscychresns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Achermann P, Jeanmonod D, Gianotti LRR, Pizzagalli D. Brain sources of EEG gamma frequency during volitionally meditation-induced, altered states of consciousness, and experience of the self. Psychiatry Res Neuroimaging. 2001;108:111–121. doi: 10.1016/S0925-4927(01)00116-0. [DOI] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practise. PNAS. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski P (2013) Neural mechanisms of attentional control in mindfulness meditation. Front Neurosci 7:8. doi:10.3389/fnins.2013.00008 [DOI] [PMC free article] [PubMed]
- Ma SH, Teasdale JD (2004) Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol 72:31–40 [DOI] [PubMed]
- Müller MM. Oscillatory cortical activities in the gamma band in the human EEG induced by visual stimuli—representation of the stimulus? Acta Neurobiol Exp. 2000;60:49–65. doi: 10.55782/ane-2000-1326. [DOI] [PubMed] [Google Scholar]
- Neff KD. Development and validation of a scale to measure self-compassion. Self Identity. 2003;2:223–250. doi: 10.1080/15298860309027. [DOI] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J Pers Soc Psychol. 1991;61:115–121. doi: 10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. J Affect Disord. 2007;104:1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamagata B, Tomioka H, Takahashi T, Yano M, Nakagome K, Mimura M. Hypofrontality in panic disorder and major depressive disorder assessed by multi-channel near-infrared spectroscopy. Depress Anxiety. 2008;25:1053–1059. doi: 10.1002/da.20463. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson DW, Haynes CT (1981) EEG phase coherence, pure consciousness, creativity, and Tm-Sidhi experiences. Int J Neurosci 13:211–217 [DOI] [PubMed]
- Palva S, Palva JM. New vistas for α-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Andrew C. Event-related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol. 1999;16:512–519. doi: 10.1097/00004691-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Jr, Neuper Ch. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/S0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. doi: 10.1111/1469-8986.3540389. [DOI] [PubMed] [Google Scholar]
- Ricardo-Garcell J, González-Olvera JJ, Miranda E, Harmony T, Reyes E, Almeida L, Galán L, Díaz D, Ramírez L, Fernández-Bouzas A, Aubert E (2009) EEG sources in a group of patients with major depressive disorders. Int J Psychophysiol 71:70–74 [DOI] [PubMed]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain Res Bull. 1998;47:297–310. doi: 10.1016/S0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sachse S, Keville S, Feigenbaum J (2010) A feasibility study for mindfulness-based cognitive therapy for individuals with borderline personality disorder. Psychol Psychother: Ther Res Prac 84:184–200 [DOI] [PubMed]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, D’Esposito M, Kleinschmidt A. Alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 2012;32:14305–14310. doi: 10.1523/JNEUROSCI.1358-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Schoenberg PLA, Speckens AEM (2014) Modulation of induced frontocentral theta (Fm-θ) event-related (de-) synchronisation dynamics following mindfulness-based cognitive therapy in major depressive disorder. Cogn Neurodyn. doi:10.1007/s11571-014-9294-0 [DOI] [PMC free article] [PubMed]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression. 2. New York: The Guildford Press; 2012. [Google Scholar]
- Segrave RA, Cooper NR, Thompson RH, Croft RJ, Sheppard DM, Fitzgerald PB. Individualised alpha activity and frontal asymmetry in major depression. Clin EEG Neurosci. 2011;42:45–52. doi: 10.1177/155005941104200110. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychitric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Siegle GJ, Condray R, Thase ME, Keshavan M, Steinhauer SR. Sustained gamma-band EEG following negative words in depression and schizophrenia. Int J Psychophysiol. 2010;75:107–118. doi: 10.1016/j.ijpsycho.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M, Berrios G (2000) The Cambridge depersonalisation scale: a new instrument for the measurement of depersonalisation. Psychiatry Res 93:153–164 [DOI] [PubMed]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk; sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelets VB, Garakh ZhV, Novototskii-Vlasov VY. Comparative study of the gamma rhythm in normal conditions, during examination stress, and in patients with first depressive episode. Neurosci Behav Physiol. 2007;37:387–394. doi: 10.1007/s11055-007-0025-4. [DOI] [PubMed] [Google Scholar]
- Suhhova A, Bachmann M, Aadamsoo K, Võhma Ü, Lass J, Hinrikus H. EEG coherence as a measure of depressive disorder. IFMBE Proceed. 2009;22:353–355. doi: 10.1007/978-3-540-89208-3_83. [DOI] [Google Scholar]
- Teasdale JD, Segal ZW, Williams JMG, Ridgeway VA, Souslby JM, Lau MA (2000) Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 68:615–623 [DOI] [PubMed]
- Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19:1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Travis F, Hagga D, Hagelin J, Arenander A, Tanner M, Schneider R. Self-referential awareness: coherence, power, and eloreta patterns during eyes-closed rest, transcendental meditation, and TM-sidhi practise. J Cogn Process. 2010;11:21–30. doi: 10.1007/s10339-009-0343-2. [DOI] [PubMed] [Google Scholar]
- van Aalderen JR, Donders ART, Giommi F, Spinhoven P, Barendregt HP, Speckens AEM (2012) The efficacy of mindfulness-based cognitive therapy in recurrent depressed patients with and without a current depressive episode: a randomized controlled trial. Psychol Med 42:989–1001 [DOI] [PubMed]
- van der Helden J, van Schie HT, Rombouts C. Observational learning of new movement sequences is reflected in fronto-parietal coherence. PLoS One. 2010;5:e14482. doi: 10.1371/journal.pone.0014482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective connectivity of the fronto-parietal network during attentional control. J Cogn Neurosci. 2010;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Watters AJ, Williams LM. Negative biases and risk for depression; integrating self-report and emotion test markers. Depress Anxiety. 2011;28:703–718. doi: 10.1002/da.20854. [DOI] [PubMed] [Google Scholar]
- Zylowska L, Ackerman DL, Yang MH, Futrell JL, Horton NI, Hale TS, Pataki C, Smalley SL (2008) Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord 11:737–746 [DOI] [PubMed]