Abstract
Women carrying BRCA1 and BRCA2 mutations have significantly elevated risk of developing breast and ovarian cancers. BRCA1-associated breast cancer likely originates from progenitors of the luminal epithelial lineage. Recent studies indicate that radiation therapy (RT) for BRCA1 cancer patients is associated with lower incidence of developing subsequent ipsilateral breast cancer. In the current study, we analyzed tumor-free breast tissue procured via prophylactic bilateral mastectomy from three BRCA1 and one BRCA2 mutation carriers, who had been previously treated with RT for unilateral breast cancers. Freshly isolated breast cells from the irradiated and nonirradiated breast tissue of the same individuals were subjected to flow cytometry, using established cell-surface markers. Two out of the three BRCA1 carriers and one BRCA2 carrier exhibited significantly diminished luminal cell population in the irradiated breast versus the nonirradiated side. There was also RT-associated reduction in the colony-forming ability of the breast epithelial cells. Our finding suggests that prior RT could result in the depletion of the luminal epithelial compartment and thus reduced incidence of BRCA1/2-associated breast cancer.
Keywords: BRCA1/2, radiation therapy, luminal epithelial cells
Background
Germ-line mutations in BRCA1 and BRCA2 predispose individuals to breast and ovarian cancers.1 At the molecular level, the best characterized BRCA1 and BRCA2 function is their activity to promote the homologous recombination (HR)-based pathway of DNA double-strand break (DSB) repair by recruiting various DNA repair proteins.2–4 The clinical relevance of BRCA1/2 function in DSB repair is highlighted by the compelling link between cancer-predisposing BRCA1/2 mutants and their compromised activity in DSB repair.
Breast epithelium consists of two layers of cells: luminal and basal epithelial cells in the inner and outer ductal layers, respectively.5 Despite the fact that BRCA1-associated breast tumors tend to fall into the basal-like subtype, emerging evidence from studies of both animal models and clinical samples from BRCA1 mutation carriers strongly suggests that BRCA1 breast tumors originate from progenitors of the luminal epithelial cells.6–8 Depletion of the cell of origin for BRCA1-associated tumors could inform the development of novel cancer-preventive measures, in addition to the currently available prophylactic mastectomy and oophorectomy for this group of at-risk women.9
Breast cancer patients with BRCA1 mutations who had received radiation therapy (RT) had reduced risk of ipsilateral breast cancer.10 The RT effect on breast cancer recurrence could be due to elimination of residual tumor cells left from the excised primary tumor.11,12 Alternatively, as BRCA1 deficiency results in compromised DSB repair and hypersensitivity to DSB-inducing agents,13,14 it is also conceivable that the RT-associated reduction in cancer incidence is caused by a diminished pool of the cell of origin for BRCA1-associated tumors. In support of the latter model, we previously showed in a case study that the luminal epithelial compartment from irradiated breast tissue of a BRCA1 mutation carrier was substantially diminished compared to the nonirradiated side of the same individual.15 In the current study, we extended our previous study by analyzing pairs of nonirradiated and irradiated breast tissue from multiple BRCA1/2 mutation carriers who underwent bilateral prophylactic mastectomy.
Materials and Methods
Tissue procurement
Fresh unfixed human breast tissue was procured from mastectomy, and subsequently digested with collagenase and hyaluronidase following the previously published procedure.16 The clinical protocol was approved by the Institutional Review Board (IRB) at the University of Texas Health Science Center at San Antonio. All donors gave consent for the use of the specimens for laboratory research.
Flow cytometry and cell sorting
Cell suspension isolated from digested breast tissue was pre-blocked and subsequently labeled with an allophycocyanin-conjugated rat antibody to human CD49f (clone GOH3, R&D Systems) and FITC-conjugated mouse antibody to human EpCAM (clone VU1-D9, StemCell Technologies), following a previously published protocol.6 Biotin-conjugated mouse antibodies to human CD45 (clone H130, eBiosciences), CD235a (clone HIR2, eBiosciences), and CD31 (clone WM59, eBiosciences) were used to label hematopoietic and endothelial cells, followed by pacific blue-conjugated streptavidin (Invitrogen). Cells were incubated with 7-ADD (BD Bioscience) before analysis to distinguish between live and dead cells. For cell sorting on a fluorescence-activated cell sorter (FACS) Aria (Becton Dickinson) and Moflo Astrios cell sorters (Beckmen Coulter), cells were separated into the following four fractions: EpCAM−CD49f− stromal cells, EpCAMlowCD49fhigh basal epithelial cells, EpCAMhigh CD49f+ luminal progenitor cells, and EpCAMhighCD49f− mature luminal epithelial cells.
Colony-forming cell assay
Fluorescence-activated cell-sorted cells were seeded with previously irradiated (30 Gy) NIH 3T3 feeder cells and cultured for 7–12 days under the condition previously described.17 Upon completion of the culturing, cells were fixed with 1:1 ratio methanol/acetone and stained with Wright’s Giemsa (Sigma). Cell colonies were imaged and enumerated under a dissecting microscope (Nikon SMZ1000).
Statistical analysis
Samples in the colony-forming cell assay were analyzed in triplicates. The P-value was calculated by Student’s t-test and was considered significant when ≤0.05.
Results and Discussion
The patient cohort used in our study consisted of three cancer-predisposing BRCA1 (BSC44, BSC88, BSC101) and one BRCA2 (BSC103) germ-line mutation carriers, aged 34–50 years (Table 1). The BRCA1 mutations were 4987C > G (BSC44), 5385insC (BSC88), and exon1–2 deletion (BSC101). The BRCA2 mutation (BSC103) was 886delGT. All four patients had been treated with RT for previous unilateral breast cancers. The patients had subsequent bilateral prophylactic mastectomy 2–9 years after RT. Following IRB-approved patient consents, we procured fresh tumor-free tissue from the nonirradiated and irradiated breasts at the time of bilateral mastectomy, and performed enzymatic digestion and single-cell isolation16 for the pair of left and right breast tissue samples from each donor.
Table 1.
Medical history of the tissue donors.
| BSC44 | BSC88 | BSC101 | BSC103 | |
|---|---|---|---|---|
| Mutation | BRCA1 (4987C > G) | BRCA1 (5385insC) | BRCA1 (exon1–2 del) | BRCA2 (886delGT) |
| Age | 34 | 40 | 42 | 50 |
| Menopause status | Post, BSO* | Post, BSO | Pre | Post, BSO |
| RT interval | 3 yrs | 2 yrs | 7 yrs | 9 yrs |
| Ethnicity | Hispanic | White/Caucasian | Hispanic | White |
| Age at 1st Preg. | 16 | 30 | 14 | 27 |
| Gravida | 6 | 3 | 3 | 1 |
| Para | 4 | 2 | 3 | 1 |
Note:
Bilateral salpingo-oophorectomy.
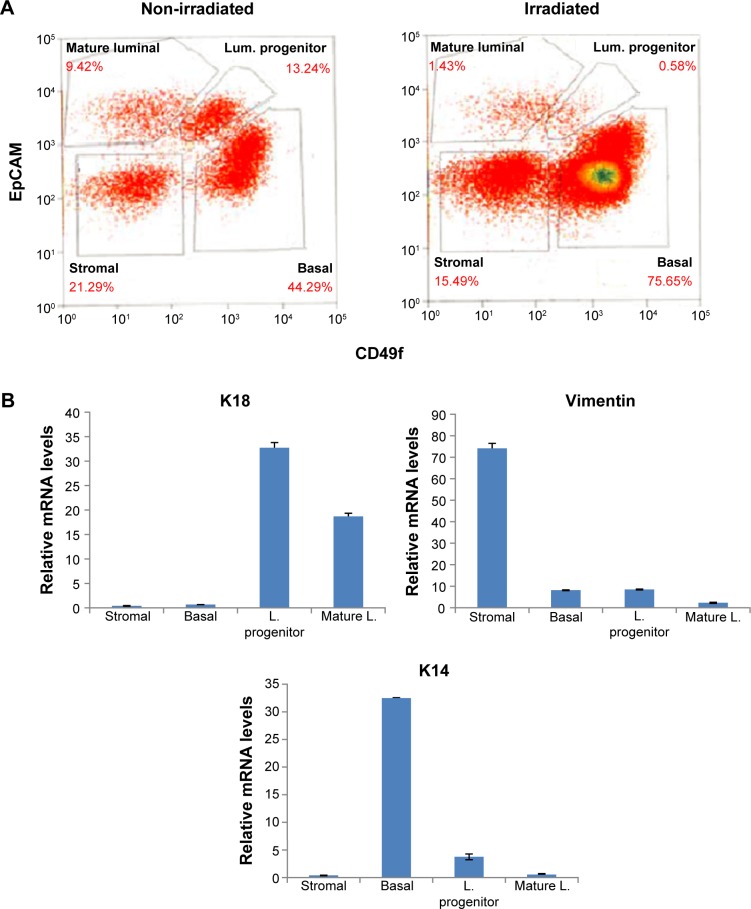
Isolated single cells were then subjected to FACS using established cell surface markers for various breast epithelial and stromal cell populations.6,17–19 As shown in Figure 1A, the procedure allowed us to distinguish the following lineage-negative cell populations: luminal progenitor cells (EpCAMhighCD49f+), mature luminal epithelial cells (EpCAMhighCD49f−), basal epithelial cells (EpCAMlow CD49fhigh), and stromal cells (EpCAM−CD49f−). We confirmed the purity of the sorted cells by assessing the mRNA levels of known markers for luminal (keratin 18), basal (keratin 14), and stromal cells (vimentin) (Fig. 1B). As shown in Table 2, two BRCA1 (BSC44 and BSC101) and one BRCA2 (BSC103) samples exhibited significantly reduced luminal progenitor cell population in the previously irradiated breast versus the nonirradiated side of the same donors (0.93% vs 21.5%, 0.58% vs 13.24%, and 10.12% vs 42.29%, respectively). This is equivalent to 89%, 96%, and 76% reduction in the RT-associated luminal progenitor epithelial population. In addition, the mature luminal fractions of the RT side from the two BRCA1 samples (BSC44 and BSC101) also showed reduced abundance (0.6% vs 5.34%, 1.43% vs 9.42%). In contrast, the remaining BRCA1 sample BSC88 did not show any substantial difference in either progenitor or mature luminal fraction between the irradiated and nonirradiated side (12.93% vs 13.66%, 10.23% vs 11.65%).
Figure 1.
RT-associated reduction of luminal epithelial compartment. (A) Flow cytometry of normal breast tissue from the nonirradiated and irradiated breasts of a BRCA1 mutation carrier (BSC101). (B) Reverse-transcriptase polymerase chain reaction of cell-type-specific markers verifies the cell sorting efficiency.
Table 2.
Enumeration of abundance of various breast cell types by flow cytometry.
| BSC44 | BSC88 | BSC101 | BSC103 | |||||
|---|---|---|---|---|---|---|---|---|
| Mutation | BRCA1 (4987C. G) | BRCA1 (5385ins C) | BRCA1 (exon1–2 del) | BRCA2 (886delGt) | ||||
| RT interval | 3 yrs | 2 yrs | 7 yrs | 9 yrs | ||||
| POPULATION (%) | NON-IRRADIATED | I RR AD I ATED | NON-IRRADIATED | IR R ADIATED | NON-IRRADIATED | I RR ADI ATED | NON-IRRADIATED | IRR AD IATED |
| Stromal | 32.4 | 32.6 | 23.93 | 23.31 | 21.29 | 15.49 | 7. 0 6 | 16.77 |
| Basal | 36.6 | 61.3 | 45.94 | 48.53 | 44.29 | 75.65 | 7. 4 4 | 11. 2 8 |
| Luminal progenitor | 21.5 | 0.93 | 13.66 | 12.93 | 13.24 | 0.58 | 42.29 | 10.12 |
| Mature luminal | 5.34 | 0.6 | 11. 6 5 | 10.23 | 9.42 | 1.43 | 31.30 | 47. 5 4 |
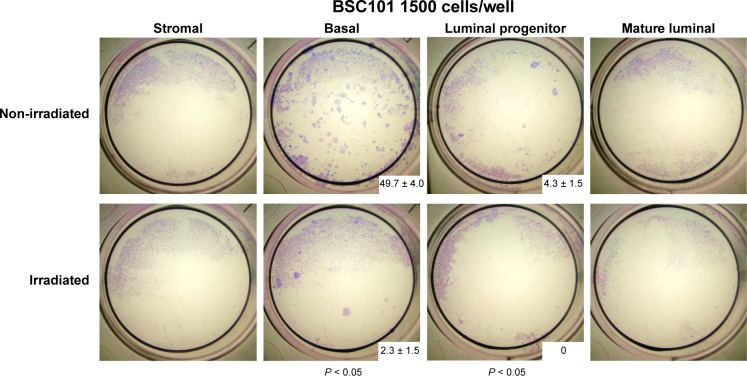
For BSC101, which yielded sufficient number of sorted cells, we also conducted in vitro mammary colony-forming cell (Ma-CFC) assay per established protocols.17,19 Upon seeding an equal number of live cells from the sorted samples with NIH 3T3 feeder cells, we cultured the cells for 7–12 days and enumerated the total colony number from triplicates of each biological sample. As expected, both luminal progenitor and basal epithelial cells, but not mature luminal or stromal cells, from the nonirradiated breast produced cell colonies (Fig. 2). However, the colony numbers from the epithelial samples of the irradiated breast were significantly diminished. This result further supports the notion that RT is associated with reduced progenitor cell activity in breast tissue from BRCA1 mutation carriers.
Figure 2.
Breast tissue previously exposed to RT has lower colony-forming activity. Sorted live epithelial cells from the nonirradiated and irradiated breast tissue (BSC101) were assessed for their ability to form colonies in vitro. Experiment was carried out in duplicate. The images were taken 7–12 days after culturing (± standard deviation). P-value was calculated by Student’s t-test.
Given the well-documented role of BRCA1 and BRCA2 in DNA DSB repair, it has been suggested that carriers of cancer-predisposing mutations of these genes are likely to be radiosensitive. However, several population-based studies of BRCA1/2 mutation carriers did not find increased RT-associated risk of developing contralateral breast cancers compared to their noncarrier counterparts,20–23 nor was there a significant association between BRCA1/2-associated breast cancer risk and mammography,24–26 where the typical dose involved is more than two orders of magnitude lower than RT.27 On the contrary, one recent report indicates that RT is associated with reduced recurrence of BRCA1-associated ipsilateral breast cancer.10 Our finding that RT, in three out of four BRCA1/2 samples analyzed, is associated with reduced luminal progenitor cell number and activity lends further support to a protective role of RT against BRCA1/2-associated tumor development. It is conceivable that intact DNA damage-responsive checkpoint mechanisms in normal breast epithelial cells, especially those proliferating progenitor cells, induce permanent cell cycle arrest and/or apoptosis in response to RT-triggered DSB, thus eliminating the damaged cells before they have the opportunity to accumulate genomic instability and undergo tumorigenesis. Following the same logic, it has been proposed that low-dose RT could be used a prophylactic measure to reduce breast cancer incidence.28 As a proof of principle, a recent study demonstrates that prophylactic mammary irradiation significantly reduces tumor incidence in a mammary tumor-prone animal model.29
A significant strength of our current study is the parallel processing and analysis of fresh bilateral breast tissue from the same donors, which allowed us to compare and contrast both the abundance and activity of irradiated and nonirradiated samples without introducing individual-based variations. Using additional clinical samples, the current finding represents an extension of our previous case study.15 However, given the exquisite nature of the rare clinical cases used in our study, our work still has the limitation of small sample size. It is prudent to continue validating the findings of our current study with more BRCA1 and BRCA2 samples that share the same rare confluence of events. In addition, when technically feasible, it is important to compare sensitivity of breast epithelial cells to RT between BRCA1/2 mutation carriers and noncarriers.
It is unclear why one BRCA1 case (BSC88) did not show any significant RT-associated difference in luminal progenitor cells. We note that, of all four donors in the study, BSC88 had the shortest interval between RT and prophylactic mastectomy (2 years). Also, the BRCA1 mutation associated with BSC88 is located further downstream of those in the other BRCA1 mutation carriers (BSC44 and BSC101), which could result in a mutant gene product with some residual DSB activity. It is also possible that additional changes in other DNA repair gene expression and/or activity could modulate the radiosensitivity of the BRCA1-mutation carrying cells. Future studies are needed to discern these and other biological factors that influence the kinetics and extent of RT-associated depletion of luminal progenitor cells in BRCA1/2 mutation carriers.
Consistent with our previous case study, the RT-associated cell depletion preferentially occurred in the luminal epithelial compartment. Neither basal epithelial nor stromal cells from the irradiated breast samples exhibited any reduction in cell number compared to the nonirradiated side. This cell-type-selective finding is reminiscent of the tissue-specific nature of BRCA-associated tumors. As the DSB repair activity of BRCA1/2 is readily demonstrable in cell lines of nonbreast or ovarian origins in vitro, it remains an enduring conundrum as to why loss of BRCA1/2 DNA repair function preferentially predisposes individuals to breast and ovarian cancers. It is tempting to speculate that epithelial cell lineage, hormonal milieu, and/or other yet to-be-defined DSB repair-independent functions of BRCA1/2 could fine-tune the cellular radiosensitivity of BRCA1/2 mutation carriers.
Conclusion
Our current work with additional BRCA1 and BRCA2 samples extends our previous case study by demonstrating RT-associated preferential depletion of luminal epithelial cells in a number of BRCA1 and BRCA2 mutation carriers studied. Our findings lend additional support to the notion that low-dose RT could effectively diminish the cell of origin of BRCA-associated breast tumors. When validated by further study, the lineage-specific epithelial cell depletion could inform the development of new approaches for cancer prevention for at-risk women.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
FUNDING: This work was supported by NIH grants to RL (CA161349 and CA184084) and YH (CA170306). The authors gratefully acknowledge the support of the Cancer Therapy and Research Center at the University of Texas Health Science Center San Antonio, an NCI-designated cancer center. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conducted the experiments: H-CC. Recruited the patients and provided clinical guidance and interpretation to the work: RE. Designed the experiments and wrote the manuscript: RL, YH. Tissue procurement: PL, IJ. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorslund T, West SC. BRCA2: a universal recombinase regulator. Oncogene. 2007;26(56):7720–7730. doi: 10.1038/sj.onc.1210870. [DOI] [PubMed] [Google Scholar]
- 3.Huen MS, Sy SM, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11(2):138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parameswaran B, Chiang HC, Lu Y, et al. Damage-induced BRCA1 phosphorylation by Chk2 contributes to the timing of end resection. Cell Cycle. 2015;4:437–448. doi: 10.4161/15384101.2014.972901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23(22):2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim E, Vaillant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux G, Geyer FC, Magnay FA, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7(3):403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Proia TA, Keller PJ, Gupta PB, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8(2):149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KL, Isaacs C. BRCA mutation testing in determining breast cancer therapy. Cancer J. 2011;17(6):492–499. doi: 10.1097/PPO.0b013e318238f579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe K, Lynch HT, Ghadirian P, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2011;127(1):287–296. doi: 10.1007/s10549-010-1336-7. [DOI] [PubMed] [Google Scholar]
- 11.Holland R, Veling SHJ, Mravunac M, Hendriks JHCL. Histologic multifocality of tis, T1–2 breast carcinomas implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8:113–118. doi: 10.1200/JCO.1990.8.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Murphy CG, Moynahan ME. BRCA gene structure and function in tumor suppression: a repair-centric perspective. Cancer J. 2010;16(1):39–47. doi: 10.1097/PPO.0b013e3181cf0204. [DOI] [PubMed] [Google Scholar]
- 14.O’Donovan PJ, Livingston DM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 2010;31(6):961–967. doi: 10.1093/carcin/bgq069. [DOI] [PubMed] [Google Scholar]
- 15.Chiang HC, Nair SJ, Yeh IT, et al. Association of radiotherapy with preferential depletion of luminal epithelial cells in a BRCA1 mutation carrier. Exp Hematol Oncol. 2012;1(1):31. doi: 10.1186/2162-3619-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eirew P, Stingl J, Raouf A, et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14:1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- 17.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 18.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67(2):93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 19.Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217(2):229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- 20.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24(16):2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 21.Shanley S, McReynolds K, Ardern-Jones A, et al. Breast Unit of the Royal Mars-den NHS Foundation Trust Late toxicity is not increased in BRCA1/BRCA2 mutation carriers undergoing breast radiotherapy in the United Kingdom. Clinical Cancer Res. 2006;12(23):7025–7032. doi: 10.1158/1078-0432.CCR-06-1244. [DOI] [PubMed] [Google Scholar]
- 22.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein JL, Thomas DC, Shore RE, et al. WECARE Study Collaborative Group Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: a WECARE study report. Eur J Cancer. 2013;49(14):2979–2985. doi: 10.1016/j.ejca.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfrank D, Chuai S, Bernstein JL, et al. Effect of mammography on breast cancer risk in women with mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2311–2313. doi: 10.1158/1055-9965.EPI-06-0176. [DOI] [PubMed] [Google Scholar]
- 25.Narod SA, Lubinski J, Ghadirian P, et al. Hereditary Breast Cancer Clinical Study Group Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet Oncol. 2006;7(5):402–406. doi: 10.1016/S1470-2045(06)70624-6. [DOI] [PubMed] [Google Scholar]
- 26.Giannakeas V, Lubinski J, Gronwald J, et al. Mammography screening and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a prospective study. Breast Cancer Res Treat. 2014;147(1):113–118. doi: 10.1007/s10549-014-3063-y. [DOI] [PubMed] [Google Scholar]
- 27.Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD., Jr Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res. 2002;158(2):220–235. doi: 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Brenner DJ, Shuryak I, Russo S, Sachs RK. Reducing second breast cancers: a potential role for prophylactic mammary irradiation. J Clin Oncol. 2007;25(31):4868–4872. doi: 10.1200/JCO.2007.11.0379. [DOI] [PubMed] [Google Scholar]
- 29.Shuryak I, Smilenov LB, Kleiman NJ, Brenner DJ. Potential reduction of contralateral second breast-cancer risks by prophylactic mammary irradiation: validation in a breast-cancer-prone mouse model. PLoS One. 2013;8(12):e85795. doi: 10.1371/journal.pone.0085795. [DOI] [PMC free article] [PubMed] [Google Scholar]