In mice lacking thyroid hormone receptors, bulge stem cells of the hair follicles present epigenetic alterations and a functional defect in their mobilization out of the niche. This is related to aberrant activation of Smad signaling and reduced nuclear β-catenin accumulation, an important component of stem cell mobilization.
Abstract
Observations in thyroid patients and experimental animals show that the skin is an important target for the thyroid hormones. We previously showed that deletion in mice of the thyroid hormone nuclear receptors TRα1 and TRβ (the main thyroid hormone–binding isoforms) results in impaired epidermal proliferation, hair growth, and wound healing. Stem cells located at the bulges of the hair follicles are responsible for hair cycling and contribute to the regeneration of the new epidermis after wounding. Therefore a reduction in the number or function of the bulge stem cells could be responsible for this phenotype. Bulge cells show increased levels of epigenetic repressive marks, can retain bromodeoxyuridine labeling for a long time, and have colony-forming efficiency (CFE) in vitro. Here we demonstrate that mice lacking TRs do not have a decrease of the bulge stem cell population. Instead, they show an increase of label-retaining cells (LRCs) in the bulges and enhanced CFE in vitro. Reduced activation of stem cells leading to their accumulation in the bulges is indicated by a strongly reduced response to mobilization by 12-O-tetradecanolyphorbol-13-acetate. Altered function of the bulge stem cells is associated with aberrant activation of Smad signaling, leading to reduced nuclear accumulation of β-catenin, which is crucial for stem cell proliferation and mobilization. LRCs of TR-deficient mice also show increased levels of epigenetic repressive marks. We conclude that thyroid hormone signaling is an important determinant of the mobilization of stem cells out of their niche in the hair bulge. These findings correlate with skin defects observed in mice and alterations found in human thyroid disorders.
INTRODUCTION
The skin, which protects organisms from the external environment, is an organ with high regenerative capacity. This is related to the existence of a very active stem cell (SC) compartment. In mice, the best-characterized SC niche is a region located at the base of hair follicle known as the bulge (Blanpain and Fuchs, 2009). Bulge cells divide several times during anagen (Rochat et al., 1994), generating and maintaining hair cycling. In addition, bulge SCs are multipotent and can also differentiate into epidermis and sebaceous glands (Oshima et al., 2001; Blanpain et al., 2004; Claudinot et al., 2005). Although under normal conditions bulge SCs do not contribute to the homeostasis of the interfollicular epidermis (Morris et al., 2004; Jaks et al., 2008), after skin wounding, these cells rapidly migrate upward to support the regeneration of the damaged epidermis (Taylor et al., 2000; Ito et al., 2005; Levy et al., 2007; Nowak et al., 2008). Bone morphogenetic protein (BMP), Wnt, and Hedgehog signaling are the main pathways converging to regulate bulge SC activation (Jamora et al., 2003; Silva-Vargas et al., 2005; Plikus et al., 2008; Greco et al., 2009; Fuchs and Chen, 2013; Kandyba et al., 2013). Smad-mediated BMP signals are involved in maintaining the quiescent nature of resting follicle SCs (Zhang et al., 2006; Kobielak et al., 2007; Yang et al., 2009), and BMP signaling inhibits SC activation, at least in part by inhibiting nuclear localization of β-catenin, which regulates the expression of genes involved in proliferation and SC mobilization (Huelsken et al., 2001; Lowry et al., 2005; Plikus et al., 2008).
An important characteristic that allows the identification of the bulge SCs is their quiescence; once they are labeled with bromodeoxyuridine (BrdU) when they are proliferating during the neonatal period, they can retain the label for a very long time (Cotsarelis et al., 1990; Morris and Potten, 1999; Braun et al., 2003). In contrast with these “label-retaining cells” (LRCs), labeling is gradually diluted and lost in other cells that divide rapidly. Hair follicle SCs express specific markers, including CD34 (Morris and Potten, 1999; Trempus et al., 2003; Blanpain et al., 2004), K15 (Morris and Potten, 1999), Lgr5 (Jaks et al., 2008), Sox 9 (Vidal et al., 2005), Lgr6 (Snippert et al., 2010), and Lhx2 (Rhee et al., 2006), and they show epigenetic changes (Benitah, 2012), presenting high levels of trimethylated histone H3 at lysine 9 and hypoacetylation of histone H4 (Frye et al., 2007). Another important feature of epidermal SCs is their high proliferative capacity, demonstrated by their colony-forming efficiency (CFE) in vitro (Barrandon and Green, 1987; Jones and Watt, 1993), which is believed to correlate with the number of SCs (Jones and Watt, 1993; Kobayashi et al., 1993), since each colony is believed to be derived from one individual SC.
Thyroid hormones have an important role in skin homeostasis (Slominski and Wortsman, 2000; Slominski et al., 2002; Paus, 2010). In hypothyroid patients, the epidermis is thin, and they frequently develop alopecia (Freinkel and Freinkel, 1972), showing that thyroid hormone signaling can regulate both skin proliferation and hair growth (Ahsan et al., 1998; Billoni et al., 2000; Messenger, 2000; van Beek et al., 2008). Not only hypothyroidism, but also hyperthyroidism, is known to cause hair loss in humans, indicating the importance of thyroid hormone signaling in this process. It has been shown that prolonged thyroid hormone stimulation can inhibit in vitro clonal expansion and promote differentiation of cultured K15-positive human progenitor cells, eventually leading to SC depletion (Tiede et al., 2010).
Most actions of the thyroid hormones are mediated by binding to nuclear receptors (thyroid hormone receptors [TRs]) that act as ligand-dependent transcription factors. TRs are encoded by two different genes, and TRα1 and TRβ are the main thyroid hormone–binding isoforms (Pascual and Aranda, 2013). We recently found reduced keratinocyte proliferation and decreased hyperplasia in response to topical application of 12-O-tetradecanolyphorbol-13-acetate (TPA) or retinoids in mice lacking these receptors, showing that the effects of the thyroid hormones on skin proliferation are mediated through interactions with TRα1 and TRβ and that both receptor genes contribute to give a normal proliferative response (Contreras-Jurado et al., 2011; Garcia-Serrano et al., 2011). Furthermore, we recently observed that mice lacking TRα1 and TRβ display impaired hair cycling associated with a decrease in follicular cell proliferation and develop alopecia after serial depilation (Contreras-Jurado et al., 2014). These mice also presented a wound-healing defect, with retarded reepithelialization, associated with impaired proliferation, in agreement with the finding that thyroid hormone administration accelerates wound healing in mice (Safer et al., 2005). In this work, we provide evidence that the observed phenotype is associated with functional defects in the bulge SCs. In TR-deficient mice, bulge SCs present a clear defect in their mobilization (exit of their quiescent state and migration out of the niche), associated with increased activation of Smad signaling. This leads to reduced nuclear β-catenin accumulation and c-Myc expression, which are essential for bulge SC activation.
RESULTS
SCs are not depleted in the bulges of TR-deficient mice
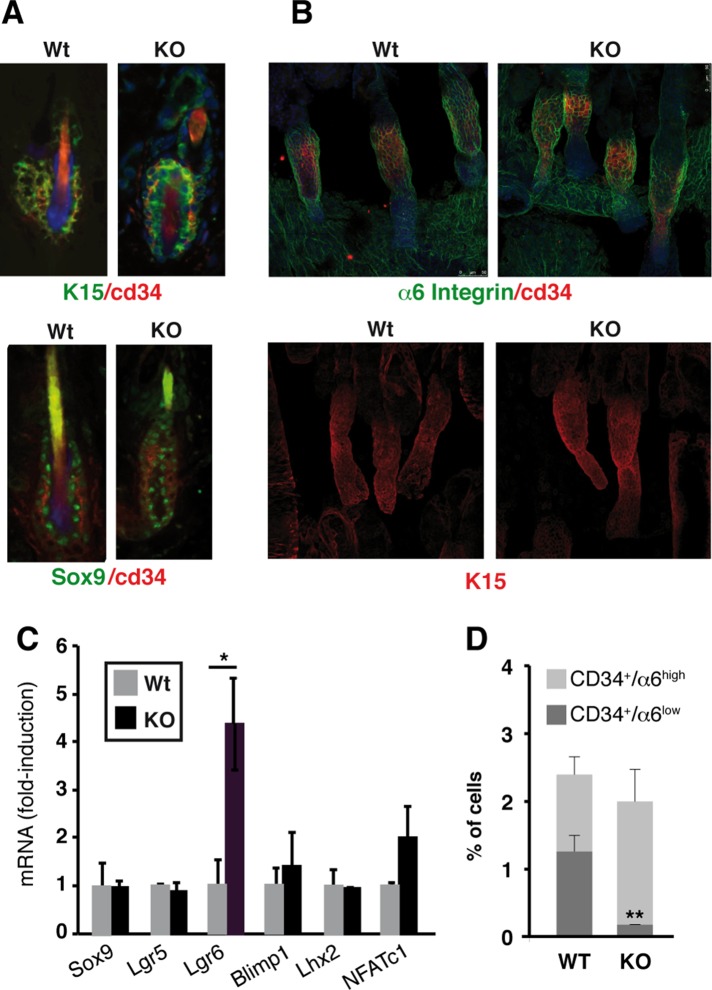
The skin phenotype of mice with defective thyroid hormone signaling (reduced response to proliferative stimuli, retarded hair growth, and impaired wound healing) suggests a defect in the number or function of the bulge SCs. This prompted us to analyze the bulge SC population in TR-deficient mice. Immunofluorescence analysis of the hair bulges of the dorsal skin or whole mounts of tail skin follicles demonstrated that the expression of the SC markers CD34, K15, and Sox9 was not reduced in TR-deficient mice (Figure 1, A and B). Furthermore, the expression of most epidermal SCs markers, determined by quantitative PCR, was not significantly altered, with the exception of Lgr6, a marker of SC in the hair infundibulum (Snippert et al., 2010), which increased in the absence of TRs (Figure 1C). We also observed a moderate increase in NFATc1 (Figure 1C), which promotes SC quiescence downstream of BMP signaling (Horsley et al., 2008). These data supported the idea that the absence of TRs did not cause SC depletion. Because Lgr6+ cells are involved in sebaceous gland generation, we used red oil staining to compare the skin of wild-type (WT) and TR-knockout (KO) mice, but we did not find differences in sebaceous gland number or morphology (Supplemental Figure S1).
FIGURE 1:
TR inactivation does not lead to SC depletion in hair bulges. (A) Double immunofluorescence of SC markers keratin 15 (K15), CD34, and Sox9 in the bulges of dorsal skin of WT and TR KO mice. Nuclei were counterstained with DAPI (blue), and merge images are shown. (B) Confocal images of α6 integrin, CD34, and K15 expression in whole mounts of tail skin in both groups. (C) Transcript levels of the indicated SC markers determined in telogen epidermis of WT and TR KO animals. Results are expressed relative to the values obtained in WT mice. (D) Flow cytometry analysis of surface levels of CD34 and α6 integrin in WT and KO mice. Subpopulations of CD34+ cells expressing high and low levels of α6 integrin were calculated. Data are means ± SD. *p < 0.05, **p < 0.01.
Two distinct populations of multipotent bulge SCs have been described, one attached and other detached from the basal lamina. They both display high surface CD34 but differ in surface α6 integrin, a component of the hemidesmosomes that mediate attachment to the basal lamina (Blanpain et al., 2004). Flow cytometry analysis indicated no major alterations in the percentage of CD34+ cells as a consequence of TR absence (Figure 1D), in agreement with the immunofluorescence analysis. Nonetheless, the analysis of integrin α6/CD34 populations revealed that TR deletion produced a significant depletion of the α6lowCD34+ subpopulation with respect to the α6highCD34+ subpopulation. In normal mice, ∼50% of the CD34+ cells were α6high, whereas this population did not exceed 10% of the total in the KO mice (Figure 1D).
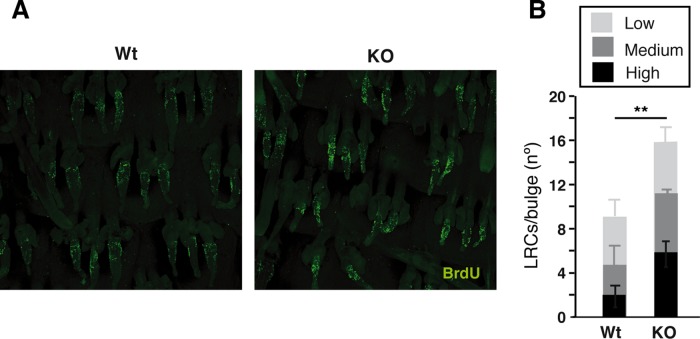
On the other hand, the impaired hair growth and delayed wound healing observed when thyroid hormone signaling is impaired may indicate defective SC replication or mobilization. Therefore we next investigated whether the LRCs in the hair bulge were affected by the absence of TRs. For this purpose, we extensively labeled the skin of neonatal WT and TR KO mice with BrdU and detected the presence of LRCs in whole mounts of mice tail epidermis after 75 d. As shown in Figure 2A, the bulges of the TR KO mice showed stronger BrdU labeling. Quantification of the confocal images demonstrated that the number of LRCs was higher in the bulges of the TR-deficient animals. This was particularly evident for cells showing high and medium BrdU label intensity, corresponding to SCs that had experienced fewer cell divisions (Figure 2B). To examine further the LRC population dynamics, we detected LRCs in dorsal skin bulges by BrdU immunohistochemistry after 30 and 75 d of labeling. As shown in Supplemental Figure S2, the percentage of hair follicle bulges containing LRCs was similar in WT and KO mice after a 30-d chase. However, whereas a significant reduction between 30 and 75 d was observed in WT animals, this did not occur in TR KO mice, and at 75 d, a significantly higher number of labeled bulges were detected in these animals. These results suggest that SCs of the hair follicles replicate less in mice lacking TRs, and, as a consequence, the number of LRCs in the niche increases. To study further the effect of TR inactivation on the epidermal SC function, we performed clonogenic assays. After 10 d in culture, TR KO SCs gave rise to a significantly larger number of colonies than those of WT animals (Figure 3), suggesting that the reduced proliferation of the SCs in TR KO epidermis is not due to a cell-autonomous effect but instead to an alteration in SC niche signaling.
FIGURE 2:
LRC quantification in whole mounts of tail epidermis. (A) WT and TR KO neonatal mice (six mice/group) were injected with BrdU, and LRCs were identified by immunofluorescence after 75 d. (B) BrdU labeling intensity in the bulges of untreated WT and TR-deficient mice was quantified, and the number of LRCs with high, medium, and low label intensity is shown. High intensity represents nuclei in which the labeled area is >55 μm2; medium intensity, >42 but <55 μm2; and low intensity, >28 but <42 μm2. **p < 0.01.
FIGURE 3:
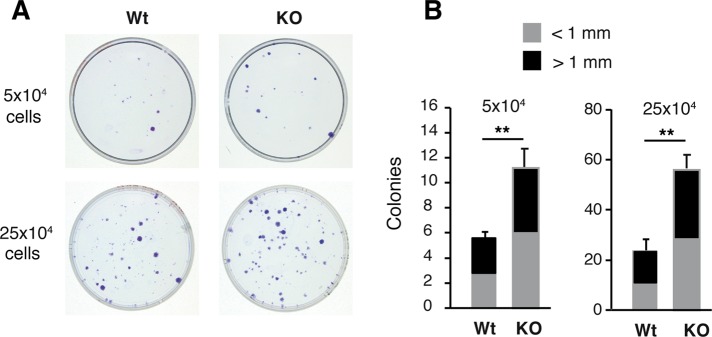
Enhanced colony formation efficiency in TR-deficient mice (A) Representative images of CFE of keratinocytes isolated from adult WT and TR KO mice plated at two different concentrations (5 × 104 and 25 × 104 cells/well). (B) Quantification of the size and number of colonies obtained in both genotypes. Data are means ± SD. **p < 0.01.
Epigenetic alterations in the hair follicles of TR-deficient mice
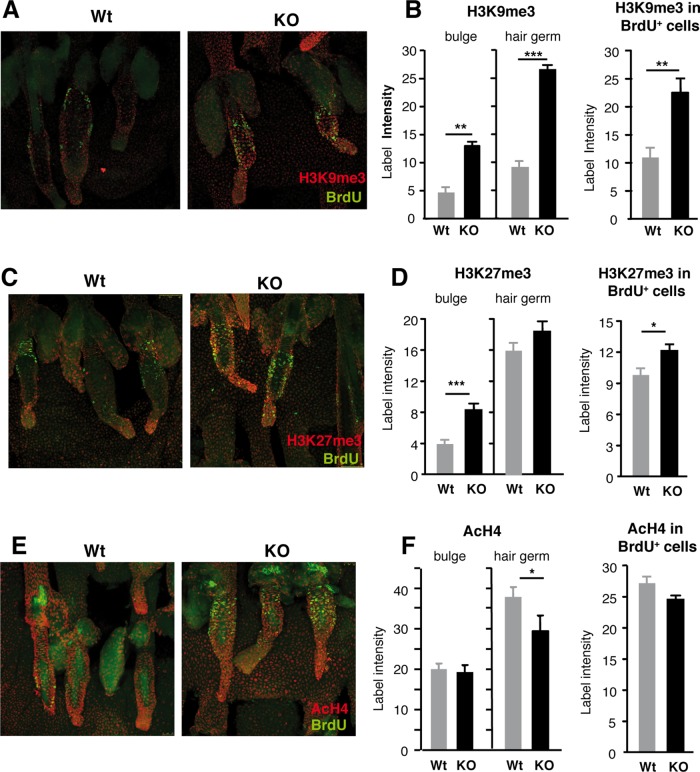
TRs and other nuclear receptors are transcription factors that regulate gene expression by recruiting coactivator and corepressor complexes that induce important epigenetic changes (Pascual and Aranda, 2013). Hair follicle bulges are characterized by presenting high levels of trimethylated histone H3 at lysine 9 (H3K9me3; Frye et al., 2007), an epigenetic mark associated with transcriptional repression (Barski et al., 2007). As illustrated in Figure 4, when we analyzed the levels of H3K9me3 in whole mounts of tail skin, we observed that the bulges of TR KO mice displayed significantly higher levels of H3K9me3 than their WT counterparts, and this increase was also found in the hair germs. Furthermore, double labeling for BrdU and the trimethylated histone demonstrated that the LRCs of the bulge of TR-deficient mice were also enriched in the histone mark. Another repressive mark, trimethylation of histone H3 at lysine 27 (H3K27me3), was also increased in the bulges of KO mice. In addition, global acetylation of H4, a mark for transcriptional activity, was similar in the bulges of WT and KO mice, and only a small reduction of AcH4 was found in the hair germs of the KO mice. Therefore the LRCs of the TR KO mice presented epigenetic alterations, affecting specifically the repressive methylation marks, that could be related to altered gene expression in these cells.
FIGURE 4:
LRCs in the bulges of TR KO mice are enriched in trimethylated histone 3 at lysines 9 and 27. (A) Neonatal mice were injected with BrdU, and after the chase period, whole mounts of tail epidermis from WT and TR KO mice were used for double immunofluorescence with H3K9me3 and BrdU antibodies. (B) Quantification of the label intensity of H3K9me3 in the bulges, hair germs, and LRCs (BrdU-positive cells in the bulges). (C) Representative images obtained as in A with BrdU and H3K27me3 antibodies. (D) Quantification of H3K27me3 in the bulges, hair germs, and LRCs. (E) Double immunofluorescence images of BrdU and acetyl histone 4 (AcH4). (F) Quantification of AcH4 in the bulges, hair germs, and LRCs of WT and TR KO mice. Data are means ± SE. *p < 0.05, **p < 0.01, and ***p < 0.001.
LRC mobilization is impaired in the absence of TRs
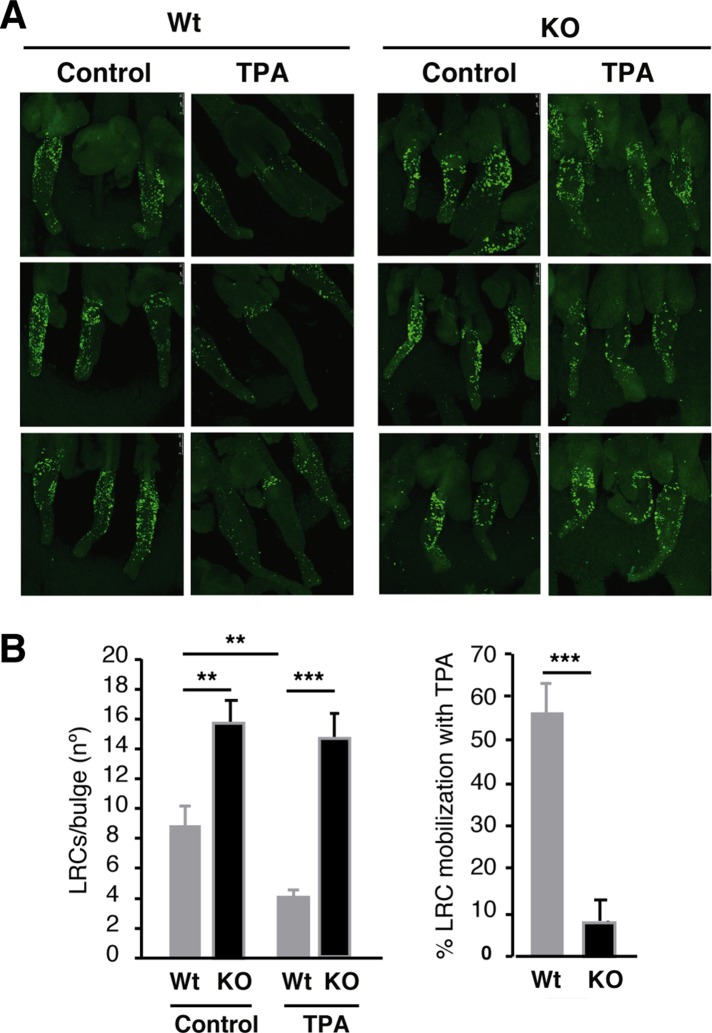
A defect in SC mobilization would be compatible with the observed phenotype in TR-deficient mice. Therefore we next examined LRC mobilization, studying the response of WT and TR KO mice to TPA treatment at the end of the chase period. This treatment activates LRCs to give numerous progeny and migrate, resulting in rapid disappearance of LRCs from the bulges (Braun et al., 2003). After TPA treatment, WT epidermis showed a strong reduction (54%) in the number of LRCs localized within the bulge. In contrast, TPA-treated TR KO mice showed a much smaller reduction (7%), indicating that the absence of TRs causes a functional defect in SC mobilization out of the niche (Figure 5).
FIGURE 5:
TR deletion blocks LRC mobilization. (A) Neonatal mice were labeled with BrdU, and 68 d later, tails were topically treated with TPA as described in Materials and Methods. Representative LRC images of BrdU immunofluorescence in whole mounts of tail epidermis from vehicle-treated (control) and TPA-treated WT and TR KO mice. (B) Number of LRCs in which the label occupied an area >28 μm2 was scored in control and TPA-treated mice. ANOVA followed by Bonferroni test was used to analyze differences among groups. (C) Percentage of LRCs mobilized upon TPA treatment in both genotypes. Data are means ± SD. **p < 0.01 ***p < 0.001.
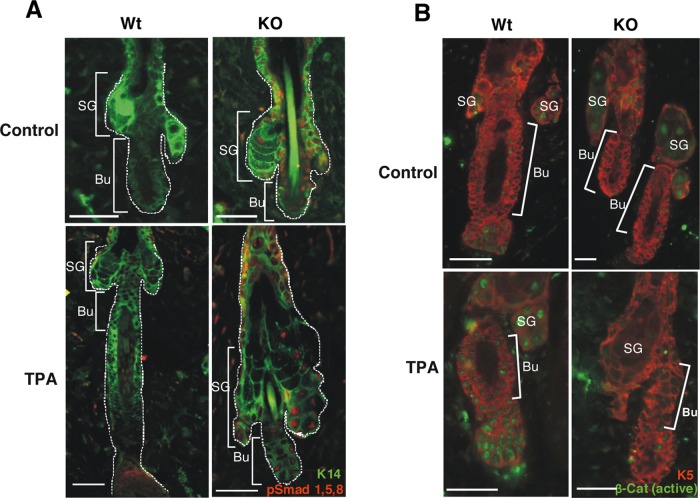
Because BMP signaling produced primarily by the SC niche plays an essential role as an inhibitor of bulge SC activation, and the expression of NFATc1, which is regulated by BMPs, is increased in TR-deficient mouse skin, we next analyzed the levels of phosphorylated Smad1,5,8 in the follicles. As shown in Figure 6A, P-Smad levels detected by immunofluorescence in the dorsal skin were strongly increased in the TR-deficient mice both under basal conditions and after TPA treatment. Nuclear accumulation of β-catenin induces activation and mobilization of the bulge SCs from the niche (Fuchs and Chen, 2013), and Smad factors act, at least in part, by inhibiting Wnt/β-catenin signaling (Kobielak et al., 2007). Therefore we next analyzed nuclear β-catenin levels in the hair follicles of WT and TR KO mice. In agreement with the increased Smad1,5,8 phosphorylation, nuclear β-catenin was basally reduced in the TR KO mice. In addition, TPA caused a strong increase of β-catenin nuclear accumulation in the follicles of WT animals while only inducing a weak increase in the TR KO mice (Figure 6B). Once it is translocated into the nucleus, β-catenin binds members of the TCF/LEF family of transcription factors, controlling the expression of several target genes, such as Cyclin D1 or c-Myc (Clevers, 2006), with important roles in follicular proliferation. As shown in Figure 7A, levels of the transcripts encoding these genes were reduced in the epidermis of the TR KO mice in both the presence and absence of TPA. Furthermore, because c-Myc is known to regulate SC mobilization (Arnold and Watt, 2001; Waikel et al., 2001; Watt et al., 2008), we also analyzed by immunofluorescence the expression of the activated oncoprotein in the follicles of WT and TR KO mice (Figure 7B). We observed that c-Myc activation after TPA treatment was prevented in TR KO mice, which may also contribute to the strong defect in LRC mobilization found in these animals.
FIGURE 6:
Smad signaling is aberrantly activated in the follicles of TR KO mice. (A) Double immunofluorescence images of K14 and pSmad1,5,8 in the skin of control and TPA-treated WT and TR KO mice, showing increased Smad phosphorylation in the hair follicles of TR-deficient mice. (B) Double immunofluorescence of nuclear β-catenin and K5 in the same groups. Hair follicles are marked by a white line, and brackets indicate the location of the bulges (Bu) and the sebaceous gland (Sg). Bars, 150 μm.
FIGURE 7:
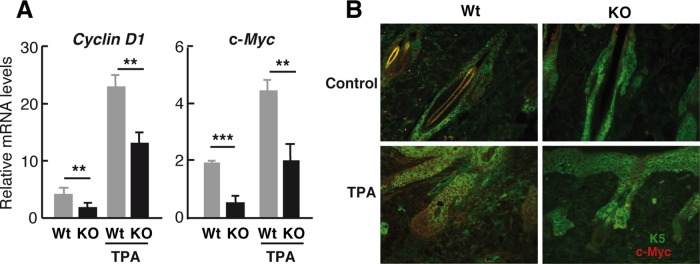
Reduced expression of β-catenin targets in TR-deficient mice. (A) Transcript levels of the β-catenin target genes Cyclin D1 and c-Myc determined in epidermis of control and TPA-treated WT and TR KO animals. Results are expressed relative to the Gus mRNA levels. Data are means ± SD. **p < 0.01, ***p < 0.001. (B) Double immunofluorescence of K5 and activated c-Myc in the skin of control and TPA-treated WT and TR KO mice.
Finally, to explore whether exogenous addition of thyroid hormones could alter BMP or Wnt signaling, we analyzed the levels of phosphorylated Smad1,5,8 and nuclear β-catenin in the bulges of dorsal skin follicles of control mice and mice that were treated with supraphysiological concentrations of the thyroid hormones T4 and T3 for 30 d. As shown in Figure 8A, the number of P-Smad–positive cells in resting follicles was significantly reduced in the hyperthyroid mice, whereas nuclear β-catenin levels were strongly increased in the anagen follicles of these animals (Figure 8B). Therefore these pathways are altered in an opposite way by TR deficiency and thyroid hormone excess.
FIGURE 8:
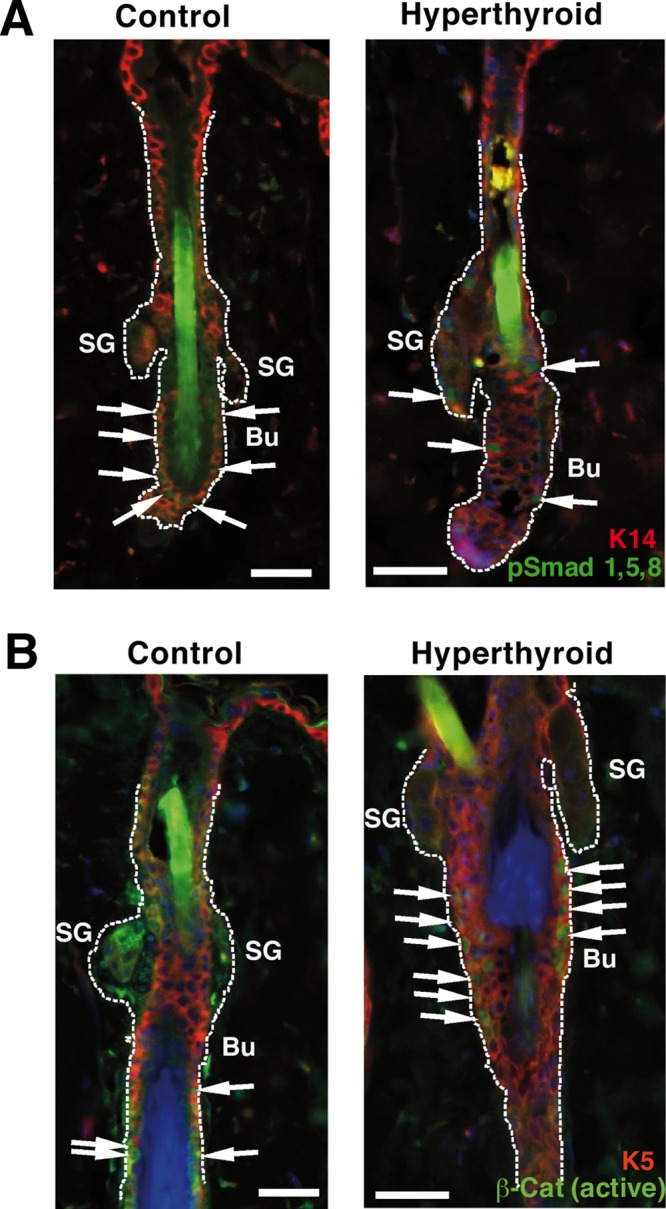
Reduced Smad signaling in the hair follicles of hyperthyroid mice. (A) Representative double immunofluorescence images of K14 and pSmad1,5,8 in the follicles of control mice and mice treated for 30 d with thyroid hormones (hyperthyroid). Arrows indicate the presence of cells positive for the phosphorylated Smad. (B) K5 and nuclear β-catenin detected by double immunofluorescence in control and hyperthyroid hair follicles. β-Catenin–positive cells are shown with arrows. Bu, bulge; SG, sebaceous gland. Bars,150 μm.
DISCUSSION
Both clinical and experimental data indicate that thyroid hormones stimulate hair growth (Freinkel and Freinkel, 1972; Ahsan et al., 1998; Billoni et al., 2000; Messenger, 2000; Safer et al., 2001; van Beek et al., 2008). We showed that disruption of TRs in mice impairs anagen entry, which is compatible with alterations in the bulge stem cells. In addition, cutaneous wounds heal more slowly in TR KO animals (Contreras-Jurado et al., 2014). Retarded reepithelialization in the absence of TRs is also compatible with a reduced contribution of SCs from the bulge, since these cells have been shown to support the regeneration of the injured interfollicular epidermis (Taylor et al., 2000; Ito et al., 2005; Levy et al., 2007; Nowak et al., 2008).
The skin phenotype of TR KO mice suggested the existence of a defect in the number or function of the bulge SCs. It was possible that in the absence of TRs, reduced SC generation, premature differentiation, or a lower survival could lead to SC depletion. However, TR ablation did not decrease the expression of bulge SC markers. Another main feature of quiescent follicle SCs, BrdU label retention, was increased after TRs disruption. This increased retention could suggest an accumulation of stem cells in the bulges of the TR KO mice. However, quantification of the number of CD34/α6 integrin–positive cells did not reveal differences between WT and KO mice. Instead, the increased numbers of LRCs might indicate that SCs divide or migrate out of the niche less frequently in the absence of TRs. In this regard, SCs from TR KO animals show increased CFE, suggesting that the absence of TRs promotes an antiproliferative signal from the SC niche, leading to reduced SC proliferation in vivo. This suggests an important role of thyroid hormone signaling in maintaining homeostasis of follicle SCs not only in a cell-autonomous manner, but also in a non–cell-autonomous manner affecting the SC niche.
We found increased Lgr6 expression in the skin of TR KO mice. This surface marker is characteristic of a SC population different from the CD34+ or the LRCs of the hair bulge, which generates sebaceous glands and interfollicular epidermis but does not contribute to hair follicle formation (Snippert et al., 2010). Augmented Lgr6 transcripts suggest that this population might be affected by the absence of TR signaling. Although the sebaceous glands appear to be normal in TR-deficient mice, the implication of increased Lrg6 expression in skin homeostasis merits further investigation.
The phenotype observed in animals lacking TRs would be compatible with inability of stem mobilization (exit of quiescent state and migration out of the niche), which was analyzed by neonatal BrdU injection and TPA administration at the end of the chase period. In these experiments, we demonstrated that TR ablation blocks mobilization of epidermal SCs, enriching the LRC population in the SC niche after TPA treatment. This occurred concurrently with a strong reduction in the number of follicular proliferating cells and in the anagen response and with the reduced skin hyperplasia in response to the tumor promoter previously observed by us (Contreras-Jurado et al., 2011; Garcia-Serrano et al., 2011). These results also suggested an altered susceptibility of TR-deficient mice to develop skin tumors. Indeed, these animals developed fewer tumors than normal mice when subjected to a two-stage chemical carcinogenesis protocol. However, the tumors formed became larger and more aggressive after chronic TPA treatment (Martinez-Iglesias et al., 2009), showing that TRs can have divergent effects on cell proliferation and tumor progression.
Signaling pathways that are essential determinants of bulge SCs function were profoundly altered in the skin of TR-deficient mice. BMP signaling plays a key repressive role in bulge SC activation. Overexpression of BMP4 or deletion of Noggin (a BMP antagonist) impairs hair cycling, causing progressive hair loss (Botchkarev et al., 1999). Furthermore, BMP signaling in the follicle SC niche inhibits follicle SC activation, whereas expression of Noggin activates the follicle SCs during anagen induction by cyclical inactivation of BMP signaling (Zhang et al., 2006). BMPs induce the phosphorylation of members of the Smad proteins, particularly Smad1,5,8. Nuclear localization of P-Smad1,5,8 together with Smad-interacting proteins and target genes is present in the resting bulge, indicating a role for Smad-BMP signals in maintaining quiescence of hair follicle SCs (Jamora et al., 2003; Plikus et al., 2008; Greco et al., 2009; Fuchs and Chen, 2013; Kandyba et al., 2013). Phosphorylation of Smad1/5/8 was strongly increased in the hair follicles of mice lacking TRs. Thus excessive BMP-Smad signaling appears to play a role on the reduced SC proliferation found in the absence of thyroid hormone signaling. Identification of the molecular mechanisms by which TRs modulate this signaling pathway will be essential to fully understand the role of the thyroid hormones in skin homeostasis and SC function.
A balance between BMP/Smad and Wnt/β-catenin pathways determines bulge SC activation, which is dependent on β-catenin stabilization (Moore et al., 1999; Jamora et al., 2003; Kobielak et al., 2007; Kandyba et al., 2013). Cycling of the hair follicle is dependent on a change in the transcriptional status of genes that are regulated by Wnt signaling. β-Catenin controls genes involved in follicle SC proliferation and their activation from the resting to the activated state. In agreement with the activation of Smad phosphorylation, we found inhibition of nuclear β-catenin accumulation (Kobielak et al., 2007) in TR KO mice, which leads, among other changes, to reduced c-Myc activation, which is an important component of SC mobilization (Arnold and Watt, 2001; Waikel et al., 2001; Watt et al., 2008).
In addition to regulation of Smad1,5,8 phosphorylation, other mechanisms could contribute to reduced β-catenin accumulation in TR-deficient mice. Thus TRα1 induces β-catenin gene transcription in the intestine in a cell-autonomous way, leading to increased expression of cyclin D1 and c-myc genes. Moreover, TRα1 interacts with the β-catenin/Tcf4 complex, and this interaction has a positive effect on the Wnt downstream response in vitro (Plateroti et al., 2006; Sirakov et al., 2012). These results are compatible with the reduced β-catenin and target gene expression we found in the skin of TR KO animals.
In contrast to the phenotype found in TR-deficient mice, treatment of euthyroid mice with an excess of thyroid hormones reduces follicular Smad phosphorylation and increases nuclear β-catenin, suggesting that follicle SCs would be overactive in these animals. These results point toward the possibility that whereas lack of thyroid hormone signaling can lead to defective SC mobilization, excessive signaling could lead to exhaustion of the SC reservoir, which could be associated with the observation that continued thyroid hormone treatment can cause depletion of K15-positive cells in humans (Tiede et al., 2010) and with the hair loss observed in hyperthyroid patients.
Another mechanism that could underlie the anomalous function of bulge SCs in the absence of TRs is an alteration in the epigenetic profile. Quiescent stem cells in the hair follicle bulge are enriched in H3K9me3 (Frye et al., 2007). The levels of this repressive mark, as well as of H3K27me3, were increased in the LRCs of the TR-deficient mice. This may indicate that chromatin silencing is increased in the SC population when the receptor is not present, suggesting that thyroid hormone signaling induces a widespread change in chromatin state that is permissive for transcription and SC mobilization.
In agreement with the finding that hair cycling and wound healing are impaired in mice lacking TRs, topical treatment with the thyroid hormone induces hair growth and accelerates wound healing (Safer et al., 2001, 2005), showing that binding to their receptors is required for the hormone effects in the skin. We observed TRα and TRβ expression in cells of the hair bulges, and both receptors appear to contribute to skin homeostasis (Contreras-Jurado et al., 2011, 2014). However, because in the mouse model used the receptors have been deleted in all cells, it is unclear which cell populations are responsible for the defective bulge stem cell function observed. TRs could directly affect the bulge SCs, but the thyroid hormones alter many metabolic processes that could regulate SC function in an indirect manner. Of interest, transcription of the deiodinase 2 gene in the hair follicles increases steadily, together with other transcripts encoding putative hair-cycling stimulating factors as telogen progresses (Greco et al., 2009). Deiodinase 2 is an enzyme that converts the thyroid hormone thyroxine in the active hormone triiodothyronine with high affinity for TR binding. Therefore local activation of thyroid hormone signaling should occur during hair growth. However, we cannot dismiss the possibility that other epidermal and/or stromal cell populations could be affected by lack of thyroid hormone signaling and regulate SC activity through BMP signaling. Future studies with conditional knockouts in which TRs are deleted only in the epidermal SC population will clarify the specific role of the receptors in these cells and their contribution to the skin anomalies found in the absence of thyroid hormone signaling.
TRs have long been known as key regulators of many physiological processes. Recent results suggest that many actions of TRs on adult tissue homeostasis could be related to regulation of proliferation and maturation of precursor/SCs. This includes, among others, correct proliferation and migration of neural SCs (Lemkine et al., 2005; Lopez-Juarez et al., 2012), proliferation of intestinal epithelial progenitors (Sun and Shi, 2012), and differentiation of myogenic SCs during muscle regeneration (Dentice et al., 2010). Our present results show an important role for thyroid hormone signaling in the normal function of follicle SCs and provide a basis for understanding the skin alterations found in human thyroid disorders.
MATERIALS AND METHODS
Animals and treatments
All animal studies were done in agreement with European Community law (86/609/EEC) and Spanish law (R.D. 1201/2005) and with the approval of the Ethics Committee of the Consejo Superior de Investigaciones Científicas. TRα1−/−/TRβ−/− double-KO mice and the corresponding WT TRα+/+/TRβ+/+ animals have been described previously (Gothe et al., 1999). Adult female age-matched animals were used in the experiments. Five 3-mo-old mice were made hyperthyroid by adding thyroxine (95 ng/g of mouse) and T3 (25 ng/g of mouse) to their drinking water.
Immunohistochemistry and immunofluorescence
Skin samples were fixed with paraformaldehyde or ethanol and embedded in paraffin. Skin sections (4 μm) were stained with hematoxylin and eosin or processed for immunohistochemistry or immunofluorescence. For frozen sections (5 μm), fresh skin samples were embedded in OCT (TissueTech, Miami, FL) and kept frozen until use. Immunohistochemistry was performed using standard protocols on deparaffinized sections (Contreras-Jurado et al., 2011; Garcia-Serrano et al., 2011). Slides were mounted and analyzed by light microscopy (Leica DM RXA2). Frozen sections from dorsal skins were used for indirect immunofluorescence of the hair bulges. The antibodies used are listed in the Supplemental Material.
Flow cytometry analysis
Keratinocytes were isolated from back skin of Wt and TR KO mice, and 2 × 105 cells were stained with biotin-labeled CD34 antibody for 30 min at 4°C and then with phycoerythrin-labeled α6-integrin antibody as described earlier (Lorz et al., 2010). Flow cytometry was performed a FACS CANTO System (BD Bioscience, Madrid, Spain) and analyzed with FlowJo software (Tree Star, Ashland, OR). The CD34 results shown were obtained in three independent experiments.
Whole mounts and LRC identification
To analyze LRCs, we used whole mounts of mice tail epidermis (Braun et al., 2003). The 3-d-old WT and KO animals (six mice/group) were injected with BrdU (50 mg/kg of body weight) every 12 h for a total of four injections, and animals were killed after 75 d. To analyze LRC mobilization, 68 d after the last BrdU injection, mice were treated topically every 48 h with TPA (20 nmol) or vehicle for a total of four doses. Twenty-four hours after the last application, the mice were killed and tails amputated. Tail skin was incubated with 5 mM EDTA for 4 h at 37ºC, and epidermal sheets were dissociated from the dermis and fixed in 4% formaldehyde. To detect LRCs, epidermal sheets were blocked, permeabilized, and immersed in 2 N HCl for 20 min at 37ºC. Epidermal sheets were incubated overnight with BrdU antibody or with the antibodies indicated in the figures, washed, and mounted in Mowiol 4-88 (Sigma-Aldrich, St. Louis, MO) with 4′,6-diamidino-2-phenylindole (DAPI). Thirty optical sections of each epidermal sheet were captured with an increment of 1 μm. Fluorescence analysis was performed with an Espectral Leica TCS SP5 confocal microscope (40×/1.25–0.75 oil), and maximum-intensity projections of the image stacks were then generated using Software LAS-AF. Quantification of label intensity of confocal images was performed with ImageJ64 (National Institutes of Health, Bethesda, MD) software, analyzing >100 follicles/mouse. Whole mounts of tail skin were also used to generate confocal images of stem cell markers or stain sebaceous glands with 100 ng/ml Nile Red (Sigma-Aldrich) for 10 min.
Colony formation assays
Keratinocytes were extracted from 8-mo-old mouse skin. Cells from three animals were pooled, and 50 × 104 cells and 250 × 104 cells were seeded in EMEM medium containing 4% Chelex-treated fetal bovine serum and 0.2 mM CaCl2. After 8 h, keratinocytes were grown in CnT-07 medium. After 10 d, dishes were washed with phosphate-buffered saline, fixed in 5% formaldehyde, and stained with 1% crystal violet for 5 min to visualize colony formation. Colony number was scored, and colony size was measured. Data were obtained from three separate experiments performed in duplicate.
Quantitative real-time PCR
Total RNA from paraffin-embedded sections from 4- to 5-mo-old mice (four telogen animals/per group) was isolated using the RNeasy FFPE Kit (Qiagen, Hilden, Germany). cDNAs were obtained from reverse transcription of RNA with specific primers listed in the Supplemental Material, using Omniscript RT Kit (Qiagen). Gene expression analysis was performed with Syber Green and analyzed in triplicate samples by quantitative real-time PCR using the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). Data analysis was done using the comparative Ct method, and data were corrected with the GusB mRNA levels.
Statistical analysis
Differences between KO and wild-type animals were assessed by analysis of variance (ANOVA) followed by the Bonferroni posttest or with the Mann-Whitney t test when only two groups were compared. Four to six animals per group were used in the different experiments. In the figures, significant differences between wild-type and TR KO mice are indicated by *p < 0.05, **p < 0.01, and ***p < 0001.
Supplementary Material
Acknowledgments
We thank B. Vennström and D. Forrest for the KO mice, E. Alonso-Merino and O. Martínez-Iglesias for samples, and M. Sánchez-Prieto for technical help. This work was supported by grants from the Ministerio de Economía y Competitividad (BFU2011-28058 to A.A. and SAF2012-34378 to J.M.P.), the Instituto de Salud Carlos III (RD12 /0036/0030 to A.A. and RD12/0036/0009 to J.M.P.), and the Comunidad de Madrid (S2011/BMD-2328 TIRONET to A.A. and S2010/BMD-2470 to J.M.P.).
Abbreviations used:
- BMP
bone morphogenetic protein
- BrdU
bromodeoxyuridine
- CFE
colony-forming efficiency
- LRC
label-retaining cell
- SC
stem cell
- TR
thyroid hormone receptor
- TPA
12-O-tetradecanolyphorbol-13-acetate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-07-1251) on February 5, 2015.
REFERENCES
- Ahsan MK, Urano Y, Kato S, Oura H, Arase S. Immunohistochemical localization of thyroid hormone nuclear receptors in human hair follicles and in vitro effect of L-triiodothyronine on cultured cells of hair follicles and skin. J Med Invest. 1998;44:179–184. [PubMed] [Google Scholar]
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Benitah SA. Defining an epidermal stem cell epigenetic network. Nat Cell Biol. 2012;14:652–653. doi: 10.1038/ncb2538. [DOI] [PubMed] [Google Scholar]
- Billoni N, Buan B, Gautier B, Gaillard O, Mahe YF, Bernard BA. Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645–652. doi: 10.1046/j.1365-2133.2000.03408.x. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Contreras-Jurado C, Garcia-Serrano L, Gomez-Ferreria M, Costa C, Paramio JM, Aranda A. The thyroid hormone receptors as modulators of skin proliferation and inflammation. J Biol Chem. 2011;286:24079–24088. doi: 10.1074/jbc.M111.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Jurado C, García-Serrano L, Martínez-Fernández M, Ruiz-Llorente L, Paramio JM, Aranda A. Impaired hair growth and wound healing in mice lacking thyroid hormone receptors. PLoS One. 2014;9:e108137. doi: 10.1371/journal.pone.0108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest. 2010;120:4021–4030. doi: 10.1172/JCI43670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349–352. [PubMed] [Google Scholar]
- Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS One. 2007;2:e763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Chen T. A matter of life and death: self-renewal in stem cells. EMBO Rep. 2013;14:39–48. doi: 10.1038/embor.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Serrano L, Gomez-Ferreria MA, Contreras-Jurado C, Segrelles C, Paramio JM, Aranda A. The thyroid hormone receptors modulate the skin response to retinoids. PLoS One. 2011;6:e23825. doi: 10.1371/journal.pone.0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci USA. 2013;110:1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci USA. 1993;90:7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prevot O, Samarut J, Levi G, Demeneix BA. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 2005;19:863–865. doi: 10.1096/fj.04-2916fje. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10:531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Lorz C, Garcia-Escudero R, Segrelles C, Garin MI, Ariza JM, Santos M, Ruiz S, Lara MF, Martinez-Cruz AB, Costa C, et al. A functional role of RB-dependent pathway in the control of quiescence in adult epidermal stem cells revealed by genomic profiling. Stem Cell Rev. 2010;6:162–177. doi: 10.1007/s12015-010-9139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]
- Messenger AG. Thyroid hormone and hair growth. Br J Dermatol. 2000;142:633–634. doi: 10.1046/j.1365-2133.2000.03521.x. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830:3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Paus R. Exploring the “thyroid-skin connection”: concepts, questions, and clinical relevance. J Invest Dermatol. 2010;130:7–10. doi: 10.1038/jid.2009.359. [DOI] [PubMed] [Google Scholar]
- Plateroti M, Kress E, Mori JI, Samarut J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26:3204–3214. doi: 10.1128/MCB.26.8.3204-3214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- Safer JD, Crawford TM, Holick MF. Topical thyroid hormone accelerates wound healing in mice. Endocrinology. 2005;146:4425–4430. doi: 10.1210/en.2005-0192. [DOI] [PubMed] [Google Scholar]
- Safer JD, Fraser LM, Ray S, Holick MF. Topical triiodothyronine stimulates epidermal proliferation, dermal thickening, and hair growth in mice and rats. Thyroid. 2001;11:717–724. doi: 10.1089/10507250152484547. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Sirakov M, Skah S, Lone IN, Nadjar J, Angelov D, Plateroti M. Multi-level interactions between the nuclear receptor TRalpha1 and the WNT effectors beta-catenin/Tcf4 in the intestinal epithelium. PLoS One. 2012;7:e34162. doi: 10.1371/journal.pone.0034162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–1455. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Sun G, Shi YB. Thyroid hormone regulation of adult intestinal stem cell development: mechanisms and evolutionary conservations. Int J Biol Sci. 2012;8:1217–1224. doi: 10.7150/ijbs.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tiede S, Bohm K, Meier N, Funk W, Paus R. Endocrine controls of primary adult human stem cell biology: thyroid hormones stimulate keratin 15 expression, apoptosis, and differentiation in human hair follicle epithelial stem cells in situ and in vitro. Eur J Cell Biol. 2010;89:769–777. doi: 10.1016/j.ejcb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA, Slominski A, Wenzel BE, Paus R. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381–4388. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation. Nat Rev Cancer. 2008;8:234–242. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang L, Yang X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol Biol Cell. 2009;20:882–890. doi: 10.1091/mbc.E08-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, Mishina Y, Feng JQ, Li L. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.