Abstract
The gastric pathogen Helicobacter pylori utilize glucose during metabolism, but the underlying mechanisms linking to oxygen-18 (18O) and carbon-13 (13C)-isotopic fractionations of breath CO2 during glucose metabolism are poorly understood. Using the excretion dynamics of 18O/16O and 13C/12C-isotope ratios of breath CO2, we found that individuals with Helicobacter pylori infections exhibited significantly higher isotopic enrichments of 18O in breath CO2 during the 2h-glucose metabolism regardless of the isotopic nature of the substrate, while no significant enrichments of 18O in breath CO2 were manifested in individuals without the infections. In contrast, the 13C-isotopic enrichments of breath CO2 were significantly higher in individuals with Helicobacter pylori compared to individuals without infections in response to 13C-enriched glucose uptake, whereas a distinguishable change of breath 13C/12C-isotope ratios was also evident when Helicobacter pylori utilize natural glucose. Moreover, monitoring the 18O and 13C-isotopic exchange in breath CO2 successfully diagnosed the eradications of Helicobacter pylori infections following a standard therapy. Our findings suggest that breath 12C18O16O and 13C16O16O can be used as potential molecular biomarkers to distinctively track the pathogenesis of Helicobacter pylori and also for eradication purposes and thus may open new perspectives into the pathogen’s physiology along with isotope-specific non-invasive diagnosis of the infection.
Helicobacter pylori (H. pylori) is a micro-aerophilic pathogen, which is known to be able to colonize the mucosal surfaces of the human stomach, where it gives rise to chronic gastritis, peptic ulcers1,2,3 and is closely linked to the development of certain types of gastric cancer4. The gastric pathogen H. pylori uses glucose as the primary energy substrate5,6, although the overall metabolism of H. pylori yet remains inadequately understood. Some early evidences, however, suggest that H. pylori has the ability to utilize glucose for metabolism through a glucokinase activity7 and enzymes of the pentose phosphate and glycolysis pathways8,9. Carbon dioxide (CO2) is usually produced as a by-product of glucose catabolism which is then transported to the lungs through the blood stream, and finally it is excreted in human breath. However, the precise role of glucose metabolism, especially in the pathogenesis of the H. pylori infection is not currently known. A new insight into the role of glucose metabolism is essential to elucidate the pathophysiology of H. pylori for its successful colonization of the gastrointestinal tract. However, to our knowledge, so far there have been no studies focused on glucose uptake for individuals harboring H. pylori infections, exhibiting the time-dependent excretion dynamics of the metabolite CO2 in exhaled breath. The purpose of this study was therefore, primarily to explore the potential links between breath CO2 and H. pylori infections in response to unlabelled and labelled 13C-enriched glucose metabolism. A complete understanding of glucose metabolism during the H. pylori infection could be of significance in the development of novel therapies for the micro-organism alongside new and better approaches for treating the most common human bacterial infection.
Furthermore, an earlier study revealed that the oxygen-16 (16O) and the oxygen-18 (18O) isotopes in 12C16O2 and water (H218O), respectively, are rapidly interchanged during the human respiration process mediated by the metalloenzyme carbonic anhydrase (CA)10,11. It is also known that H. pylori encodes two different forms of the metalloenzyme carbonic anhydrase (α-CA and β-CA)12 and this gastric pathogen plays a vital role in inter-conversion of carbon dioxide and bicarbonate (CO2 + H2O↔H+ + HCO3−), catalyzed by the CA activity12,13,14. This efficient activity suggests that investigations of breath 18O/16O isotopic fractionations of CO2 may specifically track the gastric pathogen H. pylori and hence may introduce a novel non-invasive strategy in the diagnosis of H. pylori infections living in human stomach. As a consequence, we hypothesized that simultaneous monitoring the 18O/16O and 13C/12C stable isotope ratios of exhaled breath CO2 associated with glucose metabolism in H. pylori may act as potential markers for the early detection of H. pylori infections or during the preclinical phase of the infections. In view of the fact that H. pylori is able to uptake and metabolize glucose confirmed as experimentally15 and also by analysing the genome sequence5 therefore, there is a pressing need to assess the clinical efficacy of the glucose utilization by H. pylori for large-scale screening individuals harboring the micro-organism. In addition, unravelling the precise metabolic pathways involved in causing the isotopic fractionations of 12C16O16O, 13C16O16O and 12C18O16O in human breath during the glucose uptake by H. pylori remains a major challenge, whenever an individual is at-risk of developing the disease.
In this study, we first report, the potential links of both 18O and 13C-stable isotopes of breath CO2 with the gastric pathogen H. pylori in response to glucose ingestion. We investigated simultaneously the time-dependent excretion dynamics of the 12C18O16O/12C16O16O and 13C16O16O/12C16O16O isotope ratios of breath CO2 from individuals with H. pylori positive and negative by employing a laser-based integrated cavity output spectroscopy (ICOS) method. We further explored the potential metabolic pathways underlying the glucose utilization in the pathogenesis of H. pylori infection and the mechanisms linking breath oxygen-18 and carbon-13 isotopic fractionations of CO2 to the gastric pathogen H. pylori. Finally, we determined various diagnostic parameters such as optimal diagnostic cut-off values, diagnostic sensitivity and specificity of oxygen-18 and carbon-13 stable isotopes in breath CO2 to gain a better insight into the diagnostic efficiency for the non-invasive detection of H. pylori infection in real-time.
Results and Discussion
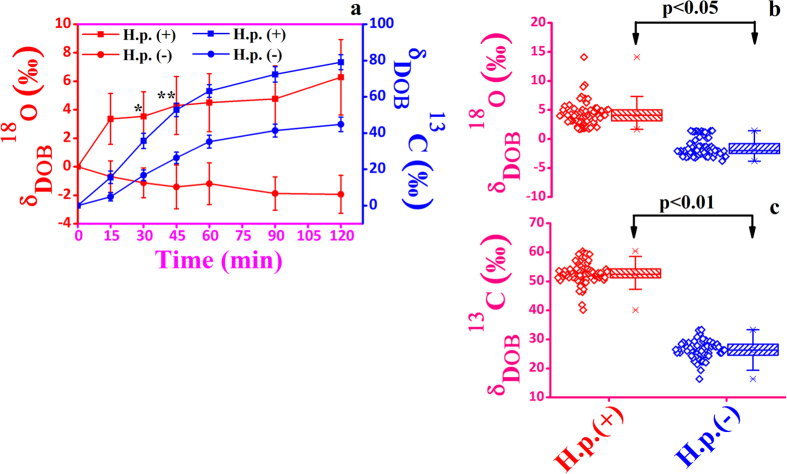
To investigate the 18O and 13C isotopic fractionations of breath CO2, we first studied the time-dependent excretion dynamics of both isotopes in exhaled breath after ingestion of an oral dose of 13C-enriched glucose for H. pylori positive (n = 72) and negative (n = 55) individuals, using a laser-based high-precision cavity-enhanced integrated cavity output spectrometer (ICOS). We explored the isotopic fractionation of CO2 by simultaneous monitoring the 18O/16O and 13C/12C stable isotope ratios in breath, expressed as delta-over-baseline (DOB) relative to the Vienna Pee Dee Belemnite standard, i.e., δDOB18O‰ = [(δ18O‰)t=t – (δ18O‰)t=basal] and δDOB13C‰ = [(δ13C‰)t=t – (δ13C‰)t=basal], respectively, associated with glucose metabolism. In this investigation (Fig. 1a), individuals with H. pylori positive exhibited significantly higher isotopic enrichments of 18O in CO2 compared with H. pylori negative during the 2h-glucose metabolism, while no significant enrichments of 18O in CO2 were manifested in individuals without H. pylori infections. These findings suggest a potential link between H. pylori infections in human stomach and the 18O-isotopic fractionations in exhaled breath and hence may open a new route for the non-invasive assessment of H. pylori infections. Carbonic anhydrase (CA) activity of H. pylori has previously been proposed to interchange the oxygen isotopes of CO2 (16O) and H2O (18O) efficiently10,11, suggesting that in our observations CA activity may play an important role in oxygen-isotope fractionations of breath CO2 in the glucose-mediated bacterial environment. Hence a statistically significant difference in the δDOB18O‰ values in excretion dynamics established a marked distinction (Fig. 1b) between H. pylori infected and non-infected individuals. Taken together, these findings indicate that the monitoring of 18O-exchange between C16O2 and H218O in response to CA activity may distinctively track the development of the gastric pathogen in human stomach and might be considered as a potential biomarker for the non-invasive detection of H. pylori infection.
Figure 1. Comparisons of δDOB 18O‰ and δDOB 13C‰ values of exhaled breath CO2 associated with 13C-labelled glucose metabolism in presence [H. p. (+)] and absence [H. p. (−)] of H. pylori infection.
(a) Excretion kinetics of δDOB18O‰ and δDOB13C‰ values for H. pylori positive [H. p. (+)] and H. pylori negative [H. p. (−)] individuals up to 120 minutes. (b,c) The Box-Whiskers plots demonstrating a statistically significant differences of δDOB18O‰ [p < 0.05] and δDOB13C‰ [p < 0.01] values at 45 minutes between H. pylori positive [H. p. (+)] and H. pylori negative [H. p. (−)] individuals. *p < 0.05 and **p < 0.01. Data are means ± SE.
We then critically assessed the excretion dynamics of δDOB13C (‰) (Fig. 1a) values in exhaled breath samples in response to 13C-enriched glucose ingestion. The isotopic enrichments of 13C in breath CO2 were significantly higher (Fig. 1c) in individuals with H. pylori positive compared with individuals with H. pylori negative. It was noticed that for H. pylori positive patients the δDOB13C (‰) values increased gradually with time at a faster rate in comparison with individuals without H. pylori infections. Several lines of evidence suggest that H. pylori can metabolize glucose in both oxidative and fermentative pathways8,9 and as a consequence the catabolism of glucose resulted in higher isotopic enrichments of 13C in exhaled breath CO2. Moreover, in some early evidences9,16, it was demonstrated the biphasic characteristics of glucose utilization by H. pylori with a slower initial period, followed by a second phase with a higher rate of glucose uptake. The transport and utilization of glucose was previously investigated into the intact micro-organism employing the radioactive tracer analysis. Therefore, the gradual increase of the δDOB13C (‰) values in the time-dependent excretion dynamics is possibly attributed to the increased rate of glucose utilization through the biphasic activity of the micro-organism. Hence our results of the δDOB13C (‰) values in exhaled breath are coincidence with the previous study16, where the uptake of glucose into H. pylori cells exhibited the biphasic patterns. Our observations therefore, point to new perspectives into the physiology of H. pylori underlying the isotopic fractionations of 13C in breath CO2 associated with glucose metabolism.
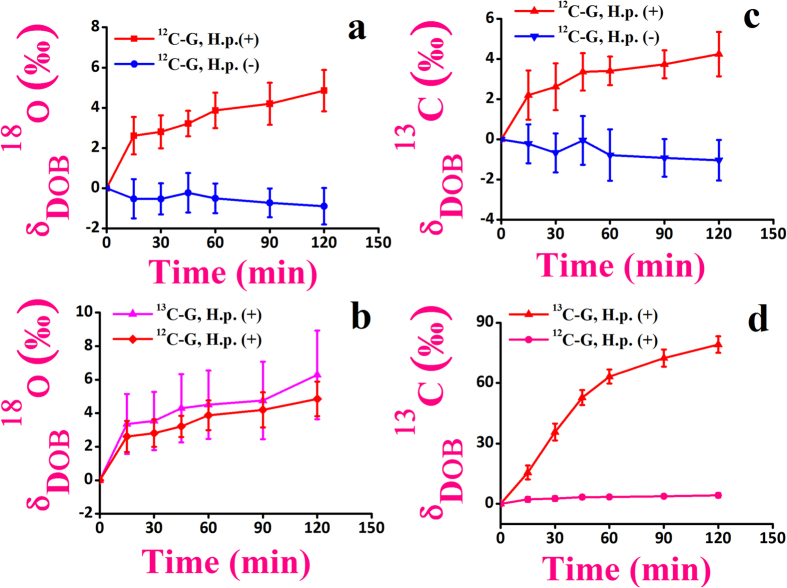
We next explored how the time-dependent excretion dynamics of isotopic breath CO2 changes after administration of unlabelled glucose (i.e. with no 13C-enriched glucose), as the potential role of glucose metabolism in response to unlabelled glucose ingestion for individuals with H. pylori infection and the possible links underlying the 18O and 13C-isotopic fractionations of breath CO2 remains unknown. To investigate this, we performed the 2-h excretion kinetics of δDOB18O‰ and δDOB13C‰ values simultaneously in breath samples for a number of 52 H. pylori infected and 45 non-infected individuals. When the unlabelled glucose was orally administered in positive H. pylori patients, the post-dose δDOB18O‰ values in breath samples manifested a significant change with time and depicted the similar excretion kinetics with that of 13C-enriched glucose (Fig. 2a,b), whereas no significant change of the post-dose δDOB18O‰ values in breath samples over time was evident for H. pylori negative individuals (Fig. 2a). These findings suggest that the mechanisms i.e. oxidation of glucose in the bacterial environment to form bicarbonate (HCO3−) and subsequently the enzyme carbonic anhydrase-mediated rapid inter-conversion of HCO3− and CO2, leading to the generation of 12C16O18O, exclusively depends on the substrate (glucose) regardless of its isotopic nature. Interestingly, although the isotopic nature of the substrate is vital to observe effectively the 13C-isotopic enrichments of breath CO2 (i.e. enhancement of δDOB13C‰ values), yet the enrichments of δDOB13C‰ values, due to natural abundances of 13C, during glucose metabolism of H. pylori are significantly distinguishable for H. pylori positive patients (Fig. 2c,d), suggesting a new step towards characterizing the transport and utilization of unlabelled glucose into the human pathogen for better understanding of its physiology in the gastric niche. In view of the breath results, we have also established the previous hypothesis12 that the bacterium requires high CO2 for growth and the interconversion of 18O (H218O) and 16O (C16O2) is vital, catalyzed by the enzyme carbonic anhydrase activity (α-CA and β-CA) of H. pylori and thus this activity might be a contributing factor for the development of the disease in the gastric environment. The summary of the detailed results has been provided in the Table 1.
Figure 2. Excretion kinetics of δDOB 18O‰ and δDOB 13C‰ values of exhaled breath CO2 during unlabelled glucose (12C-G) metabolism of H. pylori positive [H. p. (+)] and H. pylori negative [H. p. (−)] individuals.
(a) The excretion kinetics illustrating the increased δDOB18O‰ values for H. pylori positive [H. p. (+)] patients. (b) depicts the similar excretion kinetics of δDOB18O‰ values with that of 13C-enriched glucose. (c,d) enhancement of δDOB13C‰ values for H. pylori positive [H. p. (+)] patients and comparisons with the 13C-enriched glucose. Values are means ± SE.
Table 1. The summary of the detailed characteristics of the study subjects for H. pylori infected and non-infected groups. UBT and GBT correspond to the urea breath test and glucose breath test respectively.
| Parameters | H. pylori infected (mean ± SD) | H. pylori non-infected (mean ± SD) | p Value |
|---|---|---|---|
| AGE | 38.91 ± 10.43 | 39.19 ± 9.68 | 0.73 |
| HbA1c | 5.13 ± 0.12 | 5.1 ± 0.1 | 0.07 |
| 13C-UBT (30 min) | 18.12 ± 12.93 | 0.67 ± 0.64 | <0.001 |
| 13C-GBT (45 min) | |||
| δDOB13C‰ | 52.70 ± 3.71 | 26.34 ± 3.16 | <0.001 |
| δDOB18O‰ | 4.29 ± 2.03 | −1.41 ± 1.53 | <0.01 |
| 12C-GBT (45 min) | |||
| δDOB13C‰ | 3.36 ± 0.93 | −0.04 ± 1.21 | <0.01 |
| δDOB18O‰ | 3.11 ± 0.63 | −0.22 ± 0.97 | <0.01 |
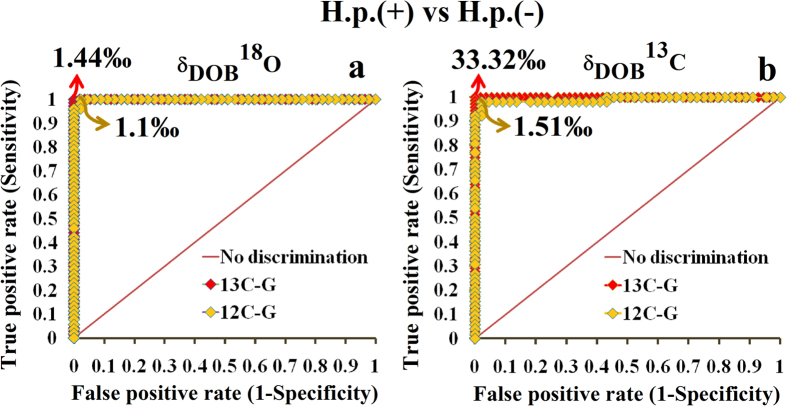
To distinctively track the H. pylori infection as well as for early detection prior to the onset of different gastric diseases, we subsequently determined numerous statistically sound optimal diagnostic cut-off points of δDOB18O‰ and δDOB13C‰ values in exhaled breath associated with 13C-labelled and unlabelled glucose metabolism, using receiver operating characteristics curve (ROC) analysis (Fig. 3). Individuals with δDOB18O‰ ≥ 1.44‰ and δDOB18O‰ ≥ 1.1‰ were considered to be H. pylori positive with and without 13C-enriched glucose metabolism respectively, and these corresponded to the diagnostic sensitivity and specificity of 100% and ~98%, respectively. On the contrary, a different optimal diagnostic cut-off point of δDOB13C‰ ≥ 33.32‰ between individuals with H. pylori positive and negative, demonstrated the sensitivity and specificity of 100% and 100%, respectively, when 13C-labelled glucose is ingested, whereas without 13C-labelled glucose, δDOB13C‰ ≥ 1.51‰ precisely diagnosed the infected and non-infected persons corresponding to the similar levels of diagnostic sensitivity (100%) and specificity (98%). It is noteworthy to mention that the uncertainty of these cut-off values is associated with the less-sensitive techniques for isotope measurements and the variation of isotopic fractionations in the test meal. However, these findings suggest that the oxygen-18 and carbon-13 isotopic fractionations of the major metabolite CO2 in human breath linked to glucose metabolism of H. pylori provide a new non-invasive approach to treat the world’s most common chronic bacterial infection of humans and hence may have a broad clinical efficacy for precise assessment of the gastric pathogen H. pylori.
Figure 3. Receiver operating characteristic (ROC) curves analysis for the optimal diagnostic cut-off points of H. pylori infection.
(a) δDOB18O values ≥1.44‰ and 1.1‰ are indicative of the H. pylori infection associated with 13C-labelled and unlabelled glucose metabolism at 45 minute, respectively, whereas (b) δDOB13C values ≥33.21‰ and 1.51‰ indicate the same for 13C-labelled and unlabelled glucose metabolism, respectively.
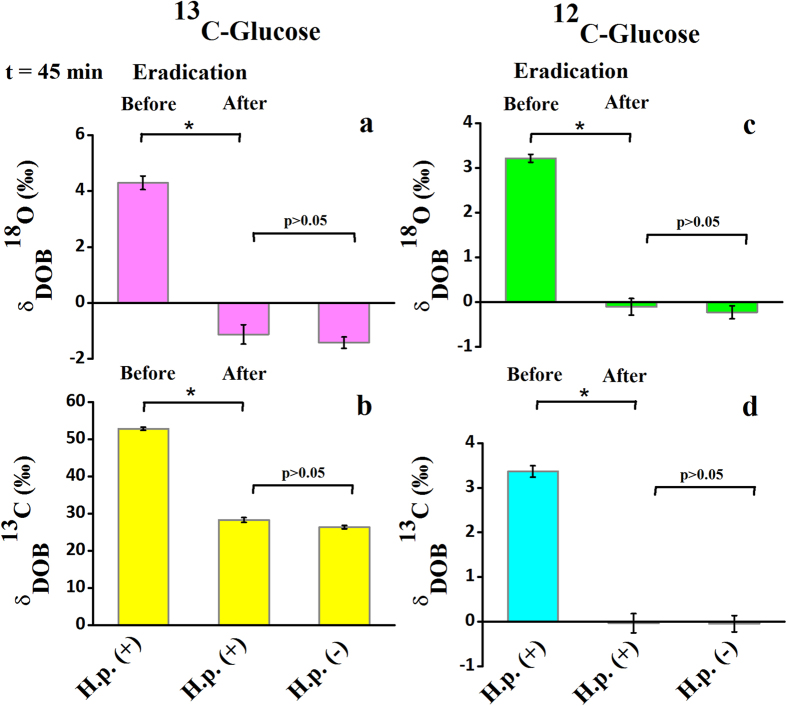
We next explored the efficacy of the glucose breath test in response to the standard eradication therapies of the infection. A marked depletions of both δDOB18O‰ and δDOB13C‰ values for H. pylori infected patients (n = 37 for 13C-glucose and n = 28 for 12C-glucose) (Fig. 4) after complete eradication of the infection were manifested, suggesting the widespread clinical significance of the glucose breath test. Our findings associated with the glucose metabolism by H. pylori infections thus point towards a considerable clinical advancement in the non-invasive diagnosis of H. pylori infection by contrast with the currently available 13C-urea breath test (13C-UBT), where 13C-enriched substrate (urea) is usually used. In view of this result, we therefore posit that the glucose breath test by ingestion of a natural substrate (unlabelled glucose) is a valid and potentially robust new-generation diagnostic tool and thus indicate great promise for comparatively less-expensive and non-toxic global technique, in comparison with the 13C-UBT, for the non-invasive assessment i.e. early detection and follow-up of patients after eradication of H. pylori infection.
Figure 4. Glucose breath test in response to the standard eradication therapies of the H. pylori infection.
(a–d) a marked distinction (*p < 0.01) for the δDOB18O and δDOB13C values at 45 minute was observed before and after the therapies in case of both 13C-glucose and 12C-glucose ingestion.
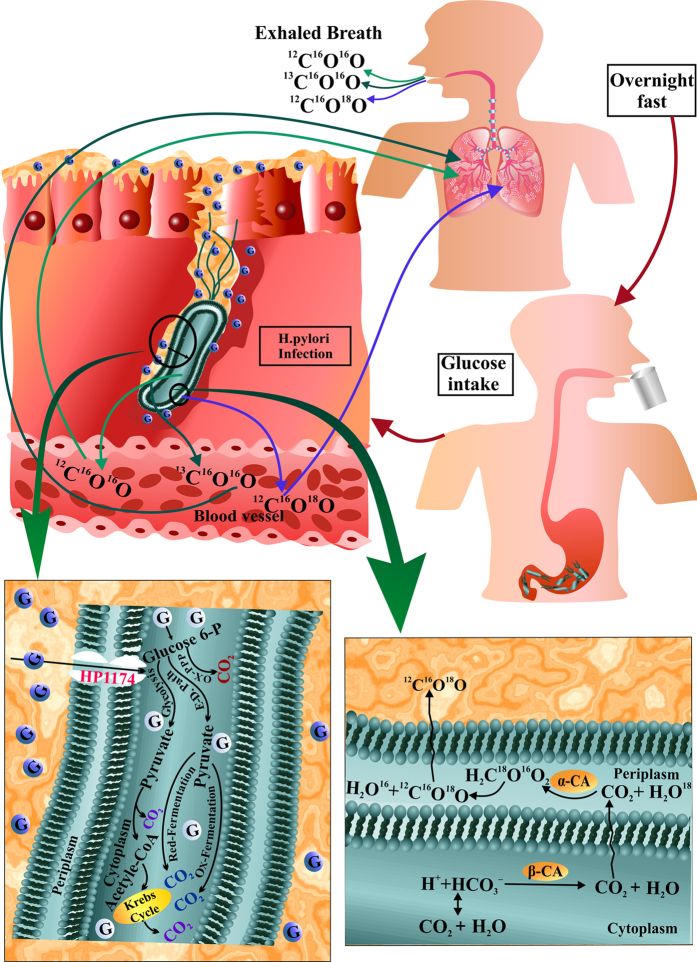
Finally, we elucidated the potential metabolic pathways (Fig. 5) underlying the mechanisms linking isotopic fractionations of breath CO2 and glucose utilization by H. pylori infection. When a dose of glucose is orally administered to the patients, the ingested glucose disposal takes place in the cytoplasm of H. pylori through the HP1174 transporter (protein)7. After glucose enters into the cytoplasm, it is phosphorylated to produce glucose-6-phosphate which subsequently incorporated with three potential metabolic routes: glycolysis, pentose phosphate and the Entner-Doudoroff pathway17,18. A part of the total glucose-6-phosphate, which goes into the pentose phosphate pathway, is predominantly oxidised into CO2. The remaining part of glucose-6-phosphate enters into the other two metabolic pathways and may lead to the generation of pyruvate17 and eventually gives rise to CO2 followed by the formation of acetate as the key metabolite through the intermediary oxidative and reductive fermentation pathways9. Another fate for pyruvate is the conversion of acetyl-CoA, which afterwards enters into the Krebs cycle and generates CO2 as a by-product18. Therefore, the administration of 13C-glucose (either from 13C-enriched exogenous glucose or naturally abundant 13C-glucose) facilitates the production of 13CO2 in the by-product CO2 in presence of H. pylori infection. Thereafter, the major metabolite CO2 (13CO2 and 12CO2) produced by all these metabolic processes is then transported through the blood streams and eventually excreted as 13C16O16O and 12C16O16O in exhaled breath. Conversely, the cytoplasmic β-carbonic anhydrase (β-CA) activity of H. pylori catalyzes the reversible interconversion between the major metabolite CO2 and HCO3− (CO2 + H2O↔H+ + HCO3−).Then the CO2 diffuses rapidly through the inner member into the periplasm of H. pylori, where it forms carbonic acid (H2CO3), catalyzed by the α-CA. Because the isotopes 16O of 12C16O2 and 18O of H218O are rapidly exchanged in response to periplasmic α-CA activity, it therefore leads to the generation of H2C18O16O2. This carbonic acid rapidly degasses to produce 12C18O16O, which is then transported to the lungs and is excreted through exhaled breath. As a result, individuals with H. pylori infections exhibit the preferential isotopic enrichments of 18O in breath CO2, whereas no significant change of 18O in CO2 was manifested in H. pylori-uninfected individuals.
Figure 5. Potential metabolic pathways for 13C and 18O-isotopic exchanges associated with glucose metabolism by H. pylori infection.
The ingested glucose in the cytoplasm of H. pylori through the HP1174 transporter is phosphorylated to produce glucose-6-phosphate which subsequently incorporated with three potential metabolic routes: glycolysis, pentose phosphate and the Entner-Doudoroff pathway to finally produce major metabolite CO2.This major metabolite CO2 is then transported through the blood streams and eventually excreted as 13C16O16O and 12C16O16O in exhaled breath. Conversely, the major metabolite CO2 diffuses rapidly through the inner member into the periplasm of H. pylori, where it forms carbonic acid (H2CO3), catalyzed by the α-CA. The isotopic exchange between 16O of 12C16O2 and 18O of H218O in response to periplasmic α-CA activity leads to the generation of 12C18O16O, which is then transported to the lungs and is excreted through exhaled breath.
In conclusion, our new findings point to a fundamental mechanism underlying both the 18O and 13C stable isotopic fractionations of the major metabolite CO2 in human breath related to glucose metabolism of H. pylori infection in humans. Subsequently, we have taken a step towards unravelling the potential metabolic pathways linking the 18O and 13C-isotopic exchange of breath CO2 and the glucose uptake by H. pylori, thus suggesting that breath 12C18O16O and 13C16O16O in response to glucose ingestion could be used as potential molecular biomarkers to distinctively track the pathogenesis of H. pylori infection in a non-invasive approach. Although many imperative gaps remain in our understanding of these processes and in the pathophysiology underlying the isotopic exchange and glucose metabolism, our studies may provide new perspectives in the isotope-specific molecular diagnosis of H. pylori infection and hence may pave the way for broad clinical applications along with eradication purposes following standard therapies. Furthermore, new insight into the mechanism linking the isotopic exchange in breath molecule CO2 to glucose metabolism of H. pylori is fostering exploration of the molecular basis of this infection and new and better approaches together with new pharmacological targets to prevent or treat the deleterious effects of the world’s most common gastric pathogen.
Materials and methods
Subjects
Two hundred and twenty four individuals (135 male and 89 female with average age of 39 ± 10 yrs (SD)) were enrolled for this study with different gastrointestinal disorders such as active peptic ulcer disease (PUD), chronic gastritis, and univestigated dyspepsia. We categorized all the human subjects in two distinct groups: infected with H. pylori (H. pylori positive patients: 124) and without the infection of H. pylori (H. pylori negative patients: 100) depending on the reports of gold standard invasive and non-invasive methods, i.e. endoscopy and biopsy based rapid urease test (RUT) and 13C-urea breath test (13C-UBT).The 13C-UBT was considered to be indicative of H. pylori positive when δDOB13C (‰) ≥ 3‰19,20,21. There were no mismatches between the two test-reports of all the subjects enrolled in this study (Supplementary Table 1). Exclusion criteria included patients with previous history of diabetes and gastric surgery, taking antibiotics, proton pump inhibitors or H2 receptor antagonists in the four week prior to endoscopy and 13C-UBT. We received the Ethical approval from the Ethics Committee Review Board of AMRI Hospital, Salt Lake, Kolkata, India (Study no.: AMRI/ETHICS/2013/1). The current protocol has also been approved by the institutional administrative of S. N. Bose Centre, Kolkata, India (Ref. no.: SNB/PER-2-6001/13-14/1769) and the methods were carried out in accordance with the approved guidelines. Informed written consents were taken from all patients participating in this study.
Breath samples collection and measurements
All the human subjects enrolled for the study completed their endoscopic examinations and 13C-UBTs, 1-2 days prior to glucose breath test (GBT). On the study day before GBT, all the patients were instructed for their mouth-washing to prevent any kind of contact of ingested test meal with the oral cavity bacteria. After an overnight fasting (10-12 hours), an initial baseline breath sample was collected in a 750 ml breath collection bag (QUINTRON, USA, SL No.QT00892) from each subject. After that a test meal of 75 mg U-13C6 labelled D-glucose (CIL-CLM-1396-CTM, Cambridge Isotope Laboratories, Inc. USA) or 75 mg unlabeled glucose dissolved in 50 ml water was orally administered to the patient and then subsequent breath samples were collected at 15 minute intervals up to 120 minute. The physical activities of the subjects were restricted inside a room during the test. For the measurements of 18O/16O and 13C/12C isotope ratios of exhaled breath CO2, a laser-based high-precision ICOS system was employed and the detailed description of the ICOS was given in the following section.
Integrated cavity output spectrometer (ICOS) for breath analysis
For high precision isotopic measurements of breath CO2, a high-resolution carbon dioxide analyzer, based on off-axis integrated cavity output spectroscopy (ICOS) method, has been utilized in this study. The detailed description and the measurement accuracy of ICOS method in comparison to the conventional isotope ratio mass spectrometry (IRMS) have been previously demonstrated elsewhere22,23. In brief, the laser-based ICOS spectrometer (CCIA 36-EP, Los Gatos research, USA) exploits a high-finesse optical cavity (~59 cm) with two high reflectivity mirrors (R ~ 99.98%) at the both ends of the cavity. This arrangement provides an effective optical path-length of around 3 km through the measuring gas sample, thus offering a high-precision measurement. A continuous wave distributed feedback diode laser operating at ~2.05 μm is repeatedly tuned over 20 GHz to scan the absorption features of 12C16O16O, 12C18O16O and 13C16O16O at the wavenumbers of 4874.448 cm−1, 4874.178 cm−1 and 4874.086 cm−1 respectively. The absorption features of 12C16O16O, 12C18O16O and 13C16O16O, corresponding to the R (27), P (36) and P (16) ro-virational lines respectively, in the (2,00,1) ← (0,00,0) vibrational combinational band of CO2, have been utilized to measure the 13C/12C and 18O/16O isotope ratios simultaneously. The isotopic enrichments of 13CO2 and 12C18O16O have been expressed as the conventional notations i.e., δ13C (‰) and δ18O (‰) respectively, relative to the international standard Pee Dee Belemnite (PDB).
 |
 |
where the standard PDB values for (13C/12C)Standard and (18O/16O)Standard are 0.0112372 and 0.0020672, respectively. The accuracy and precision of ICOS method for the δ13C‰ measurements were determined by measuring three calibration standards, containing 5% CO2 in air analyzed by IRMS (Cambridge Isotope Laboratory, USA), with δ13C values ranging from baseline-level (−22.8‰) to high-level (−7.33‰) including the mid-level (−13.22‰) whereas a standard NOAA air tank was used for the calibration of δ18O (‰) measurements (Supplementary Table 2 and Supplementary Table 3). A 25 mL breath sample was injected into the ICOS cell with a syringe/stopcock for the measurements. High-purity dry nitrogen (HPNG10-1, F-DGSi SAS, France, purity >99.99%), as the carrier gas, was used to purge the cavity and dilute the breath samples.
Statistical method
All the data were presented as mean ± SE (Standard Error). For statistical analyses, we performed non-parametric Mann-Whitney test and one way ANNOVA test. A two sided p value <0.05 was taken account as statistically significant of data. Box-Whiskers plots were utilized to demonstrate the statistical distribution of isotopic enrichments of exhaled breath CO2. To obtain the optimal diagnostic cut-off values for δDOB18O‰ and δDOB13C‰ associated with 13C-labelled (13C-G) and unlabelled glucose (12C-G) metabolism, we performed receiver operating characteristic curve (ROC) analysis (Supplementary Table 4, Supplementary Table 5, Supplementary Table 6 and Supplementary Table 7). All the data were analysed using Origin Pro 8.0 (Origin Lab Corporation, USA) and Analyse-it Method Evaluation software (Analyse-it Software Ltd, UK, version 2.30).
Additional Information
How to cite this article: Som, S. et al. Mechanisms linking metabolism of Helicobacter pylori to 18O and 13C-isotopes of human breath CO2. Sci. Rep. 5, 10936; doi: 10.1038/srep10936 (2015).
Supplementary Material
Acknowledgments
M. Pradhan acknowledges the ‘Rapid Grant for Young Investigators (No. BT/PR6683/GBD/27/477/2012)’ from the Department of Biotechnology (DBT, India) for this work. S. Som and C. Ghosh acknowledge Bose Centre for PhD fellowship, whereas A. De, G. D. Banik, A. Maity and M. Pal thank to the Department of Science & Technology (DST, India) for Inspire Fellowships. We also thank all the volunteers participated in this study.
Footnotes
Author Contributions M.P. (Manik Pradhan) provided the funding and conception of the study; M.P. (Manik Pradhan), S.C., S.B.D. and S.J. supervised the whole study; M.P. (Manik Pradhan), S.C. and S.S. designed the study; S.S., A.D., G.D.B., A.M., C.G. and M.P. (Mithun Pal) collected and analysed the samples; All authors drafted the manuscript and critically reviewed.
References
- Covacci A., Telford J. L., Guidice G. D., Parsonnet J. & Rappuoli R. Helicobacter pylori virulence and genetic geography. Science 284, 1328–1333 (1999). [DOI] [PubMed] [Google Scholar]
- El-omar E. M. et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402 (2000). [DOI] [PubMed] [Google Scholar]
- Som S. et al. Excretion kinetics of 13C-urea breath test: influences of endogenous CO2 production and dose recovery on the diagnostic accuracy of Helicobacter pylori infection. Anal. Bioanal. Chem. 406, 5405–5412 (2014). [DOI] [PubMed] [Google Scholar]
- Polk D. B. & Peek R. M. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer. 10, 403–414 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J-F. et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388, 539–547 (1997). [DOI] [PubMed] [Google Scholar]
- Park S. A., Ko A. & Lee N. G. Simulation of growth of the human gastric pathogen Helicobacter pylori by atmospheric level of oxygen under high carbon dioxide tension. BMC Microbiol. 11, 96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psakis G. et al. The sodium-dependent D-glucose transport protein of Helicobacter pylori. Mol Microbiol. 71, 391–403 (2009). [DOI] [PubMed] [Google Scholar]
- Marais A., Mendz G. L., Hazell S. T. & Megraud F. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol. Mol. Biol. Res. 63, 642–674 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk P. A., Roberts A. D. & Blows W. M. Metabolism of pyruvate and glucose by intact cells of Helicobacter pylori studied by 13C NMR spectroscopy. Microbiology. 140, 2085–2092 (1994). [DOI] [PubMed] [Google Scholar]
- Epstein S. & Zeiri L. Oxygen and carbon isotopic compositions of gases respired by humans. Proc. Natl. Acad. Sci. USA. 85, 1727–1731 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh C. et al. Oxygen-18 isotope of breath CO2 linking to erythrocytes carbonic anhydrase activity: a biomarker for pre-diabetes and type 2 diabetes. Scientific Reports 5, 8137 (2015). 10.1038/srep08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirica L. C. et al. Expression and localization of α- and β-carbonic anhydrase in Helicobacter pylori. Biochim Biophys Acta. 1601, 192–199 (2002). [DOI] [PubMed] [Google Scholar]
- Marcus E. A., Moshfegh A. P., Sachs G. & Scott D. R. The periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 187, 729–738 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity A. et al. Oxygen-18 stable isotope of exhaled breath CO2 as a non-invasive marker of Helicobacter pylori infection. J. Anal. At. Spectrom. 29, 2251–2255 (2014). [Google Scholar]
- Mendz G. L., Burns B. P. & Hazell S. L. Characterisation of glucose transport in Helicobacter pylori. Biochim Biophys Acta. 1244, 269–276 (1995). [DOI] [PubMed] [Google Scholar]
- Mendz G. L., Hazell S. L. & Burns B. P. Glucose utilization and lactate production by Helicobacter Pylori. J. Gen. Microbiol. 139, 3023–3028 (1993). [DOI] [PubMed] [Google Scholar]
- Mendz G. L., Hazell S. L. & Burns B. P. The Entner-Doudoroff pathway in Helicobacter pylori. Arch. Biochem. Biophys. 312, 349–356 (1994). [DOI] [PubMed] [Google Scholar]
- Pitson S. M., Mendz G. L., Srinivasan S. & Hazell S. L. The tricarboxylic acid cycle of Helicobacter Pylori. Eur. J. Biochem. 260, 258–267 (1999). [DOI] [PubMed] [Google Scholar]
- Goddard A. F. & Logan R. P. H. Review article: urea breath tests for detecting Helicobacter pylori. Aliment Pharmacol Ther. 11, 641–649 (1997). [DOI] [PubMed] [Google Scholar]
- Gisbert J. P. & Pajares J. M. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection-a critical review. Aliment Pharmacol Ther. 20, 1001–1017 (2004). [DOI] [PubMed] [Google Scholar]
- Maity A. et al. Residual gas analyzer mass spectrometry for human breath analysis: a new tool for the non-invasive diagnosis of Helicobacter pylori infection. J. Breath Res. 8, 016005 (2014). [DOI] [PubMed] [Google Scholar]
- Crosson E. R. et al. Stable isotope ratios using cavity ring-down spectroscopy: determination of 13C/12C for carbon dioxide in human breath. Anal. Chem. 74, 2003–2007 (2002). [DOI] [PubMed] [Google Scholar]
- Banik G. D. et al. Diagnosis of small intestinal bacterial overgrowth in irritable bowel syndrome patients using high-precision stable 13CO2/12CO2 isotope ratios in exhaled breath. J. Anal. At. Spectrom. 29, 1918–1924 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.