Abstract
Endocannabinoids (eCBs) are key activity-dependent signals regulating synaptic transmission throughout the CNS. Accordingly, eCBs are involved in neural functions ranging from feeding homeostasis to cognition. There is great interest in understanding how exogenous (e.g. cannabis) and endogenous cannabinoids affect behavior. As behavioral adaptations are widely considered to rely on changes in synaptic strength, the prevalence of eCB-mediated long term depression (eCB-LTD) at synapses throughout the brain merits close attention. The induction and expression of eCB-LTD, while remarkably similar at various synapses, is controlled by an array of regulatory influences which we are just beginning to uncover. This complexity endows eCB-LTD with important computational properties, such as coincidence detection and input specificity, critical for higher CNS functions like learning and memory. In this article, we review the major molecular and cellular mechanisms underlying eCB-LTD, as well as the potential physiological relevance of this widespread form of synaptic plasticity.
Keywords: Cannabinoid, CB1, LTD, LTP, synaptic transmission, STDP
Terms/Definitions: Endocannabinoid system, Endocannabinoid synthesis/release, Spike Timing-Dependent Plasticity
Introduction
Retrograde signaling by endocannabinoids (eCBs) has emerged as a major theme in the study of synaptic plasticity. Neuronal activity releases these neuromodulators, which activate the presynaptic type 1 cannabinoid receptor (CB1R, a G protein-coupled receptor), suppressing neurotransmitter release at both excitatory and inhibitory synapses in a short- and long-term manner (1-6). Examples of eCB-mediated long-term depression (eCB-LTD) have been reported throughout the brain. The discovery of eCB-LTD demonstrates that eCBs can have a long-term impact on neural function and it has certainly expanded our view on the role of eCB signaling in the CNS. As a result of the last 10 years of research on eCB-signaling and synaptic plasticity, eCBs have become the most prominent example of retrograde signaling molecules in the CNS. Moreover, eCB-LTD is by now one of the best examples of presynaptic forms of long-term plasticity. Since our previous review of eCB-mediated synaptic plasticity (3), a number of exciting developments have prompted a fresh appraisal of this field. Thorough review articles on eCB signaling and synaptic transmission have recently been published (5, 6), and the breadth and depth of studies focusing primarily on eCB-LTD warrants its own dedicated review. In this article, we will review the major developments regarding eCB-LTD's induction, expression, computational properties, developmental regulation, and physiological relevance.
Overview of eCB-LTD
Endocannabinoids and the CB1 receptor
Endogenous cannabinoids, or endocannabinoids (eCBs) are so called because of their close relationship to exogenous cannabinoids, the most famous being Δ9-tetrahydrocannabinol (THC), a major psychoactive component of marijuana (7). One of the most surprising discoveries came from studies revealing a unique, previously unknown receptor for THC within the mammalian central nervous system (CNS). The Type 1 Cannabinoid receptor (CB1R) was ultimately isolated, cloned, and characterized as a G protein-coupled receptor (GPCR) that signals via the αi/o family of G-proteins (8, 9). Soon after, CB1Rs were not only found to bind THC, but they also appeared to have an endogenous ligand; anandamide (AEA) was the first of several eCBs isolated, among which 2-arachidonyl glycerol (2-AG) is most prevalent in brain (10, 11). The physiological relevance of eCBs quickly emerged, as neuronal activity was identified as a potent stimulus for eCB release, and eCBs were found to act as retrograde messengers, regulating synaptic transmission through presynaptic CB1Rs (1). Since 2001, eCBs have been identified as triggers for short and long-term plasticity at synapses throughout the brain (3), in agreement with the ubiquitous expression of CB1Rs (12, 13).
Activation of the CB1R, and subsequent long-term reduction of transmitter release at the same synapse, defines eCB-LTD. CB1Rs also mediate short-term plasticity exemplified by Depolarization-induced Suppression of Inhibition or Excitation (DSI or DSE, respectively). DSI/DSE is well characterized in the hippocampus and cerebellum, and has been observed in other brain areas (2, 5). CB1Rs can engage a wide range of effector molecules, including (but not limited to) voltage-dependent Ca2+ channels (VDCCs), K+ channels, PKA and MAPK (recently reviewed in 14).
eCB-LTD: a widespread phenomenon in the brain
The first evidence implicating eCB signaling in LTD emerged in 2002 at excitatory synapses in dorsal striatum (15). Since then, eCB-LTD has been reported in several other brain structures such as the nucleus accumbens (16), amygdala (17-19), hippocampus (20-25), visual cortex (26-29), somatosensory cortex (30, 31), prefrontal cortex (32), cerebellum (33), ventral tegmental area (VTA) (34), brain stem (35) and superior colliculus (36). As shown in Table I, it is now clear that eCB-LTD is a widely expressed phenomenon in the brain that can be observed at both excitatory and inhibitory synapses. This prevalence strongly suggests that eCB-LTD may be a fundamental mechanism for making long-term changes to neural circuits and behavior.
Table I. eCB-LTD is a widespread phenomenon the brain.
Examples of eCB-LTD confirmed using CB1R antagonists, CB1R knockout mice or both. STDP, spike timing-dependent plasticity; HFS, high-frequency stimulation (100 Hz); LFS, low-frequency stimulation (1 Hz).
| Brain Structure | Synapse | Induction protocol | References |
|---|---|---|---|
| Neocortex | |||
| Visual | Excitatory inputs, L5 pyramidal cell-pairs | STDP (postsynaptic bursts) STDP and LFS |
(26, 27) (28) |
| Excitatory inputs, L4 → L2/3 pyramidal neurons (immature visual cortex) | TBS | (29) | |
| Somatosensory (barrel cortex) | Excitatory inputs to L2/3 pyramidal neurons | STDP (postsynaptic bursts) | (30, 31) |
| Prefrontal | L2/3 → L5/6 | Moderate 10 Hz stimulation for 10 min | (32) |
| Hippocampus | Inhibitory inputs to CA1 pyramidal cells | HFS, TBS | (20-22, 24, 25) |
| Excitatory inputs to CA1 pyramidal cells (immature hippocampus) | HFS | (23) | |
| Amygdala | Inhibitory inputs to basolateral amygdala | LFS | (17-19) |
| Dorsal Striatum | Excitatory inputs to medium spiny neurons | LFS, STDP | (15, 39, 49, 50) |
| Nucleus Accumbens | Excitatory inputs to medium spiny neurons | Moderate 13 Hz stimulation for 10 min | (16, 142) |
| Cerebellum | Excitatory inputs to Stellate Interneurons | 4 bouts of 25 stimuli at 30 Hz, delivered at 0.33 Hz | (33) |
| Ventral Tegmental Area (VTA) | Inhibitory inputs to dopamine neurons | Moderate 10 Hz stimulation for 5 min | (34) |
| Dorsal Cochlear Nucleus | Excitatory inputs to Cartwheel cells | STDP | (35) |
| Superior Colliculus | Inhibitory inputs to tectal neurons in vitro | HFS | (36) |
Induction of eCB-LTD
Strong similarities in the pattern of eCB-LTD induction and expression are evident at both excitatory and inhibitory synapses from brainstem to cortex (3). The main objective of this section will be to define 1) synaptic events which trigger eCB production/release, 2) how eCB production, release and degradation may be regulated, and 3) which presynaptic events are required for successful induction of eCB-LTD.
Synaptic events triggering eCB-mediated synaptic plasticity
eCB-LTD induction typically begins with a transient increase in activity at glutamatergic afferents and a concomitant release of eCBs from a target (postsynaptic) neuron (Fig. 1). eCBs then travel backwards (retrogradely) across the synapse, activating CB1Rs on the presynaptic terminals of either the original afferent (homosynaptic eCB-LTD), or nearby afferents (heterosynaptic eCB-LTD) (3). In the past few years, mounting evidence indicates that eCB-LTD induction requires presynaptic activity of the target afferent, independent of its role in triggering eCB release (see below).
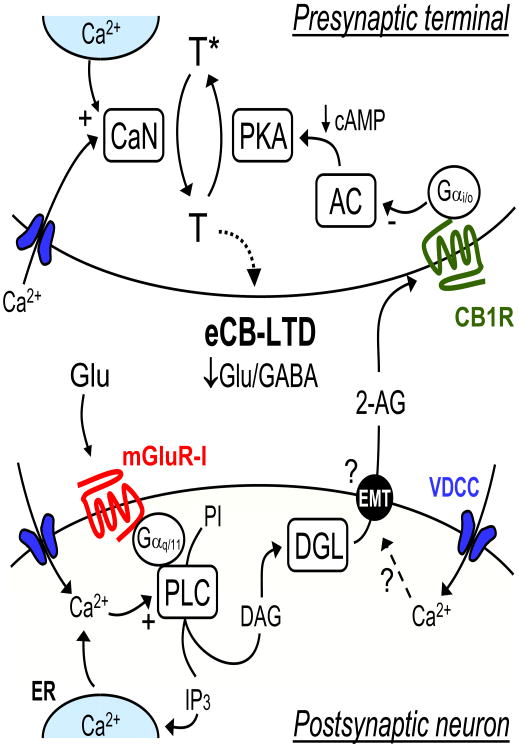
Figure 1. Schematic summary of the eCB-LTD induction mechanism.
One of the most common initial steps of induction is the activation of postsynaptic group I metabotropic glutamate receptors (mGluR-I), following repetitive activation of excitatory inputs. These receptors couple to Phopholipase C (PLC) via Gαq/11 subunits and promote diacylglycerol (DAG) formation (from Phosphatdylinositol, PI), which is then converted into the eCB 2-arachidonoylglycerol (2-AG) by Diacylglycerol Lipase (DGL). 2-AG is then released from the postsynaptic neuron by a mechanism that presumably requires an eCB membrane transporter (EMT), and binds presynaptic CB1Rs. Postsynaptic Ca2+ can contribute to eCB mobilization either by stimulating PLC, or in a PLC-independent, uncharacterized manner. This Ca2+ rise can be through voltage-dependent Ca2+channels (VDCC) actived by action potentials (e.g. during spike timing-dependent protocols), NMDARs, or released from the Endoplasmic Reticulum (ER), e.g. by the PLC product, inositol 1,4,5-trisposphate (IP3). In some synapses, induction of eCB-LTD by “afferent-only” stimulation protocols can occur independently of postsynaptic Ca2+. At the presynaptic terminal, the CB1R inhibits adenylyl cyclase (AC) via Gαi/o, reducing PKA activity. Induction of eCB-LTD may also require a presynaptic Ca2+ rise through presynaptic VDCCs, NMDARs (not shown) or release from Ca2+ internal stores. Activation of the Ca2+-sensitive phosphatase calcineurin (CaN), in conjunction with the reduction in PKA activity, shifts the kinase/phosphatase activity balance, thereby promoting dephosphorylation of a presynaptic target (T) that mediates a long-lasting reduction of transmitter release. For clarity, eCB-LTD mediated by AEA is not shown.
Induction protocols differ widely across examples of eCB-LTD (Table I). Some forms of eCB-LTD are induced by the tetanic stimulation of afferents, an approach used extensively in the study of synaptic plasticity. A number of induction protocols effectively produce eCB-LTD, from 100 pulses at 1 Hz to 100 Hz, or the more patterned theta burst stimulation (TBS). These afferent-only induction protocols for eCB-LTD have not been rigorously compared at most synapses, but at least for eCB-LTD at hippocampal inhibitory synapses, induction is effective over a broad range of frequencies (24, 37). eCB-LTD has also been found by repetitively firing presynaptic and postsynaptic neurons at fixed intervals with respect to each other. This induction protocol can yield “spike timing-dependent plasticity” (STDP), where the order and interval of the two spikes dictates the direction (i.e. t-LTD or t-LTP) and magnitude of plasticity (for a recent review, see 38). At this time, several instances of STDP are known to feature a mechanistically distinct t-LTD and t-LTP, the former being CB1R dependent (eCB-t-LTD). eCB-t-LTD's presynaptic locus of expression is indistinguishable from those forms induced with “afferent-only” stimulation protocols described above. A given synapse may well be able to support eCB-LTD induced by both afferent-only and spike timing-dependent protocols (15, 39).
Stimuli for eCB production
Activity-dependent release of eCBs from the postsynaptic cell is the common trigger to all forms of eCB-LTD. Extensive studies have established that two separate processes, neurotransmitter release and neuronal depolarization, mediate eCB release (for comprehensive reviews on this topic, see (5, 40)). For example, glutamate can stimulate eCB release through activating Type I metabotropic glutamate receptors (mGluR-I), which can initiate eCB synthesis “on demand” through effectors of the Gαq/11 subunit (41, 42). Neuronal depolarization is also a potent trigger of eCB release, through a less understood Ca2+-dependent process that does not depend on G proteins (1, 40).
1. Metabotropic receptor-dependent eCB release
Studies of neural tissue conducted in vivo or in acutely prepared brain slices indicate that eCB release need not be linked to special metabotropic receptors, or a particular route of Ca2+ entry. In addition to mGluR-I, metabotropic dopamine (Type 2; D2), muscarinic acetylcholine (type 1/3; M1/M3), serotonin (type 2; 5-HT2), orexin, and cholecystokinin receptors are all effective stimuli for eCB release (43-48). Most of these GPCRs signal through Gαq/11, engaging phospholipase C (PLC) and diacylglyerol lipase (DGL), which form the eCB 2-AG (Fig. 1). However, alternative mechanisms coupling these receptors to eCB release have also been proposed. For example, in basolateral amygdala, mGluR1 appears to stimulate AEA production through a pathway involving adenylyl cyclase (AC) and cAMP-dependent protein kinase (PKA) (18). Despite the marked differences in eCB synthetic route and even eCB identity, eCB-LTD is expressed in a nearly identical fashion in amygdala and hippocampus (19), reinforcing the notion that eCB release, by any means, is a core component of eCB-LTD.
D2Rs differ from the other metabotropic receptors named above as they typically signal through the Gαi/o pathway and stimulate formation of AEA, rather than 2-AG, although the precise synthetic pathway is not known (40, 43). D2Rs are required for corticostriatal eCB-LTD (39, 49, 50), and several lines of evidence from slice electrophysiology suggest a role for AEA in this process (50, 51). In part due to the paucity of pharmacological tools for manipulating AEA biosynthesis, it has been difficult to determine the precise mechanism by which D2Rs mediate eCB-LTD. D2Rs may directly stimulate eCB release from medium spiny neurons (MSNs), or, as recently suggested, D2Rs exert an indirect effect on eCB release via cholinergic interneurons in dorsal striatum (52). However, a recent study has shown that the presence or absence of D2Rs on MSNs determine whether eCB-LTD can be induced at an afferent glutamatergic synapse (50), owing to a D2R-mediated facilitation of eCB release (49). Indeed, using spike timing-dependent induction protocols, it has been recently reported that corticostriatal synapses onto D1-expressing neurons can undergo eCB-LTD, provided induction occurs in the presence of a D1R antagonist (39). Although these corticostriatal afferents are capable of expressing CB1R agonist-induced LTD (49, 53), it seems that specific postsynaptic factors can pose intrinsic limitations on eCB-LTD.
2. Calcium-dependent eCB release
While not universally required for eCB-LTD, Ca2+-dependent mechanisms play a pivotal role in many forms of eCB-LTD (15, 16, 26, 28, 30-33, 35, 54). Like metabotropic signaling pathways, which converge through multiple pathways onto eCB release to ultimately trigger eCB-LTD, Ca2+-dependent eCB release also has a similarly broad set of initiating mechanisms. Ca2+ influx through NMDARs, L-type and T-type VDCCs, and Ca2+ release from intracellular stores have all been reported to drive eCB release in brain slices (30, 31, 54-57). The source of postsynaptic Ca2+ differs among individual examples of eCB-LTD. Although no specific mode of Ca2+ rise stands as a universal requirement, it is not clear to what extent different Ca2+ sources can compensate for each other.
Integrating signals for eCB release
The metabotropic and Ca2+-driven mechanisms can operate independently - mGluR-I can release substantial amounts of eCB even under strong Ca2+-buffering conditions (18, 20, 42, 58) - but they can also act synergistically (25, 49, 59-61). Some of the enzymes mediating this cooperativity have been characterized. For example, the PLCβ isoform is critical for mGluR-I-triggered release of 2-AG (61). Gαq/11, through PLCβ, provides the substrate for DGL, leading to 2-AG formation (62). In CA1 pyramidal cells of the hippocampus, activity of the isoform PLCβ1 is significantly enhanced by raising Ca2+ concentrations, allowing for a potential coincidence detection mechanism in the postsynaptic neuron, gating eCB release and possibly eCB-LTD (61). Another isoform of this enzyme, PLCβ4, mediates a similar process in the cerebellum, at parallel fiber to Purkinje cell synapses (59, 63). Here, activation of mGluR-I renders eCB release more sensitive to intracellular Ca2+ levels, raised either by direct Purkinje cell depolarization, or through stimulating powerful climbing fiber inputs to the Purkinje cell. Although eCB-LTD has not been assessed in transgenic animals lacking the various PLCβ enzymes, pharmacological blockade of PLC effectively blocks eCB-LTD in hippocampus, VTA and prefrontal cortex (20, 32, 34, 64). It remains to be seen whether similar synergy of eCB release between metabotropic and Ca2+ driven signals occurs with enzymes in the AEA synthetic pathway, as suggested for phospholipase D (40).
Spatial constraints on eCB production
Investigations into PLCβ have revealed a compelling picture of how the temporal pattern of synaptic input can amplify or constrain eCB production. In principle, this mechanism by PLCβ can also provide some spatial specificity to the eCB signal. Neuronal depolarization presumably triggers eCB synthesis/release wherever Ca2+ influx occurs; only active synaptic inputs engage the metabotropic facilitation of the eCB signal, while silent afferents would not. Even so, eCBs can trigger both homo- and heterosynaptic plasticity. How else, then, might a synapse limit the spatial impact of eCB release?
Another strategy neurons may employ to provide spatial specificity to the eCB signal is to confine the Ca2+ signal itself, thereby restricting the lateral extent of eCB release. This hypothesis has been explored in the context of eCB-LTD at cerebellar parallel fiber to stellate cell synapses (33). Stellate cells are especially notable for their absence of structural specializations, such as the spine head, which in other neurons effectively compartmentalize second messenger signals (65). Rather, the Ca2+ signal is biochemically “confined” through interactions with the Ca2+-buffering protein parvalbumin as well as a generalized reduction of small molecule mobility within the dendrite (33). These mechanisms can isolate the eCB release generated by a single glutamatergic synapse from its neighboring synapse, 15 μm away.
The spatial distribution of eCB-synthetic machinery may also impact synaptic transmission. As discussed above, DGL plays a key role in mGluR-dependent eCB production and several forms of eCB-LTD. Anatomical studies have revealed differential sub-cellular localization with respect to the synapse in various brain structures (66-69). The relevance of these findings to eCB-LTD induction remains unexplored.
eCB degradation
Enzymes regulating the eCB lifetime in the synaptic cleft are also potentially significant targets for regulating eCB-LTD induction. The degradation of 2-AG and AEA largely depends on the enzymes monoacylglycerol lipase (MGL) and FAAH, respectively (40). Other enzymes may also participate in eCB catabolism, although with less specificity (e.g. COX-2) (70, 71). In hippocampus, where 2-AG is very likely to be the dominant functional eCB, electron microscopic studies reveal a predominantly presynaptic pattern of MGL expression, supporting a role in regulating the effective concentration of eCBs near their site of action (72). Consistent with this idea, MGL inhibition can transform a subthreshold tetanus into an effective stimulus for eCB-LTD in the prefrontal cortex (32), mirroring similar results with FAAH inhibitors in eCB-LTD in neocortex, amygdala and dorsal striatum (18, 26, 50). At present, it is not known if MGL or FAAH activity is dynamically regulated in neurons, although some data have also shown that elevated intracellular Ca2+ levels can inhibit MGL activity in microglia (73). Regulating MGL in this way offers a potential mechanism whereby presynaptic activity, and Ca2+ within the terminal, might hold sway over the duration of CB1R activation, and possibly eCB-LTD.
A regulated eCB efflux step?
Several aspects of eCB release are still poorly understood, but may prove to be important points of control over eCB-LTD. A recent study has suggested that efflux of newly synthesized eCBs can be regulated by postsynaptic activity. Adermark and Lovinger (2007) loaded postsynaptic striatal MSNs with AEA or 2-AG and found that pairs of test stimuli (0.1 Hz) could induce a CB1R-dependent suppression of excitatory transmission, independent of mGluR-I activation, postsynaptic Ca2+ or postsynaptic membrane potential. Since afferent stimulation did not modulate direct presynaptic inhibition by a CB1R agonist, the authors hypothesized that afferent activity regulates a late step in eCB release/mobilization, prior to CB1R activation. Accordingly, postsynaptic loading with blockers of the putative eCB membrane transporter (EMT), prevented the synaptic depression induced by combined postsynaptic AEA application and afferent activation (74). Indeed, Lovinger and co-workers have previously shown that postsynaptic loading of EMT blockers prevents eCB-LTD in dorsal striatum (75), an observation also confirmed by others in somatosensory cortex (31). Whether postsynaptic activity governs other forms of eCB-LTD through the putative EMT is unknown, as this type of regulation may be a synapse specific phenomenon. In contrast to the situation at excitatory synapses, postsynaptically loaded eCBs suppresses inhibitory synapses onto MSNs regardless of afferent activity (74).
Presynaptic activity and eCB-LTD
The foregoing discussion has primarily focused on presynaptic (afferent) activity as a regulator of eCB release. However, several lines of evidence suggest that eCB-LTD requires presynaptic activity as a cofactor for induction that is independent of eCB release and CB1R activation. Coincident activation of CB1Rs and presynaptic activity provides an additional mechanism to ensure synapse specificity, such that only active fibers undergo eCB-LTD.
Spike timing-dependent eCB-LTD and presynaptic NMDARs
In a series of elegant experiments using cell-pair recordings in L5 of visual cortex, Sjöstrom and co-workers (2003) provided the first evidence that presynaptic activation of NMDARs is required for the induction of eCB-LTD, here induced by spike timing-dependent protocols (or eCB-t-LTD). They showed that exogenous activation of CB1Rs, a manipulation that short-cuts eCB release from the postsynaptic pyramidal neuron, induces LTD only if the presynaptic neuron is activated at a relatively high frequency, and importantly, this LTD is blocked by the NMDAR antagonist D-APV (26). Sjöstrom et al (2003) postulated that coincident activation of presynaptic NMDA and CB1 receptors is required for the induction of eCB-t-LTD. Further support for the presynaptic NMDAR requirement has been independently provided by other groups studying somatosensory cortex (30, 31) and visual cortex (28, 76), showing that bath application of NMDAR antagonists blocks eCB-LTD, but blockade disrupting postsynaptic NMDAR function with either hyperpolarization or the inclusion of MK-801 in the postsynaptic recording pipette, does not affect the induction of eCB-LTD. A recent study in somatosensory cortex has provided direct evidence that presynaptic NMDARs are required for t-LTD induction. Indeed, loading presynaptic neurons with MK-801 in synaptically coupled cell-pairs selectively abolished t-LTD, without affecting t-LTP (77). A still open question is how presynaptic NMDARs and CB1Rs interact to induce a long-lasting reduction in transmitter release. It is tempting to postulate that Ca2+ influx through presynaptic NMDARs might be required to activate some metabolic process at the presynaptic terminal. Alternatively, presynaptic NMDAR could signal in a Ca2+-independent manner. These possibilities remain to be tested.
CB1Rs are insufficient for eCB-LTD and presynaptic activity is required
The involvement of presynaptic NMDARs may represent a unique aspect linking the various forms of eCB-t-LTD (26, 28, 31). However, the fact that several examples of eCB-LTD are NMDAR-independent immediately raises the question: is presynaptic Ca2+ entry, or even presynaptic activity, a general requirement of eCB-LTD induction? Experiments performed with eCB-t-LTD showed that CB1R agonists alone do not trigger long-term plasticity without coincident activity (26, 31). CB1R activation, alone, is similarly unable to trigger eCB-LTD in hippocampus, dorsal striatum and VTA (22, 34, 53, 64, 75, 78-80). Data from hippocampus and striatum show that eCB-LTD requires minutes of CB1R activation after a brief induction stimulus (20, 75), suggesting that another induction signal, such as presynaptic activity, may be integrated during that period to induce eCB-LTD. This issue has been investigated in some detail for eCB-LTD in dorsal striatum and at CA1 inhibitory synapses.
Recent evidence shows that eCB-LTD in the dorsal striatum requires low-frequency presynaptic activity during the period of CB1R activation (53). The authors propose that this dual requirement confers synapse specificity onto eCB-LTD under conditions of (e.g.) eCB spillover. This finding is consistent with a previous report showing that CB1R activation alone cannot induce striatal eCB-LTD (75). The requirement for presynaptic activity fits well with the fact that CB1R activation for more than 5 minutes after the induction tetanus is also necessary for eCB-LTD in the hippocampus (20) and striatum (75). In these studies, the role of afferent stimulation may either have been 1) to supply activity at the test synapse, or 2) to release a cofactor crucial for induction. This latter concern is not trivial, given that this eCB-LTD may involve a complex interplay between dopamine receptors and cholinergic interneurons, in addition to events at the glutamatergic corticostriatal synapse (52, 81). Our own experiments in the hippocampus using interneuron-pyramidal cell pairs provide direct experimental evidence that eCBs and presynaptic activity at the test synapse interact to induce eCB-LTD. Under conditions of maximal eCB release (i.e. minutes of mGluR-I activation), we found that firing these interneurons produces eCB-LTD, whereas holding neurons silent during eCB release effectively prevents induction of eCB-LTD (78).
Timing of afferent activity – milliseconds vs minutes
Most evidence indicates that eCB-LTD induction requires presynaptic activity, in addition to CB1R activation. The timing requirements for presynaptic activity, however, seem to differ significantly among synapses. There is some evidence to suggest that these timing requirements reflect signal integration at the presynaptic terminal, rather than special conditions needed for eCB release. In L5 pyramidal cells of visual cortex, even manipulations which extended the lifetime of eCBs in the synaptic cleft did not remove spike timing dependence; rather they only broadened the time window where post-before-pre firing could trigger eCB-LTD (26). Similarly, in the dorsal cochlear nucleus, blocking the t-LTP component broadened the effective time window for eCB-LTD induction without changing the required order of spikes (35). It is tempting to speculate that the requirement of a presynaptic NMDAR, which as yet occurs exclusively in eCB-t-LTD, confers a timing property (82). eCB-t-LTD mediates coincidence detection on the order of milliseconds. For eCB-t-LTD in somatosensory cortex, this strict timing requirement may reflect its proposed physiological role in rodent whisker desensitization (83). In hippocampus and dorsal striatum, eCB-LTD seems to integrate aggregate events occurring over minutes to yield eCB-LTD. At present, the significance of this timing requirement is not known, but in principle it allows for an associativity between synaptic events on two very different time scales.
Presynaptic calcium and eCB-LTD
As suggested previously, the known involvement of presynaptic NMDARs in some forms of eCB-t-LTD may indicate a requirement for presynaptic Ca2+ in the induction of eCB-LTD. Our data (78) and that of Singla et al (2007), although indirect, support a role for presynaptic Ca2+ in the induction of eCB-LTD. Support for this hypothesis comes from experiments in both dorsal striatum (53) and in hippocampal inhibitory synapses, several methods of disrupting presynaptic Ca2+ dynamics all blocked eCB-LTD, strongly suggesting that interneuron spikes regulate this form of plasticity by raising Ca2+ at the nerve terminal. In addition, induction of eCB-LTD at hippocampal synapses also requires activation of the Ca2+-sensitive phosphatase calcineurin (78). Together, these observations lead us to propose a mechanism where firing, by raising Ca2+ levels at the presynaptic terminal, activates calcineurin, thereby shifting the balance of kinase and phosphatase activity in the presynaptic terminal, finally inducing eCB-LTD (see below).
In conclusion, most evidence indicates that both pre- and postsynaptic activity can control eCB-LTD induction. These regulatory mechanisms endow eCB-LTD with at least two forms of associativity as summarized in Figure 2. In the postsynaptic compartment, glutamate release and activity-dependent Ca2+ rise facilitates eCB mobilization, a process mediated by PLC. Presynaptic associativity involves CB1R activation by eCBs and presynaptic firing, which increases Ca2+ concentration, both required to engage long-term suppression of transmitter release.
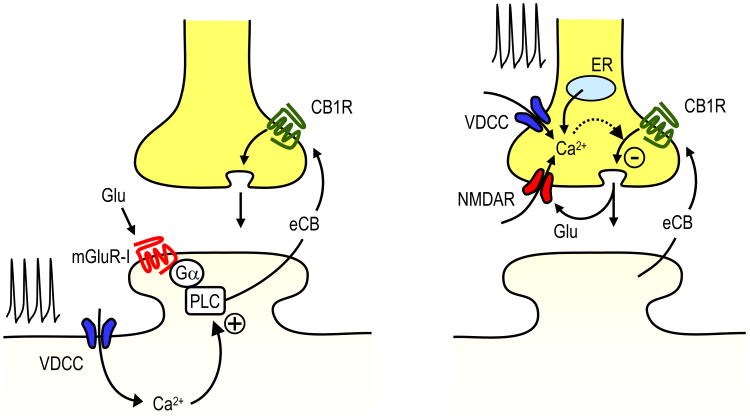
Figure 2. Potential mechanisms of associativity involved in eCB-LTD induction.
Postsynaptic compartment (left): the postsynaptic neuron can integrate action potential firing (which promotes Ca2+ rise via VDCCs) and synaptic release of glutamate (which activates mGluR-I) to facilitate eCB mobilization and eCB-LTD induction. Other Ca2+ sources (e.g. NMDARs and Ca2+ internal stores) could also contribute. In this model, PLC operates as a coincidence detector (61). Presynaptic compartment (right): the presynaptic terminal can also integrate two signals, eCBs (which activate presynaptic CB1Rs) and presynaptic firing (which promotes a Ca2+ rise via VDCCs and NMDARs). Ca2+ stores (endoplasmic reticulum, ER) may also contribute to this process. The presynaptic NMDAR may operate as a coincidence detector during a brief time window (26). In addition, the activity-dependent Ca2+ rise that occurs during minutes of CB1R activation likely promotes dephosphorylation of a presynaptic target downstream of the CB1R, the latter of which is an essential step for eCB-LTD induction (78).
Expression Mechanisms
The molecular mechanisms of eCB-LTD's expression and maintenance are just beginning to emerge. It was recognized early on that CB1R activation is necessary for induction but not during expression of eCB-LTD. Accordingly, washing in a CB1R antagonist after eCB-LTD is established fails to reverse plasticity in all synapses where this manipulation has been tested (20, 26, 75). Here we will discuss what processes downstream CB1R activation can explain the long-term reduction of transmitter release reported during eCB-LTD. Changes in the amount of transmitter release can result from modifications of the presynaptic action potential-induced Ca2+ influx and/or the downstream release machinery, as well as changes in excitability of the afferent axon.
Role of cAMP/PKA cascade in eCB-LTD
One of the most striking requirements distinguishing eCB-LTD induction from eCB-dependent short-term depression such as DSI/DSE is the extended duration of CB1R activity (several minutes) (3). This relatively long induction period suggests that CB1Rs may engage a different signaling process than the one mediating transient suppression of transmitter release. We have recently investigated this possibility in the hippocampus, where inhibitory synapses can undergo both short- and long-term eCB-mediated plasticity (DSI and I-LTD, respectively) (20). Here, a direct comparison of the expression mechanisms underlying these two forms of depression can be made at synapses impinging on the same target cell (19).
Compelling evidence indicates that the transient suppression of transmitter release occurring during DSI/DSE is mainly due to CB1R-dependent inhibition of presynaptic VDCCs (84-87), a process likely mediated by the Gβγ subunits (84). The relatively fast decay of DSI (<1 min) likely reflects the transient profile of eCB production and CB1R engagement. We hypothesized that the increased time requirement for eCB-dependent I-LTD induction could reflect the recruitment of the αi/o effector limb of the CB1R G protein signaling cascade, which inhibits the cAMP/Protein Kinase A (PKA) pathway (88, 89). Indeed, we found that inhibiting PKA activity with selective blockers, or raising cAMP levels by continuous activation of adenylyl cyclase, prevents I-LTD without affecting DSI (19). More recently, we reported that presynaptic phosphatase activity may complement the CB1R-dependent inhibition of PKA during eCB-LTD induction. Specifically, inhibiting the Ca2+-activated phosphatase, calcineurin, fully blocked I-LTD, once again leaving DSI intact (78). In addition, postsynaptic blockade of PKA and calcineurin had no effect on either I-LTD or DSI, supporting the idea that these kinase and phosphatase activities occur presynaptically. Altogether these findings indicate that 1) the signaling pathways downstream CB1Rs that mediate transient vs. long- lasting depression of transmitter release differ, and 2) a CB1R-mediated reduction of cAMP/PKA activity underlies I-LTD.
The involvement of the cAMP/PKA pathway in eCB-LTD has also been reported in other brain structures. As in the hippocampus, inhibiting PKA or raising cAMP levels interfere with eCB-LTD at glutamatergic synapses in the nucleus accumbens (90). In addition, protein kinase inhibitors reportedly prevent striatal eCB-LTD (91), although in this case, PKA may also be required postsynaptically for AEA production, as suggested for eCB-LTD in the amygdala (18). The cAMP/PKA cascade could also mediate the eCB-LTD recently reported at excitatory hippocampal synapses in neonatal rats (23). Thus, most evidence suggests that CB1R-dependent inhibition of the cAMP/PKA signaling pathway is a critical step in eCB-LTD at both excitatory and inhibitory synapses. Whether this pathway also mediates eCB-LTD in other brain areas remains to be tested; it is formally possible that other signaling pathways downstream CB1Rs could also participate.
eCB-LTD expressed by changes in the release machinery
Modulation of the cAMP/PKA pathway has been previously implicated in presynaptic forms of LTP and LTD (for a review, see 92). A common theme in these forms of plasticity is that their expression mechanism relies on changes in the transmitter release machinery (93-95). In particular, the active zone protein RIM1α, which is phosphorylated by PKA in vitro (94), is a demonstrated requirement for PKA-dependent LTP at several excitatory synapses, including the mossy fiber to CA3 pyramidal cell synapse (93), the parallel fiber to Purkinje cell synapse (93, 94), and the Schaffer collateral to CA1 pyramidal synapse (96). Using RIM1α knockout mice, we have recently found that I-LTD in the hippocampus and amygdala also requires RIM1α (19). In addition, the reduction of mIPSC frequency (but not amplitude) mediated by a CB1R agonist or a PKA blocker was markedly reduced in these mice, strongly suggesting that CB1Rs and PKA can target the release machinery in a RIM1α-dependent manner. Thus, changes in the release machinery via cAMP/PKA signaling and RIM1α may represent a general mechanism underlying presynaptic forms of long-term plasticity (LTP and LTD) at both excitatory and inhibitory synapses.
According to the PKA/RIM1α model of presynaptic plasticity a reduction of PKA activity (e.g. via CB1R activation) enables RIM1α dephosphorylation, which translates into depression of transmitter release. Conversely, increases in PKA activity and RIM1α phosphorylation lead to LTP (19, 93, 94, 97). A previous report suggested that phosphorylation of RIM1α at Serine 413 is necessary for presynaptic LTP in parallel fiber synapses formed in vitro by cultured cerebellar neurons (94). To test whether RIM1α phophorylation/dephosphorylation is indeed required for PKA-dependent forms of plasticity reported in acute brain slices, Thomas Südhof and co-workers have recently generated knock-in mice in which serine 413 is mutated to alanine. Surprisingly, mossy fiber LTP, cerebellar LTP, and hippocampal I-LTD, are all normal in these transgenic mice (98). These findings strongly suggest that although the RIM1α complex is essential for presynaptic long-term plasticity, PKA regulates this type of plasticity by a mechanism distinct from RIM1α phosphorylation at S413. Indeed, PKA-dependent modulation of transmitter release can occur as a result of the phosphorylation/desphosphorylation of several other presynaptic proteins involved in exocytosis (for a review, see 99). Future studies to identify the nature of such presynaptic protein(s) in PKA/RIM1α-dependent forms of plasticity, including eCB-LTD, are warranted.
eCB-LTD expressed by changes in voltage-dependent calcium channels
Manzoni and co-workers have recently reported that blockade of P/Q-type, but not L- or N-type VDCCs, occludes eCB-LTD at excitatory synapses in the nucleus accumbens (90). Previous work by this group showed that these synapses can undergo an mGluR2/3-dependent form of LTD that also depends on the cAMP/PKA pathway, and whose expression mechanism likely involves a selective reduction in the contribution of P/Q-type VDCCs to glutamate release (100). Importantly, mGluR2/3-dependent LTD and eCB-LTD mutually occlude, supporting the idea that both forms of plasticity could share a common mechanism (101). Together, these observations suggest that a PKA-dependent reduction of presynaptic Ca2+ influx via P/Q-type VDCCs could underlie both forms of LTD. The involvement of P/Q-type VDCCs in the expression of a presynaptic form of LTD has been previously demonstrated at mossy fiber synapses onto stratum lucidum interneurons in the hippocampus (102). Indeed, using two-photon Ca2+ imaging from anatomically defined mossy fiber terminals, Pelkey and co-workers (2006) have directly shown that expression of mGluR7-dependent LTD is due to a long-lasting reduction of presynaptic Ca2+ influx via P/Q-type VDCCs. Whether eCB-LTD in nucleus accumbens or other brain structures is also associated with a long-term reduction of presynaptic Ca2+ signals has not been examined. Selective depression of P/Q-type VDCC function has also been reported in amphetamine-induced, CB1R-dependent LTD of excitatory synaptic transmission in the amygdala (103). Interestingly, this form of plasticity is accompanied by a change in the frequency, but not amplitude, of miniature EPSCs, suggesting a second (parallel) mechanism of expression downstream Ca2+ influx. Whether eCB-LTD at a given presynaptic terminal can express both RIM1α-dependent and P/Q-type VDCC-dependent mechanisms remains unknown.
eCB-LTD expressed by changes in presynaptic excitability
A third expression mechanism has been suggested by a recent study describing a heterosynaptic eCB-LTD of glutamatergic synaptic transmission in the developing CA1 area of the hippocampus (23). This form of eCB-LTD is associated with a decrease of fiber volley amplitude, suggesting a reduction of presynaptic excitability. Importantly, LTD of both synaptic transmission and fiber volley amplitude are mimicked by a CB1R agonist, and blocked by a CB1R antagonist. Moreover, depression of fiber volleys is impaired by K+ channel blockers (23). These observations strongly suggest that heterosynaptic eCB-LTD at excitatory synapses in the immature hippocampus is likely due to a CB1R-dependent reduction of presynaptic excitability through activating K+ channels at presynaptic fibers. It will be interesting to see whether a similar expression mechanism also underlies the heterosynaptic eCB-LTD recently reported at glutamatergic synapses in the immature visual cortex (29).
Developmental Regulation of eCB-LTD
The refinement of neural circuits during postnatal development is believed to involve activity-dependent changes in synaptic strength. Presumably paralleling the ongoing changes in pre- and postsynaptic properties, induction mechanisms of synaptic plasticity adjust over development. Consistent with this notion, several recent studies have shown that eCB-LTD can be developmentally regulated. From a mechanistic perspective, regulation of eCB-LTD likely reflects developmental changes in eCB signaling (e.g. eCB production/degradation, CB1R number/function). For example, the probability that high frequency stimulation induces striatal LTD increases in brain slices from rats during the third and fourth postnatal week (104), a change that has been associated with a developmental increase in AEA levels in striatal tissue (51). In the hippocampus, the magnitude of eCB-mediated I-LTD is reportedly greater in adolescent rats (postnatal day 28-35) than in adults (postnatal day 75-110), in tandem with the reduced ability of a CB1R agonist to suppress inhibitory neurotransmission (22). It is unclear whether this developmental regulation reflects differences in CB1R number and/or function at GABAergic terminals, although binding studies have reported maximal CB1R expression levels on postnatal day 30-40 (105).
As described above, glutamatergic synapses in the immature hippocampus (postnatal day 2-10) can express a form of heterosynaptic eCB-LTD, reportedly due to a reduction of presynaptic excitability (23). This form of plasticity becomes less prominent during development and it cannot be induced in the mature hippocampus. At present, the mechanism of this developmental change is unknown. Similar heterosynaptic eCB-LTD has been recently shown in the immature visual cortex (29). High frequency stimulation of excitatory inputs to L2/3 neurons induces heterosynaptic eCB-LTD in most mice at postnatal day 7–20 but not at postnatal day 35–41. Interestingly, brain-derived neurotrophic factor (BDNF), which may be released from strongly activated presynaptic sites, appears to prevent the induction of homosynaptic eCB-LTD. Presumably, an interaction between the CB1R and TrkB (the high-affinity receptors for BDNF) at active presynaptic terminals leads to the blockade of CB1R signaling. A developmental upregulation of BDNF expression, diffusion of BDNF in cortical tissues, and a concomitant reduction in CB1R expression, have been proposed as potential mechanisms underlying the developmental regulation of heterosynaptic eCB-LTD in visual cortex (29).
Developmental changes in any of the major pre- and postsynaptic determinants of eCB-LTD could regulate this form of plasticity. A developmental loss of presynaptic NMDARs has been reported in visual cortex, occurring abruptly at the onset of the critical period for receptive field plasticity (>P23 in rats) (76). During the peak of the critical period for ocular dominance (P23-30), t-LTD in L2/3 of visual cortex requires activation of postsynaptic NMDARs (76). Given that most studies on eCB-dependent t-LTD in neocortex have been performed in young rodents, and show a requirement fo presynaptic NMDARs, future studies will be necessary to determine whether older animals retain this form of plasticity.
eCB-LTD Metaplasticity
Metaplasticity, the plasticity of synaptic plasticity, refers to an enduring change in the ability of neurons or synapses to generate synaptic plasticity (for a recent review, see 106). eCBs are known to mediate metaplasticity by triggering long-term suppression of inhibitory transmission (i.e. I-LTD) and facilitating subsequent induction of LTP at excitatory inputs (18, 37). Recent work suggests that eCB signaling itself can be subject to metaplasticity. Indeed, repetitive stimulation in CA1 produces a long-term up-regulation of eCB signaling as indicated by an enduring enhancement of the magnitude of DSI (24, 107). In one study, DSI potentiation could be triggered by low-frequency stimulation (LFS) (24), whereas in another study induction required strong HFS, as well as the co-activation of mGluRs, AMPA/kainate receptors, and CB1Rs (107). While the mechanism underlying DSI potentiation induced by LFS has not been explored, an up-regulation of CB1R number likely mediates eCB plasticity induced by strong HFS (107). This mechanism has previously been implicated in the DSI potentiation that occurs following febrile seizures in rats (108). A subsequent study by Alger and co-workers has shown that the long-lasting potentiation of DSI can be triggered by brief activation of mGluR-I (25). This study showed that the transient postsynaptic Ca2+ rise that occurs by a single episode of DSI can facilitate subsequent mGluR-I-dependent mobilization of eCBs and the induction of I-LTD (25). The Ca2+-dependent process that causes this remarkable form of metaplasticity is unknown. In summary, these examples underscore that eCB signaling, and as a result eCB-mediated plasticity, can be dynamically regulated. Because some induction protocols can trigger simultaneous LTP and LTD, regulation of eCB signaling can be important in modulating the balance between the two.
Functional Relevance of eCB-LTD
Reflecting the widespread distribution of the CB1R in the CNS (12, 13), exogenous ligands for CB1R impact a broad range of CNS functions, ranging from homeostatic control of feeding to associative memory formation; some of these CB1R ligands have also been associated with neuropsychiatric disorders. However, ascribing specific roles for eCB-LTD in these behaviors has been challenging. Here we will discuss the most promising avenues of investigation linking eCBs and eCB-LTD to normal and pathological physiology.
Sensory deprivation
LTD is a leading model to explain the synaptic weakening that occurs at cortical synapses after a period of sensory deprivation (109-111), but see (112, 113). Studies in both somatosensory and visual cortices have revealed that this LTD-like phenomenon mechanistically resembles eCB-LTD reported in vitro. Feldman and co-workers have recently shown that whisker removal weakens L4-L2/3 synapses in rat barrel cortex by decreasing presynaptic function (114), mirroring the expression mechanism of eCB-t-LTD described at these synapses (31) (see above). Similarly, monocular deprivation (MD) depresses visually evoked responses within multiple layers of visual cortex (115, 116). Bear and co-workers found that a previously described LTD at L4-L2/3 synapses in vitro (117), thought to contribute to this MD-induced synaptic depression, is CB1R dependent (28). Most notably, in brain slices prepared after whisker deprivation or MD, eCB-t-LTD is abolished, suggesting that sensory deprivation may have occluded eCB-t-LTD and therefore may share a common mechanism (28, 114).
Data from genetic and pharmacological interference with CB1Rs support a role for eCB-LTD in sensory-driven cortical plasticity. Anatomical analysis of the barrel cortex in CB1-/- mice revealed abnormal barrel maps (118). During a brief period of MD, systemic application of the CB1R antagonist AM251 fully prevented the depression of inputs to L2/3 of visual cortex in vivo, the same area which expresses eCB-LTD (28, 119). Whether eCB-t-LTD causally drives the loss of cortical responses to deprived sensory inputs remains to be determined. In addition, short-term plasticity mediated by eCBs, such as DSI/DSE (120, 121), could also contribute to these forms of cortical plasticity.
Associative learning
Long-term changes in synaptic strength are also believed to underlie associative memory formation in hippocampus and amygdala (122). The first studies to show a role for the eCB/CB1R system in hippocampus and amygdala-specific learning tasks (the Morris Water Maze and cued fear conditioning, respectively) used CB1-/- mice or systemically applied CB1R antagonists (17, 123). These and subsequent studies found that eCBs and CB1Rs were specifically required for extinguishing an established fear or spatial memory, but were not needed to acquire these types of memories (17, 123-126). In addition, FAAH inhibitors, which enhance eCB signaling, facilitated extinction of cue-shock associations as well as memories of a hidden platform's former position in the Morris Water Maze (126, 127). While these findings may point to an underlying involvement of eCB-LTD, a number of eCB-mediated effects on synaptic transmission have been noted in these two main structures. eCB-LTD has been described at inhibitory synapses in CA1 and basolateral amygdala (17, 20), and, in developing hippocampus, at excitatory synapses (23). Hippocampal synapses also express DSI and DSE (1, 3). In addition, in both hippocampus and amygdala, eCB-LTD also modulates excitability and the induction of LTP at excitatory synapses, a process widely thought to underlie memory acquisition (18, 20, 37). Marsicano and colleagues have developed a promising strategy to address the contribution of CB1Rs at excitatory and inhibitory terminals in the context of epilepsy, generating a line of transgenic mice with selective deletions of the CB1R in either forebrain GABAergic interneurons or in principal neurons (128). Identifying unique molecular determinants of eCB-LTD versus DSI/DSE will further aid in resolving these issues.
Parkinson's Disease
Parkinson's Disease (PD) is a neurodegenerative disorder characterized by hallmark motor symptoms such as rigidity, bradykinesia and akinesia, as well as the progressive loss of the dopaminergic neurons in the substantia nigra pars compacta. In addition to dopamine depletion, PD is also thought to involve a reactive increase in corticostriatal excitatory drive in the basal ganglia via the indirect (striatopallidal) pathway, a circuit identified by MSNs exclusively expressing the D2R rather than D1R (129). Accordingly, strategies to treat these symptoms are aimed not only at replacing the missing dopamine, but also at reducing transmission through the indirect pathway. As a D2R-dependent mechanism which suppresses corticostriatial transmission (4, 39, 50, 130), striatal eCB-LTD is a potential therapeutic target in PD. In fact, a recent study has shown that in vivo administration of the FAAH inhibitor URB597, reduces parkinsonian motor deficits in dopamine depleted animal models of PD, but only when administered together with a D2R agonist, which presumably triggers eCB release (43, 50). A direct demonstration that this effect is mediated by eCBs awaits confirmation. Interestingly, striatal eCB-LTD was also abolished in these animal models, but rescued in the presence of URB597 or the D2R agonist quinpirole (50). Motor abnormalities observed in animal models of PD can also be corrected by treatment with an mGluR5 antagonist (131). Interestingly, MSNs express high levels of mGluR5 (but not mGluR1) (68), whose activation can mobilize eCB release (42) and trigger “chemical” eCB-LTD at striatopallidal MSNs (50). Together, these studies suggest that a reduction of eCB production, particularly in the indirect pathway, could be an important mechanism contributing to the pathophysiology of PD, presumably by eliminating eCB-LTD. Therefore, manipulations of this pathway by modulating eCB production may provide an alternative approach for the treatment of striatal-based brain disorders, such as PD.
Drug Addiction
Like PD, drug addiction is a robust behavioral phenomenon particularly amenable to analysis in animals, as its major pathological features are reproducible across species. The rewarding properties of exogenous cannabinoids are well known (132, 133). However, the process of addiction not only involves the acute, rewarding effects of a drug, but may also involve long term neural adaptations induced by the drug of abuse (134, 135). These adaptations are thought to include changes of synaptic strength, especially in the VTA, a midbrain area densely populated by dopaminergic neurons, and the NAc, which receives prominent projections from the VTA (136-138). At least one such behavioral adaptation, sensitization to the rewarding properties of cocaine, is known to require eCBs and CB1Rs, based on experiments with CB1R antagonists and CB1R-/- mice (recently reviewed in 139). In addition, eCB-LTD in both VTA and NAc can be modulated by in vivo exposure to drugs of abuse, including cocaine (34, 140-142). Repetitive cocaine exposure in vivo fully occludes eCB-LTD at GABAergic synapses in VTA (34). This reduced GABAergic inhibition has been linked to a facilitation of LTP at nearby excitatory synapses in VTA (143), believed to be critical for inducing behavioral sensitization (136). A similar process has been described in NAc, where a single dose of cocaine abolishes eCB-LTD at excitatory synapses (140), however, diminished mGluR5-driven eCB release appears to account for that effect, rather than true occlusion of eCB-LTD at the presynaptic terminal. The role of the eCB/CB1R signaling system in addiction appears to vary depending on the specific drug of abuse (139), indicating a need to examine the interaction of other drugs of abuse with eCB-LTD in these critical brain areas.
Future Directions and Concluding Remarks
eCBs are now well established as mediators of synaptic plasticity. Extensive work demonstrates that eCB-LTD is a widespread phenomenon in the brain expressed by both excitatory and inhibitory synapses. As the list of synapses expressing eCB-LTD increases, so does our understanding of the induction/expression mechanisms and computational power of this form of plasticity. eCBs also mediate short-term plasticity and, by regulating inhibitory transmission, they can also modulate the induction of eCB-independent forms of plasticity such as LTP (3). All these actions combined clearly highlight the unique position of eCBs as regulators of synaptic function.
In addition to eCB-LTD, eCBs have been implicated in other forms of long-term plasticity. For example, in the cerebellum, Safo & Regehr found that a well studied form of postsynaptically expressed LTD between parallel fibers and Purkinje cells depends on retrograde release of 2-AG and activation of presynaptic CB1Rs (144). The authors suggest that a second (anterograde) signal is interposed between terminals bearing the CB1R and the Purkinje cell, where LTD is ultimately expressed. Interestingly, a similar situation has been observed in the VIIIth nerve to Mauthner cell mixed (electrical and chemical) synapse in goldfish (145), where presynaptic CB1Rs stimulate dopamine release from immunocytochemically identified dopaminergic terminals, triggering a postsynaptic LTP of both electrical and chemical neurotransmission. This CB1R-dependent regulation of a second neuromodulator might also account for an eCB-dependent LTP of excitatory transmission described in lamprey spinal cord (146). These cases possibly represent a novel class of eCB-mediated plasticity; however, they also join an existing literature where eCB-dependent processes modulate an otherwise eCB-independent form of plasticity (3). Here, the familiar retrograde eCB-CB1R signaling system forms a component of a more complex induction process. The most striking departure from eCB-LTD is the novel functional role for CB1Rs, which, rather than suppressing neurotransmitter release, stimulate release of a key factor for inducing synaptic plasticity. Other novel roles for CB1R in long-term plasticity may come to light, given evidence of functional CB1Rs at postsynaptic sites in neocortex (147).
Although protein synthesis is a known requirement in some forms of LTD (148, 149), thus far, only one study has directly examined the contribution of protein synthesis in eCB-LTD. Yin et al. (2006) found that inhibition of protein translation, but not transcription, prevents striatal eCB-LTD, implicating new protein synthesis in this form of plasticity (150). The authors provide evidence that rapid protein translation, likely occurring at the presynaptic axon/terminal, is critical for the expression of striatal LTD. Future studies will have to determine whether presynaptic protein translation is a general requirement for the maintenance of eCB-LTD in other brain structures. It will also be important to identify the presynaptic signaling pathways (e.g. Ca2+, PKA) that promote local protein synthesis, as well as the nature of the newly-synthesized protein(s) required for the long-lasting reduction in transmitter release.
Recent progress in eCB signaling and eCB-mediated synaptic plasticity provides new targets for developing experimental tools and potential therapies for neuropsychiatric disorders. The existing literature on eCB and synaptic plasticity raises several important questions, as well. For example, it is unclear how long eCB-LTD can persist. No study has yet addressed whether structural alterations of the synapse and active zone accompany eCB-LTD in the maintenance phase. The reversal of eCB-LTD is another critically underexamined issue, which has been extensively explored in other forms of plasticity. In the laboratory, induction paradigms are commonly selected based on their effectiveness rather than their physiological significance. Further research will have to determine whether the two induction paradigms commonly used to trigger eCB-LTD can occur in vivo. Of equal importance will be elucidating the functional and computational roles of eCB-LTD. Given the widespread importance of the eCB-CB1R signaling pathway in both synaptic physiology and behavior we predict tremendous interest in this field in the coming years.
Summary Points.
Endogenous cannabinoids (eCBs) can have a long-term impact in neural function by mediating eCB-LTD. This form of plasticity has now been observed in several brain structures at both excitatory and inhibitory synapses.
Despite the variety of stimulation protocols and experimental conditions used for its induction, nearly every synapse studied still expresses eCB-LTD as a long lasting reduction of neurotransmitter release. eCB-LTD is by now one of the best examples of presynaptic forms of long-term plasticity
The emerging molecular details of induction and expression of eCB-LTD at various synapses reveal many points of regulation. Important determinants of eCB-LTD induction are the enzymes involved in eCB synthesis, degradation, and (perhaps) release, as well as the cAMP/PKA signaling pathway downstream CB1Rs.
CB1R activation per se is not sufficient for eCB-LTD induction at a number of synapses. Rather, the presynaptic terminal seems to integrate multiple signals to generate eCB-LTD. At several synapses, presynaptic regulatory mechanisms of eCB-LTD induction translate into key computational properties, such as synapse specificity and associativity.
Acknowledgments
We wish to thank all members of the Castillo lab and all scientists whose data are reviewed in this review article. We apologize to all the investigators whose work could not be cited due to space limitations. Supported by the National Institutes of Health/National Institute on Drug Abuse grants, a National Institutes of Health training grant (to B.D.H.), the Irma T. Hirschl Career Scientist Award (to P.E.C.), and the National Alliance for Research on Schizophrenia and Depression.
Acronyms
- 2-AG
2-arachidonylglycerol
- AEA
Anandamide
- eCB
Endocannabinoid
- LTD/LTP
Long-term depression/potentiation
- EPSC/IPSC
Excitatory/Inhibitory Postsynaptic Current
- HFS
High-frequency stimulation (∼100Hz)
- LFS
Low-frequency stimulation (∼1Hz)
- t-LTD
LTD induced by spike-timing dependent protocols
- I-LTD
Long-term depression at inhibitory synapses
- STDP
Spike timing-dependent plasticity
Footnotes
Disclosure Statement: The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 2.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–86. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 3.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 4.Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–9. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol Rev. 2008 doi: 10.1152/physrev.00019.2008. in press. [DOI] [PubMed] [Google Scholar]
- 6.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–77. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 7.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- 8.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 9.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- 10.Devane WA, Breuer A, Sheskin T, Jarbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35:2065–9. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 11.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 12.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 14.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 16.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. PNAS. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 18.Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–61. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–12. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 21.Lafourcade CA, Alger BE. Distinctions among GABA(A) and GABA (B) responses revealed by calcium channel antagonists, cannabinoids, opioids, and synaptic plasticity in rat hippocampus. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential Sensitivity of GABAA Receptor-Mediated IPSCs to Cannabinoids in Hippocampal Slices from Adolescent and Adult Rats. J Neurophysiol. 2007 doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda H, Huang Y, Tsumoto T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc Natl Acad Sci U S A. 2008;105:3106–11. doi: 10.1073/pnas.0708349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–9. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- 25.Edwards DA, Zhang L, Alger BE. Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc Natl Acad Sci U S A. 2008;105:8142–7. doi: 10.1073/pnas.0803558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöstrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 27.Sjöstrom PJ, Turrigiano GG, Nelson SB. Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking. J Neurophysiol. 2004;92:3338–43. doi: 10.1152/jn.00376.2004. [DOI] [PubMed] [Google Scholar]
- 28.Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1383–8. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Yasuda H, Sarihi A, Tsumoto T. Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. J Neurosci. 2008;28:7074–83. doi: 10.1523/JNEUROSCI.0899-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–13. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–77. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- 34.Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–97. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henneberger C, Redman SJ, Grantyn R. Cortical efferent control of subcortical sensory neurons by synaptic disinhibition. Cereb Cortex. 2007;17:2039–49. doi: 10.1093/cercor/bhl112. [DOI] [PubMed] [Google Scholar]
- 37.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–81. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Caporale N, Dan Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu Rev Neurosci. 2008 doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 39.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 41.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188:1–5. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–63. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 44.Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–15. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–91. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007 doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 48.Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–16. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- 49.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–45. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 51.Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–9. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–4. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–7. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isokawa M, Alger BE. Ryanodine receptor regulates endogenous cannabinoid mobilization in the hippocampus. J Neurophysiol. 2006;95:3001–11. doi: 10.1152/jn.00975.2005. [DOI] [PubMed] [Google Scholar]
- 56.Beierlein M, Regehr WG. Local interneurons regulate synaptic strength by retrograde release of endocannabinoids. J Neurosci. 2006;26:9935–43. doi: 10.1523/JNEUROSCI.0958-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007;584:407–18. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–74. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–31. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 60.Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–61. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- 61.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–68. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–21. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 63.Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–35. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–21. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 66.Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–51. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–76. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–8. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 72.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–58. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 73.Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A. 2004;101:3214–9. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc Natl Acad Sci U S A. 2007;104:20564–9. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–9. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–45. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008;11:744–5. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- 78.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–5. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–6. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–48. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci. 2003;14:207–16. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- 82.Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–22. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 83.Jacob V, Brasier DJ, Erchova I, Feldman D, Shulz DE. Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J Neurosci. 2007;27:1271–84. doi: 10.1523/JNEUROSCI.4264-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–62. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 85.Varma N, Brager D, Morishita W, Lenz RA, London B, Alger B. Presynaptic factors in the regulation of DSI expression in hippocampus. Neuropharmacology. 2002;43:550–62. doi: 10.1016/s0028-3908(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 86.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 87.Lenz RA, Wagner JJ, Alger BE. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol. 1998;512(Pt 1):61–73. doi: 10.1111/j.1469-7793.1998.061bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol. 1996;52:819–27. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- 89.Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–13. [PubMed] [Google Scholar]
- 90.Mato S, Lafourcade M, Robbe D, Bakiri Y, Manzoni OJ. Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca(++) channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology. 2008;54:87–94. doi: 10.1016/j.neuropharm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 91.Calabresi P, Pisani A, Mercuri NB, Bernardi G. Post-receptor mechanisms underlying striatal long-term depression. J Neurosci. 1994;14:4871–81. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang CC, Chen YL, Liang YC, Hsu KS. Role for cAMP and protein phosphatase in the presynaptic expression of mouse hippocampal mossy fibre depotentiation. J Physiol. 2002;543:767–78. doi: 10.1113/jphysiol.2002.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–30. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 94.Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 95.Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–45. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 96.Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Sudhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 2005;102:9365–70. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaeser PS, Sudhof TC. RIM function in short- and long-term synaptic plasticity. Biochem Soc Trans. 2005;33:1345–9. doi: 10.1042/BST0331345. [DOI] [PubMed] [Google Scholar]
- 98.Kaeser PS, Kwon HB, J B, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1 phosphorylation at serine 413 by rotein kinase A is not required for presynaptic long-term potentiation and learning. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0806679105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–42. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 100.Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–56. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mato S, Robbe D, Puente N, Grandes P, Manzoni OJ. Presynaptic homeostatic plasticity rescues long-term depression after chronic Delta 9-tetrahydrocannabinol exposure. J Neurosci. 2005;25:11619–27. doi: 10.1523/JNEUROSCI.2294-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron. 2006;52:497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 103.Huang YC, Wang SJ, Chiou LC, Gean PW. Mediation of amphetamine-induced long-term depression of synaptic transmission by CB1 cannabinoid receptors in the rat amygdala. J Neurosci. 2003;23:10311–20. doi: 10.1523/JNEUROSCI.23-32-10311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–9. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–8. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 107.Chen K, Neu A, Howard AL, Foldy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 109.Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–29. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- 110.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–50. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 111.Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–9. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- 112.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 113.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–8. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 114.Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–65. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–32. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- 117.Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–21. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 118.Deshmukh S, Onozuka K, Bender KJ, Bender VA, Lutz B, Mackie K, Feldman DE. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience. 2007;145:279–87. doi: 10.1016/j.neuroscience.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–5. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 120.Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–56. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol. 2004;92:2105–12. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- 122.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 123.Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–24. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- 124.Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–72. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- 125.Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–86. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–24. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 127.Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–41. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- 128.Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–66. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 130.Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997;17:4536–44. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oueslati A, Breysse N, Amalric M, Kerkerian-Le Goff L, Salin P. Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur J Neurosci. 2005;22:2765–74. doi: 10.1111/j.1460-9568.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- 132.Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005;81:285–99. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 134.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 135.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 136.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 137.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 138.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–7. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 139.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–83. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–6. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- 142.Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–20. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Safo PK, Regehr WG. Endocannabinoids Control the Induction of Cerebellar LTD. Neuron. 2005;48:647–59. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 145.Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–47. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–74. doi: 10.1523/JNEUROSCI.3174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–6. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 148.Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25:7199–209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–7. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 150.Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–20. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]