Abstract
The peak sodium current underlies excitability and conduction in heart muscle, but a late sodium current flowing after the peak contributes to maintaining and prolonging the action potential plateau, and also to intracellular sodium loading, that in turn increases intracellular calcium with consequent effects on arrhythmia and diastolic function. Late sodium current is pathologically increased in both genetic and acquired heart disease, making it an attractive target for therapy to treat arrhythmia, heart failure, and angina. This review provides an overview of the underlying bases for the clinical implications of late sodium current block.
Keywords: Sodium Current, Long QT syndrome, Antiarrhythmic Drugs
Introduction
Late sodium current (INa) is the residual INa flowing after the large peak INa during an action potential (AP) or voltage clamp (Fig. 1). Although under “normal” conditions it is a small current (~0.5%) relative to peak INa, it is sufficiently large during the AP plateau to affect the duration, and the flow over hundreds of milliseconds during the AP contributes more to Na+ loading than the brief transient of peak INa [1]. With the recognition that the mechanism of action for the antianginal drug ranolazine was through a relatively specific block of late INa, a role for late INa as a mechanism for pathogenesis of angina, heart failure, and arrhythmia has attracted much attention [2,3]. This article offers perspectives and observations on late INa and human cardiac disease with selective references focusing on late INa, its causes and regulation, an account of pathogenesis of cardiac disease through electrophysiology and altered Na-Ca homeostasis, and a consideration of clinically available drugs that block or increase late INa. The role of late INa in angina, arrhythmia, and heart failure is speculative and subject to on-going studies, and the reader is referred to key and recent comprehensive reviews of late INa and ranolazine that cover the wealth of experimental and clinical data [2-8] providing additional detail and supporting references.
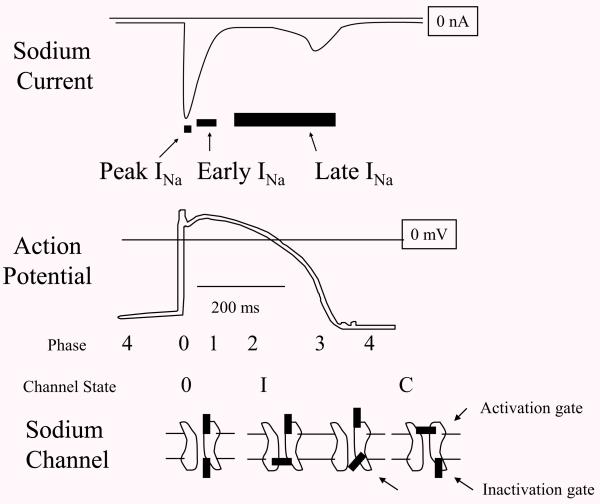
Fig. 1.
Peak, Early, and Late INa. Diagrams of cardiac sodium current (INa) action potential (AP), and sodium channel cartoon, lined up with time on the horizontal. Peak INa occurs in less than a millisecond and underlies the rapid upstroke or phase 0 of the AP. At this time, the activation gate and the inactivation gate on the channel are both open. Then over several milliseconds the current begins to decay contributing to a notch in the AP called phase 1. At this time, some of the channels have inactivated (shown as “I” with the inactivation gate closed). There is no commonly accepted name for this phase of INa but here it is labeled “Early INa”. After several milliseconds INa normally decays to <1% of peak INa, but a residual current flows as late INa and this depolarizing current along with calcium currents support phase 2 or the plateau of the AP. The mechanisms for late INa at the sodium channel level are multiple but can generally be thought of as incomplete inactivation. Eventually, activating potassium currents repolarize the membrane (phase 3 of the AP) and when the voltage decreases below the sodium channel threshold, the activation gate closes.
Background
The recognition that late INa plays a role in cardiac physiology goes back ~50 years when it was shown that the selective Na+ channel blocker tetrodotoxin shortened the AP plateau [9]. This late INa and its effect on the AP were subsequently studied as “window-current” [10], “steadystate” current [11], “slow inactivation” current [12,13], “late current” [14], “slow current” [15] and “persistent current” [16]. As noted below, the characteristics and mechanisms for late INa are heterogeneous, therefore the term “late INa” is preferred as the most general term not invoking particular mechanisms or characteristics. An important issue when reading the literature is to note how long after the depolarization late INa is measured. Because INa in cardiac tissue is subject to slow decay over tens of milliseconds a measure of late INa at 10 ms will give a higher value than later measurement at 20 ms or 50 ms. Also note that for comparison purposes, late INa is often expressed as a percentage of the peak INa or normalized to cell size as measured by cell capacitance.
Several key features of late INa are important for understanding the clinical manifestations. An important biophysical distinction is the late INa produced by overlap of the voltage dependence of activation and inactivation to produce what is called “window current” that occurs over the voltage range of this overlap that affects AP duration. Window current is the consequence of the voltage dependence of more or less normal gating in the overall population of sodium channels, and is regulated by the protein kinase C (PKC) pathway [17]. In contrast, increased late INa may involve a fundamental change or abnormality in the inactivation gate in a select population of channels. This abnormality may have diverse underlying causes and mechanisms. Another important feature of late INa addressed in more detail below is whether or not it is subject to “slow inactivation”, which will lead to a frequency dependent inactivation of the late INa. Finally, it is important to note that late INa is heterogeneous in amplitude by region in the heart [18]. Several comprehensive reviews [4,5] provide additional detail and references for these topics.
Sodium channel macromolecular complex
Cardiac INa flows through a channel formed by the α-subunit NaV1.5 encoded by the gene SCN5A. Although the α-subunit alone accounts for major features of INa, it is part of a macromolecular complex consisting of auxiliary subunits and associated channel interacting proteins or ChIPs. Identification of components of the macromolecular complex and how they regulate INa is a rapidly evolving field [19]. Although NaV1.5 underlies the majority of INa, other “non-cardiac” isoforms may make up an important part of cardiac INa. In particular, the nerve/brain isoforms NaV1.1 [20] and NaV1.8 [21] may underlie an important part of late INa in human heart. Other components of the complex that may play important roles in regulating late INa in human heart include α1-syntrophin (Snta1) [22], Caveolin 3 (Cav3) [23] and Calmodulin Kinase 2(CaM-KII) [24]. The β1 subunit [25,26] and β4 subunit [27] also play a role in late INa. The biophysical mechanisms by which α-subunit structure and interacting proteins in the complex affect late INa are not completely understood. Late INa is generally thought to be a modification of or failure in the inactivation process. A structure responsible for fast inactivation of INa resides in “IFM” residues on the DIII-DIV linker as a “ball” or “lid” and on the bottom of the S4-S5 linker as a receptor, but myriad mutations throughout the NaV1.5 topology cause long QT syndrome Type 3 (LQT3), so it seems diverse perturbations in NaV1.5 structure are associated with late INa. One way to think of it is that the complex for inactivation is a fine-tuned precise machine, and there are many different ways to disrupt normal gating to make late INa. If this is true, a single final common structure-function pathway for the cell signaling and biophysical mechanisms for regulating and causing late INa may be elusive.
Causes of increased late INa
Increased late INa has been characterized under a wide variety of experimental conditions. Table 1 in a recent review [7] lists and references conditions, drugs, toxins, and diseases associated with increased late cardiac INa. Table 1 in this article lists causes most relevant to mechanisms and treatment of cardiac disease, along with possible mechanisms for their effect. It is important to note that these “causes” are not mutually exclusive, but may be upstream or downstream elements in a regulatory or pathological pathway. For example, increased late INa in ischemia may be caused by acidosis and ischemic metabolites such as lysophosphatidylcholine (LPC), and late INa in LQT9 and LQT12 is caused by enhanced nitrosylation. Possible mechanisms (Table 1) at the cellular level (such as altered signaling pathways) for the increased late INa cardiac diseases are coming into focus, but as noted above discovery of the mechanisms at the biophysical level appear less tractable.
Table 1.
Clinically relevant “causes” of late INa and possible mechanisms. Remodeling means alteration of the Na channel complex in a way that favors late INa by for example the disappearance of subunits [49]. References for these clinically relevant, and many other experimentally relevant causes of late INa have been recently reviewed [7]
| Genetic | |
| LQT3 | Direct Biophysical |
| LQT9 | Enhanced Nitrosylation |
| LQT10 | ? |
| LQT12 | Enhanced Nitrosylation |
| Acquired | |
| Hypertrophy | Stretch, altered signaling, remodeling |
| Heart Failure | Stretch, altered signaling, remodeling |
| Ischemia | Acidosis, metabolites (LPC) |
| Diabetes | Cardiomyopathy, altered signaling |
| Molecules | |
| Carbon Monoxide | ? |
| Acidosis | ? |
| LPC (ischemic metabolite) | PKC? |
| ROS (H2O2) | CamK? |
| Drugs | |
| Dofetilide | PI3K inhibition |
| Sotalol | PI3K inhibition |
| Erythromycin | PI3K inhibition |
| Thioridazine | PI3K inhibition |
| Nilotinib | PI3K inhibition |
| Cell signaling | |
| nNOS Nitrosylation | direct on NaV1.5 |
| CamK Phosphorylation | direct on NaV1.5 |
| PI3K-Akt pathway inhibition | ? |
| PKC Phosphorylation | direct on NaV1.5 |
Regulation of late INa by cell signaling and post-translational modification
Late INa is present physiologically and is regulated by multiple pathways including nitrosylation via nNOS and by phosphorylation via a number of kinases (Table 1). Specificity of nitrosylation for the NaV1.5 channel can be achieved because the syntrophin/dystrophin complex interacts with the c-terminus of NaV1.5 through PDZ domains [28], and α1-syntrophin (Snta1) is a member of the family of dystrophin-associated proteins containing multiple protein interaction motifs that act as molecular scaffolds for nNOS, and plasma membrane Ca-ATPase (PMCA). Plasma membrane Ca-ATPase subtype 4b (Pmca4b) interacts with Snta1 between the PH2 and SU domains to inhibit nitric oxide (NO) production [29]. These three proteins, Snta1, nNOS, and Pcma4b associate with the C-terminus of NaV1.5, and the mutation A390V in Snta1, discovered in a patient with long QT syndrome, specifically disrupts binding of Pcma4b from the complex [22]. NO increases late INa [30], and thus this finding is consistent with the mutation increasing late INa through locally increased production of NO.
Phosphorylation of NaV1.5 at specific residues S516 and T594 by calcium-calmodulin kinase (CamK) dependent mechanisms also increases late INa [24]. The Ca2+-sensing protein calmodulin (CaM) activates CamKIIδ, the predominant isoform in heart, and regulates many aspects of excitation-contraction coupling [31]. CaM binds near the isoleucine and glutamine at residues 1908 and 1909 on the carboxy-terminus of Nav1.5 in a Ca2+-dependent manner where it slows inactivation of INa [32]. CaM also interacts with the DIII-DIV linker with similar functional effects [33]. CaMKIIδ was also shown to associate with and phosphorylate NaV1.5 and cause late INa [34]. Possible interactions or synergy between nitrosylation and this phosphorylation are not known.
PKC phosphorylation pathways are also involved in regulation of late INa. PKC-dependent phosphorylation of NaV1.5 at S1503 causes alterations in INa kinetics that open up a window current [35], and PKC inhibition blocked increased late INa that was caused by calcium loading the cell [36] suggesting invovlement of PKC in CaM pathway causes of late INa
The phosphonositide 3-kinase (PI3K) signaling pathway including tyrosine kinase (TK) as an upstream activator and the protein kinase Akt as a downstream effector has been recently reviewed as a modulator of cardiac ion channels including INa [37]. TK inhibitors such as the anti-cancer drug nilotinib were found to increase the QT interval in patients, and the drugs were shown to increase late INa by inactivating PI3K [38]. The antiarrhythmic drugs dofetilide and sotalol as well as the drugs thioridazine and erythromycin were also found to increase late INa by inhibiting this pathway [39]. It is important to note that the full effect to increase late INa took up to 48 hours. The detailed mechanism and the phosphorylation target(s) by which late INa is decreased by TK/PI3K/Akt is unclear, but it is important to note that unlike regulation by nNOS and CamK which increase late INa, the PI3K pathway suppresses late INa. This pathway affects many other ion currents, and so far it is not known if the machinery for this pathway is part of the NaV1.5 macromolecular complex to allow specificity for INa, in a way analogous to Cav3/SNTA1 and nitrosylation, and to the CaM-dependent phosphorylation pathway.
Drugs that increase late INa
Drugs that increase late INa such as DPI 201-106 were studied as positive inotropes but did not reach clinical practice because of toxicity [40]. As noted above, PI3K inhibition by anticancer [38] and by antiarrhythmic and other drugs [39] increase late INa and prolong APD by this mechanism in addition to their effects to reduce potassium currents. This suggests that drug safety screening for long QT might need to include testing for effects on late INa.
Increased late INa in Genetic Cardiac Disease – LQT3, 9, 10, 12
Late INa causes QT prolongation and arrhythmia for mutations in NaV1.5 causing the LQT3 syndrome. Late INa may be increased by several biophysical mechanisms such as failure to inactivate completely and increased window current. Depending upon the underlying mutation, late INa undergoes a gradual voltage-dependent decay because of slow inactivation into a state from which recovery is slow, as for late INa in the LQT3 mutation ΔKPQ [41], or it can be flat and persistent as for E1784K [42]. Slow inactivation of late INa accumulates with faster heart rates, causing a decrease in late INa, shortening of the AP plateau, and a correspondingly enhanced rate-adaptation of the QT interval on the surface ECG. About half of LQT3 mutations studied exhibit slow decay of late INa. Mutations in NaV1.5 implicated in SIDS did not have this decay [43] and perhaps this lack of decay leads to increased Na loading and underlies the greater lethality compared to LQT3. This possibly clinically important feature is not often characterized or noted in studies of late INa. This finding also suggests an underappreciated heterogeneity in the behavior of late INa that may be clinically important.
As noted above, LQT3 mutations are scattered throughout NaV1.5 and not localized in specific domains making it difficult to propose a detailed biophysical mechanism for how the mutations cause late INa, although some LQT3 mutations may mimic phosphorylation [44]. Mutations in sodium channel complex proteins other than NaV1.5 also result in late INa. LQT10 was associated with a mutation in the β4 subunit, but the mechanism for increased late INa is not known. On the other hand, increased late INa associated with mutations in Cav3 (LQT9) and Snta1 (LQT12) are associated with enhanced direct nitrosylation of NaV1.5 [22,23]. Late INa has also been noted in both dilated cardiomyopathy and hypertrophic cardiomyopathy that are or can be genetic diseases, but it is unclear if the cause of late INa is specific to the mutation, or if it should be considered acquired as a non-specific consequence of the hypertrophy or failure
Direct electrophysiological effect of late INa and arrhythmia
Increased late INa is often called a “gain of function”, and decreased peak INa is called a “loss of function”. In heart failure, peak INa is decreased but late INa is increased, posing an apparent contradiction that heart failure causes both a gain in function as well as a loss of function. The contradiction is resolved by recognizing the timing of these effects and that they are not mutually exclusive. Increased late INa is a persistent depolarizing force during the plateau which opposes repolarizing currents and lengthens the AP, corresponding to a prolonged QT on the surface ECG (Fig 1). Loss of function as a decrease in peak and early INa is arrhythmogenic through mechanisms diagrammed (Fig. 2), whereas increased late INa is arrhythmogenic through AP duration prolongation, and long QT arrhythmia which has both elements of triggered activity (early afterdepolarizations or EADs) and effects on reentry as diagrammed (Fig. 2)
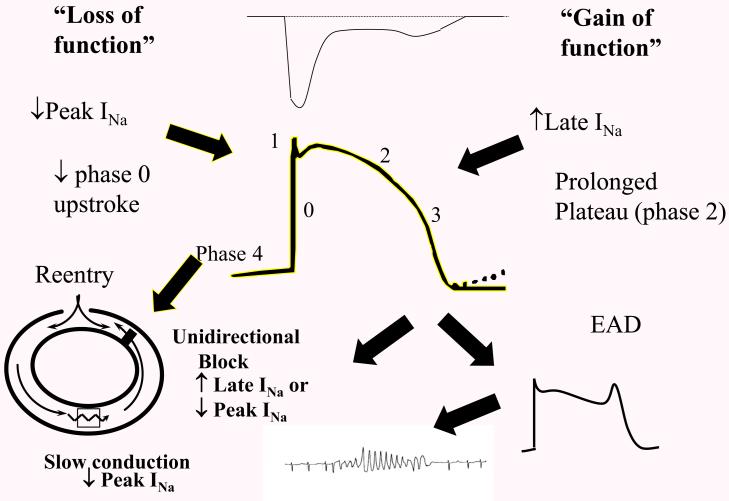
Figure 2.
The role of late INa in arrhythmia. An increase in late INa (gain of function) provides a depolarizing current during the plateau, prolonging the AP, and at the cellular level produces an early afterdepolarization (EAD) which at the tissue/organ level triggers Torsades des Pointes arrhythmia. It is important to point out that late INa might also contribute to re-entrant arrhythmia by prolonging refractoriness and producing unidirectional block. For completeness and contrast, “loss of function” generally refers to a loss of peak INa and can contribute to the substrate for reentry as shown, or to a loss of early INa that might lead to early repolarization and phase 2 reentry (not shown) thought to underlie Brugada syndrome arrhythmia. An increase in late INa is often called a “gain of function” especially when referring to mutations that increase late INa as in LQT3. Generally, the increase in late INa is out of proportion to the gain, if any, in peak INa.
Indirect effect of late INa on Na-Ca homeostasis on arrhythmia, heart failure, and angina
Na+ enters the cell through INa, Na-Ca exchange, and Na-H exchange, and this “Na+ loading” under normal circumstances is balanced by Na+ leaving the cell through Na-K ATPase. Na+ loading by INa depends upon the amplitude and duration of INa during each phase of the AP [1]. Under normal conditions the majority of Na+ loading from INa occurs during phase 2, followed by phase 1, phase 0, phase 3, and phase 4. Even though INa amplitude is highest during phase 0, the duration is short so Na+ loading is low. INa amplitude is low during phase 2, usually < 0.5% of peak, but the duration is much longer making phase 2 dominant in Na+ loading. An increase in late INa amplitude as found in pathological states [45] increases Na+ loading sufficiently to raise intracellular Na+. An increase in late INa amplitude also prolongs phase 2 duration, further increasing Na+ loading by sustaining the driving force for a longer time. Increased intracellular Na+ will cause increased intracellular Ca2+ because less energy is available in the Na+ gradient to extrude Ca2+ through Na-Ca exchange (Fig. 3), and if intracellular Na+ is sufficiently increased then Na-Ca exchange may operate in reverse mode with Ca2+ actually entering the cell [46], although it is important to point out that Ca2+ loading would tend to increase with any decrease in extrusion and without an actual reversal of Na-Ca exchange.
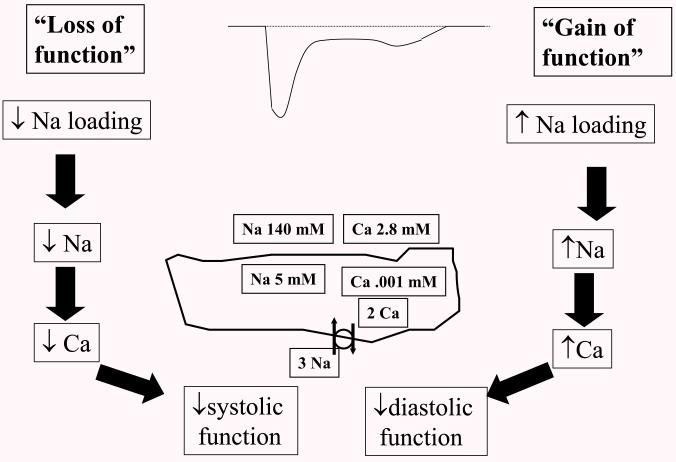
Figure 3.
The role of late INa in contractility – Sodium calcium exchange (pictured as the circle with two arrows on the diagram of the cell at center) uses the energy stored in the sodium gradient to drive calcium out of the cell against its gradient, and thus balance the calcium that enters the cell through calcium channels during each AP. With a “loss of function”, internal Na will decrease, increasing the Na gradient and driving force for Ca extrusion through Na-Ca exchange, with a subsequent decrease in systolic function. On the other hand, increased late INa or “gain of function) will increase Na, decreased the driving force for Ca extrusion and cause increased internal Ca and subsequent effects on diastolic relaxation.
Increased intracellular Ca2+ can contribute to arrhythmia by the mechanism of delayed afterdepolarizations, and could have effects on reentrant mechanisms. More direct effects occur through effects on contractility where decreased Ca2+ can cause decreased systolic function, and increased Ca2+ can cause impaired relaxation or decreased diastolic function (Fig. 3). Increased late INa in ischemia and the subsequent calcium loading can elevate left ventricular end-diastolic pressure, causing a “vicious feedback” on both limbs of the supply-demand mismatch worsening angina (Fig 4). Block of late INa can interrupt this feedback and is the proposed mechanism for the proven effectiveness and clinical indication for ranolazine treatment of effort angina. It is interesting to note that amiodarone, a potent late INa blocker (Table 2), was initially developed as an antianginal drug before its antiarrhythmic properties came to the forefront.
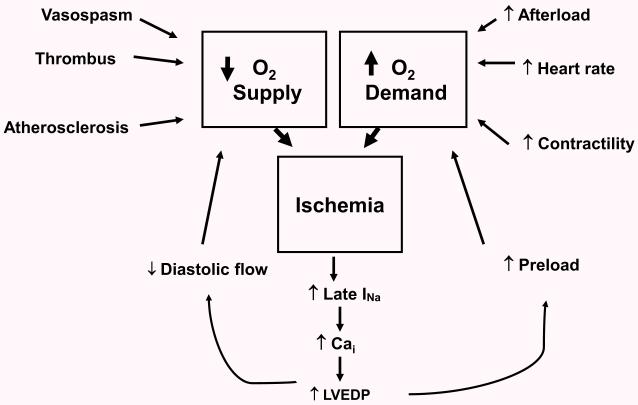
Figure 4.
The role of late INa in ischemia/angina - Ischemia is represented as the classic paradigm of supply-demand which can be caused by any number of external factors. Once started, ischemia causes an increased late INa, which by mechanisms noted earlier, causes internal Ca loading and increase in left ventricular end-diastolic pressure (LVEDP). Increased LVEDP operates to exacerbate ischemia by actions on both limbs of the supply-demand mismatch. Increased LVEDP further decreases O2 supply by decreasing the pressure gradient in coronary arteries for diastolic flow. It increases demand by augmenting preload.
Table 2.
Clinically available drugs that affect late INa. The blockers are in order of selectivity for late INa with concentrations for half block IC50 [6] and therapeutic concentrations [50]. For IC50 for drugs that increase late INa we report the concentration for half maximal increase from control for dofetilide [39] and the concentration to double late INa for Nilotonib [38]
| Drug | Ratio Late:Peak IC50 |
Late INa
IC50 μM |
Therapeutic concentration range μM |
|---|---|---|---|
| Block of late INa | |||
| Ranolazine | 38 - 61 | 7 | 0.4 - 6.1 |
| Amiodarone | 13 - 60 | 3 - 6.7 | 0.8 - 3.9 |
| Mexilitene | 9 - 51 | 3 - 5 | 3.4 - 9.5 |
| Lidocaine | 3 - 12 | 25 | 0.8 - 4.2 |
| Propranalol | 7 | 3 | 0.1 – 0.4 |
| Flecainide | 3 - 7 | 1.4 | 0.5 - 2.4 |
| Vernakalant | 3 - 5 | 30 | 7.2 - 14.3 |
| Quinidine | 1 | 12 | 9.3 - 24.7 |
| Increased Late INa | |||
| Dofetilide | .103 | .005-.023 | |
| Nilotinib | 1.0 | 1.7-62 | |
Pharmacology of late INa
Because of the role for increased late INa in the pathogenesis of arrhythmia, diastolic heart failure, and ischemia, it is an attractive target for treatment in these conditions. It is an especially attractive target because it exists as a larger target under the pathological conditions where it is increased, thus reducing the chances of on-target toxic effects. Moreover, block of late INa in normal hearts has been shown to have no deleterious effects on contractility or conduction [47]. A wealth of experimental data with ranolazine, a relatively specific blocker of late INa, in cellular and animal models of arrhythmia support a role for late INa in the treatment of arrhythmia [2] and raises the question of whether block of late INa may be a mechanism for the effectiveness of clinically available antiarrhythmic drugs (Table 2). It is important to note, however, that none of the drugs in the table are specific blocker of INa and all have effects on other ion channels, receptors, and other targets at therapeutic concentrations.
No drug known is completely specific for late INa over peak INa, probably because they produce both types of block by binding to the same site on NaV1.5 [48]; a key question is the relative selectivity of the drug to block late INa versus peak INa. The mechanisms for relative selectivity to block late INa probably arises from state dependence of the block (open and inactivated states) and the on and off rate kinetics of the drug, which varies from drug to drug, and which varies with study conditions. A simple way to portray selectivity is to take a ratio of the inhibitory concentration at 50% block (IC50) for late INa compared to peak INa. Table 2 lists clinically available drugs that have been shown to block late INa in order of their selectivity for late INa. All of these drugs have other targets for their action, how much of the therapeutic effect is block of late INa? Table 2 also shows the IC50 for block of late INa and the therapeutic concentration range to address this question. It is important to note that the IC50 for late INa block is obtained under much different conditions than would apply in humans treated with these drugs. Often they are determined with NaV1.5 expressed in heterologous cell expression systems, and under tonic and not use-dependent protocols. In many cases, the IC50 may be higher than it is in vivo so that the block of late INa may be greater than suggested. Overall, based on the correspondence of the IC50 with the therapeutic range of the drug, it seems likely that block of late INa may be clinically important for the action of ranolazine, amiodarone, mexiletine, flecainide and quinidine (Table 2). Drugs in development such as Gilead’s GS-6615 that even more specifically target late INa [7] may tell us more about late INa as therapeutic target.
As noted above in discussing PI3K regulation of late INa, clinically available drugs including anti-cancer agents such as Nilotinib [38] and antiarrhythmic agents dofetilide and other drugs [39] increase late INa by blocking the PI3K pathway. This increased late INa has the potential to underlie long QT arrhythmia and other deleterious effects of increased late INa. Increased late INa may take its place with potassium channel block as an off target cardiac safety issue.
Summary
Late INa is a physiological phenomenon where INa continues to flow through the cardiac sodium channel complex during the AP plateau. It is subject to regulatory pathways including phosphorylation and nitrosylation, and it is increased with many genetic and acquired cardiac diseases. Increased late INa in the heart can lead to arrhythmia by direct electrophysiological action to prolong the AP duration and by indirect action to cause calcium overload. The calcium overload can also lead to diastolic dysfunction contributing to heart failure, and increased wall stress in ischemia leading to angina. Block of late INa to treat these conditions is supported by data from many experimental models as well as an expanding list of clinical data, and it is likely to be at least part of the mechanism of action of some clinically used drugs to treat arrhythmia, angina and arrhythmia. Increased late INa has many underlying causes and diverse behavior; the mechanisms for increased late INa are multiple and complex and remain under investigation. Understanding these mechanisms may lead to improved and more selective therapy.
Acknowledgements
Thanks to Dr. John W. Kyle for reading and commenting on the manuscript.
Supported by NIH grants HL R56 HL71092 and R01HL128076
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trends in Cardiovascular Medicine
The author has no financial conflicts of interest to disclose.
Reference List
- [1].Makielski JC, Farley AL. Na(+) current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- [2].Gupta T, Khera S, Kolte D, Aronow WS, Iwai S. Antiarrhythmic properties of ranolazine: A review of the current evidence. Int.J.Cardiol. 2015;187:66–74. doi: 10.1016/j.ijcard.2015.03.324. [DOI] [PubMed] [Google Scholar]
- [3].Sossalla S, Maier LS. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacology & Therapeutics. 2012;133:311–323. doi: 10.1016/j.pharmthera.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [4].Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006;92(Suppl 4):iv1–iv5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog.Biophys.Mol.Biol. 2008;96:421–451. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Antzelevitch C, Nesterenko V, Shryock JC, Rajamani S, Song Y, Belardinelli L. The role of late I Na in development of cardiac arrhythmias. Handb.Exp.Pharmacol. 2014;221:137–168. doi: 10.1007/978-3-642-41588-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Belardinelli L, Giles WR, Rajamani S, Karagueuzian HS, Shryock JC. Cardiac late Na(+) current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12:440–448. doi: 10.1016/j.hrthm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- [8].Shryock JC, Song Y, Rajamani S, Antzelevitch C, Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovascular Research. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dudel J, Peper K, Rudel R, Trautwein W. Effect of tetrodotoxin on membrane currents in mammalian cardiac fibres. Nature. 1967;213:296–297. doi: 10.1038/213296a0. [DOI] [PubMed] [Google Scholar]
- [10].Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Archives - European Journal of Physiology. 1979;379:137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- [11].Colatsky TJ. Mechanisms of action of lidocaine and quinidine on action potential duration in rabbit cardiac Purkinje fibers. An effect on steady state sodium currents? 1982;50:17–27. doi: 10.1161/01.res.50.1.17. Circ Res. [DOI] [PubMed] [Google Scholar]
- [12].Gintant GA, Datyner NB, Cohen IS. Slow inactivation of a tetrodotoxin-sensitive current in canine cardiac Purkinje fibers. Biophys J. 1984;45:509–512. doi: 10.1016/S0006-3495(84)84187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carmeliet E. Slow inactivation of the sodium current in rabbit cardiac Purkinje fibres. Pflugers Archives - European Journal of Physiology. 1987;408:18–26. doi: 10.1007/BF00581835. [DOI] [PubMed] [Google Scholar]
- [14].Wasserstrom JA, Salata JJ. Basis for tetrodotoxin and lidocaine effects on action potentials in dog ventricular myocytes. American Journal of Physiology. 1988;254:H1157–1166. doi: 10.1152/ajpheart.1988.254.6.H1157. [DOI] [PubMed] [Google Scholar]
- [15].Liu YM, DeFelice LJ, Mazzanti M. Na channels that remain open throughout the cardiac action potential plateau. Biophys J. 1992;63:654–662. doi: 10.1016/S0006-3495(92)81635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. J Physiol.(Lond.) 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ono K, Fozzard HA, Hanck DA. A direct effect of forskolin on sodium channel bursting. Pflugers Archives - European Journal of Physiology. 1995;429:561–569. doi: 10.1007/BF00704162. [DOI] [PubMed] [Google Scholar]
- [18].Qi D, Yang Z, Robinson VM, Li J, Gao C, Guo D, et al. Heterogeneous distribution of INa-L determines interregional differences in rate adaptation of repolarization. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.02.013. Epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- [19].Kyle JW, Makielski JC. Diseases caused by mutations in Nav1.5 interacting proteins. Cardiac Electrophysiology Clinics. 2015;6:797–809. doi: 10.1016/j.ccep.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mishra S, Reznikov V, Maltsev VA, Undrovinas NA, Sabbah HN, Undrovinas A. Contribution of sodium channel neuronal isoform Nav 1.1 to late sodium current in ventricular myocytes from failing hearts. J.Physiol. 2015;593:1409–1427. doi: 10.1113/jphysiol.2014.278259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circulation Research. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc.Natl.Acad.Sci.U.S.A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng J, Valdivia CR, Vaidyanathan R, Balijepalli RC, Ackerman MJ, Makielski JC. Caveolin-3 suppresses late sodium current by inhibiting nNOS-dependent S-nitrosylation of SCN5A. J.Mol.Cell Cardiol. 2013;61:102–110. doi: 10.1016/j.yjmcc.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, et al. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. Journal of Biological Chemistry. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valdivia CR, Nagatomo T, Makielski JC. Late Na currents affected by alpha subunit isoform and beta1 subunit co-expression in HEK293 cells. J.Mol.Cell Cardiol. 2002;34:1029–1039. doi: 10.1006/jmcc.2002.2040. [DOI] [PubMed] [Google Scholar]
- [26].Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its beta1 subunit. J.Physiol Sci. 2009;59:217–225. doi: 10.1007/s12576-009-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, et al. SCN4B-Encoded Sodium Channel {beta}4 Subunit in Congenital Long-QT Syndrome. Circulation. 2007;116:136–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ou YJ, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, et al. Syntrophin gamma 2 regulates SCN5A Gating by a PDZ domain-mediated interaction. Journal of Biological Chemistry. 2003;278:1915–1923. doi: 10.1074/jbc.M209938200. [DOI] [PubMed] [Google Scholar]
- [29].Williams JC, Armesilla AL, Mohamed TM, Hagarty CL, McIntyre FH, Schomburg S, et al. The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. Journal of Biological Chemistry. 2006;281:23341–23348. doi: 10.1074/jbc.M513341200. [DOI] [PubMed] [Google Scholar]
- [30].Ahern GP, Hsu SF, Klyachko VA, Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- [31].Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovascular Research. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [32].Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, et al. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- [33].Potet F, Chagot B, Anghelescu M, Viswanathan PC, Stepanovic SZ, Kupershmidt S, et al. Functional Interactions between Distinct Sodium Channel Cytoplasmic Domains through the Action of Calmodulin. Journal of Biological Chemistry. 2009;284:8846–8854. doi: 10.1074/jbc.M806871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J.Clin.Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qu Y, Rogers JC, Tanada TN, Catterall WA, Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. J Gen.Physiol. 1996;108:375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu Y, Wang L, Ma J, Song Y, Zhang P, Luo A, et al. Protein kinase C and Ca(2+) -calmodulin-dependent protein kinase II mediate the enlarged reverse INCX induced by ouabain-increased late sodium current in rabbit ventricular myocytes. Exp.Physiol. 2015;100:399–409. doi: 10.1113/expphysiol.2014.083972. [DOI] [PubMed] [Google Scholar]
- [37].Ballou LM, Lin RZ, Cohen IS. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circulation Research. 2015;116:127–137. doi: 10.1161/CIRCRESAHA.116.303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, et al. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci.Transl.Med. 2012;4:131ra50. doi: 10.1126/scitranslmed.3003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130:224–234. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Flesch M, Erdmann E. Na+ channel activators as positive inotropic agents for the treatment of chronic heart failure. Cardiovascular Drugs & Therapy. 2001;15:379–386. doi: 10.1023/a:1013329203750. [DOI] [PubMed] [Google Scholar]
- [41].Nagatomo T, Ye B, Valdivia CR, Fan Z, January CT, Makielski JC. Frequency-dependent reduction of late Na current by ultra-slow recovery in the LQT3 D KPQ mutant Na channel. Circulation. 1998;98:469. [Google Scholar]
- [42].Wei J, Wang DW, Alings M, Fish F, Wathen M, Roden DM, et al. Congenital long-QT syndrome caused by a novel mutation in a conserved acidic domain of the cardiac Na+ channel. Circulation. 1999;99:3165–3171. doi: 10.1161/01.cir.99.24.3165. [DOI] [PubMed] [Google Scholar]
- [43].Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, et al. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- [44].Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, et al. Ca2+/Calmodulin-Dependent Protein Kinase II-Based Regulation of Voltage-Gated Na+ Channel in Cardiac Disease. Circulation. 2012;126:2084–2094. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac "late sodium current.". Pharmacology & Therapeutics. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [46].Bers DM. 2nd Kluwer Academic Press; 2001. Excitation-contraction coupling and cardiac contractile force. [Google Scholar]
- [47].Fernandes S, Hoyer K, Liu G, Wang WQ, Dhalla AK, Belardinelli L, et al. Selective inhibition of the late sodium current has no adverse effect on electrophysiological or contractile function of the normal heart. Journal of Cardiovascular Pharmacology. 2014;63:512–519. doi: 10.1097/FJC.0000000000000075. [DOI] [PubMed] [Google Scholar]
- [48].Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of Ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. British Journal of Pharmacology. 2006 doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mishra S, Undrovinas NA, Maltsev VA, Reznikov V, Sabbah HN, Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am.J.Physiol Heart Circ.Physiol. 2011;301:H1596–H1605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Campbell TJ, Williams KM. Therapeutic drug monitoring: antiarrhythmic drugs. Br.J.Clin.Pharmacol. 2001;52(Suppl 1):21S–34S. doi: 10.1046/j.1365-2125.2001.0520s1021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]