Abstract
Non-Hodgkin's lymphomas constitute a heterogeneous group of hematologic malignancies with varying aggressiveness and many therapeutic options. Non-myeloablative conditioning has been the cornerstone of allogeneic adoptive immunotherapy for these diseases. To optimize this therapeutic approach, we must determine its association with disease characteristics (histologic disease type, new prognostic factors, and conventional chemotherapy response), patient characteristics, and transplantation characteristics (conditioning type, donor source, graft-versus-host disease prophylaxis, donor lymphocyte infusion, and relapse prevention methods). In this review, we discuss the current status of non-myeloablative allogeneic transplantation in the major lymphoma subgroups.
Keywords: Allogeneic, nonmyeloablative, graft-versus lymphoma, lymphoma
Allogeneic hematopoietic stem cell transplantation (SCT) offers advantages over autologous SCT—specifically, lymphoma-free grafts and the immunologic graft-versus-lymphoma (GVL) effect, which have been found to confer long-term remission.1,2 Allogeneic SCT also permits the use of high-dose cytotoxic therapy and cell products devoid of tumor cells and prior chemotherapy-induced DNA damage. The widespread use of myeloablative allogeneic SCT in non-Hodgkin lymphoma (NHL), however, is limited by upfront mortality rates of up to 40%, occurring in part because myeloablative allogeneic SCT has traditionally been used in patients with more advanced, chemorefractory disease. However, in the past 15 years, we have witnessed a dramatic shift in how allogeneic SCT is performed, with increasing numbers of patients receiving less toxic non-myeloablative (NMA) or reduced-intensity (RI) conditioning regimens that promote donor cell engraftment and rely primarily on GVL induction.3 As a result, allogeneic SCT is feasible in a larger group of patients, and it is common knowledge that it would benefit older patients and patients with comorbid conditions. However, the increasing use of allogeneic SCT has made treatment decisions in NHL more complex. In this review, we describe the principles behind NMA conditioning and its effectiveness in the major lymphoma subgroups.
RATIONALE FOR ALLOGENEIC SCT IN NHL
Unlike allogeneic SCT, autologous SCT is associated with a treatment-related mortality (TRM) rate of <5%; however, it is also associated with a higher risk of relapse, especially in patients with relapsed follicular lymphoma (FL) and mantle cell lymphoma (MCL).4 The concurrent administration of rituximab, before and after autologous SCT, is associated with a significant improvement in overall (OS) and disease-free survival rates in relapsed, chemosensitive diffuse large-B-cell lymphoma (DLBCL).5 The addition of rituximab has also improved OS and decreased the risk of relapse in MCL patients who underwent autologous SCT during first remission, as a consolidation to their response with conventional chemotherapy.4,6 Rituximab was administered during stem cell mobilization (375 mg/m2 1 day before chemotherapy and 1000 mg/m2 7 days after chemotherapy). A dose of 1000 mg/m2 was administered again on days 1 and 8 after SCT. The use of monoclonal anti-CD20 antibody with autologous SCT has not led to improved outcomes in relapsed FL and MCL. Furthermore, the observed 8- to 15-fold increased incidence of secondary myelodysplasia is of major concern after autologous SCT.7
The only prospective comparison of autologous and allogeneic hematopoietic SCT in NHL was conducted in relapsed FL; it closed early because of poor accrual,8 which was in part due to the advent of several therapeutic approaches. The other comparisons were based on retrospective analyses of single-center or registry data; they demonstrated lower relapse rates after allogeneic SCT than after autologous SCT in both FL and DLBCL.1,9 The high TRM rate associated with myeloablative allogeneic SCT, however, offsets any potential survival benefits. The results of these retrospective comparisons are confounded by imbalances in patients characteristics and differences in treatment protocols. Other important determinants, such as serum lactate dehydrogenase (LDH) levels, 18F-fluoro-deoxyglucose positron emission tomography (PET) scan results, or bulky disease, are rarely included. In addition, most studies report on patients accrued over long periods of time, during which better donor selection (with improvements in histocompatibility typing procedures), the use of new prophylactic antifungal agents, and the early detection of cytomegalovirus reactivation by sensitive methods have contributed to a lower TRM risk. By contrast, no major changes have occurred in autologous SCT over the past decade.
GVL EFFECTS
The early clinical evidence of GVL activity was largely based on the important but indirect observation that immunosuppression withdrawal restored remission after relapse in some cases10 and that T-cell depletion was associated with an increased risk of relapse.
The most definitive evidence of GVL's effects remains the clinical observation of a marked donor lymphocyte response in patients who experience relapse after allogeneic SCT11,12 and a decreased relapse risk after T-cell depletion with prophylactic donor lymphocyte infusion (DLI) infusion.13 Immunomodulation responses have been described in several histologic subtypes of lymphoma, including DLBCL, FL, MCL, and peripheral T-cell lymphoma (TCL).10,14-16 In general, however, responses are more common in indolent histologic disease types with low tumor burdens than in those with aggressive histologic types and rapidly progressive disease. The natural culmination of GVL has been the development of NMA and RI regimens for allogeneic SCT that promote donor cell engraftment and rely primarily on GVL induction rather than dose-intensive chemotherapy.
NMA ALLOGENEIC SCT IN NHL: LONG-TERM RESULTS
The use of allogeneic SCT for NHL depends on several factors, including disease status, age, performance status, comorbidities, and donor availability. In addition, because NHL is a heterogenous group of tumors with varied biologic behavior, the histologic tumor type is an important factor.
Numerous series have reported promising response rates for NMA allogeneic SCT in patients with lymphoid malignancies. The most encouraging results have been in FL and MCL.
Follicular Lymphoma (FL)
The most compelling results on NMA SCT's role in FL originated at our institution. In 2008, we reported our 8-year experience with fludarabine, cyclophosphamide, and rituximab regimen in 47 FL patients who underwent sibling donor (n=45) or matched unrelated donor (n=2) allogeneic NMA SCT.14 We used a high-dose rituximab schedule of 375 mg/m2 on day −13, followed by 1000 mg/m2 on days −6, +1, and +8. Tacrolimus and methotrexate were used for GVHD prophylaxis. The median patient age was 53 years (range, 33–68 years), and all patients had chemosensitive disease. At the time of SCT, 62% of patients were in partial remission; after SCT, 100% had experienced a complete response (CR). Acute and chronic extensive GVHD occurred in 11% and 36% of patients, respectively. A unique finding in that report was that 20 of 28 (71%) patients who experienced chronic GVHD had de novo onset, which had no major negative effect on survival. The 1-year TRM rate was 13%. Furthermore, only 6 of the 47 patients died of infections, even though the regimen targets both cellular and humoral immunity. At a median of 60 months of follow-up, only 2 cases of disease progression after CR had occurred (4%). The progression-free survival (PFS) and OS rates were 83% and 85%, respectively. Since the last update, 1 patient developed recurrent disease. With a median follow-up of 107 months (range, 72-142 months), the 11-year overall OS and PFS rates were 78% and 72% (Table 1).17 These results suggest that NMA allogeneic SCT is curative for relapsed FL. At our institution, this strategy is reserved for patients with refractory or recurrent disease after the best chemo-immunotherapy available and who have a matched sibling or unrelated donor.
Table 1.
Non-myeloablative allogeneic SCT in NHL post fludarabine, cyclophosphamide and anti-CD20-containing conditioning.
| Histology | N | Conditioning regimen | Median Age (range) | %Sensitive %Refractory | %Sib %Unrelated | Median Follow-up mos (range) | TRM | %PFS | %OS |
|---|---|---|---|---|---|---|---|---|---|
| FL17 | 47 | FCR | 53 (33-68) | 100; - | 100; - | 107 (72-142) | 13 (1y) | 72 (11y) | 78 (11y) |
| 26 | 90YFC | 55 (29-66) | 61; 39 | 62; 38 | 33 (17-94) | 8 (1y) | 85 (3y) | 88 (3y) | |
| MCL4 | 30 | FCR | 58 (43-62) | 70; 30 | 83; 17 | 56 (11-110) | 9 (1y) | 46 (6y) | 53 (6y) |
FL, follicular lymphoma; MCL, mantle cell lymphoma; FCR, fludarabine, cyclophosphamide and rituximab; 90YFC, yittrium-90, ibritumomab tiuxetan, fludarabine, cyclophosphamide; Sib, sibling; Mos, months; TRM, treatment-related mortality; PFS, progression-free survival; OS, overall survival; y, year.
Mantle Cell Lymphoma (MCL)
MCL is now recognized as a distinct clinicopathological subtype of B-cell NHL that continues to pose a significant challenge to oncologists because there is currently no standard therapy for newly diagnosed or relapsed disease. The clinically inadequate results of conventional chemotherapy have led to the exploration of autologous SCT during the first remission. In the pre-rituximab era, early autologous SCT extended the median remission duration by 1-2 years, but most patients eventually experienced relapse.4,18 With the advent of rituximab and its incorporation into stem cell mobilization and conditioning regimens, we and other research groups have reported improved outcomes.4,19 In an updated analysis, we recently described the emergence of an early survival curve plateau after 3 years, with a projected lymphoma-free-survival duration at 10 years of 65% (95% CI, 44%-80%), suggesting a cured fraction.6 We also found that ki-67 of >30% at diagnosis was associated with a higher risk of relapse. In the relapsed or refractory disease setting, the clinical results of autologous SCT remain inadequate. This has led to exploring the use of allogeneic SCT, which has been reserved to treat patients with relapsed or recurrent disease.
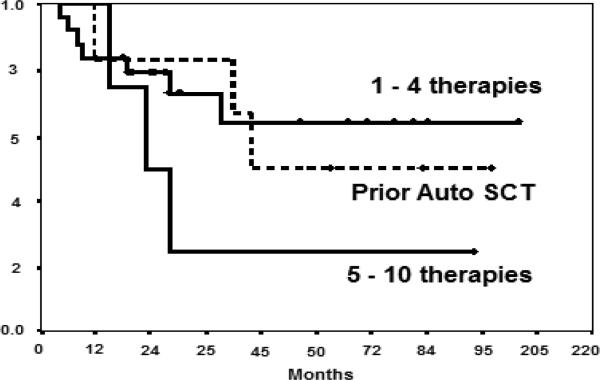
We recently reported the mature results of an allogeneic study after NMA in 35 patients (median age, 58 years [range, 43–68 years]) using fludarabine, cyclophosphamide, and high-dose rituximab.4 Patients were heavily pretreated with a median of 3 lines of therapy (range, 1-10), although most (88%) had chemosensitive disease at the time of NST. With a median follow-up of 56 months (range, 19-110 months), the median PFS duration was 60 months, and the median OS had not yet been reached. The 6-year actuarial PFS rate was 46%, and the 6-year actuarial OS rate was 53% (Table 1). Importantly, plateaus in the survival curves were observed for both PFS and OS, with no relapses or deaths occurring in 9 patients followed up for 63 to 110 months. The factors associated with favorable PFS on multivariate analysis were a peripheral blood graft with ≥ 95% chimerism and ≤ 4 prior therapies (Fig.1). These outcomes were significantly superior to those of patients who underwent autologous SCT for relapsed or refractory disease at our institution (P = 0.01 for both PFS and OS).
Figure 1.
Progression-free survival after NMA allogeneic SCT in relapsed MCL patients according to the number of prior therapies
Maris et al.20 published the results of SCT after NMA conditioning in MCL patients at Fred Hutchinson Cancer Center. Thirty-three patients with relapsed or refractory disease underwent conditioning with fludarabine and 2 Gy total body irradiation, followed by allogeneic SCT from a related (48%) or unrelated (51%) donor. The 2-year PFS rate was 60%. Similar to the MD Anderson results, the number of prior therapies was a major determinant of disease control, with no relapses in the 14 patients with <4 prior lines of therapy compared with 4 relapses in the 19 patients with ≥ 4 prior therapies (P = 0.01).
The results of these 2 series suggest that a significant proportion of patients with relapsed MCL will be cured with NMA allogeneic SCT. At present, this remains the only treatment associated with a long-term remission in relapsed MCL. The demonstration of a GVL effect through observed responses to DLI is encouraging.15 Early referral for SCT is certainly warranted, especially in patients with elevated ki-67 levels who do poorly with conventional chemotherapy and autologous SCT.
Diffuse Large B-cell Lymphoma (DLBCL)
DLBCL is the most common subtype of NHL. The indications for autologous and allogeneic SCT have generally been based on disease response to salvage chemotherapy. The PARMA21 prospective, randomized multicenter trial provided the strongest evidence supporting the use of autologous SCT over salvage chemotherapy in patients with chemoresponsive, relapsed DLBCL. A significant improvement in OS and PFS rates occurred with the addition of anti-CD20 antibody, especially in PET-negative patients at the time of SCT;22 thus, autologous SCT is the treatment of choice for these patients. However, new treatment strategies are needed to improve outcomes in patients with a poor prognosis and in those who experience a relapse after autologous SCT. Patients with high serum LDH levels or International Prognostic Index (IPI) scores or positive 18F-fluoro-deoxyglucose PET scans at the time of autologous SCT have inferior outcomes.22 Allogeneic SCT is the alternative approach.
Overall, the use of allogeneic SCT for DLBCL is still limited. The numbers of patients and published studies in which this strategy has been used are small and thus do not allow us to form definitive conclusions. Most DLBCL patients who are referred for allogeneic SCT have relapsed high-risk or refractory disease, have relapsed disease after autologous SCT, or are precluded autologous SCT because of stem cell collection difficulties. Studies have shown a lower relapse rate and longer disease-free survival duration after allogeneic SCT than after autologous SCT.1,9 The high TRM rate offsets any potential survival benefits. However, the response to DLI and withdrawal of immunosuppression in some patients lends credence to the existence of a GVL effect in DLBCL.10 Little evidence exists, however, that allogeneic SCT improves outcomes in refractory DLBCL. At least 1 research group found that patients with low-volume, stable disease have a better outcome after allogeneic SCT than do those with progressive disease, especially with high LDH levels.23,24
No clear-cut indication exists for allogeneic SCT for DLBCL, possibly because the prognostic features need to be studied at the first sign of relapse rather at the time of SCT after salvage chemotherapy. Patients with high IPI scores and LDH levels at relapse should be considered for allogeneic SCT, especially if PET remains positive after salvage treatment. Several investigators have found that PET positivity, which is usually associated with poor outcomes after autologous SCT, has no effect on response to allogeneic SCT.25
Other biological characteristics of the disease may also be important determinants of outcomes after allogeneic SCT. DLBCL is not a single disease; it can be categorized on the basis of its molecular features as germinal center B-cell-like, non-germinal center B-cell-like, or primary mediastinal B-cell lymphoma.26 Furthermore, the double-hit subtype, with bcl2-Myc translocation, is increasingly reported as having poor prognostic features.27 The effects of this sub-classification on allogeneic SCT are unclear at present.
Several reports have assessed the effectiveness of allogeneic SCT in DLBCL patients who have not experienced a response to a prior autologous SCT. We demonstrated the feasibility of this approach in 2004.28 Since then, several studies have demonstrated durable remissions in 20%-40% of patients.29 Chemosensitivity, co-morbidities, and time to relapse after autologous SCT are the most significant determinants of outcome. Patients who experience a relapse in 6-12 months have a high TRM rate. As a result, less than 20% of patients are usually eligible for this procedure.
T-Cell Lymphomas (TCL)
TCL is a heterogeneous group of cancers that represents 15% of NHLs.30 Compared with B-cell lymphoma, TCLs are more resistant to conventional chemotherapy and are generally associated with a poor outcome, except for anaplastic kinase-positive large-cell lymphoma. Treatment with new agents leads to improved responses,30 but relapse is common, especially in patients with advanced or recurrent disease. Continuous remissions of more than 5 years have been observed in patients who underwent autologous SCT during their first remission.31 Poor outcomes have been observed in patients with relapsed disease.
Several small reports have described responses to DLI or immunosuppression withdrawal, which suggest that T cells are a good target for donor-derived immune systems. NMA or RI conditioning may lead to decreased TRM rates and improved results. This strategy led to favorable outcomes in a small series of advanced mycosis fungoides patients who had previously not experienced a response to several lines of conventional treatment.32
Corradini et al16 found a 3-year PFS rate of 80% in a study of 17 relapsed, chemosensitive peripheral T-cell (not otherwise specified) patients. A recent report from Dana Farber suggested that allogeneic SCT is particularly successful in patients with nodal TCL and less so in those with extranodal disease.33 Whether allogeneic SCT is truly superior to autologous SCT continues to be debated. A recent retrospective study from our institution demonstrated similar survival rates between autologous and allogeneic SCT for relapsed TCL, with the exception of lymphoblastic lymphoma patients, who fared better after allogeneic SCT.31 In both, an IPI score for TCL of > 0 was associated with poor survival rates.
NMA CONDITIONING: DISEASE SPECIFIC OR DISEASE INTENSE
The development of NMA or RI conditioning has been one of the most important advances in allogeneic SCT for NHL. Numerous regimens of various intensities are being developed to minimize SCT toxicity while maximizing GVL activity. In the absence of randomized trials, it is difficult to demonstrate the superiority of 1 regimen over the other. However, some general conclusions can be drawn from the published results.
Conditioning regimens allow engraftment of donor cells with the least toxicity and provide early disease control, allowing time for the donor cells to generate GVL activity. Dose intensity may be particularly important in patients with aggressive histologic disease types and less important in patients with indolent diseases and low tumor burdens. The most commonly used regimens are the combination of fludarabine, cyclophosphamide, and rituximab; low-dose total body irradiation plus fludarabine; and the more intense melphalan plus fludarabine or carmustine, etoposide, cytarabine, and melphalan. The more intense regimens have been reserved for patients with aggressive histologic disease types and are usually associated with a higher risk of GVHD. However, none of these regimens seems to be effective against bulky refractory disease.
Various strategies are being investigated to improve the efficacy of conditioning without additional toxicity. One strategy is to incorporate novel agents in allogeneic conditioning that are effective against lymphoma. We34 recently reported the preliminary results of bendamustine, a novel compound that is effective in patients whose disease is refractory to alkylating agents. To improve outcomes in NMA SCT patients, we substituted cyclophosphamide with bendamustine in the conditioning regimen. Bendamustine was given intravenously in escalated doses of 70, 90, 110, and 130 mg/m2 daily on days −5 to −3 prior to SCT, together with 30 mg/m2 of fludarabine on days −5 to −3 and 375 mg/m2 of rituximab on day −13 and 1000 mg/m2 on days −6, +1, and +8. The study included 23 patients with various lymphoid diseases that had relapsed after the best conventional therapy available. Fifteen patients (65%) underwent SCT from human leukocyte antigen-compatible siblings and 8 (35%) from unrelated donors. Their median age was 60 years (range, 30-70 years). Two, 3, 3, and 15 patients received 70, 90, 110, and 130 mg/m2 daily doses of bendamustine, respectively. No dose-limiting toxicity was observed. Fourteen patients (61%) did not experience a nadir of their absolute neutrophil count to < 0.5 ×109/L; 3 did not require filgrastim for neutrophil recovery; and 19 (83%) did not experience a platelet count < 20,000 ×109/L. All patients experienced donor cell engraftment. The median donor T cell level at day 30 was 93%. Only 1 patient developed acute GVHD (grade 3). Chronic extensive GVHD was observed in 2 of 22 (9%) evaluable patients. Fungal infection was the cause of the only death observed. With a median follow-up time of 8 months (range, 3-25 months), the OS and PFS rates were 92% and 79%, respectively.
The monoclonal anti-CD20 antibody has significant single-agent activity in B-cell lymphoid malignancies and may enhance GVL through antibody-dependent cytotoxicity and by increasing dendritic cell uptake and presentation of tumor cell-derived peptides.35,36 We recently demonstrated the effectiveness of this strategy in chronic lymphocytic leukemia, in which it led to increased natural killer (NK) cell lysis against the tumor, enhancing the complete remission rate.37 We hypothesized that the lower risk of GVHD in our studies was attributable to rituximab. In a recent study by Arai et al,38 the use of prophylactic anti-B cell therapy delivered 2 months after SCT decreased allogeneic donor B cell immunity and resulted in low incidences of acute (6%) and chronic GVHD (20%).
Various strategies are being investigated to improve the outcome of chemorefractory FL patients after NMA allogeneic SCT. One of the most compelling studies of targeted therapy was the incorporation of radioimmunotherapy with anti-CD20 antibody and yttrium-90-ibritumomab tiuxetan (90YIT) in the NMA conditioning in patients with refractory FL.17
90YIT is associated with a higher response rate than is rituximab in relapsed or chemorefractory FL patients. Because of its beta emission, 90Y delivers radiation not only to the tumor cells that bind the antibody but also to neighboring tumor cells that are inaccessible to the antibody or have insufficient antigen expression as a result of a cross-fire effect. Thus, we hypothesized that adding 90YIT to the NMA conditioning regimen would enhance initial disease control and that remission could later be sustained via the GVL effect. Patients received single doses of 90YIT (0.4 mCi/kg on day −14) with fludarabine and cyclophosphamide. The cohort included 26 relapsed FL patients: 10 (38%) had disease that was refractory to chemoimmunotherapy and 11 (44%) were PET positive at study entry. Sixteen (62%) patients had sibling donors and 10 (38%) had unrelated donors. The toxicity and GVHD profiles were similar to those observed for fludarabine, cyclophosphamide, and rituximab: unlike the 90YIT patients, all patients had instead chemosensitive disease and sibling donors. With a median follow-up of 33 months (range, 17-94 months), the 3-year PFS rates of patients with chemorefractory and chemosensitive disease were 80% and 87%, respectively (P = 0.7) (Table 1).
DONOR SOURCE, GVHD PROPHYLAXIS, AND DLI
Allogeneic SCT is no longer considered as a sole procedure of stem cell infusion after NMA or RI conditioning. It is now an integral part of comprehensive treatment programs, including intensive debulking, donor cell choice, GVHD prophylaxis type and duration, and use of DLI.
Earlier studies of allogeneic SCT for NHL were restricted to recipients of matched sibling SCT. Advances in unrelated donor selection, including high-risk human leukocyte antigen typing,39 have resulted in major improvements in outcome after unrelated donor SCT. Several recent studies have demonstrated that unrelated donor SCT results in similar or even better survival rates than do matched sibling SCT.40 In a recent FL study at our center, the addition of 1 mg/kg thymoglobulin on days −2 and −1 to the 90YIT conditioning regimen resulted in similar engraftment rates and acute and chronic GVHD risks in sibling and unrelated SCT patients. The OS and PFS rates were also similar.17
The increasing accuracy of donor typing has led to fewer matching donors, especially in patients of minority descent. Alternative donor SCT, including cord blood41 or haplo-identical SCT42 are increasingly being considered. One limiting factor of haplo-identical SCT is the prohibitive incidence of severe GVHD. The use of high-dose post-SCT cyclophosphamide by the investigators at John Hopkin's group has promising results.42
Because the incidence of GVHD can be significant after DLI, studies of prophylactic DLI must include a careful assessment of GVHD risk and the clinical setting. Selected patients receive DLI for persistent disease and relapse, but it is commonly provided for mixed chimerism after NMA and RI SCT, even in the absence of measurable disease. This leads to a high incidence of GVHD and is a major cause of morbidity and mortality after allogeneic SCT. We have recently demonstrated that this practice is not routinely needed in patients with FL who continue to have stable mixed chimera after non-T cell-depleted NMA allogeneic SCT because there is no effect on the relapse rate or final responses, even molecularly.14 Whether this strategy can be used for other histologic types remains to be studied. On the other hand, prophylactic adoptive immunotherapy may be useful for restoring GVL activity after T cell-depleted SCT.43
STRATEGIES TO ENHANCE GVL
It has been widely accepted that GVL is the most potent in indolent lymphoid histologic disease types. Innovative approaches are needed to maximize GVL's activity in patients with other histologic types and limit GVHD.
One such approach is combining DLI with novel antibodies that can direct and activate effector cells directly at the tumor site. The use of rituximab in this setting has been associated with a CR rate of 47% in chronic lymphocytic leukemia patients with recurrent or persistent disease after NMA allogeneic SCT. Patients who experienced a response had increased NK cell lysis.37 Methods to enhance this activity in other histologic disease types are ongoing.
Several strategies are used to enhance donor T-cell activity, including using donor T cells that have been expanded and activated through ex vivo co-stimulation.44 It is also possible to genetically modify T cells to express chimeric antigen receptors and redirect T-cell specificity. Kochenferder45 recently reported the use of donor T cells expressing an anti-CD19 chimeric antigen receptor to treat patients with chronic lymphocytic leukemia and other lymphoid malignancies. NK cells can have potent antitumor activity. Several trials have demonstrated the safety and feasibility of donor NK cell infusions after haploidentical SCT,46 and the results of recent trials suggest that graft-versus-tumor activity is optimized by donor NK cells expressing appropriate killer-cell immunoglobulin-like receptor genes.47 New techniques that isolate and expand NK cells ex vivo will allow us to use novel strategies to test NK cell immunotherapy.
SUMMARY
NMA or RI allogeneic SCT can have a significant GVL effect in advanced NHL patients. The applicability of this approach appears to depend on the histologic disease type and individual patient and disease characteristics. Several strategies are available to enhance the GVL effect, but it will ultimately require a better understanding of important target antigens. The field offers significant opportunity for clinical research, and we strongly encourage patients to participate in clinical trials whenever possible.
Footnotes
Conflict of interest: Research grants from Cephalon, Spectrum Pharmaceuticals.
REFERENCES
- 1.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–668. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 2.Hosing C, Saliba RM, McLaughlin P, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin's lymphoma. Ann Oncol. 2003;14:737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 3.Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 4.Tam CS, Bassett R, Ledesma C, et al. Mature results of the MD Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khouri IF, Saliba RM, Hosing C, Okoroji GJ, et al. Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:2240–2247. doi: 10.1200/JCO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Chakhachiro ZT, Saliba RM, Okoroji GJ, et al. 12-year experience with high-dose rituximab-containing autologous stem cell transplantation for SOX11-positive mantle cell lymphoma patients in first remission: Emerging lymphoma-free survival plateau after 3 years. Blood (American Society of Hematology) 2011:118. Abstract 662. [Google Scholar]
- 7.Metayer C, Curtis RE, Vose J, et al. Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: a multicenter case-control study. Blood. 2003;101:2015–2023. doi: 10.1182/blood-2002-04-1261. [DOI] [PubMed] [Google Scholar]
- 8.Laport G, Bredeson CN, Tomblyn M, et al. Autologous versus reduced intensity allogeneic hematopoietic cell transplantation for patients with follicular non-Hodgkin's lymphoma (FL) beyond first complete response or first partial response. J Clin Oncol (American Society of Clinical Oncology) 2008:26. doi: 10.1016/j.bbmt.2010.11.004. Abstract 7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Besien KW, de Lima M, Giralt SA, et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graft-versus-lymphoma effects. Bone Marrow Transplant. 1997;19:977–982. doi: 10.1038/sj.bmt.1700781. [DOI] [PubMed] [Google Scholar]
- 11.Mandigers CM, Verdonck LF, Meijerink JP, et al. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32:1159–1163. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 12.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp. Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Thomson KJ, Morris EM, Milligon D, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khouri IF, Lee M-S, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for advanced/recurrent mantle cell lymphoma. J Clin Oncol. 2003;21:4407–4412. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 16.Corradini P, Dodero A, Zallio F, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Khouri IF, Saliba RM, Erwin WD, et al. Nonmyeloablative allogeneic transplantation with or without 90 yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012 doi: 10.1182/blood-2012-03-417808. [Epub ahead of print, May 14, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 19.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression- free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 21.Philip T, Guglielmi C, Hagenbeek A, et al. ABMT as compared with salvage chemotherapy in relapses of chemosensitive NKL. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 22.Alousi AM, Saliba RM, Okoroji GJ, et al. Disease staging with positron emission tomography or gallium scanning and use of rituximab predict outcome for patients with diffuse large B-cell lymphoma treated with autologous stem cell transplantation. Br J Haematol. 2008;142:786–792. doi: 10.1111/j.1365-2141.2008.07277.x. [DOI] [PubMed] [Google Scholar]
- 23.Bishop MR, Dean RM, Steinberg SM, et al. Correlation of pretransplant and early posttransplant response assessment with outcomes after reduced-intensity allogeneic hematopoietic stem cell transplantation for non-Hodgkin's lymphoma. Cancer. 2010;116:852–862. doi: 10.1002/cncr.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean RM, Fowler DH, Wilson WH, et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy-refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:593–599. doi: 10.1016/j.bbmt.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Lambert JR, Bomanji JB, Peggs KS, et al. Prognostic role of PET scanning before and after reduced-intensity allogeneic stem cell transplantation for lymphoma. Blood. 2010;115:2763–2768. doi: 10.1182/blood-2009-11-255182. [DOI] [PubMed] [Google Scholar]
- 26.Alizadeh A, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 27.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 28.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic haematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin's lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 29.van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–1348. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 30.Foss FM, Zinzani PL, Vose JM, et al. Peripheral t-cell lymphoma. Blood. 2011;117:6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 31.Beitinjaneh A, Saliba RM, Okoroji GJ, et al. Autologous and allogeneic stem cell transplantation for t-Cell Lymphoma: the M.D. Anderson Cancer Center experience. Blood (American Society of Hematology) 2011:118. Abstract 4118. [Google Scholar]
- 32.Duvic M, Donato M, Dabaja B, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28:2365–2372. doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;11:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khouri IF, Saliba RM, Korbling M, et al. Nonmyeoablative allogeneic conditioning with bendamustine in combination with fludarabine and rituximab for lymphoid malignancies: immunosuppression without myelosuppression and without acute GVHD. Blood (American society of Hematology) 2011:118. Abstract 894. [Google Scholar]
- 35.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 36.Hsu FJ, Komarovskaya M. CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells. J Immunother. 2002;25:455–468. doi: 10.1097/00002371-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011 doi: 10.1002/cncr.26091. [Epub ahead of print, March 31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B cell alloimmunity with low chronic GVHD incidence. Blood. 2012 doi: 10.1182/blood-2011-12-395970. [Epub ahead of print, May 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 40.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1196–1204. doi: 10.1016/j.bbmt.2010.12.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunstein CG, Cantero S, Cao Q, et al. Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:214–222. doi: 10.1016/j.bbmt.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peggs KS, Kayani I, Edwards N, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin's lymphoma. J Clin Oncol. 2011;29:971–978. doi: 10.1200/JCO.2010.32.1711. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein SC, Levine B, Smith J, et al. First report of the prophylactic administration of ex vivo co-stimulated donor lymphocyte infusion (DLI) from Related and unrelated donors after reduced intensity conditioning for high risk hematologic malignancies [abstract]. Blood. 2009;112:468. [Google Scholar]
- 45.Kochenderfer JN, Dudley M, Maric I, et al. Dramatic regression of chronic lymphocytic leukemia in the first patient treated with donor derived genetically engineered anti-CD 19 chimeric antigen receptor expressing T cells after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:S158. [Google Scholar]
- 46.Passweg JR, Tichelli A, Meyer-Monard S, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 47.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]