Abstract
Astrocytes have been found to play important roles in physiology being fundamental for ionic homeostasis and glutamate clearance from the synaptic cleft by their plasma membrane glutamate transporters. Astrocytes are electrically non-excitable, but they exhibit Ca2+ signaling, which now has been demonstrated to serve as an indirect mediator of neuron-glia bidirectional interactions through glio-transmission via tripartite synapses and to modulate synaptic function and plasticity. Spontaneous astrocytic Ca2+ signaling was observed in vivo. Intercellular Ca2+ waves in astrocytes can be evoked by a variety of stimulations. Astrocytes are critically involved in many pathological conditions including ischemic stroke. For example, it is well known that astrocytes become reactive and form glial scar after stroke. In animal models of some brain disorders, astrocytes have been shown to exhibit enhanced Ca2+ excitability featured as regenerative intercellular Ca2+ waves. This chapter briefly summarizes astrocytic Ca2+ signaling pathways under normal conditions and in experimental in vitro and in vivo ischemic models. It discusses the possible mechanisms and therapeutic implication underlying the enhanced astrocytic Ca2+ excitability in stroke.
Keywords: Astrocytes, Ca2+ signaling, Ischemic stroke, Hypoxia
10.1 Astrocytes in the Central Nervous System
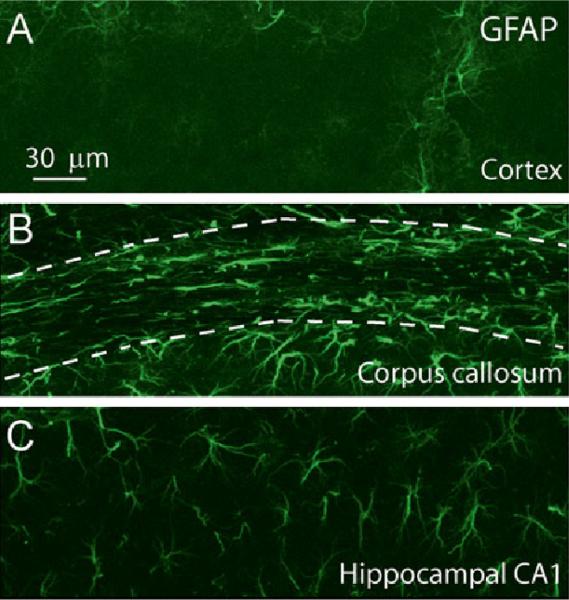
Astrocytes are specialized and the most numerous glial cell type in the central nervous system (CNS) (Agulhon et al. 2008; Barres and Barres 2008; Haydon 2001). There are two major types of astrocytes: protoplasmic astrocytes in grey matter and fibrous astrocytes in white matter (Fig. 10.1). Morphologically, protoplasmic astrocytes are complex and highly branched with numerous fine processes and their endfeet wrap around blood vessels. Fibrous astrocytes are less complex and have thicker and less branched processes. It is well known that astrocytes control CNS homeostasis by regulating ionic concentration (e.g., astrocytes act as a K+ sink to maintain extracellular K+ homeostasis) (Djukic et al. 2007) and removing glutamate from the synaptic cleft by their plasma membrane glutamate transporters to avoid glutamate toxicity (Huang et al. 2004; Bergles et al. 1999; Danbolt 2001). Astrocytes provide nutritional and structural support for neurons. Some astrocytes express the unique intermediate filament protein, glial fibrillary acidic protein (GFAP), which is used to distinguish them from other cell types in the CNS; however, its expression levels in astrocytes are different in different regions. For example, under normal conditions, protoplasmic astrocytes in the mouse cortex express much lower levels of GFAP than do protoplasmic astrocytes in the hippocampus (Fig. 10.1), although the densities of astrocytes in these two regions are similar (Ding 2013). It is also clear now that not all astrocytes express GFAP and vice versa, not all cells that express GFAP are astrocytes (Oberheim et al. 2012). Electrophysiological recordings also showed that even in the same region astrocytes have differential patterns of current-voltage relationship; for instance, one type of astrocytes termed outward rectifying astrocytes and the other termed variably rectifying astrocytes (Zhou and Kimelberg 2000). In vivo Ca2+ imaging also demonstrates that astrocytes in the cortical layer 1 (L1) exhibited distinct Ca2+ dynamics in vivo from astrocytes in the cortical layer 2/3 (L2/3) in rats anesthetized with urethane (Takata and Hirase 2008). Astrocytes in L1 nearly doubled the Ca2+ activity of astrocytes in L2/3. Furthermore, Ca2+ fluctuations of processes within an astrocyte were independent in L1, while those in L2/3 were more synchronous (Takata and Hirase 2008). Thus, astrocytes in the CNS are heterogeneous in function, morphology and molecular expression.
Fig. 10.1.
Heterogeneity of morphology and GFAP expression of astrocytes in the mouse brain under normal conditions. (a) GFAP expression in protoplasmic astrocytes in the cortex layer 2-3. (b) GFAP expression in fibrous astrocytes in the corpus callosum (dashed outline, middle). (c) GFAP expression in protoplasmic astrocytes in the CA1 region of the hippocampus. Notice the thick process and high express levels of GFAP in the corpus callosum and highly branched astrocytes in the hippocampal CA1 region. All the mages were taken by confocal microscopy and are the maximum projection images. Adapted from Ding (2013)
10.2 Ca2+ Signaling in Astrocytes
10.2.1 GPCR-Mediated Ca2+ Signaling
More than two decades ago, it was discovered that astrocytes could mediate Ca2+ signaling (i.e., transient Ca2+ increase) (Cornell-Bell et al. 1990), which suggested that they can play more active roles in the CNS than previously delineated. Astrocytes express a variety of G-protein coupled receptors (GPCRs), e.g., for glutamate, γ-aminobutyric acid (GABA), ATP, serotonin, norepinephrine, and dopa-mine. These receptors can all mediate astrocytic Ca2+ signaling and intercellular waves in vivo by the activation of metabotropic glutamate receptors (mGluRs) (Ding et al. 2007; Fellin et al. 2004; Sun et al. 2013), P2Y receptors (Ding et al. 2009; Thrane et al. 2012; Sun et al. 2013; Wang et al. 2006; Nizar et al. 2013), GABAB receptors (GABABRs) (Ding et al. 2009; Meier et al. 2008), noradrenergic receptors (Bekar et al. 2008), and dopamine receptors (Haydon and Carmignoto 2006; Ni et al. 2007). mGluRs are classified into three groups (Pin and Duvoisin 1995; Schoepp et al. 1999; Niswender and Conn 2010). Group I mGluRs includes mGluR1 and 5 which are coupled to phospholipase-C/inositol 1,4,5-triphosphate (PLC/IP3) pathway to mobilize Ca2+ from the internal store. Group II (mGluR2 and 3) and III (mGluR4-6 and 7-8) are negatively coupled to adenylyl cyclase. Cortical and hippocampal astrocytes predominantly express mGluR5 and mGluR3 (Schools and Kimelberg 1999). GABABRs were also reported to be expressed in the cortical and hippocampal astrocytes (Meier et al. 2008; Nilsson et al. 1993; Oka et al. 2006; Charles et al. 2003), GABABRs can mediate Ca2+ elevations in astrocytes in response to interneuron activation in brain slices (Meier et al. 2008; Kang et al. 1998). Similarly, GABABR agonist baclofen can stimulate Ca2+ elevations in astrocytes in brain slices (Meier et al. 2008) and in vivo (Ding et al. 2009). It is likely that GABABR-stimulated Ca2+ signaling in astrocyte is mediated through the release from the internal Ca2+ store although the mechanism is not fully studied (Doengi et al. 2009). ATP has been extensively used to stimulate Ca2+ release in vivo through P2Y receptors (Ding et al. 2007, 2009; Sun et al. 2013; Wang et al. 2006; Thrane et al. 2012; Nizar et al. 2013). Ca2+ signaling is now considered as a primary form of cellular excitability in astrocytes that can be determined by fluorescent imaging using different Ca2+ indicators.
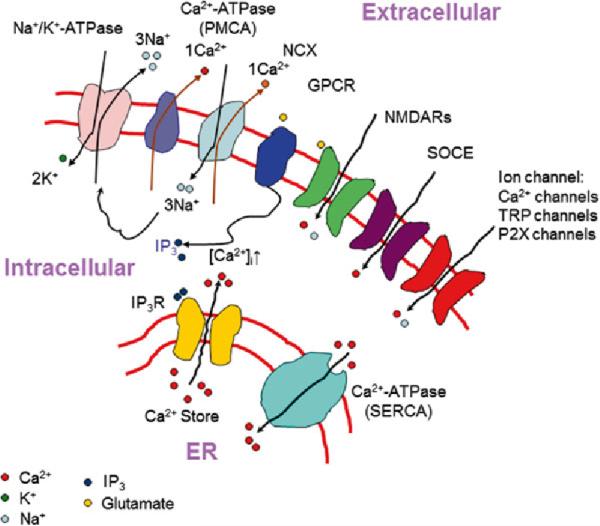
GPCR stimulation activates PLC with subsequent IPr mediated Ca2+ release (Agulhon et al. 2008; Haydon 2001; Petravicz et al. 2008) (Fig. 10.2). Among the three types of IP3R (IP3R1-3), IP3R2 seems to be the predominant type in astrocytes in the rodent brain (Hertle et al. 2007; Holtzclaw et al. 2002; Sharp et al. 1999). IP3R2 knock-out (IP3R2 KO) mice do not exhibit GPCR agonists-evoked Ca2+ increase in astrocytes in brain slice and in vivo, demonstrating that IP3R2 is a key mediator of intracellular Ca2+ release in astrocytes, but IP3R2 has no effect on long-term plasticity and vascular tone (Nizar et al. 2013; Petravicz et al. 2008).
Fig. 10.2.
Ca2+ signaling pathway and routes of Ca2+ entry. ER endoplasmic reticulum; SOCE store-operated Ca2+ entry. NCX sodium-calcium exchanger, forward mode operation; PMCA plasma membrane Ca2+ ATPase; SERCA sarco/endoplasmic reticulum Ca2+-ATPase; NMDARs N-methyl-D-aspartate receptors; TRP transient receptor potential channel
10.2.2 Ca2+ Influx Through the Plasma Membrane
A number of plasma membrane proteins can also regulate Ca2+ homeostasis by controlling Ca2+ influx from the extracellular space. Those proteins include Na+/Ca2+ exchanger (Reyes et al. 2012; Takuma et al. 2013; Kirischuk et al. 2012), plasma membrane Ca2+ ATPase (Reyes et al. 2012), store-operated channels (Linde et al. 2011), P2X purinoceptors (Illes et al. 2012; Palygin et al. 2010), transient receptor potential A (TRPA) channels (Shigetomi et al. 2012), C (TRPC) channels (Linde et al. 2011; Shirakawa et al. 2010; Malarkey et al. 2008; Reyes et al. 2013), and N-methyl-D-aspartate (NMDA) receptors (Palygin et al. 2010) (Fig. 10.2).
10.2.3 Ca2+-Dependent Gliotransmission and the Tripartite Synapse
Studies using cultured astrocytes (Parpura et al. 1994; Parpura and Haydon 2000) and brain slice preparations (Ding et al. 2007; Fellin et al. 2004; D'Ascenzo et al. 2007; Angulo et al. 2004) indicate that an increase in astrocytic Ca2+ leads to the release of chemical transmitters, the process referred to as gliotransmission. Thus, Ca2+ signaling in astrocytes serves as a mediator of bidirectional interactions between neurons and astrocytes which are the integral part of a tripartite synapse. A tripartite synapse is composed of presynaptic nerve terminal, postsynaptic terminal, and astrocytic processes (Haydon 2001). Gliotransmission is predominantly mediated by mGluRs (Fellin et al. 2004; D'Ascenzo et al. 2007; Pasti et al. 1997; Porter and McCarthy 1996). Glutamate (Ding et al. 2007; Fellin et al. 2004; D'Ascenzo et al. 2007; Angulo et al. 2004; Jourdain et al. 2007; Parri et al. 2001; Parpura and Haydon 2000; Parpura et al. 1994), ATP (Cotrina et al. 2000; Pascual et al. 2005; Newman 2001), and D-serine (Mothet et al. 2005; Henneberger et al. 2010; Martineau et al. 2013; Stobart et al. 2013; Takata et al. 2011) can all be released from astrocytes. Thus, astrocytes can send signal back to neurons through gliotransmission to modulate neuronal activity (Ding et al. 2007; Fellin et al. 2004; D'Ascenzo et al. 2007; Angulo et al. 2004) and synaptic plasticity in brain slice and in vivo (Pascual et al. 2005; Takata et al. 2011; Chen et al. 2012; Navarrete et al. 2012). Under pathological conditions, enhanced astrocytes Ca2+ signaling may consequently increase excitotoxicity through gliotransmission.
10.2.4 Imaging Intracellular Ca2+ in Astrocytes
Astrocytic Ca2+ signaling has been extensively studied in cultured astrocytes using fluorescence imaging. Organic acetoxymethyl (AM) ester form of Ca2+ indicators such as fluo-4, Oregon green BAPTA-1 (OGB), Rhod-2, or x-Rhod-1 have been largely used for in vitro and in vivo Ca2+ imaging due to their high sensitivity and speed (Wang et al. 2006; Takano et al. 2006; Ding et al. 2007, 2009; Ding 2012; Hirase et al. 2004; Takata and Hirase 2008; Takata et al. 2011; Thrane et al. 2012; Sun et al. 2013; Nimmerjahn et al. 2004; Aguado et al. 2002; Porter and McCarthy 1996). The selective labeling of astrocytes by organic Ca2+ indicators in vivo can be confirmed by an astrocyte selective dye sulforhodamine 101 (SR101), (Nimmerjahn et al. 2004). Recently developed genetically encoded Ca2+ indicators (GECis) including FRET-based GECIs such as YC3.6 and single-ftuorophore GECIs such as GCaMP provide an alternative way to label astrocytes in vivo. The major advantage for using GECIs is that they can be expressed in astrocytes for long-term imaging. The GECIs can be expressed in astrocytes using viral transduction, in utero electro-poration or transgenic mice (Tian et al. 2009; Zariwala et al. 2012; Akerboom et al. 2012; Shigetomi et al. 2013). Astrocyte-specific expression of GECIs using adeno-associated viral vectors can be achieved by astrocyte-specific promoter (Xie et al. 2010; Tong et al. 2013). Transgenic mice expressing floxed GCaMP3 can cross with Cre mice with specific promoters for neuronal and astrocytic Ca2+ imaging (Zariwala et al. 2012). For astrocytic specific expression of GCaMP3, GFAP-Cre mice are commercially available. Since GECIs can be expressed in astrocytes for prolonged times, it is feasible to perform long-term and repeated in vivo Ca2+ imaging in astrocytes, inducing that in ischemic conditions. Usually, for in vivo imaging, a cranial window in the cortex must be prepared for loading organic Ca2+ dyes. For detailed procedures and protocols of the surgery procedures and dye loading for in vivo Ca2+ imaging in astrocytes, readers can consult detailed reviews (Ding 2012; Tian et al. 2006).
10.3 In Vivo Ca2+ Signaling in Astrocytes Under Normal Conditions
In the past decade, the application of two-photon (2-P) laser-scanning fluorescence microscopy in in vivo imaging provided a valuable tool to understand the behavior of astrocytic Ca2+ signaling in live animals. Before I discuss astrocytic Ca2+ signaling in stroke, I briefly summarize the current studies on in vivo Ca2+ signaling in astrocytes in rodents under normal conditions. Detailed discussion about this topic can be found in a recent review (Ding 2013). Ca2+ signaling in cultured astrocytes will not be discussed as the morphology and gene profiles of cultured astrocytes are generally, but not always, altered as compared with those of astrocytes in vivo.
10.3.1 In Vivo Spontaneous Ca2+ Signaling in Astrocytes
Spontaneous Ca2+ signaling of astrocytes in the cortex and hippocampus in rodents has been observed in vivo using 2-P microscopy. Data from published studies show that Ca2+ signaling properties are affected when different preparations are used.
Cortical astrocytes in adult mice (more than 2-month old) usually exhibit low frequency spontaneous Ca2+ oscillations in cell body and processes when anesthetized with urethane (Ding et al. 2007, 2009), ketamine/xylazine (Wang et al. 2006), or isoflurane (Thrane et al. 2012). The duration of Ca2+ signal in the cell body and processes is short (somewhat between 10 and 25 s). Importantly, Ca2+ signals in the cell bodies of different astrocytes and in the different processes are independent. Thus, spontaneous Ca2+ signaling in cortical astrocytes in anesthetized adult mice do not exhibit intercellular wave-like behavior, and Ca2+ signals in the processes are limited within microdomains of the processes.
Astrocytes exhibit regional heterogeneity in spontaneous Ca2+ signaling. Astrocytes in the cortical layer 1 (L1) exhibited distinct Ca2+ dynamics in vivo from astrocytes in the cortical layer 2/3 (L2/3) in rats anesthetized with urethane; astrocytes in L1 nearly doubled the Ca2+ activity of astrocytes in L2/3 (Takata and Hirase 2008). Furthermore, Ca2+ fluctuations in the processes within an astrocyte were independent in L1, while those in L2/3 were more synchronous (Takata and Hirase 2008). On the other hand, in urethane-anesthetized young mice (P9-25), hippocampal astrocytes in the network exhibited synchronized Ca2+ oscillations and intercellular waves (Kuga et al. 2011; Sasaki et al. 2011). The difference in Ca2+ activities in astrocytes between the cortex and the hippocampus in mice might reflect the functional heterogeneity, which possibly resulted from the different neuronal activities, and microenvironment and/or different properties of astrocytes per se. The fact that GFAP levels are higher in hippocampal astrocytes than in the cortical astrocytes supports the latter reason (see Fig. 10.1). However, since the mice used in the study were young (P9-25), it is not clear whether astrocytes in adult mice would exhibit similar Ca2+ signaling.
In awake mice, cortical astrocytes exhibit much higher frequency of spontaneous Ca2+ signaling in the cell body and processes than in mice anesthetized by isoflurane, ketamine, and urethane (Thrane et al. 2012). In addition, somatic Ca2+ signals in astrocytes are highly synchronized, suggesting that synchronization of cortical astrocytic Ca2+ activity is a hallmark of wakefulness of an animal.
In summary, the properties of spontaneous Ca2+ signaling in astrocytes could be affected by animal species (e.g., mice, rats or other rodents), animal ages (e.g., young or adult animals), locations of astrocytes (e.g., in the cortex, hippocampus or other regions), anesthesia (e.g., isoflurane, ketamine/xylazine or urethane), and wakefulness of animals.
10.3.2 In Vivo Stimulated Ca2+ Signaling in Astrocytes
Astrocytic Ca2+ signals can be stimulated by a variety of approaches including GPCR agonists, sensory stimulations, mechanical stimulations, and photolysis of caged compounds (Ding et al. 2007; Sun et al. 2013; Wang et al. 2006; Takano et al. 2006; Tian 2005). ATP is a powerful agonist that can induce intercellular Ca2+ waves in a large number of astrocytes (Ding et al. 2009; Sun et al. 2013; Wang et al. 2006; Thrane et al. 2012; Nizar et al. 2013). Regenerative intercellular Ca2+ waves with high frequency can be elicited with continuous presence of ATP in the cortex (Ding et al. 2007, 2009; Ding 2012), while the group I mGluR agonists (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) and (RS)3,5-dihydroxyphenylglycine (DHPG) stimulate intercellular Ca2+ waves with low frequency (Ding et al. 2007). Sensory stimulations, including whisker deflection (Wang et al. 2006), locomotion (Nimmerjahn et al. 2009; Dombeck et al. 2007), limb stimulation (Bekar et al. 2008; Winship et al. 2007; Ghosh et al. 2013), light illumination of visual cortex (Chen et al. 2012; Schummers et al. 2008), and odor stimulation of olfactory glomeruli (Petzold et al. 2008), can also induce intercellular Ca2+ waves in astrocytes in vivo in anesthetized animals. Ca2+ signals of Bergmann glial (BG) cells were associated with motor behavior and neuronal activity in awake and behaving mice (Nimmerjahn et al. 2009). Interestingly, BG cells exhibit three different forms of Ca2+ excitation: flares, bursts, and sparkles. Bursts and sparkles are ongoing in awake mice at rest, whereas flares are initiated during locomotor behavior. Locomotor performance initiates synchronized Ca2+ signals arising within the network of BG cells. The macroscopic flare events are correlated with blood perfusion levels, suggesting that they are sufficient to potentially regulate blood flow. Thus, the specific animal behaviors dictate Ca2+ excitability.
10.3.3 Effect of Ca2+ Signaling in Astrocytes on Neuronal Function and Cerebral Blood Flow In Vivo
From in vivo studies, Takata et al. reported that combined stimulation of mouse whisker and the nucleus basalis of Meynert (NBM), the principle source of cholinergic innervation to the cortex, enhances whisker-evoked local field potential (LFP) (Takata et al. 2011). This plasticity is dependent on Ca2+ increase in astrocytes and Ca2+ dependent D-Serine release from astrocytes as IP3R2 KO mice abolish the plasticity. In visual cortex, paired visual and electrical stimulation of the nucleus basalis (NB) induces significant potentiation of visual responses in the primary visual cortex in mice (Chen et al. 2012). By removing the cortical tissue, Navarrete et al. studied astrocyte-dependent plasticity in hippocampal CA3-CA1 synapses in vivo (Navarrete et al. 2012). Their results show that cholinergic-induced long-term potentiation (LTP) requires astrocyte Ca2+ elevation and Ca2+-dependent release of glutamate from astrocytes to act on mGluR5 presynaptic terminals. Thus, in addition to the wealth of information from in vitro studies, astrocytes are actively involved in synaptic plasticity through Ca2+ signaling in in vivo setting.
Astrocytic Ca2+ signaling has been shown to affect neuronal function and vasculature tone in brain slice preparations. Hence, Ca2+ elevation in astrocytes by neuronal afferent stimulation and photolysis of caged Ca2+ compound induced vasodilation (Stobart et al. 2013; Zonta et al. 2003), while other studies suggested that the polarity of astrocytic Ca2+-dependent regulation of blood flow was dictated by tissue metabolism of brain and retina slices (Gordon et al. 2008; Mishra et al. 2011). These studies suggest complex mechanisms of blood flow regulation by astrocytic Ca2+ signals (Attwell et al. 2010). In in vivo studies, photolysis of caged Ca2+ induced rapid dilation of the artery in the cortex (Takano et al. 2006). Motor behavior induced astrocytic Ca2+ flares were correlated with blood flow increase (Nimmerjahn et al. 2009). Odor stimulation induces mGluR5-dependent Ca2+ transients in astrocyte endfeet and an associated dilation of upstream arterioles. These processes are also dependent on cyclooxygenase activation (Petzold et al. 2008). Electrical stimulation of a forepaw induced vasodilation and astrocytic Ca2+ increase in the somato-sensory cortex (Nizar et al. 2013). However, the stimulus-induced vasodilation is independent of IP3R-mediated Ca2+ increase since IP3R2 KO mice exhibit normal functional hyperemia. In addition, the onset of vasodilation precedes astrocytic Ca2+ increase. The results suggest that functional hyperemia induced by forepaw stimulation is mainly neuron-dependent, and the astrocytic Ca2+ increase may not be sufficient enough to exert an additional effect on the onset of vasodilation. Similarly, inhibition of group I mGluRs does not affect transient hemodynamic responses following a brief whisker stimulation in rats under isoflurane anesthesia (Calcinaghi et al. 2011). The results suggest that group I mGluRs, regardless of their expression in neurons and/or astrocytes, do not play a role in early hemodynamic responses following sensory stimulation in rats.
10.4 Astrocytic Ca2+ Signaling in Ischemic Stroke and Hypoxia
10.4.1 Astrocytes in Stroke
Stroke causes severe brain damage and is the leading cause of human death and long-term disability. It has a major impact on public health. Cerebral ischemia accounts for approximately 80 % of all human strokes. Cerebral ischemia initiates from vasculature dysfunction (disease) and begins with the mechanical occlusion of blood vessels by thrombus or embolus that reduces or blocks blood flow. The degree to which blood flow is reduced largely determines the damage to the brain. Due to the loss of glucose and oxygen, synthesis of ATP through glycolysis and oxidative phosphorylation is impaired in the ischemic region. Thus, ischemia results in energy depletion, which leads to loss of ionic gradients and membrane potential depolarization in neurons and astrocytes. Consequently, this induces the release of gluta-mate and other neurotransmitters from presynaptic terminals to the extracellular space. The energy depletion further contributes to the malfunction of astrocytic and neuronal glutamate transporters, which are critical in clearing the glutamate released into the synaptic cleft. A variety of potential mechanisms by which ischemia leads to neuronal death and brain damage have been proposed from experimental studies of stroke. These mechanisms include glutamate and Ca2+ toxicity, oxidative stress, acidosis, inflammation, and mitochondrial dysfunction. Glutamate excitotoxicity, largely resulting from influx and intracellular overloading of Ca2+ through over-stimulation of glutamate receptors (especially NMDARs), is the primary mediator of acute neuronal death (Choi 1988). The excessive accumulation of glutamate in the extracellular space leads to the overstimulation of ionotropic and metabotropic glutamate receptors, especially ionotropic NMDARs, and results in pathological overloading of intracellular Ca2+ in neurons after a short latency period (Medvedeva et al. 2009; Tanaka et al. 1997). The overloading of intracellular Ca2+ during ischemia is linked (along with glutamate excitotoxicity) to the triggering of activations of downstream phospholipases and proteases that cause degradation of membranes and proteins and eventually neuronal death (Lo et al. 2003; Dirnagl et al. 1999). Thus glutamate excitotoxicity is the primary mediator of neuronal death; it does not only cause acute neuronal death, but also initiates molecular events that lead to delayed neuronal death and brain damage (Dirnagl et al. 1999).
After ischemia, the brain experiences temporal- and spatial-dependent changes of pathological processes. In the ischemic core region, cells die rapidly from the onset of ischemia due to severely impaired energy production and the ensuing breakdown of ionic homeostasis. In the penumbra, the region surrounding the core of a focal ischemic locus, the brain tissue is hypoperfused with collateral blood flow and energy metabolism is partially preserved. The partial energy reduction does not cause severe and terminal disruption of ionic gradients and immediate cell death. This region can completely or partially recovered after reperfusion, but can progress to infarction without treatment due to the ongoing excitotoxicity (Rossi et al. 2007). Astrocytes play an important role in reducing glutamate excitotoxicity by glutamate uptake through glutamate transporters in the acute phase of ischemia. However, in severe ischemia, reversed glutamate uptake could contribute to glutamate elevation (Rossi et al. 2000).
Compared with neurons, the role of astrocytes in ischemia is less understood. After the onset of ischemia, astrocytes undergo numerous pathological processes (Nedergaard and Dirnagl 2005; Barber and Demchuk 2003; Swanson et al. 2004). It is well known that glutamate transporters in astrocytes, which normally function to remove glutamate from the synaptic cleft to avoid glutamate toxicity, are impeded due to energy depletion. Glutamate can be even released from astrocytes by transporter reversal during in vivo and in vitro ischemia (Rossi et al. 2000; Phillis et al. 2000). This leads to extracellular glutamate elevations and aggravates glutamate excitotoxicity. Astrocytes rapidly swell after ischemia, to which a number of factors contribute (Kimelberg 2005; Zheng et al. 2010; Risher et al. 2012). Swelling gradually spreads outward from the ischemic core to the adjacent tissue, and eventually many of swollen astrocytes lyse, while astrocytes in the penumbral region exhibit reversible swelling (Zheng et al. 2010). In general, astrocytes are more resistant to ischemia than neurons (Lo et al. 2003) largely because they can use glycogen stores as an alternative energy source (Kasischke et al. 2004; Brown 2004; Rossi et al. 2007; Gurer et al. 2009). Most glycogen in adult brain is found in astrocytes, not in neurons (Brown 2004). Astrocytes can convert glycogen to lactate and pass lactate to neurons to supplement neuronal energy requirement during the pathological shortage of glucose (Brown and Ransom 2007). Astrocytes can also convert glycogen to glucose, thus delay energy depletion after ischemia through producing ATP by glycolysis (Brown 2004). Astrocytes may also provide glucose to neurons because both astrocytes and neurons express glucose transporters, thereby allowing neurons to delay their own ATP depletion by glycogen-derived glucose (Brown and Ransom 2007). Astrocytes are known to become reactive over time after ischemia, as characterized by an excessive expression of GFAP (i.e., astrogliosis) and eventually form a glial scar around the area of the injury (Nedergaard and Dirnagl 2005; Panickar and Norenberg 2005; Li et al. 2013; Barreto et al. 2011; Pekny and Nilsson 2005). Reactive astrocytes in glial scar could result from the upregulation of GFAP in existing astrocytes or newly generated astrocytes with a common feature of high GFAP expression levels (Barreto et al. 2011; Li et al. 2013). Glial scar formation is associated with substantial tissue shrink and morphological changes of reactive astrocytes (Fig. 10.3) (Li et al. 2013). Spontaneous recovery of brain injury in the chronic phase of ischemia may involve astrocyte reactivation (Cramer 2008).
Fig. 10.3.

Reactive astrocytes and the glial scar formation after ischemia. (a) GFAP staining images in the ipsilateral side of the brain 2 weeks after photothrombosis (PT). (b) is the high resolution image of the boxed region in (a). The dashed outline in (a) indicates the boundary of glial scar. IC ischemic core. Adapted from Li et al. (20 13)
Current strategies to treat ischemic stroke are aimed at restoring blood supply by administration of thrombolytic drugs, which is effective within a narrow window of about 3 h following ischemic stroke (Stapf and Mohr 2002; Huang and McNamara 2004). Thus, identifying novel therapeutic targets and elucidating cellular and molecular mechanisms by which ischemia induces neuronal death and brain damage are of great importance to provide effective therapeutic avenues. Although neuronal gluta-mate and Ca2+ excitotoxicity is widely acknowledged (Szydlowska and Tymianski 2010; Choi 1988), whether or how astrocytic Ca2+ is altered after ischemia has not been studied well. Since astrocytic Ca2+ signaling is the major feature of astrocytic excitability and due to the tripartite nature of synapse, Ca2+-dependent gliotransmitter release will expectedly affect neuronal excitability and toxicity in stroke.
10.4.2 Ca2+ Signaling in Astrocytes in In Vitro Stroke
Astrocytic Ca2+ signaling was studied after in vitro ischemia using cultured astrocytes, acutely isolated astrocytes and brain slices using an oxygen/glucose deprivation (OGD), a common in vitro ischemia model. Using live cell imaging, it was reported that 2 h OGD did not cause changes in cytosolic Ca2+ ([Ca2+]cyt) increase in cultured astrocytes, but led to a significant increase in endoplasmic reticulum Ca2+ ([Ca2+]ER) (Liu et al. 2010). However, cultured astrocytes exhibit [Ca2+]cyt increase about 2 h after reoxygenation (REOX) following 2 h OGD. Inhibition of IP3R with its specific blocker xestospongin largely blocked the delayed rise of [Ca2+]cyt, suggesting that the ER Ca2+ store is the source for post-OGD Ca2+ elevation in the cytosol. The study further showed that the delayed Ca2+ increase can be suppressed by the inhibition of Na+/Ca2+ exchanger (NCX) and Na+–K+–Cl- cotransporter isoform 1 (NKCC1), suggesting that the Ca2+ is provided from the extracellular space through the delivery by these transporters. Mitochondrial Ca2+ (Ca2+m) content was increased significantly within 15 min REOX followed by a secondary rise (~4.5-fold) and a release of mitochondrial cytochrome c (Cyt c). Cell death assay showed that the majority of cell death occurred during the 125–180 min of reoxygenation when the cytosolic Ca2+ dysregulation was triggered. These results illustrate that OGD/REOX triggers a time-dependent loss of Ca2+ homeostasis in the cytosol and organelles (ER and mitochondria) in astrocytes. Collective stimulation of NKCC1 and the reverse mode function of NCX contribute to these changes. These findings suggest that IP3R-mediated Ca2+ depletion in astrocytes may occur in ischemic brain, especially during the brain reperfusion, and exacerbate astrocyte damage.
In isolated astrocytes, OGD induces a small and slowly developing Ca2+ increase in the presence of extracellular Ca2+; this increase is completely reversible by the removal of extracellular Ca2+ (Duffy and MacVicar 1996). Thus, the results from isolated astrocytes can be interpreted by Ca2+ influx through functional Ca2+ channels.
Cultured and acutely isolated astrocytes cannot mimic tissue environment after ischemia with release of extracellular messengers including neurotransmitters. Acute brain slices provide a better preparation for studying Ca2+ signaling in astrocytes in ischemia. Duffy and Mac Vicar (1996) studied the presence of Ca2+ signals in hippocampal astrocytes in response to in vitro ischemia. To study Ca2+ signaling in astrocytes, astrocytes were loaded with a Ca2+ indicator using iontophoretic loading techniques. Using rat brain slices, they found that a short episode (5 min) of simultaneous hypoxia and hypoglycemia can induce intracellular Ca2+ increase within an average of 7.5 min and it took 2.5 min to reach a peak. After reoxygenation, astrocytic Ca2+ remained elevated for a highly variable period of time ranging from several minutes to 1 h. In the absence of extracellular Ca2+, Ca2+ increase in astrocytes could be still observed with a relative consistent duration. Electrophysiological recordings show that hypoxia and hypoglycemia also depolarize astrocytes (Duffy and MacVicar 1996). The data from brain slice experiments suggest that astrocytes can mediate Ca2+ increase from the internal store release and influx of voltage-dependent Ca2+ influx. Alternatively, internal Na+ accumulation could lead to Ca2+ influx through reversal operation of the NCX.
Another study found that OGD induced slow inward currents (SICs) mediated by extrasynaptic NMDA receptors in rat CA1 pyramidal neurons brain slices (Dong et al. 2013). Moreover, dialysis of the Ca2+ chelator BAPTA into astrocytic network decreased the frequency of OGD induced SICs, indicating that the activation of extrasynaptic NMDA receptors depended on astrocytic Ca2+ activity. Using two-photon Ca2+ imaging, it was found that astrocytes exhibited rare spontaneous Ca2+ elevation under control conditions, but frequent astrocytic Ca2+ elevations were observed during OGD. Strikingly, over 60 % of astrocytes displayed detectable Ca2+ elevations within the 10 min of OGD. In addition, most astrocytes displayed more than two transients. To further demonstrate the importance of astrocytic Ca2+ activity, hippocampal slices from IP3R2 knock-out mice were used. Astrocytes exhibited rare Ca2+ elevations during OGD and OGD induced lower frequency of SICs in hippo-campal CA1 neurons from IP3R2 knock-out mice than that from wild-type mice.
10.4.3 In Vivo Ca2+ Signaling in Astrocytes in Acute Stroke
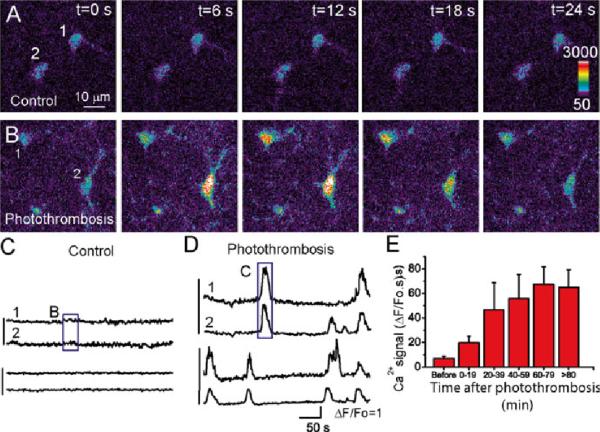
Although in vitro studies on astrocytic Ca2+ signaling using cultured astrocytes and brain slice preparations with OGD model can derive insights into the role of astrocytes in neuronal injury in ischemia, in vivo study using live animals is essential to elucidate the mechanism of astrocytic Ca2+ signaling in ischemia. Photothrombosis (PT)-induced ischemia model has been used for in vivo imaging of neuronal and astrocytic responses because of the following advantages (Zhang et al. 2005; Winship and Murphy 2008; Ding et al. 2009). First, the surgical procedures of PT are relatively easy and noninvasive. Second, ischemia can be induced using light illumination through a microscopy objective and 2-P in vivo Ca2+ imaging can be performed conveniently after ischemia. Third, the location of ischemic core and penumbral region can be controlled and determined easily (Ding et al. 2009; Risher et al. 2010, 2012; Winship and Murphy 2008; Zhang et al. 2005). Using this model, Ding et al. studied in vivo astrocytic Ca2+ signaling in urethane-anesthetized adult mice using 2-P microscopy (Ding et al. 2009). In the study, they found that astrocytes exhibit enhanced Ca2+ signaling characterized as intercellular Ca2+ waves starting ~20 min after PT and Ca2+ signals reach the plateau 60 min after PT (Fig. 10.4). Both amplitude and frequency of PT-induced astrocytic Ca2+ signals were significantly increased as compared to relative quiescent Ca2+ signaling prior to ischemia. An important feature of the Ca2+ signals was that most of them initiated and returned to the basal level at the same time among astrocytes in the imaging field, i.e., they were highly synchronized (Fig. 10.4d). This was confirmed by a large cross correlation coefficient between adjacent astrocyte under ischemic condition (Ding et al. 2009). To further determine the nature of astrocytic Ca2+ signals in ischemia, antagonists for GPCRs including mGluR5, GABABR and P2Y receptors were applied after emergence of Ca2+ signals. 2-methyl 6 (phenylethynyl)pyridine hydrochloride (MPEP), an antagonist of mGluR5, significantly reduced Ca2+ signals (~62 %), while CGP54626, the antagonist of GABAB receptor, also significantly reduced Ca2+ signals (~55 %). When MPEP and CGP54626 were co-administered, the astrocytic Ca2+ signals were reduced. However, suramin, a non-specific inhibitor for P2Y receptors, did not reduce the PT-induced Ca2+ signals. Pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), a general P2 receptor antagonist, did not attenuate the Ca2+ signal either. Similarly, inhibition of adenosine receptor A1 by its antagonist 8-cyclopentyltheophylline (CPT) did not inhibit PT-induced astrocytic Ca2+ signals. The pharmacological study suggests that glutamate and GABA are likely to be released to the extracellular space following PT to stimulate intercellular Ca2+ waves in astrocytic network. It is surprising that neither P2Y nor A1 receptor contributes to the enhanced Ca2+ signaling after PT, presumably due to the rapid degradation of ATP and adenosine by enzymes such as ectonucleotidases after ischemia (Dunwiddie and Masino 2001). The results represent the first direct study of astrocytic Ca2+ response after ischemia in in vivo setting. In this study, overloading of astrocytic Ca2+ was not observed; this is probably because the recording time was too short, so that Ca2+ homeostasis was not disrupted. In future study, it will be interesting to compare the time course of neuronal and astrocytic Ca2+ signaling and overloading after ischemia as neuronal Ca2+ overloading is one of the major events that causes neuronal excitotoxicity followed by neuronal death.
Fig. 10.4.
Enhanced Ca2+ signaling in astrocytes in the mouse cortex in vivo after photothrombosis. (a, b) Represent images of astrocytes loaded with ftuo-4 before (a) and after (b) photothrombosis. (c, d) Time courses of somatic Ca2+ oscillation of individual astrocytes expressed as ΔF/Fo before (c) and after photothrombosis (d). The box regions correspond to the images in (a) and (b) as indicated. (e) Time course of somatic Ca2+ signal in astrocytes developed after photothrombosis. Adapted from Ding et al. (2009)
10.4.4 In Vivo Ca2+ Signaling in Astrocytes in Chronic Stroke
In addition to the acute changes in astrocytic Ca2+ signaling, the responses of astrocytes are also altered in the chronic phase after ischemia. The functional recovery starts 2 weeks after ischemia in both ipsilateral and contralateral hemispheres. Winship et al. studied the neuronal and astrocytic Ca2+ signaling and functional rewiring in somatosensory neurons in ipsilateral hemisphere after stroke (Winship and Murphy 2008). Astrocytic Ca2+ responses were examined in anesthetized mice to study whether the prevalence of these responses was altered in peri-infarct cortex half, one, and 2 months after PT. Increased astrocyte responses to both preferred and nonpreferred limbs after stroke were observed. Data analysis revealed that there was a significant increase in the prevalence of responses to preferred limb stimulation in the primary somatosensory cortex. The selectivity of astrocyte responses between regions of overlap between contralateral hindlimb- and contralateral forelimb-evoked intrinsic optical signals and regions without intrinsic optical signals overlap were also significantly different. These results suggest that mechanisms for rapid neuron–astrocyte communication are preserved, or even enhanced, after stroke in the penumbra.
It is known that ischemia also causes changes at molecular level in contralateral hemisphere. To study the role of astrocytes in the functional recovery in contralateral hemisphere, Takatsuru et al. performed in vivo Ca2+ imaging to examine the neuronal and astrocytic Ca2+ responses in the region contralateral to the site of stroke at different times following PT (Takatsuru et al. 2013). The results showed that the number of astrocytes with a response to limb stimulation was small in the sham group, but in the stroke groups the number was significantly increased in the contralateral somatosensory cortex responding to ipsilateral limb stimulation at the first and second week after infarction. A significantly larger number of astrocytes responded only to the single-limb stimulation in the sham group as compared with stroke groups, but a smaller number of astrocytes responded to multiple-limb stimulation in the sham group as compared to the stroke groups. Interestingly, unlike neurons, astrocytes showed no preference in response to contralateral and ipsilateral limb stimulation (Takatsuru et al. 2013). The peak amplitude of Ca2+ response was also increased in stroke groups. Microdialysis study showed a large increase in glutamate concentration 2 weeks after the stroke compared with the sham and 1-week group; however, glutamine concentration was much higher in the contralateral side in the 1-week group than in the sham and the 2-week group. Further study suggested that astrocytic plasma membrane glutamate transporter 1 (GLT-1) may contribute to the uptake of glutamate in the 1-week groups. Together, these findings demonstrate that activated astrocytes increase the uptake of glutamate by glutamate transporters during the first week, and indicate that astrocytes play an important role in functional recovery and cortical remodeling in the area contralateral to ischemic lesion in the post-ischemic period.
10.4.5 Ca2+ Signaling in Astrocytes in Hypoxia
Hypoxia is a pathological condition in which the body or a region of the body has reduced oxygen supply that is insufficient to maintain cellular functions (Howard et al. 2011). The brain is the most hypoxia-sensitive organ because of its need for high oxygen supply. Hypoxia does not cause severe brain injury provided the system circulation is preserved, but it can cause malfunction of neurons and astrocytes. BG cells are a special type of astrocytes in the cerebellum and they exhibit radially transglial Ca2+ waves mediated by P2Y receptors (Hoogland et al. 2009). A recent study found that ATP-induced Ca2+ increase in BG cells was correlated with the reduction of local tissue O2 tension in the cerebellum (Mathiesen et al. 2013). The incidence and frequency of spontaneous Ca2+ waves were significantly higher in aging mice than those in adult mice. Furthermore, the aging mice had lower basal O2 tension than adult mice, but they had comparable blood oxygen saturation. The results suggest that aging-increased incidence and frequency of Ca2+ waves are correlated with decreased tissue O2 tension. Whether the increase in the frequency of Ca2+ waves could be mimicked by hypoxia was further studied. Indeed, acute hypoxia of the reduction of O2 tension by 20 % for as short as 20 min could increase spontaneous Ca2+ wave in BG. The study suggests that the cortical O2 tension may play a role in inducing glial Ca2+ waves in aging mice, and ATP levels in brain tissue might be higher in aging brain. Whether the increased Ca2+ waves in BG is beneficial or detrimental to neurons is unclear and the mechanisms of hypoxia-induced increase in Ca2+ signal in BG cells need to be further studied.
10.4.6 The Mechanisms and Implications of Ca2+ Signaling in Astrocytes in Stroke
All the aforementioned in vivo studies on astrocytic Ca2+ signaling in ischemia used the PT model. Usually, the infarction by PT was much smaller than that using middle cerebral artery occlusion (MCAO) model. Given the different severities of brain injury, it is likely that the responses of astrocytes and neurons to ischemic insult will differ between the two models. Due to the complicated surgical procedure, it is difficult to perform in vivo imaging on mice using MCAO model to study astrocyte and neuronal Ca2+ signaling before, during, and after ischemia although it is still feasible to study their structural changes because of its low temporal resolution (Li and Murphy 2008).
The mechanisms of increased Ca2+ waves in ischemia and hypoxia might be different. Ischemia can cause the release of a large amount of glutamate or GABA and immediate cell death in the brain; hence, mGluR5 and GABABR can play a major role in astrocytic Ca2+ signaling after ischemia (Ding et al. 2009). Compared with ischemia, hypoxia does not cause immediate neuronal death. The study from BG cells suggests that hypoxia can release ATP to induce Ca2+ hyperexcitability in astrocytes. On the other hand, glial Ca2+ can cause a reduction of O2 tension in the brain, which means that the glial Ca2+ waves are closely related to brain energy metabolism in hypoxia. In chronic phase of ischemia, the altered response of astrocytic Ca2+ signaling to limbic stimulation in contralateral hemisphere suggests the change of astrocytic Ca2+ plasticity after ischemia and may contribute to brain remodeling in the recovery process. In summary, elucidation of the mechanisms of Ca2+ signaling in astrocytes may provide insights for potential therapeutic targets in stroke.
Synchronized and intercellular Ca2+ waves in astrocytes were also observed in other experimental disease models in the CNS. Using pilocarpine-induced SE model and 2-P in vivo imaging, Ding et al. found that 3 days following SE a significant increase in astrocytic Ca2+ oscillation was observed in mice that had entered SE compared to the control mice which either received a saline injection or sub-threshold injection of pilocarpine (Ding et al. 2007). This prolonged enhancement of Ca2+ excitability was detected at day 2 and 3 following SE, then returned to control levels thereafter (Ding et al. 2007). They further found that mGluR5 antagonist MPEP (1 mg/kg weight) injected through the tail vein significantly reduced the astrocytic Ca2+ oscillations 3 days following SE.
A recent study by Choo et al. showed that traumatic brain injury (TBI) using a micromechanical impact of exposed cortex to a depth of 150–200 μm immediately elicits Ca2+ waves in astrocytes in vivo (Choo et al. 2013). The Ca2+ increase can be inhibited by apyrase that hydrolyzes ATP to AMP and inorganic phosphate, but not by flufenamic acid, a gap junction inhibitor. The results demonstrated that mechanical impact to astrocytes in vivo triggers ATP-mediated Ca2+ waves, which propagated beyond the initial epicenter of mechanical trauma.
Astrocytes also exhibit altered Ca2+ signaling in in vitro and in vivo Alzheimer's disease models. In neuronal-glial co-culture, application of Aβ triggers Ca2+ elevation in astrocytes that is initiated from a focal rise (Abramov et al. 2003). The Ca2+ elevation can maintain for a long period in the presence of Aβ. In transgenic APP/PS1 mice which express mutant human Aβ precursor protein (APP) and mutant presenilin (PS1) in neurons, but not in astrocytes, it was found that the resting astrocytic Ca2+ in mice with plaques is higher than in wild-type mice; nevertheless, the resting Ca2+ concentration is independent of the proximity of astrocytes to the plaques (Kuchibhotla et al. 2009). Remarkably, the frequency of spontaneous astrocytic Ca2+ activity in mice with cortical Aβ deposition is much higher than in younger mice before developing senile plaques or wild-type mice. The increased spontaneous Ca2+ activity is also independent on the plaque proximity.
The common observation in these disease models is that the cortical astrocytes exhibit increased frequency of Ca2+ elevation characterized with synchronized intercellular Ca2+ waves in astrocytic network. Such dramatic changes may represent the micro-environmental changes in brain tissue. Thus, astrocytes exhibit distinct Ca2+ signaling in health versus diseases. Although the patterns of Ca2+ responses in the discussed disease models are similar, the mediators of the synchronized Ca2+ waves might be different. Precipitating invasive brain injury after stroke, epilepsy, and TBI could cause release of large amount of glutamate and other released neurotransmitters to stimulate GPCR in astrocytes and induce enhanced Ca2+ hyperexcitability in astrocytes. This was supported by results where mGluR5 antagonist was shown to inhibit Ca2+ excitability and reduce neuronal deaths after SE (Ding et al. 2007) and PT (Ding et al. 2009), and P2Y1 antagonist was shown to reduce neuronal loss in the CA3 subregion of the hippocampus after TBI (Choo et al. 2013). In a less severe condition such as hypoxia, released ATP from brain tissue may induce astrocytic Ca2+ waves (Mathiesen et al. 2013). In chronic neurodegenerative diseases, the proinflammatory factors released from microglia and astrocytes might be potential candidates. In AD mice, Aβ-plaques or plaque-induced or -associated bioactive species might induce intercellular Ca2+ waves in astrocytes (Kuchibhotla et al. 2009); on the other hand, the modification of the proteins that maintain Ca2+ homeostasis might change the resting Ca2+ levels in astrocytes. Candidate proteins for this change include NCX, P2X receptors, and TRP channels (Reyes et al. 2012; Shigetomi et al. 2012; Shirakawa et al. 2010).
Although enhanced astrocytic Ca2+ excitability is found in different disease models, the observation of this phenomenon can be dependent on various factors. For example, the time from disease onset and the distance from disease core will likely affect the magnitude and frequency of Ca2+ increase in astrocytes. The enhanced intercellular Ca2+ waves in astrocytes could cause the release of vast amounts of gliotransmitter in disease; hence, Ca2+-dependent gliotransmission might impose a detrimental effect on neuronal excitotoxicity, and inhibition of astrocytic Ca2+ signals could represent a potential target for therapeutic intervention in the treatment of brain disorders.
Footnotes
Conflict of interest The author declares no conflict of interest.
References
- Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD, Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Ward ill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SSH, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber PA, Demchuk AMH. Biochemistry ischemic stroke. Adv Neural. 2003;92:151–164. [PubMed] [Google Scholar]
- Barres BA, Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Sun X, Xu L, Giffard RG. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS One. 2011;6:e27881. doi: 10.1371/journal.pone.0027881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE, Bergles DE, Diamond JS, Jahr CE. Clearance of gluta-mate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–127. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Calcinaghi N, Jolivet R, Wyss MT, Ametamey SM, Gasparini F, Buck A, Weber B. Metabotropic glutamate receptor mGluR5 is not involved in the early hemodynamic response. J Cereb Blood Flow Metab. 2011;31:e1–e10. doi: 10.1038/jcbfm.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles KJ, Deuchars J, Davies CH, Pangalos MN, Charles KJ, Deuchars J, Davies CH, Pangalos MN. GABA B receptor subunit expression in glia. Mol Cell Neurosci. 2003;24:214–223. doi: 10.1016/s1044-7431(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA. 2012;109:E2832–E2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;I:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choo AM, Miller WJ, Chen YC, Nibley P, Patel TP, Goletiani C, Morrison B, Kutzing MK, Firestein BL, Sui JY, Haydon PG, Meaney DF. Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain. 2013;136:65–80. doi: 10.1093/brain/aws286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JHC, Lopez-GarCia JC, Naus CCG, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neuro. 2008;l63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ding S. Methods Mol Biol. Vol. 814. Humana Press; 2012. In vivo imaging of Ca2+ signaling in astrocytes using two-photon laser scanning fluorescent microscopy. pp. 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. In vivo astrocytic Ca2+ signaling in health and brain disorders. Future Neurol. 2013;8:529–554. doi: 10.2217/fnl.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia. 2009;57:767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doengi M, Hirnet D, Coulon P, Pape HC, Deitrner JW, Lohr C. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci U S A. 2009;106:17570–17575. doi: 10.1073/pnas.0809513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Mac Vicar BA. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. J Neurosci. 1996;16:71–81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Wyss MT, Weber B. Somatotopic astrocytic activity in the somatosensory cortex. Glia. 2013;61:601–610. doi: 10.1002/glia.22458. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurer G, Gursoy-Ozdemir Y, Erdemli E, Can A, Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol. 2009;19:630–641. doi: 10.1111/j.1750-3639.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: Listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle DN, Yeckel MF, Hertle DN, Yeckel MF. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience. 2007;150:625–638. doi: 10.1016/j.neuroscience.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G, Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw LA, Pandhit S, Bare DJ, Mignery GA, Russell IT, Holtzclaw LA, Pandhit S, Bare DJ, Mignery GA, Russell JT. Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia. 2002;39:69–84. doi: 10.1002/glia.10085. [DOI] [PubMed] [Google Scholar]
- Hoogland TM, Kuhn B, Gobelc W, Huang W, Nakai J, Helmchen F, Flint J, Wang SSH. Radially expanding trans glial calcium waves in the intact cerebellum. Proc Natl Acad Sci U S A. 2009;106:3496–3501. doi: 10.1073/pnas.0809269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RS, Holmes PA, Koutroumanidis MA. Hypoxic-ischaemic brain injury. Pract Neurol. 2011;11:4–18. doi: 10.1136/jnnp.2010.235218. [DOI] [PubMed] [Google Scholar]
- Huang Y, McNamara JO. Ischemic stroke: “acidotoxicity” is a perpetrator. Cell. 2004;ll8:665–-666. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE, Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Illes P, Verkhratsky A, Burnstock G, Franke H. P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist. 2012;18:422–438. doi: 10.1177/1073858411418524. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, SanteHo M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10(33):1–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–505. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28:11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang N, Sun G, Ding S. Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN Neuro. 2013;5:195–207. doi: 10.1042/AN20130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde CI, Baryshnikov SG, Mazzocco-Spezzia A, Golovina VA. Dysregulation of Ca2+ sig naling in astrocytes from mice lacking amyloid precursor protein. Am J Physiol Cell Physiol. 2011;300:C1502–C1512. doi: 10.1152/ajpcell.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kintner DB, Begum G, Algharabli J, Cengiz P, Shull GE, Liu XJ, Sun D. Endoplasmic reticulum Ca2+ signaling and mitochondrial Cyt c release in astrocytes following oxygen and glucose deprivation. J Neurochem. 2010;114:1436–1446. doi: 10.1111/j.1471-4159.2010.06862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V, Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC l channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP. Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci. 2013;33:3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Brazhe A, Thomsen K, Lauritzen M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab. 2013;33:161–169. doi: 10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. ProcNatl Acad Sci US A. 2011;108:17827–17831. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the glio-transmitter D-serine. Proc Natl Acad Sci US A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, de Sevilla DF, Gomez-Gonzalo M, Nufiez A, Martfn ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Bio1. 2012;10:e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci 2. 2001;1:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Ronnback L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54:605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1(3):1–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim N, Goldman S, Nedergaard M. Heterogeneity of astrocytic form and function. In: Milner R, editor. Astrocytes. Humana Press; New York: 2012. pp. 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Wada M, Wu Q, Yamamoto A, Fujita T. Functional expression of metabotropic GABAB receptors in primary cultures of astrocytes from rat cerebral cortex. Biochem Biophys Res Commun. 2006;341:874–881. doi: 10.1016/j.bbrc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2Xl/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium. 2010;48:225–231. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general onsiderations. Glia. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons.[see comment]. Proc Natl Acad Sci US A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sui JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN, Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood How in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Ren J, O'Regan MH. Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex: studies with DL-threo-beta-benzyloxyaspartate. Brain Res. 2000;868:105–112. doi: 10.1016/s0006-8993(00)02303-9. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Porter IT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong QP, He JQ, Chai Z. Astrocytic Ca2+ waves mediate activation of extrasynaptic NMDA receptors in hippocampal neurons to aggravate brain damage during ischemia. Neurobiol Dis. 2013;58:68–75. doi: 10.1016/j.nbd.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012;4:e00075. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. TRPC1-mediated Ca2+ and Na+ signalling in astroglia: differential filtering of extracellular cations. Cell Calcium. 2013;54:120–25. doi: 10.1016/j.ceca.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci. 2010;30:9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Croom D, Kirov SA. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia. 2012;60:1709–1720. doi: 10.1002/glia.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kuga N, Namiki S, Matsuki N, Ikegaya Y. Locally synchronized astrocytes. Cereb Cortex. 2011;21:1889–1900. doi: 10.1093/cercor/bhq256. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabo-tropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. J Neurosci Res. 1999;58:533–543. doi: 10.1002/(sici)1097-4547(19991115)58:4<533::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA, Sharp AH, Nucifora FO, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPAl channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adena-associated viruses. J Gen Physiol 14. 2013;1:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, Kusano A, Hashimoto E, Nakagawa T, Kaneko S. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapf C, Mohr JP. Ischemic stroke therapy. Annu Rev Med. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Lu L, Anderson HDI, Mori H, Anderson CM. Astrocyte-induced cortical vaso-dilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2013;110(8):3149–54. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. [Review] [241 refs]. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood How. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One. 2008;3:e2525. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuru Y, Eto K, Kaneko R, Masuda H, Shimokawa N, Koibuchi N, Nabekura J. Critical role of the astrocyte for functional remodeling in contralateral hemisphere of somatosensory cortex after stroke. J Neurosci. 2013;33:4683–4692. doi: 10.1523/JNEUROSCI.2657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Ago Y, Matsuda T. The glial sodium-calcium exchanger: a new target for nitric oxide-mediated cellular toxicity. Curr Protein Pept Sci. 2013;14:43–50. doi: 10.2174/1389203711314010007. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yamamoto S, Kudo Y, Mihara S, Higashi H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CAl neurons in vitro. J Neurophysiol. 1997;78:891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A. 2012;109:18974–18979. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GFH. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Takano T, Lin JH, Wang X, Bekar L, Nedergaard M, Tian GF, Takano T, Lin JHC, Wang X, Bekar L, Nedergaard M. Imaging of cortical astrocytes using 2-photon laser scanning microscopy in the intact mouse brain. Adv Drug Deliv Rev. 2006;58:773–787. doi: 10.1016/j.addr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist. 2013;19:274–291. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. J Neurosci. 2008;In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke.28:6592–6606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adena-associated viral transduction. Neuroscience. 2010;170:992–1003. doi: 10.1016/j.neuroscience.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw O, Zeng H, Looger LL, Svoboda K, Chen TW. ACre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, Grass EH, Walter CA, Lechleiter JD. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5:el4401. doi: 10.1371/journal.pone.0014401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K+]o uptake capabilities. J Neurophysiol. 2000;84:2746–2757. doi: 10.1152/jn.2000.84.6.2746. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]