Abstract
Allogeneic stem cell transplantation (alloSCT) can overcome the adverse prognosis of chronic lymphocytic leukemia with 17p deletion (17p- CLL). However, its applicability remains unclear. Since 2007, our leukemia service has referred 17p- CLL patients for alloSCT at presentation. In this study, the outcomes of these patients were reviewed retrospectively to determine whether they underwent alloSCT and why patients did not undergo alloSCT. Fifty-two patients with 17p- CLL, who were referred to the transplant service from 2007 to 2010, were identified. Of these patients, 32 (62%) patients did not undergo alloSCT, mainly because of treatment- or disease-related complications (n=15). The 2-year post-referral overall survival rates of the alloSCT and non-SCT groups were 64% and 25%, respectively (p = 0.001). These findings suggest that while alloSCT is an effective therapy in 17p- CLL patients, pre-SCT complications may preclude a significant proportion of patients from undergoing the procedure.
Keywords: CLL, 17p-, allogeneic transplantation
Introduction
The clinical course of chronic lymphocytic leukemia (CLL) varies, with survival durations after diagnosis ranging from months (in patients with aggressive disease) to years (in those with indolent disease) [1, 2]. In addition to classic prognostic markers such as staging or lymphocyte doubling time, new markers have been developed that have important prognostic significance. Of these, karyotypic and genetic abnormalities are a principal predictor of relapse-free survival and overall survival (OS). In particular, 17p deletions and TP53 mutations have been associated with significantly worse overall response rates and a shorter duration of response compared to conventional chemotherapy [1–3]. Additionally, the median progression-free survival (PFS) and OS rates are significantly lower in this group of patients.
Allogeneic stem cell transplantation (alloSCT) has been advocated for 17p- CLL patients [4] and has been found in several studies to induce survival plateaus in approximately 40% to 50% of cases [5–8]. However, selection bias may lead to only the “best” patients with 17p- abnormalities being selected for alloSCT: patients in some of these studies are generally selected for age, performance status, and remission status, which may lead to skewed outcomes. Prospective comparative studies of alloSCT and chemotherapy are difficult, because of the relative rarity of 17p deletion in CLL. The number of 17p- CLL patients who are eligible for alloSCT after standard treatment and the outcomes of those who do not undergo alloSCT are unknown.
Since 2007, the leukemia service at our center has referred all 17p- CLL patients for alloSCT consultation at the time of presentation. In this study, a retrospective review was performed to describe the outcomes of all patients who were referred to the transplant service between 2007 and 2010. Patients were reviewed to determine whether they underwent alloSCT and the reasons for not undergoing alloSCT. Demographic, clinical characteristics and survival rates were compared for those who underwent alloSCT versus those who did not proceed to transplantation.
Patients and methods
Patients
All patients with 17p- CLL who were referred to the transplant service were identified through a SCT database from September 2007 through December 2010. Demographic and clinical data were obtained from electronic medical records. The SCT service initiated a search for donors for all patients who were suitable candidates for alloSCT. Patients then underwent conventional therapy for reduction of disease in preparation for alloSCT after treatment. Patients were considered refractory to conventional therapy if they experienced progression while on treatment, or had less than 50% reduction of lymph nodes’ size and bone marrow involvement. There was no limit on the number of lines of treatment patients could receive pre-SCT. Patients who remained refractory to conventional treatments could proceed to alloSCT if they had lymph nodes’size of <5 cm.
Post-SCT responses were scored according to the recommendations of the National Cancer Institute-Sponsored Working Group [9]. Disease extent had been assessed by computed tomography scans of the chest, abdomen, and pelvis and positron emission tomography (PET) scans. Lymph node biopsies were undertaken, whenever possible, in patients with PET-positive scans and lactate dehydrogenase (LDH) levels to rule out the possibility of transformation. Patients were evaluated 1, 3, 6, and 12 months after alloSCT and every 6 months thereafter.
Approval for the data review as well as waiver of informed consent was obtained from our institutional review board.
Fluorescence-in-situ-hybridization (FISH)
Interphase FISH studies were performed on 200 nuclei obtained from freshly collected bone marrow samples after culturing cells for 24 hours without stimulation, using the Vysis CLL probe panel (Vysis) according to the manufacturer’s recommendations. The panel includes probes specific to TP53 (17p13.1), ATM (11q22.3), D13S319 (13q14.3),
LAMP1 (13q34), and the centromeric region of chromosome 12 (12p11.1-q11).
Statistical analysis
The median, minimum, and maximum of each continuous variable and the counts and percentages of each categorical variable by transplant status were tabulated. The Wilcoxon rank-sum test was used to assess the association between transplant status and continuous variables, with p-values computed using the normal approximation. Fisher’s exact test and its generalizations were used to assess the association between transplant status and categorical variables. Kaplan-Meier survival curves and univariate Cox proportional hazards regression analysis were used to estimate survival and the effects of clinical and demographic variables on survival. The cumulative incidence of graft-versus-host disease (GVHD) was estimated using the method of Fine and Gray [10] accounting for the competing risk of death. All statistical analyses were performed using SAS software version 9.2 for Windows (SAS Institute, Inc., Cary, NC) and R version 2.13.0 (The R Foundation for Statistical Computing). P values < 0.05 were considered statistically significant.
Results
AlloSCT consultations and rates
Fifty-two patients were referred to the transplant service from 2007 to 2010. At the time of referral, 21 (40%) patients were untreated, and 31 (60%) had undergone prior therapy with conventional chemotherapy or immunotherapy. All patients met established criteria to initiate therapy for CLL [9]. Twenty (38%) of these patients underwent alloSCT, and 32 (62%) did not.
Reasons for not undergoing alloSCT
Thirty-two patients in our study were not transplanted due to various reasons. Fifteen (47%) had persistently refractory disease or died of complications after salvage chemotherapy or disease progression. On further follow-up with the transplant service, we found that a donor had been identified for 12 of these patients (related donor = 6, matched unrelated donor = 5, and cord blood = 1). Further chemotherapy was given for cytoreduction of disease burden. The main cause of death in these patients was bacterial or fungal in origin sepsis after chemotherapy (n = 13); 2 died of disease-related complications or progression.
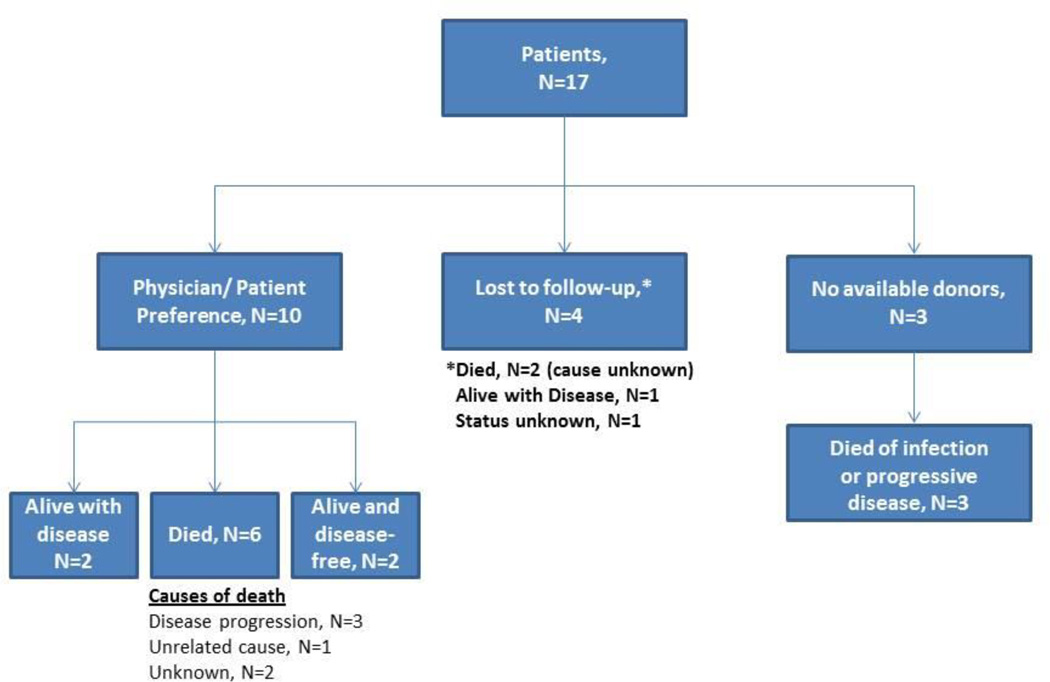
Ten (31%) patients did not undergo alloSCT because of referring physician or patient preference (Figure 1); these patients were newly diagnosed and had disease that was sensitive to salvage chemo-immunotherapy including four patients with complete responses to chemotherapy. Three (9%) other patients experienced a partial response to initial therapy but had no matched sibling or unrelated donors and experienced disease progression while awaiting further searches. Four patients (13%), three of whom had experienced disease response and one who did not receive any further treatment here were lost to follow up.
Figure 1.
Outcomes of patients (n = 17) who did not undergo alloSCT for reasons other than disease progression or treatment-related complications.
The median time from referral to alloSCT was 9.8 months (range, 3–20.5 months). The median follow-up durations from diagnosis and referral for all patients were 5.3 years (range, 0.7–22 years) and 1.5 years (range, 0.1–4.8 years), respectively. The median follow-up duration from alloSCT was 1.6 years (range, 0.3–3.4 years).
Treatment
The preparative alloSCT regimen used depended on institutional protocols at the time of transplant. Nine (45%) patients underwent reduced intensity conditioning, whereas 11 underwent non-myeloablative conditioning. The predominant non-myeloablative preparative regimen included 30 mg/m2 fludarabine and 750 mg/m2 of cyclophosphamide given intravenously on days −5 to −3 before transplantation, and 375 mg/m2 of rituximab on day −13 and 1000 mg/m2 rituximab on days −6, −1, and +8, as previously described [7]. One patient was treated with thymoglobulin and total lymphoid irradiation. The 9 patients with reduced-intensity conditioning received melphalan, fludarabine, rituximab (n = 4), carmustine, etoposide, cytarabine, and melphalan,(n = 3); or fludarabine and busulfan (n = 2).
Nine patients received grafts from human leukocyte antigen-histocompatible siblings. Another 9 received transplants from matched unrelated donors, 1 received a cord blood transplant, and one received a transplant from a haploidentical donor. The median CD34 dose was 6×106/kg (range, 1.3–16.0). GVHD prophylaxis consisted of tacrolimus and mini-methotrexate (n = 18) or mycophenolate mofetil (n = 2). Supportive care was provided as previously described [7].
The 32 patients who did not undergo alloSCT continued to undergo chemotherapy, depending on disease response; the regimens were determined by their primary oncologists. They underwent a median of 4 (range, 1–14) lines of treatment.
Comparison between alloSCT and non-SCT patients
Table I summarizes the patients’ characteristics. There were no significant differences observed between the groups who underwent alloSCT and those who did not. This includes age at diagnosis (p = 0.10) or at time of referral (p = 0.20). There were also no significant differences in disease-related features, in including the percentage of 17p- CLL diagnosed before initial therapy (“de novo 17p deletion”), and not due to the effects of treatment. The median percentage of nuclei with 17p- was also similar in both groups.
Table I.
Patient Characteristics
| Characteristic | No-SCT Group |
AlloSCT+ Group |
p-value |
|---|---|---|---|
| No. of patients | 32 | 20 | |
| Age at diagnosis, years (range) | 55 (41–68) | 49 (35–69) | 0.10 |
| Age at referral, years (range) | 60 (42–71) | 54 (37–72) | 0.20 |
| FISH 17p deletion: de novo/acquired, n (%) | 16 (50)/16(50) | 7(35)/13(65) | 0.39 |
| Median FISH 17p deletion, (range) | 71 (8–100) | 68.5 (10.5–96) | 0.63 |
| Male sex, n (%) | 23 (66) | 15 (75) | 0.55 |
| Number of prior chemotherapy regimens | 4 (1–10) | 4 (1–14) | 0.92 |
| Median β2-microglobulin at referral (range) | 4.4 (2.3–14.7) | 3.8 (2.0–9.4) | 0.53 |
| ZAP 70 positive, n (%) | 21 (75) | 16 (89) | 0.49 |
| IGHV, unmutated, n (%) | 20 (77) | 13 (93) | 0.39 |
| Additional cytogenetics abnormalities, n (%) | 15 (50) | 11 (55) | 0.78 |
| Richter’s transformation, n (%) | 8 (25) | 3 (15) | 0.5 |
| Secondary MDS, n (%) | 1 (3) | 3 (15) | 0.29 |
| PET positive at referral, (no. tested, %) | 16/16 (100) | 5/5 (100) | NA |
Legend: IGHV: Immunoglobulin heavy chain variable, MDS: Myelodysplastic syndrome, PET: Positron emission tomography
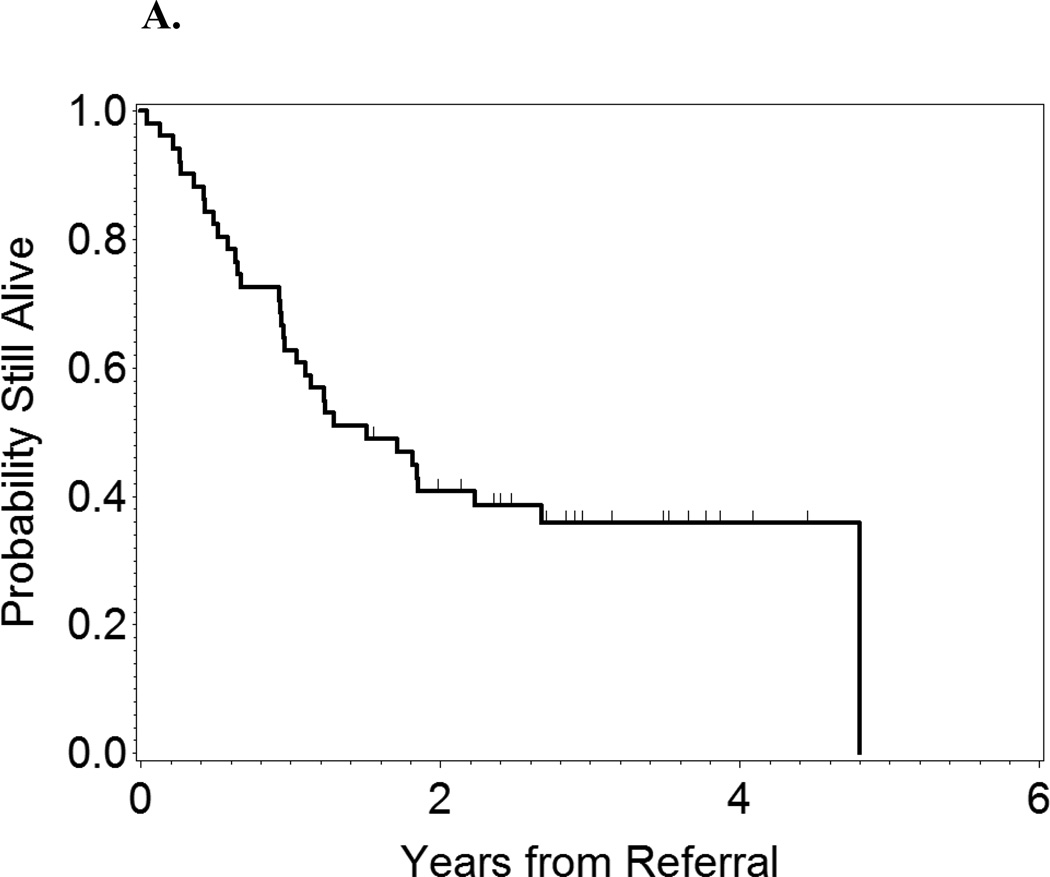
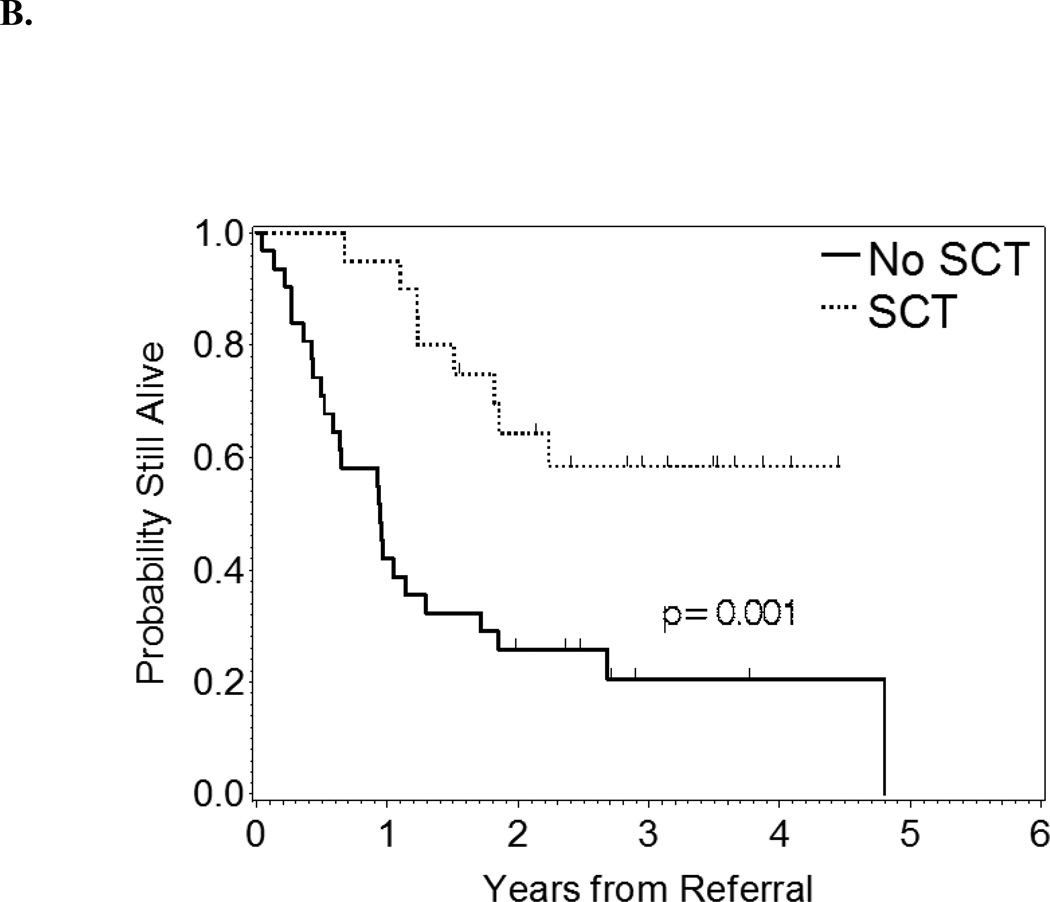
At 2 years after referral, the OS rate of the entire group of patients was 40.5%. Patients who underwent alloSCT had longer survival durations from the time of referral than did those who did not (p = 0.001, Figure 2). At 2 years after referral, the OS rate in alloSCT patients was 64% compared with 25% in non-SCT patients.
Figure 2.
A: OS from time of referral of all patients.
B: OS from time of referral by alloSCT status.
Clinical outcomes in alloSCT patients
Nineteen alloSCT patients experienced donor cell engraftment, and 1 experienced secondary graft failure secondary to a herpes simplex virus 6 infection and later had an autologous hematopoietic recovery. More than half the alloSCT patients had advanced and heavily pre-treated disease at the time of SCT. Immunomanipulation with tacrolimus withdrawal and donor lymphocyte infusion with rituximab were provided, as previously described [7] in 8 of 20 patients after SCT because of persistent (n = 4) or progressive disease (n = 4).
We analyzed patient and disease characteristics to determine whether they were associated with OS in alloSCT patients. In univariate comparisons, age > 60 years (p = 0.04), β-2 microglobulin level > 4 mg/L at SCT (p = 0.02), number of prior therapies (p = 0.02), bulky disease (> 5 cm) at SCT (p = 0.03), CD34 level of ≤ 5×106 CD34 cells/kg) (p = 0.02), and a reduced-intensity conditioning regimen (p = 0.04) were associated with lower OS rates. Refractoriness to fludarabine did not impact survival. We found a strong correlation between a β-2 microglobulin level and lymph node size (Spearman rho = 0.68, p = 0.0018).
The incidences of grade II–IV and III–IV acute GVHD were 20% and 10%, respectively. The incidences of chronic and extensive chronic GVHD were 54% and 34%, respectively. Eight patients in the alloSCT group died. Infections were the major cause of death (n=3); 2 of these patients had active GVHD. One patient died of organ failure, and 1 patient was lost to follow-up and died of an unknown cause 14 months after alloSCT. Only 1 patient died of disease progression.
Discussion
Optimal treatment for 17p- CLL remains a clinical challenge. Related studies are generally hampered by the rarity of this condition. It is intuitive that challenges further increase in patients with acquired 17p deletion due to the effects of treatment in relapsed/refractory patients. In this retrospective study, we were able to report our center’s experience with a relatively large group of 17p- (de novo 44.3%, acquired 55.7%) CLL patients, who were referred for consideration of alloSCT. Only 38% of patients did ultimately undergo their transplant. Better outcomes were observed in 17p- CLL patients who underwent alloSCT than in those who did not. Patients who underwent alloSCT had a higher 2-year OS rate from the time of referral (64%) than did those who underwent chemotherapy (25%).
We acknowledge the limitations of the retrospective nature of our study. A more effective strategy in determining the role of alloSCT in 17p- CLL would be to consider patients who have suitable donors for a prospective randomized trial of transplant vs no transplant strategy.
An important factor influencing long-term outcome of alloSCT is the response achieved prior to transplantation. The Seattle group [6] suggest that a lymph node size of >5 cm to be associated with a worse OS and PFS rates. In this study, we concurred with these conclusions. We also found a high correlation between the level of β-2 microglobulin and lymph node size measured by CT scans. These results further establish the need for better treatment patients with 17p- CLL before alloSCT.
These findings confirm the results of published reports [5–8] that suggest that alloSCT is an effective therapy in 17p- CLL patients. However, only about one-third of patients who were referred to our transplant service underwent alloSCT. Death as a result of disease progression or relapse was the main reason for not undergoing alloSCT, despite an availability of donors for 12 of these patients. Methods to extend the applicability of this procedure in all 17p- CLL patients are needed. Patients with relapsed 17p- CLL generally have a poor prognosis because of low response rates to salvage chemotherapy and death from therapy-related infective complications. At this stage, conventional chemotherapy with fludarabine, cyclophosphamide, rituximab combination, or bendamustine, plus rituximab have dismal outcomes (complete remission 0% and 7.1%; PFS 5 and 6.8 months) [11, 12]. An alternative strategy would be to consider alloSCT for 17p- CLL patients as consolidation of response in first remission, a recommendation that is consistent with current guidelines of the European Group for Bone Marrow Transplantation [5] and others [13]. This strategy could lessen the risk in some patients from being disqualified from transplantation because of bulky or resistant disease and the subsequent toxicities of salvage chemotherapy.
Despite advances, acute and chronic GVHD remain a clinically significant barrier to more widespread application of allogeneic transplantation. Furthermore, consideration of alloSCT should take into consideration current advancements in targeted therapy of CLL. A recent study [14] with an alemtuzumab-containing regimen has shown a complete response rate of 65% as first line treatment for 17p-CLL patients; however the small number of patients included (n=17) and a median PFS of only 18.3 months preclude any firm conclusions. Newer drugs such as the Bruton’s tyrosine inhibitor, ibrutinib [15] have shown a response rate 68% in 17p-CLL and a favorable toxicity profile, but only 1of 28 patients (3.5%) achieved CR. Idelalisib, a phosphoinositide 3-kinase delta inhibitor, causes rapid lymph node shrinkage and improve PFS rates in CLL [16]. Other treatment strategies involve enhancing the immune recognition on CLL cells by T cells either via vaccination [17] or by engineering chimeric antigen-receptor-modified T cells aimed at orphan receptors on CLL tumors or using other markers such as Cluster of Differentiation 19 [18]. A longer follow-up however is needed to assess the long-term benefit of these agents; especially that clonal evolution has been described in CLL patients who became resistant to BTK inhibition [19]. Patients with 17p-CLL undertaking these therapies should be monitored carefully. In the absence of randomized trials, alloSCT should be considered in poor responders or in those who lack subsequent cytoreduction options that could render alloSCT impossible in the event of failing these drugs.
In an analysis of patients with de novo 17p- CLL, Tam et al [20] reported that 1 subgroup of patients can have an indolent clinical course over a prolonged period of time. These were early-stage (78% Rai stage 0–2) patients with de novo 17p deletion. This suggests that treatment for 17p- CLL patients should be initiated when disease becomes active or symptomatic according to established criteria [9]. All the patients included in our study met those criteria.
In conclusion, our findings demonstrate that for 17p- CLL patients, alloSCT can negate the negative prognostic effect of this genetic aberration. However, disease- and treatment-related complications may preclude a significant proportion of relapsed patients from undergoing the procedure. Controlled trials addressing the role of alloSCT in the era of novel targeted therapeutic agents for CLL are needed.
Supplementary Material
Footnotes
Potential conflict of interest:
The authors declare no competing financial interests.
References
- 1.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 2.Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III Trial E2997. J Clin Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 3.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 4.Dreger P, Corradini P, Kimby E, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–17. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 5.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–5100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 6.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: Long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 9.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 11.Badoux XC, Keating MJ, Wang X, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011;118:2085–2093. doi: 10.1182/blood-2011-03-341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: A multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 13.Gribben JG, O'Brien S. Update on therapy of cgronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–550. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettitt AR, Jackson R, Carruthers S, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia of TP53: final results of the national cancer research institute CLL 206 trial. J Clin Oncol. 2012;30:1647–1655. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 15.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibritunib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhardt UE, Hainz U, Goldstein NR, et al. Autologous CLL vaccination early after transplant induces leukemia-specific T cells. J Cli Invest. 2013;123:3756–3765. doi: 10.1172/JCI69098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter D, Levine B, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphocytic leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger JA, Landau D, Hoellenriegel J, et al. Clonal evolution in patients with chronic lymphocytic leukemia (CLL) developing resistance to BTK inhibition. Blood. 2013;122 doi: 10.1038/ncomms11589. Abstract 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CS, Shanafelt TD, Wierda WG, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–964. doi: 10.1182/blood-2009-03-210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.