Abstract
Enolase enzymes are abundantly expressed, cytosolic carbon-oxygen lyases known for their role in glucose metabolism. Recently, enolase has been shown to possess a variety of different regulatory functions, beyond glycolysis and gluconeogenesis, associated with hypoxia, ischemia, and Alzheimer disease (AD). AD is an age-associated neurodegenerative disorder characterized pathologically by elevated oxidative stress and subsequent damage to proteins, lipids, and nucleic acids, appearance of neurofibrillary tangles and senile plaques, and loss of synapse and neuronal cells. It is unclear if development of a hypometabolic environment is a consequence of or contributes to AD pathology, since there is not only a significant decline in brain glucose levels in AD, but also there is an increase in proteomics identified oxidatively modified glycolytic enzymes that are rendered inactive, including enolase. Previously, our laboratory identified α-enolase as one the most frequently up-regulated and oxidatively modified proteins in amnestic mild cognitive impairment (MCI), early-onset AD (EOAD), and AD. However, the glycolytic conversion of 2-phosphoglycerate to phosphoenolpyruvate catalyzed by enolase does not directly produce ATP or NADH; therefore it is surprising that, among all glycolytic enzymes, α-enolase was one of only two glycolytic enzymes consistently up-regulated from MCI to AD. These findings suggest enolase is involved with more than glucose metabolism in AD brain, but may possess other functions, normally necessary to preserve brain function. This review examines potential altered function(s) of brain enolase in MCI, EOAD, and AD, alterations that may contribute to the biochemical, pathological, clinical characteristics, and progression of this dementing disorder.
Keywords: Enolase, plasminogen (PGn), tissue-plasminogen activator (tPA), plasmin, MAPK/ERK1/2, amyloid β-peptide, c-Myc, hypoxia-inducible protein-1α (HIF-1α), Alzheimer disease (AD), amnestic mild cognitive impairment (MCI), early-onset AD (EOAD), excitotoxicity, hypoxia, hypometabolism
1.0 Introduction
Enolase enzymes (EC 4.2.1.11) are a superfamily of abundantly expressed carbon-oxygen lyases known for their role in glycolysis and gluconeogenesis. Glycolytic enzymes, like enolase, are among some of the most well-characterized proteins to date; yet, enolase isoforms were previously believed to perform exclusively “house-keeping” functions for the cell. However, recent studies have demonstrated that enolase possesses a variety of different regulatory properties, in addition to their glycolytic functions in the brain (Pancholi 2001). In particular, enolase has been reported to be a neurotrophic factor, 14-3-2 (Hattori et al. 1994, Hattori et al. 1995, Takei et al. 1991), a hypoxic stress protein (Aaronson et al. 1995), c-Myc binding protein and transcription factor (Subramanian & Miller 2000, Ray & Miller 1991b), and a strong plasminogen (PGn) binding protein (Pancholi & Fischetti 1998, Nakajima et al. 1994). Furthermore, many of these non-glycolytic functions have been implicated in hypoxia and ischemia (Mizukami et al. 2004, Sousa et al. 2005), as well as Alzheimer disease (AD) (Dotti et al. 2004, Parnetti et al. 1995).
A largely sporadic, age-associated neurodegenerative disorder, AD typically affects populations over the age of 60. Pathologically, AD can be characterized by elevated oxidative stress and subsequent damage to brain proteins, lipids, and nucleic acids, the appearance of neurofibrillary tangles and senile plaques, and eventual loss of synapse and neuronal cells that result in a progressive decline in cognitive function. In rare instances, autosomal dominant mutations in the amyloid-β precursor protein (AβPP) or presenilin genes 1 and 2 (PS-1/-2) cause a familial form of AD (FAD) that produces the same clinical and pathological consequences as sporadic AD, but at a much earlier age (~30 years old) (Scheuner et al. 1996, Sturchler-Pierrat et al. 1997, Citron et al. 1992, Wisniewski et al. 1998). Recently, α-enolase has been identified as one of the most frequently identified differentially expressed brain proteins in human and animal tissues (Petrak et al. 2008). As described below, previous studies by our laboratory have found α-enolase to be the most consistently up-regulated and oxidatively modified proteins in brain of subjects with early-onset AD (EOAD), AD, and amnestic mild cognitive impairment (MCI) (Butterfield and Sultana, 2007; Butterfield et al., 2007) , arguably the earliest form of AD (Winblad et al. 2004, Petersen et al. 1999). These findings suggest that enolase may possess one or more ulterior functions, beyond simple glucose metabolism, that could be integral to both normal and pathological brain function. Therefore, the intent of this review is to examine potential function(s) of α- and γ-enolase isoforms in AD brain.
2.0 Enolase Functional Diversity
Beyond glucose metabolism (Fig. 1), enolase enzymes have been reported to have a number of other non-glycolytic functions, such as the ability to bind polynucleotides (al-Giery & Brewer 1992), being a τ-crystallin protein (Wistow et al. 1988), neurotrophic factor 14-3-2 (Hattori et al. 1994, 1995, Takei et al. 1991), heat-shock protein 48 (HSP48) (Iida & Yahara 1985), hypoxic-stress protein (Aaronson et al. 1995), c-Myc binding and transcription protein (Subramanian & Miller 2000, Ray & Miller 1991a), and a strong PGn binding protein (Pancholi & Fischetti 1998, Nakajima et al. 1994), among others (Table 1). This wide array of functions can be attributed to different DNA base sequences within enolase genes. For example, the promoter region of ENO1 contains a copy of the viral core consensus sequence (GTGG(A/T)(A/T)(A/T)G) (Jones et al. 1988), two copies of the octanucleotide sequence (ATTTGCAT) found in immunoglobulin (Ig) gene enhancers and promoters (Jones et al. 1988), a C2 binding site (CATGTG) present in Ig heavy chain enhancers (Peterson & Calame 1989), part of the liver-specific enhancer binding site sequence (TCNTACTC) (Grayson et al. 1988), a cAMP response element (CRE) sequence (position −298) (Grayson et al. 1988), and SP1 transcription factor binding site (Briggs et al. 1986). Although the functional significance of many of these elements is unknown, other sequences are better-characterized, particularly the carbohydrate response element (ChoRE) motif (59−CACGTG−39). Upon glucose stimulation, transcription factors, such as hypoxia-inducible factor-1α (HIF-1α) and c-Myc, bind the ENO1 ChoRE motif and initiate transcription of the enolase enzyme (Thompson & Towle 1991, Towle 1995, Dang 1999).
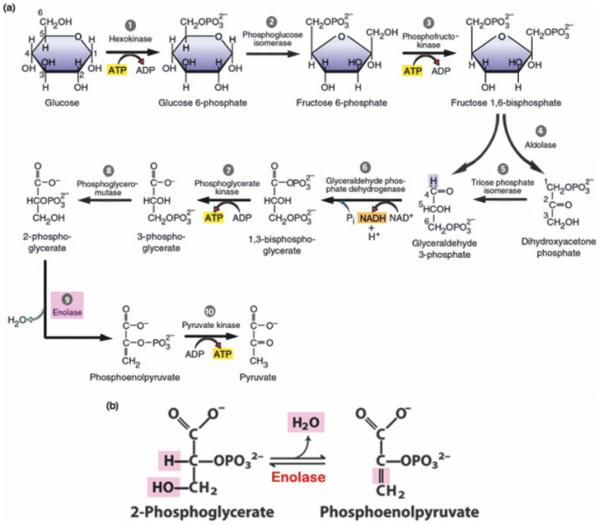
Figure 1.
a) Glycolysis. Schematic representation of aerobic glycolysis. Because two 3-carbon triose chains are produced from the reaction between aldolase and fructose-1,6-bisphosphate, steps 5-10 are completed twice (not shown). ATP production in steps 7 and 10 is thought to be the chief fuel-source for plasma membrane ion pumps, including the Na+/K+-ATPase and Ca2+-ATPase, rather than ATP produced by mitochondrial oxidative phosphorylation [adapted from (Karp 2003)]. b) Enolase reaction. Step 9 in the glycolytic chain involves conversion of 2-phosphoglycerate (2-PGA) to phosphoenolpyruvate (PEP) by enolase [adapted from (Nelson & Cox 2009)].
Table 1.
Enolase functional diversity and/or involvement in disease:
| Functional Diversity: |
|
| Other Roles/Disease Involvement: |
|
(?), signifies unknown/debated enolase functions and/or disease involvement; [adapted from(Pancholi 2001)]
2.1 Enolase & c-Myc
Typically, functional diversity of proteins originates during transcriptional regulation. For example, Giallongo, et al. (Giallongo et al. 1990) discovered multiple transcription start sites in ENO1 that are consistent with the lack of a canonical TATA box 19 to 27 base-pairs upstream of its CAP-site, which is primarily responsible for accurately positioning the correct mRNA start site. The significance of this finding is illustrated in its effects on translation initiation, which usually occurs at the first in-frame, 5’-AUG codon, representing the optimal context (Kozak 1999). However, reinitiation, direct internal initiation, and leaky scanning caused, in part, by the lack of a start site-directing TATA box, can produce more than one protein from a single mRNA, such as in the case of ENO1 (Feo et al. 2000, Kozak 1999, Giallongo et al. 1990). Thus, the two ENO1 gene products, α-enolase (48 kDa) and c-Myc binding protein-1 (MBP-1; ~37 kDa), share 97% sequence similarity (Giallongo et al. 1986). Two single-base insertions in MBP-1 result in a reading frame shift affecting its N-terminus as compared to the α-enolase coding region, while the C-terminal regions of both enzymes are identical (Ray & Miller 1991b, Onyango et al. 1998). Furthermore, α-enolase and MBP-1 have similar function, although quite different subcellular fates.
Normally, α-enolase is directed to the cytoplasm, in which it carries out its metabolic role, while MBP-1 is located in the nucleus where it is involved in transcriptional regulation of the c-Myc protooncogene. c-Myc is a DNA-binding phosphoprotein critical in the control of cell proliferation, differentiation, and apoptosis (Evan et al. 1992, Marcu et al. 1992, Spencer & Groudine 1991) that is commonly overexpressed in tumor cells. Like most “housekeeping” genes, ENO1 mRNA translation is primarily under developmental control, significantly up-regulated during cellular growth and practically undetectable during quiescent phases (Holland et al. 1983, Giallongo et al. 1990). In transformed cells, the overexpression of c-Myc stimulates abnormal cell proliferation and up-regulation of several glycolytic enzymes, including α-enolase, in order to accommodate the mounting energy deficit (Hurlin & Dezfouli 2004, Osthus et al. 2000, Kim & Dang 2005). In turn, MBP-1 negatively regulates c-Myc transcription (Chaudhary & Miller 1995, Ray & Miller 1991b, Ray 1995), acting as a tumor suppressor and completing a regulatory “feedback-loop” of both c-Myc and glycolytic activity (Sedoris et al. 2007). Interestingly, although MBP-1 does not have enolase enzyme activity, both α-enolase and MBP-1 are able to act as tumor supressors because c-Myc down-regulating activity lies within two hydrophobic N- and C-terminal regions present in both ENO1 translation products (Subramanian & Miller 2000, Bentley & Groudine 1986).
2.2 Enolase & the Plasminogen System
The PGn system is best known for the pivotal role it plays in maintenance of vascular potency and thromolysis, by dissolving fibrin (Plow et al. 1991, Collen 1999). In order to elicit function, the glycoprotein PGn binds cell surface receptors via kringle domains that recognize exposed C-terminal lysine residues. Interestingly, PGn binds these receptors with low affinity, yet is more readily activated than free PGn (Plow et al. 1995), and subsequently produced plasmin has greater enzymatic activity and is protected from inactivation by inhibitors, such as α2-antiplasmin (Plow et al. 1991). Therefore, virtually any surface protein exposing C-terminal lysines has the potential to bind and activate PGn processes, in addition to contributing to the high density and broad distribution of the many heterogenous PGn binding sites (Plow et al. 1995). Gangliosides (Miles et al. 1989), glycosaminoglycans (Andrade-Gordon & Strickland 1986), annexin II (Cesarman et al. 1994, Hajjar et al. 1994), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Lottenberg et al. 1992), and α-enolase (Redlitz et al. 1995, Pancholi & Fischetti 1998, Miles et al. 1991) are just a few examples of the different types of proteins reported to be PGn surface receptors. Most remarkable, however, is the discovery that glycolytic enzymes, GAPDH and α-enolase, are able to integrate into the cell membrane without possessing a signal sequence and retain enzymatic activity (Pancholi & Fischetti 1998). Some researchers speculate that a hydrophobic domain (33−AAVPSGASTGIY−44) within α-enolase might serve as an internal signal sequence, allowing its integration (von Heijne et al. 1991), while others suggest postranslational acylation (Bottalico et al. 1993) or phosphorylation (Cooper et al. 1984) may be a means of membrane association. Nevertheless, these two cytosolic enzymes are now part of a growing group of proteins that lack signal sequences, but are transported to the cell surface by an unknown mechanism.
However, binding surface receptors, like α-enolase, alone cannot activate PGn conversion to plasmin; the PGn-proteolytic cascade must begin with cleavage by either tissue-PGn activator (tPA) or urokinase-PGn activator (uPA) (Bergman et al. 1997), both of which, can be found in human brain (Carmeliet et al. 1994, Sappino et al. 1993). tPAs and/or uPAs initiate fibrinolysis by binding fibrin aggregates, which leads to a conformational change that dramatically increases their affinity for PGn. As a result, PGn is cleaved by tPA/uPA into proteolytic plasmin (Tucker et al. 2000b). Of particular interest in human brain is tPA, a serine protease correlated with hippocampal late-phase long term potentiation (L-LTP) (Kandel 2001, Pang & Lu 2004). Transgenic mice overexpressing tPA exhibit enhanced L-LTP and improved spatial learning (Madani et al. 1999). Induction of L-LTP, in turn, enhances neuronal expression of tPA within the hippocampus (Qian et al. 1993), perpetuating a cycle of secretion and growth necessary for brain development. In addition, PGn has also been implicated in wound healing and inflammation through its involvement in cell proliferation and migration (Kalderon 1979, 1982, Tarui et al. 2002, Plow et al. 1991), as well as many intracellular signaling events by activation of proenzymes (Liotta et al. 1981, Blasi et al. 1987, Nagase et al. 1990), prohormones (Virji et al. 1980), progrowth factors (Rifkin et al. 1990, De Sousa et al. 2005), and procytokines (Nakagawa et al. 1991, Konakova et al. 1998). Thus, in this review, the role enolase plays in conjunction with the tPA/PGn system will be analyzed with respect to subjects with AD.
3.0 Enolase & Alzheimer Disease
AD is an age-associated neurodegenerative disorder that typically affects the elderly, aged 60 and above. However, in rare instances it can affect younger populations, as early as 30 years old, in FAD (Scheuner et al. 1996, Sturchler-Pierrat et al. 1997, Citron et al. 1992, Wisniewski et al. 1998). Both AD and FAD can be characterized clinically by a progressive decline in cognitive function, and pathologically by synapse and neuronal cell loss, as well as the appearance of neurofibrillary tangles and senile plaques. Other hallmarks of AD and FAD pathology are oxidative stress and damage that induce protein and nucleic acid oxidation, lipid peroxidation, and apoptosis, which lead to declining brain function and loss of synapses and neurons (Butterfield & Lauderback 2002, Butterfield et al. 2001, 2002, Markesbery 1999, Aksenov et al. 2001, Butterfield 2002, Bader Lange et al. 2008). In the same way, MCI, considered a transition point between normal cognitive aging and probable AD (Petersen et al. 1999, Winblad et al. 2004), has also been reported to have elevated oxidative stress levels (Butterfield et al. 2006b, 2007, Butterfield & Sultana 2007, Keller et al. 2005, Markesbery et al. 2005, Markesbery & Lovell 2007, Lovell & Markesbery 2007). Oxidative modification of proteins during disease progression, in turn, results in diminished and/or complete loss of protein function, as indexed by levels of protein carbonyls, 3-nitrotyrosine, protein-/lipid-bound 4-hydroxy-2-nonenal (HNE), and S-glutathionylation (Butterfield & Stadtman 1997).
According to a recent report, α-enolase has been identified as differentially expressed in about 30% of all 2D-gel electrophoresis (2-DE)-based experiments in human and animal tissues published in recent issues of Proteomics (Petrak et al. 2008), rendering it one of the top 15 most frequently identified differentially expressed proteins. Our laboratory has reported that enolase is oxidatively modified in MCI, EOAD, and AD. In these studies compared to control brain, α- and γ-enolase were found to be excessively carbonylated (Sultana et al. 2006a, Butterfield et al. 2006a, 2006b, Castegna et al. 2002), nitrated (Reed et al. 2008b, Sultana et al. 2006b, Castegna et al. 2003), HNE-modified (Reed et al. 2008a, Perluigi et al. 2009), and S-glutathionylated (Newman et al. 2007) in brain areas such as the inferior parietal lobule (IPL), hippocampus, and frontal cortex, but not in cerebellum, a brain region essentially devoid of pathology in AD. Whether the extensive oxidative modification of enolase is simply due to its proximity to the many redox reactions occurring throughout the cell or a result of structural susceptibility to oxidation is unknown. However, both possibilities are conceivable since enolase can be found in numerous regions of the cell and possesses many active-site Lys and His residues that are extremely susceptible to Michael addition by compounds such as HNE (Butterfield & Stadtman 1997). For example, γ-enolase has been identified as a component of the NADH-dichlorophenol-indophenol (DCIP) reductase complex, one of several trans-plasma membrane oxidoreductases (PMOs) located within synaptic plasma membranes and recycling vesicles (Bulliard et al. 1997). PMOs function as antioxidant enzymes and extracellular redox sensors that regulate cell proliferation and axonal guidance in response to external pro- or anti-oxidants (Toole-Simms et al. 1991, Fuhrmann et al. 1989), thereby placing enolase in direct contact with reactive oxygen species (ROS) that could readily modify its activity and structure. Furthermore, our laboratory has also reported that enolase is present within mitochondria, one of the largest ROS producers within the cell, and contributes this organelle’s function (Poon et al. 2005).
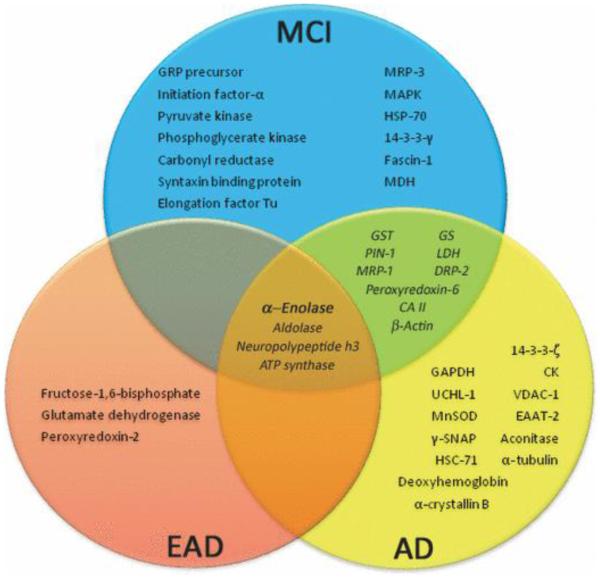
Another predominant feature in MCI, EOAD, and AD is the manifestation of glucose hypometabolism (Mielke et al. 1996), associated with the oxidative-inactivation of several glycolytic enzymes, including enolase (Sultana et al. 2007, Butterfield et al. 2006b, Castegna et al. 2002) (Fig. 2). Because the brain is one of the greatest consumers of glucose, hypometabolism can cause the up-regulation of glycolytic enzymes in an effort to combat the mounting energy deficit and hypoxic environment (Mielke et al. 1996). Moreover, previous studies have shown that cells resistant to Aβ toxicity had a greater flux of glucose through glycolysis and the hexose monophosphate shunt (Soucek et al. 2003). Interestingly, although the glycolytic function of enolase does not directly produce ATP or the reduced energy carrier NADH (Fig. 1), in all studies of MCI, EOAD, and AD brain from our laboratory, enolase levels are increased (Castegna et al. 2002, Sultana et al. 2007), while levels of pyruvate kinase, phosphoglycerate kinase, and GAPDH vary throughout disease progression (Fig. 2). Furthermore, our laboratory has shown that α-enolase was one of only four proteins, glycolytic or otherwise, consistently up-regulated and oxidatively modified in the progression from MCI to AD (Fig. 2). These remarkable findings suggest that enolase may well be involved in more than just metabolic processing of glucose, but perhaps possesses other critical functions vital to preserving brain function, which are discussed subsequently in this review.
Figure 2.
Oxidatively modified and/or glutathionylated proteins in MCI, EOAD, and AD brain identified by redox proteomics studies from our laboratory (Perluigi et al. 2009, Butterfield et al. 2006a, 2006b, Sultana et al. 2006a, 2006b, Castegna et al. 2002, 2003, Reed et al. 2008a, 2008b, Newman et al. 2007). This diagram shows the interrelation of all proteins found to be oxidatively modified in MCI, EOAD, and AD brain from our laboratory. Abbreviations: GRP precursor, Glucose-regulated protein precursor; MRP-1, Multidrug-resistant protein; MAPK, Mitogen-associated protein kinase; HSP-70, Heat-shock protein-70; MDH, Malate dehydrogenase; GST, Glutathione S-transferase; GS, Glutamine synthetase; PIN-1, Peptidyl-prolyl cis/trans isomerase (PPIase) ; LDH, Lactate dehydrogenase; DRP-2, Dihydropyrimidinase-related protein-2; CAII, Carbonic anhydrase II; HSC-71, Heat-shock cognate-71; γ-SNAP, Soluble N-ethylmaleimide-sensitive factor attachment protein-γ ; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; UCHL-1, Ubiquitin carboxy-terminal hydrolase L-1; VDAC-1, Voltage dependent anion channel-1; CK, Creatine kinase; EAAT-2, Excitatory amino acid transporter-2; MnSOD, Manganese superoxide dismutase.
3.1 Enolase, the Plasminogen System, & Glutamate Excitotoxicity
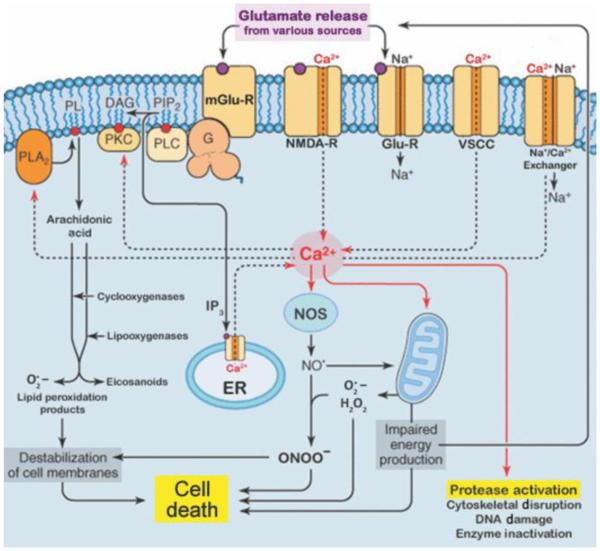
Glutamate excitotoxicity, a well-known phenomenon in AD brain, is characterized by the increased release and impaired uptake of glutamate, which mediates the toxic build-up of extracellular glutamate, leading to overstimulation of glutamate receptors, N-methyl-D-asparatate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and kainite receptors. Collapsing cellular ATP reserves and Na+ ion gradients exacerbate this process, and eventually lead to rising intracellular Ca2+ levels, due to the opening of voltage-gated Ca2+ channels and release of Ca2+ from the endoplasmic reticulum (ER) (Fig. 3). Increased cytoplasmic Ca2+, in turn, further depolarizes the cell membrane and activates cytotoxic intracellular pathways that lead to neuronal death, such as inducing the Ca2+-dependent secretion of the serine protease, tPA (Gualandris et al. 1996, Fernandez-Monreal et al. 2004b, Baranes et al. 1998). During glutamate excitoxicity, excessive neuronal activity and extracellular secretion of tPA can cause neuronal death by augmenting microglial activation (Rogove & Tsirka 1998), increasing plasmin activation and degradation of laminin (Tsirka et al. 1995, Chen & Strickland 1997), and potentiating NMDA receptor signaling processes (Nicole et al. 2001, Tsirka et al. 1995, 1997).
Figure 3.
Glutamate excitotoxicity. This diagram depicts the many intracellular signaling events elicited by excess release and impaired uptake of glutamate, leading to neuronal death. Glu-R, AMPA/Kainate receptors; mGlu-R, metabotropic glutamate receptor; NMDA-R, N-methyl-D-aspartate receptor; VSCC, voltage-sensitive Ca2+ channel; PL, phospholipids; DAG, Diacylglycerol; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-trisphosphate; G, G-protein; PLA2, phospholipase A2; PLC, phospholipase C; PKC, protein kinase C; ER, endoplasmic reticulum; H2O2, hydrogen peroxide; NO•, nitric oxide; ONOO-, peroxynitrite; NOS, nitric oxide synthase; O2•−, superoxide radical [adapted from (Siegel et al. 2006)].
Apoptotic cells often secrete and/or exhibit signal molecules within the membrane that allow activated microglia to scavenge, recognize, and bind damaged cells that require clearance, in order to prevent further injury to surrounding areas. In a study by Siao et al. (Siao & Tsirka 2002), glutamate-injured neurons were shown to release sufficient tPA to activate tPA-/- microglia in a cytokine-like manner (Rogove & Tsirka 1998). Furthermore, activated microglia were shown to secrete tPA in a proteolytic-independent manner, activating neighboring microglia, thereby, effectively amplifying the signal for microglial activation. Ultimately, this effect can lead to recruitment of microglia to the site of injury and can promote a timely resolution of cellular injury, an overly sensitive inflammatory response, or both (Siao & Tsirka 2002). For example, after Ca2+-induced neuronal secretion, tPA cleaves an N-terminal residue on the NR1 subunit of the NMDA receptor, exacerbating NMDA receptor-evoked Ca2+ influx during excitotoxic processes (Fernandez-Monreal et al. 2004a, Nicole et al. 2001), and consequently, propagating an overly sensitive inflammatory response. Alternatively, activated microglia and apoptotic neuronal cells also synthesize PGn, in addition to tPA (Tsirka et al. 1997, Nakajima et al. 1992a, 1992b, 1992c). In general, tPA secretion alone is not sufficient to cause neuronal degeneration during excitotoxic insult (Tsirka et al. 1996, 1997); therefore, presenting PGn on the membrane surface could either provide a way to further localize activated microglia to areas in which neurons have been injured, or suggests that neuronal secretion of tPA is not necessarily intended to be detrimental.
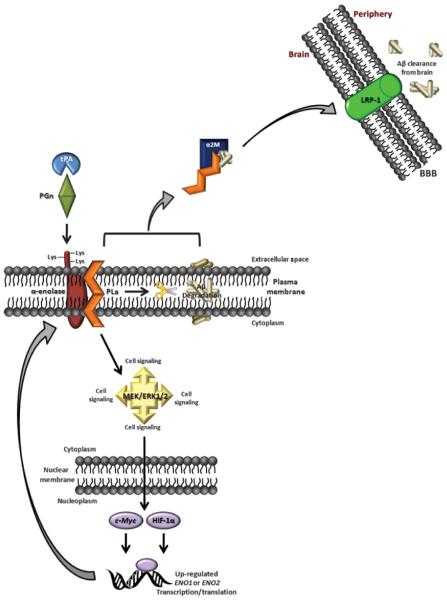
As described previously, PGn has been implicated in inflammation, as well as many intracellular signaling events by activation of proenzymes, prohormones, progrowth factors, and procytokines (Section 2.2). Studies by Nagata et al. (Nagata et al. 1992, 1993) have shown that microglia-derived PGn has a neurotrophic effect on neurons, since purified rat PGn enhanced neurite outgrowth and dopamine uptake of mesencephalic neurons. The effects elicited by PGn were found to be mediated by α-enolase on the neuronal surface (Nagata et al. 1993, Nakajima et al. 1994). Enolase has frequently been reported as a strong PGn binding protein within the brain (Section 2.2), due to its extracellular C-terminal Lys residues, and is known to be up-regulated in MCI, EOAD, and AD brain (Section 3.0). When microglial and/or neuronal PGn binds membrane-integrated enolase, PGn is rapidly activated through tPA proteolytic cleavage (Section 2.2). Consequent production of plasmin endows neurons with the catalytic amplification of tPA/PGn signaling, due to the broad spectrum of substrates affected by proteolytic plasmin (Redlitz et al. 1995). Moreover, binding enolase protects plasmin from inactivation from inhibitors, like α2-antiplasmin (Bergman et al. 1997, Plow et al. 1991). Therefore, it can be speculated that the up-regulation of enolase, in addition to enhanced membrane-resident PGn and extracellular secretion of tPA, by excitotoxic neurons and/or activated microglia during AD progression may initially be an attempt to propagate neuronal-preservation pathways, that ultimately go awry (Fig. 4).
Figure 4.
Possible role of enolase in MCI, EOAD, and AD. This scheme illustrates an alternate role for enolase, in addition to glucose metabolism, in normal and/or MCI, EOAD, and AD brain. In this model, up-regulation and membrane integration of α-enolase, promotes surface-binding of the tPA/PGn complex, which produces the protease plasmin (PLa). Plasmin, in turn, can degrade Aβ peptides associated with the bilayer and activate the MAPK/MEK/ERK1/2 pathway, promoting up-regulation of ENO1 transcription, and therefore, production of α-enolase. In this way, up-regulation of enolase would catalytically amplify an internal signal for cell survival during AD progression. Moreover, by complexing with a2M, plasmin may also be involved with Aβ clearance from the brain via LRP-1 at the blood-brain barrier (BBB). Unfortunately, due to significant oxidative modification, it is hypothesized that enolase becomes unable to facilitate the initiation of these pathways, which would lead to the augmentation of neuronal death in brain of subjects with MCI, EOAD, and AD versus normal aged brain.
3.2 Enolase, the Plasminogen System, & MAPK/MEK/ERK1/2 Signaling
As previously mentioned, a predominant feature in MCI, EOAD, and AD is the manifestation of glucose hypometabolism, which generally causes the up-regulation of glycolytic enzymes, including enolase, to combat the mounting energy deficit (Section 3.0). Thus, the lack of aerobic metabolism in AD brain is indicative of a widespread hypoxic environment. Many studies have found glycolytic ATP to be of utmost importance to the maintanence of ER Ca2+ stores, by acting as the chief ATP fuel-source for plasma membrane ion pumps, such as the Na+/K+-ATPase and Ca2+-ATPase, rather than ATP produced by mitochondrial oxidative phosphorylation (Kahlert & Reiser 2000, Kauppinen et al. 1988, Brorson et al. 1999, Silver & Erecinska 1997, Xu et al. 1995). However, neither glycolysis nor oxidative phosphorylation alone is capable of sustaining power to these pumps; therefore, a constant ATP supply must be provided by both pathways in order to prevent membrane depolarization (Kauppinen et al. 1988). In addition, collapsing cellular ATP reserves and Na+ ion gradients can exacerbate glutamate excitotoxicity, lead to rising intracellular Ca2+ levels, and activate intracellular signal pathways that either lead to neuronal death or survival (Fig. 3).
Specifically, intracellular pathways involving activation of the mitogen-activated protein kinase (MAPK) cascade are of particular interest on account of one downstream target, enolase. Among many different proteins that MAPKs can activate by phosphorylation, the extracellular signal-regulated kinase 1/2 (ERK1/2) is known to function in cell survival responses by translocating to the nucleus and inducing rapid gene expression (Davis 1993, Karin 1995, 1998, Chang & Karin 2001), often in response to ROS (Jimenez et al. 1997, Chuang et al. 2000, Kishida et al. 2005, Conde de la Rosa et al. 2006, Kulich et al. 2007), a well-known initiator and/or consequence of AD pathology (Section 3.0). In a study by Mizukami, et al. (Mizukami et al. 2004), ERK1/2 was shown to be involved in the maintenance of intracellular ATP through induction of α-enolase expression, resulting in cardiomyocyte survival in ischemic hypoxia and re-oxygenation (Mizukami et al. 2004). Sousa, et al. (Sousa et al. 2005) provided additional insight into ERK1/2-induced expression of ENO1 mRNA, revealing that active PGn on the cell surface activates MAPK and ERK1/2 in fibroblasts, through the proteolytic action of plasmin, which leads directly to the transcriptional regulation of ENO1. Their data also demonstrate that PGn-regulated ENO1 expression is not only restricted to fibroblast cells, suggesting that this signaling cascade, involving up-regulation of α-enolase via MAPK and ERK1/2, probably exists in the brain, as well (Sousa et al. 2005). Further evidence to support this notion is that all MAPK pathways, including ERK1/2, are known to be activated in AD brain, as ERK 1/2 immunoreactivity can be found in tangle-bearing and non-tangle-bearing neurons (Hyman et al. 1994), as well as in dystrophic neurites of senile plaques (Trojanowski et al. 1993).
Because over 25 proteins have been identified to be downstream targets of ERK1/2 signaling (Lewis et al. 2000), the exact intracellular mechanism by which the MAPK/ERK1/2 survival pathway induces ENO1 expression has yet to be established. However, two possible mechanisms suggested by Mizukami, et al. (Mizukami et al. 2004) in the heart, involving c-Myc and HIF-1α (Sections 2.0-2.1), may provide insight into α-enolase regulation in the brain. In their 2004 study, Mizukami, et al. (Mizukami et al. 2004) found that ischemic hypoxia and re-oxygenation induced α-enolase expression in cells transfected with c-Myc cDNA, suggesting that ERK1/2 may induce α-enolase expression through c-Myc. Induction of c-Myc mRNA expression is dependent upon Ets transcription factor binding sites located within the c-Myc promoter region that are activated by ERK1/2 phosphorylation (Brunner et al. 1994, McCarthy et al. 1997, Cheng et al. 1999, Gupta et al. 1993). Furthermore, like MAPK and ERK1/2, strong, active c-Myc immunoreactivity has been noted in a subpopulation of reactive astrocytes, dystrophic neurites of senile plaques, and neurons with neurofibrillary degeneration in AD (Ferrer & Blanco 2000), implicating a role for MAPK/ERK1/2 activation of c-Myc, that would, in turn, induce expression of α-enolase in AD. Since the ENO1 promoter region contains two c-Myc binding motifs within the ChoRE sequence (Section 2.0), c-Myc can directly transactivate production of α-enolase. Interestingly, activation of c-Myc by the ERK1/2 pathway selectively up-regulates α-enolase production over MBP-1, as MBP-1 would inhibit c-Myc by binding its promoter (Section 2.1).
Alternatively, the MAPK/ERK1/2 pathway can also induce α-enolase transcription through the hypoxia-inducible factor, HIF-1α, which up-regulates glycolytic gene expression during hypoxia, a common phenomenon in AD pathology (Semenza et al. 1996, Wang et al. 1995, Aaronson et al. 1995) (Section 3.0). Cells adapt to hypoxic conditions by inducing activation of transcription factors such as HIF-1, a key regulator of oxygen homeostasis that accumulates in response to low cellular oxygen levels (Wang & Semenza 1993a, 1993b, Wang et al. 1995). Previous studies indicate that embryonic stem cells deficient in HIF-1α expressed decreased levels of mRNAs encoding over ten different glucose transporters and glycolytic enzymes, including α-enolase (Iyer et al. 1998), insinuating HIF-1α protective effects are largely attributable to increased metabolic flow. Yang et al. (Yang et al. 2005) suggest that HIF-1α up-regulation of glucose transporters and glycolytic enzymes during hypoxia may favor glycolytic ATP over ATP produced by oxidative phosphorylation, thereby compensating for diminished ATP supplies resulting from oxygen-deprived mitochondria. This explanation is also consistent with studies that suggest the chief ATP-fuel-source for many cellular functions comes directly from glycolysis, rather than mitochondrial-produced ATP, as mentioned above (Kahlert & Reiser 2000, Kauppinen et al. 1988, Brorson et al. 1999, Silver & Erecinska 1997, Xu et al. 1995).
During MAPK/ERK1 signaling, ERK1 is reported to phosphorylate the C-terminal transactivation domains of HIF-1α in hypoxic HMEC-1 endothelial cells, stimulating HIF-1α transcriptional activity (Richard et al. 1999, Minet et al. 2000), thus, demonstrating that up-regulation of enolase by HIF-1α can be controlled by MAPK/ERK1/2 signaling (Semenza et al. 1996). Moreover, a study by Soucek et al. (Soucek et al. 2003) noted that overexpression of a non-degradeable form of HIF-1α prevents Aβ(1-42)-induced neurotoxicity. Considering all MAPK pathways, including ERK1/2 and HIF-1α, are activated in AD brain, it is possible that up-regulation of enolase through MAPK/ERK1/2 signaling serves a direct neuroprotective function in AD. However, it should be noted that although there are multiple neuroprotective benefits to MAPK/ERK1/2 activation, the over-activation of these kinases, especially with respect to cell type, varying apoptotic signals, and diverse downstream targets, can increase sensitivity to neurodegeneration, especially during oxidative insult (Chu et al. 2004, Slevin et al. 2000, Zhu et al. 2002a, 2002b).
Because the ChoRE sequence of many glycolytic genes, including ENO1, is analogous to the binding site for c-Myc and HIF-1α (Section 2.0), both of these transcription factors are able to up-regulate glucose metabolism via ERK1/2 signaling under hypoxic/hypometabolic conditions (Semenza et al. 1996). For that reason, it is quite possible that both mechanisms are utilized under hypometabolic/hypoxic conditions, either separately or simultaneously, in MCI, EOAD, and AD brain. Therefore, we speculate that the increased levels of enolase found in MCI, EOAD, and AD brain are attributable to neuronal and/or glial intracellular survival pathways induced by excitotoxic, hypoxic, and/or oxidative stress. Extracellular tPA cleavage of PGn bound to membrane-resident enolase stimulates plasmin activation of the MAPK ERK1/2 pro-survival pathway, that, in turn, up-regulates transcription of glycolytic enzymes, like enolase, in an effort to counteract the hypometabolic imbalance of ATP and critical ion gradients, and perhaps saving the cell from an apoptotic death (Fig. 4). Enhancing the translation of α-enolase, in turn, would allow for additional tPA/PGn binding, thus, perpetuating catalytic amplification of not only MAPK/ERK1/2 survival signaling, but perhaps other self-preservation pathways as well.
3.3 Enolase, the Plasminogen System, & Aβ
AD is pathologically characterized by increased levels of oxidative stress and damage, as well as the accumulation of neurofibrillary tangles and amyloid plaques, that ultimately lead to synapse and neuronal cell loss (Section 3.0). Amyloid plaques are the result of an over-accumulation of the amyloid-β (1-40) and/or (1-42) peptides (Aβ), derived from β- and γ-secretase cleavage of the AβPP. As AD pathology progresses, the more toxic Aβ(1-42), in particular, rapidly aggregates into fibrils in a β-sheet conformation, similar to the cross-β-structure that fibrin peptides adopt during fibrinolysis (Kranenburg et al. 2002). Interestingly, it is this β-sheet conformation that endows fibrin the ability to bind and activate tPA in the PNS (Kranenburg et al. 2002); yet, thus far, fibrin has not been found in the brain (Dotti et al. 2004). Although fibrin and Aβ(1-42) have no relative sequence similarity, Aβ(1-42) is able to bind and activate tPA through its aggregated β-sheet structure, thereby substituting for fibrin in PGn activation by tPA, but not uPA, in the brain (Wnendt et al. 1997, Kingston et al. 1995).
Studies by Tucker et al. (Tucker et al. 2000a, 2000b), suggest that Aβ accumulation ultimately leads to the activation of the tPA/PGn system by inducing tPA expression in vitro and in vivo, in a positive feedback-loop manner. Through tPA cleavage of PGn, activated plasmin can degrade oligomeric and fibrillar Aβ, effectively blocking Aβ neuronal toxicity. Van Nostrand et al. (Van Nostrand & Porter 1999) further demonstrated that plasmin cleavage yields an N-terminal truncated form of Aβ with altered β-sheet properties that enhanced stimulation of tPA activity in a positive feedback-loop manner. Interestingly, plasmin has been noted to preferentially increase α-cleavage of AβPP (forming neurotrophic sAβPPα), either by cleaving AβPP directly, or by activating other proteases (Ledesma et al. 2000). Considering that plasmin is known to have an affinity for Lys residues (Weinstein & Doolittle 1972), and can activate metalloproteinases (Kleiner & Stetler-Stevenson 1993), such as candidate α-secretases, ADAM 10 and TACE (Buxbaum et al. 1998, Lammich et al. 1999), plasmin could contribute to Aβ degradation in two ways. First, plasmin may enhance AβPP α-cleavage, increasing the production the non-toxic sAβPPα over the more toxic Aβ(1-42), and/or, secondly, by directly degrading all forms of Aβ produced, including sAβPPα, p3, Aβ(1-40), and Aβ (1-42) in the form of oligomers and fibrils (Ledesma et al. 2000). In contrast, Melchor et al. (Melchor et al. 2003) demonstrated a significant decrease in tPA activity in the hippocampus and amygdala of AD patients, implying that diminished plasmin levels are not a consequence of Aβ deposition, but, rather, a cause. These researchers also reported that Aβ accumulation exacerbates diminishing tPA activity by inducing expression of PAI-1 (Melchor et al. 2003), a potent tPA inhibitor (Gils & Declerck 1997).
However, these papers do not discuss the effects of alternatively spliced AβPP derivatives on PGn processing in AD brain, specifically isoforms containing the kunitz-type serine protease inhibitor (KPI) domains. Alternative splicing of the gene encoding AβPP on chromosome 21 yields three AβPP isoforms of 695 (KPI(−)AβPP), 751, and 770 amino acids; of which, the 751 and 770 amino acid species (KPI(+)AβPP) contain a 56 amino acid KPI domain (Ponte et al. 1988, Tanzi et al. 1988). KPI domains are highly analogous to the proteinase inhibitor, protease-nexin II and are known to potently inhibit serine proteases, such as prothrombic enzymes and plasmin, but not uPA or tPA (Shimokawa et al. 1993, Xu et al. 2005, 2009, Van Nostrand et al. 1989, 1990, Smith et al. 1990, Schmaier et al. 1993, Mahdi et al. 1995, Konduri et al. 2001). In AD subjects, KPI(+)AβPP mRNA and protein levels are significantly elevated in many areas of the brain and cerebral spinal fluid (CSF), found in senile plaques, and are associated with increased production of Aβ, while KPI(−)AβPP levels are significantly reduced (Palmert et al. 1989a, 1989b, Saito et al. 1993, Kitaguchi et al. 1990, Hyman et al. 1992, Moir et al. 1998, Zhan et al. 1995, Willoughby et al. 1995, Preece et al. 2004).
Therefore, the above-mentioned models of tPA and plasmin regulation (Shimokawa et al. 1993, Van Nostrand & Porter 1999, Melchor et al. 2003, Tucker et al. 2000a, 2000b, Konduri et al. 2001, Van Nostrand et al. 1990, Menendez-Gonzalez et al. 2005) are not necessarily incompatible, since there might be a negative feedback-loop mechanism between tPA and/or plasmin activity and Aβ deposition. For example, plaque formation may, indeed, trigger the up-regulation of PGn, but the loss of tPA activity (i.e., by PAI-1) prior to AD onset might render a positive feedback-loop (as mentioned above) ineffective (Cacquevel et al. 2007). Moreover, increased levels of KPI(+)AβPP in AD brain would serve to exacerbate this negative feedback-loop, wherein both tPA and plasmin activity are inhibited by AD pathology. Thus, oxidative modification of α-enolase and/or other proteins results in the up-regulation of α-enolase during AD progression and may be an integral part of a system in which neurons attempt to degrade accumulating Aβ. However, the oxidative modification of α-enolase in MCI, EOAD, and AD may render this enzyme either incapable of binding PGn/plasmin while membrane-integrated, or completely unable to integrate into the plasma membrane in order to initiate a PGn/plasmin proteolytic survival cascade. In either case, plasmin would be unable to effectively degrade Aβ or initiate the MAPK/ERK1/2 survival pathway. Consequently, free plasmin proteolytic activity could be inhibited by α2-antiplasmin (Section 2.2; Fig. 4).
Lastly, Aβ accumulation in MCI, EOAD, and AD brain can arise from overproduction, decreased degradation (i.e., by plasmin, neprolysin, or other proteases), and/or by a third mechanism: decreased efflux from the brain. Efflux of brain-resident Aβ is primarily facilitated by the low-density lipoprotein-related receptor-1 (LRP-1), which mediates endocytic processing of both secreted and transmembrane forms of AβPP through the blood-brain barrier (BBB) (Kounnas et al. 1995, Knauer et al. 1996). Interestingly, studies demonstrate that plasmin interacts with α2-macroglobulin (α2M), a “pan-protease inhibitor” (Kovacs 2000, Bu et al. 1992) that is bound and internalized by LRP when complexed with proteases, such as plasmin (Qiu et al. 1996, Rebeck et al. 1995). α2M is an atypical protease inhibitor, in that cleavage of its “bait region” traps proteases, but does not block or alter the protease active-site or proteolytic ability (Kovacs 2000, Borth 1992). Studies by Qiu et al. (Qiu et al. 1996) demonstrate that a 700 kDa α2M-serine protease complex is responsible for significant Aβ(1-40) and Aβ(1-42) degradation and clearance from the brain. Although, the identity of this particular serine protease is unknown, it is conceivable that plasmin, a serine protease known to bind and degrade Aβ proteins, may be a likely candidate.
Hence, oxidative dysfunction and consequent altered binding of membrane-integrated enolase by PGn in MCI, EOAD, and AD brain could conceivably lead to decreased efflux of brain-resident Aβ. Altered binding of PGn to enolase at the cell surface could significantly reduce or completely inhibit production of the tPA/PGn cleavage product, plasmin, precluding the potential association of α2M with plasmin, and, therefore, Aβ clearance via LRP. Furthermore, since LRP is also a receptor for free and/or α2M-complexed KPI(+)AβPP, which competitively inhibits clearance of Aβ when bound to LRP (Kounnas et al. 1995, Ulery et al. 2000, Moir & Tanzi 2005, Conboy et al. 2005), elevated KPI(+)AβPP levels in AD brain, in addition to altered PGn-enolase binding, may exacerbate decreased efflux of Aβ. Studies to test these notions are now underway in our laboratory.
4.0 Enolase in Other Neurodegenerative Diseases
AD is just one of many age-related neurodegenerative disorders exhibiting a progressive decline in cognitive function, as well as extensive synapse and neuronal cell loss. Shared clinical characteristics between diseases such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), Huntington’s disease (HD), amyolateral sclerosis (ALS), and Niemann-Pick disease (PiD) involve common pathologies including oxidative stress and damage, mitochondrial dysfunction, hindered protein degradation, abnormal intracellular signaling, and cell-cycle arrest (Beal 1995, Duda et al. 2000, Jenner & Olanow 1998, McNaught & Jenner 2001, Cassarino et al. 2000). Unfortunately, not much research to date has focused on the expression, function, and/or oxidation state of the enzyme enolase in these other neurodegenerative diseases, although many studies have utilized NSE as a marker for global neuronal and glial cell loss. Studies conducted by our laboratory, among a few others, however, have shown decreased activity, as well as increased expression and oxidative modification of α- and γ-enolase in mouse models of HD (Perluigi et al. 2005b, Sorolla et al. 2008), PD (Stauber et al. 2008, Poon et al. 2005, Gomez & Ferrer 2009, De Iuliis et al. 2005), DLB (Gomez & Ferrer 2009), and fALS (Perluigi et al. 2005a, Casoni et al. 2005), in addition to AD (Section 3.0). Conversely, it is interesting to note that α-enolase expression is down-regulated in the striatum of maneb- and paraquat-induced PD mouse models (Patel et al. 2007), revealing that the specific route of pathologic cell signaling, oxidative modification, and protein expression may depend upon which factors, genetic and/or environmental, ultimately induce onset of disease pathology.
Considering that hypometabolism/hypoxia, excitotoxicity, the plasminogen system, MAPK/ERK1/2 signaling, HIF-1α, and c-Myc have all been implicated as being both protective and deleterious in the pathologies of HD, PD, ALS, DLB, and PiD (Demestre et al. 2006, Varma et al. 2007, Zhu et al. 2002a, 2003, Berding et al. 2001, Kulich et al. 2007, Ferrer et al. 2001a, 2001b, 2001c, Apostol et al. 2006, Beal 2008, Glas et al. 2007, Yang et al. 2005, Ferrer & Blanco 2000), it is quite likely that enolase dysfunction, dysregulation, and oxidative modification reported by our laboratory in the aforementioned tauopathies and Lewy body variants is a result of similar, if not identical, protective pathways suggested for MCI, EOAD, and AD above. However, more studies are needed to investigate this notion/theory since the plasminogen system, MAPK/ERK1/2, HIF-1a, and c-Myc signaling may all act differently in each neurodegenerative disease, in response/accordance to different cell types, apoptotic stimuli, and available downstream targets.
5.0 Conclusion
Although the main cause(s) of AD remain unknown, it is evident that up-regulation of glycolytic enzymes, like enolase, is significant to disease progression. Results from our laboratory support the view that enolase is more than just a glycolytic enzyme, but possesses other functions critical to brain cell survival. In this review we propose an expanded role for enolase that occurs concurrently with up-regulation of this enzyme in AD brain, a role to promote neuronal protection from Aβ accumulation and possibly glutamate excitotoxicity via action of the PGn and MAPK/ERK1/2 systems. However, given that oxidative modification of enzymes generally leads to dysfunction (Butterfield et al. 2007), these putative roles of enolase to protect against neuronal death in AD brain fail. Moreover, noting that both harmful and neuroprotective effects of both the MAPK/ERK1/2 and PGn systems in the brain are known, it is evident that the ability of tPA/PGn and plasmin to modulate neuronal death or survival via MAPK/ERK1/2 and enolase in MCI and/or AD would depend upon the cell type and apoptotic stimulus (Tucker et al. 2000b). In addition, since our laboratory has identified enolase to be one of the most oxidatively modified proteins in MCI, EOAD, and AD (Sultana et al. 2006a, 2006b, Butterfield et al. 2006a, 2006b, Castegna et al. 2002, 2003, Reed et al. 2008a, 2008b, Perluigi et al. 2009, Newman et al. 2007), as well as in response to Aβ(1-42) (Boyd-Kimball et al. 2005), it is possible that this enzyme becomes either completely unable to integrate into the cell membrane in order to bind PGn, or the oxidative dysfunction of enolase renders this enzyme incapable of binding to PGn/plasmin while membrane-integrated. These scenarios are consistent with the reported decreased levels of plasmin in AD brain (Ledesma et al. 2000). Taken together, whether or not the up-regulation of enolase in AD brain stretches beyond the basic need for metabolic ATP remains unknown, but it is highly likely that oxidative dysfunction of multifunctional enolase extends beyond altered glucose metabolism in ways that contribute to biochemical, pathological, and clinical characteristics of AD. If sustained by ongoing studies, this hypothesis would suggest that enolase is a promising therapeutic target of this devastating dementing disorder.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants to D.A.B. [AG-10836; AG-029839; AG-05119].
References
- Aaronson RM, Graven KK, Tucci M, McDonald RJ, Farber HW. Non-neuronal enolase is an endothelial hypoxic stress protein. J. Biol. Chem. 1995;270:27752–27757. doi: 10.1074/jbc.270.46.27752. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- al-Giery AG, Brewer JM. Characterization of the interaction of yeast enolase with polynucleotides. Biochim. Biophys. Acta. 1992;1159:134–140. doi: 10.1016/0167-4838(92)90017-8. [DOI] [PubMed] [Google Scholar]
- Andrade-Gordon P, Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986;25:4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Apostol BL, Illes K, Pallos J, et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum. Mol. Genet. 2006;15:273–285. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- Bader Lange ML, Cenini G, Piroddi M, Abdul HM, Sultana R, Galli F, Memo M, Butterfield DA. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol. Dis. 2008;29:456–464. doi: 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Beal MF. The urokinase system of plasminogen activator plays a role in amyotrophic lateral sclerosis (ALS) pathogenesis. Exp. Neurol. 2008;211:332–333. doi: 10.1016/j.expneurol.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Bentley DL, Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986;321:702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Berding G, Odin P, Brooks DJ, et al. Resting regional cerebral glucose metabolism in advanced Parkinson's disease studied in the off and on conditions with [(18)F]FDG-PET. Mov. Disord. 2001;16:1014–1022. doi: 10.1002/mds.1212. [DOI] [PubMed] [Google Scholar]
- Bergman AC, Linder C, Sakaguchi K, et al. Increased expression of α-enolase in c-jun transformed rat fibroblasts without increased activation of plasminogen. FEBS Lett. 1997;417:17–20. doi: 10.1016/s0014-5793(97)01247-7. [DOI] [PubMed] [Google Scholar]
- Blasi F, Vassalli JD, Dano K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J. Cell Biol. 1987;104:801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth W. α2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- Bottalico LA, Kendrick NC, Keller A, Li Y, Tabas I. Cholesteryl ester loading of mouse peritoneal macrophages is associated with changes in the expression or modification of specific cellular proteins, including increase in an α-enolase isoform. Arterioscler. Thromb. 1993;13:264–275. doi: 10.1161/01.atv.13.2.264. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA. Proteomic identification of proteins oxidized by Aβ(1-42) in synaptosomes: implications for Alzheimer's disease. Brain Res. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- Briggs MR, Kadonaga JT, Bell SP, Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986;234:47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Schumacker PT, Zhang H. Nitric oxide acutely inhibits neuronal energy production. The Committees on Neurobiology and Cell Physiology. J. Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Bu G, Williams S, Strickland DK, Schwartz AL. Low density lipoprotein receptor-related protein/α2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc. Natl. Acad. Sci. USA. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulliard C, Zurbriggen R, Tornare J, Faty M, Dastoor Z, Dreyer JL. Purification of a dichlorophenol-indophenol oxidoreductase from rat and bovine synaptic membranes: tight complex association of a glyceraldehyde-3-phosphate dehydrogenase isoform, TOAD64, enolase-γ and aldolase C. Biochem. J. 1997;324 ( Pt 2):555–563. doi: 10.1042/bj3240555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid β-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic. Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid β-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol. Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol. Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer's disease: an initial assessment. J. Alzheimers Dis. 2006a;10:391–397. doi: 10.3233/jad-2006-10407. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol. Dis. 2006b;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic. Biol. Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv. Cell. Aging Gerontol. 1997;2:161–191. [Google Scholar]
- Butterfield DA, Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer's disease and mild cognitive impairment: insights into the progression of this dementing disorder. J. Alzheimers Dis. 2007;12:61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, et al. Evidence that tumor necrosis factor-α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Cacquevel M, Launay S, Castel H, et al. Ageing and amyloid-β peptide deposition contribute to an impaired brain tissue plasminogen activator activity by different mechanisms. Neurobiol. Dis. 2007;27:164–173. doi: 10.1016/j.nbd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Schoonjans L, Kieckens L, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Casoni F, Basso M, Massignan T, Gianazza E, Cheroni C, Salmona M, Bendotti C, Bonetto V. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J. Biol. Chem. 2005;280:16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr., Sturgill TW, Bennett JP., Jr. Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-κB in cellular models of Parkinson's disease. J. Neurochem. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, α-enolase and heat shock cognate 71. J. Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer's disease brain. J. Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator (tPA). II. Annexin II-mediated enhancement of tPA-dependent plasminogen activation. J. Biol. Chem. 1994;269:21198–21203. [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chaudhary D, Miller DM. The c-myc promoter binding protein (MBP-1) and TBP bind simultaneously in the minor groove of the c-myc P2 promoter. Biochemistry. 1995;34:3438–3445. doi: 10.1021/bi00010a036. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Cheng M, Wang D, Roussel MF. Expression of c-myc in response to colony-stimulating factor-1 requires mitogen-activated protein kinase kinase-1. J. Biol. Chem. 1999;274:6553–6558. doi: 10.1074/jbc.274.10.6553. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Collen D. The plasminogen (fibrinolytic) system. Thromb. Haemost. 1999;82:259–270. [PubMed] [Google Scholar]
- Conboy L, Murphy KJ, Regan CM. Amyloid precursor protein expression in the rat hippocampal dentate gyrus modulates during memory consolidation. J. Neurochem. 2005;95:1677–1688. doi: 10.1111/j.1471-4159.2005.03484.x. [DOI] [PubMed] [Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J. Hepatol. 2006;44:918–929. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Esch FS, Taylor SS, Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine kinases in vivo and in vitro. J. Biol. Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- De Iuliis A, Grigoletto J, Recchia A, Giusti P, Arslan P. A proteomic approach in the study of an animal model of Parkinson's disease. Clin. Chim. Acta. 2005;357:202–209. doi: 10.1016/j.cccn.2005.03.028. [DOI] [PubMed] [Google Scholar]
- De Sousa LP, Brasil BS, Silva BM, Freitas MH, Nogueira SV, Ferreira PC, Kroon EG, Bonjardim CA. Plasminogen/plasmin regulates c-fos and egr-1 expression via the MEK/ERK pathway. Biochem. Biophys. Res. Commun. 2005;329:237–245. doi: 10.1016/j.bbrc.2005.01.123. [DOI] [PubMed] [Google Scholar]
- Demestre M, Howard RS, Orrell RW, Pullen AH. Serine proteases purified from sera of patients with amyotrophic lateral sclerosis (ALS) induce contrasting cytopathology in murine motoneurones to IgG. Neuropathol. Appl. Neurobiol. 2006;32:141–156. doi: 10.1111/j.1365-2990.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Galvan C, Ledesma MD. Plasmin deficiency in Alzheimer's disease brains: causal or casual. Neurodegener. Dis. 2004;1:205–212. doi: 10.1159/000080987. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Chen Q, et al. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Feo S, Arcuri D, Piddini E, Passantino R, Giallongo A. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1) FEBS Lett. 2000;473:47–52. doi: 10.1016/s0014-5793(00)01494-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, et al. Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J. Biol. Chem. 2004a;279:50850–50856. doi: 10.1074/jbc.M407069200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, et al. Is tissue-type plasminogen activator a neuromodulator. Mol. Cell. Neurosci. 2004b;25:594–601. doi: 10.1016/j.mcn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R. n-Myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res. Mol. Brain Res. 2000;77:270–276. doi: 10.1016/s0169-328x(00)00062-0. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Puig B. Phosphorylated c-myc expression in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Neuropathol. Appl. Neurobiol. 2001a;27:343–351. doi: 10.1046/j.1365-2990.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Puig B, Barrachina M, Gomez C, Ambrosio S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and Dementia with Lewy bodies. J. Neural Transm. 2001b;108:1383–1396. doi: 10.1007/s007020100015. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, et al. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 2001c;11:144–158. doi: 10.1111/j.1750-3639.2001.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann GF, Fehlau R, Schneider H, Knauf PA. The effect of ferricyanide with iodoacetate in calcium-free solution on passive cation permeability in human red blood cells: comparison with the Gardos-effect and with the influence of PCMBS on passive cation permeability. Biochim. Biophys. Acta. 1989;983:179–185. doi: 10.1016/0005-2736(89)90231-9. [DOI] [PubMed] [Google Scholar]
- Giallongo A, Feo S, Moore R, Croce CM, Showe LC. Molecular cloning and nucleotide sequence of a full-length cDNA for human α-enolase. Proc. Natl. Acad. Sci. USA. 1986;83:6741–6745. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo A, Oliva D, Cali L, Barba G, Barbieri G, Feo S. Structure of the human gene for α-enolase. Eur. J. Biochem. 1990;190:567–573. doi: 10.1111/j.1432-1033.1990.tb15611.x. [DOI] [PubMed] [Google Scholar]
- Gils A, Declerck PJ. Proteinase specificity and functional diversity in point mutants of plasminogen activator inhibitor 1. J. Biol. Chem. 1997;272:12662–12666. doi: 10.1074/jbc.272.19.12662. [DOI] [PubMed] [Google Scholar]
- Glas M, Popp B, Angele B, Koedel U, Chahli C, Schmalix WA, Anneser JM, Pfister HW, Lorenzl S. A role for the urokinase-type plasminogen activator system in amyotrophic lateral sclerosis. Exp. Neurol. 2007;207:350–356. doi: 10.1016/j.expneurol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Gomez A, Ferrer I. Increased oxidation of certain glycolysis and energy metabolism enzymes in the frontal cortex in Lewy body diseases. J. Neurosci. Res. 2009;87:1002–1013. doi: 10.1002/jnr.21904. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Costa RH, Xanthopoulos KG, Darnell JE. One factor recognizes the liver-specific enhancers in α1-antitrypsin and transthyretin genes. Science. 1988;239:786–788. doi: 10.1126/science.3257586. [DOI] [PubMed] [Google Scholar]
- Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Seth A, Davis RJ. Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc. Natl. Acad. Sci. USA. 1993;90:3216–3220. doi: 10.1073/pnas.90.8.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J. Biol. Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- Hattori T, Ohsawa K, Mizuno Y, Kato K, Kohsaka S. Synthetic peptide corresponding to 30 amino acids of the C-terminal of neuron-specific enolase promotes survival of neocortical neurons in culture. Biochem. Biophys. Res. Commun. 1994;202:25–30. doi: 10.1006/bbrc.1994.1888. [DOI] [PubMed] [Google Scholar]
- Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci. Res. 1995;21:191–198. doi: 10.1016/0168-0102(94)00849-b. [DOI] [PubMed] [Google Scholar]
- Holland JP, Labieniec L, Swimmer C, Holland MJ. Homologous nucleotide sequences at the 5' termini of messenger RNAs synthesized from the yeast enolase and glyceraldehyde-3-phosphate dehydrogenase gene families. The primary structure of a third yeast glyceraldehyde-3-phosphate dehydrogenase gene. J. Biol. Chem. 1983;258:5291–5299. [PubMed] [Google Scholar]
- Hurlin PJ, Dezfouli S. Functions of Myc:Max in the control of cell proliferation and tumorigenesis. Int. Rev. Cytol. 2004;238:183–226. doi: 10.1016/S0074-7696(04)38004-6. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Elvhage TE, Reiter J. Extracellular signal regulated kinases. Localization of protein and mRNA in the human hippocampal formation in Alzheimer's disease. Am. J. Pathol. 1994;144:565–572. [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Tanzi RE, Marzloff K, Barbour R, Schenk D. Kunitz protease inhibitor-containing amyloid-β protein precursor immunoreactivity in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 1992;51:76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Iida H, Yahara I. Yeast heat-shock protein of Mr 48,000 is an isoprotein of enolase. Nature. 1985;315:688–690. [Google Scholar]
- Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann. Neurol. 1998;44:72–84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am. J. Physiol. 1997;273:1029–1035. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- Jones NC, Rigby PW, Ziff EB. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988;2:267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Reiser G. Requirement of glycolytic and mitochondrial energy supply for loading of Ca2+ stores and InsP(3)-mediated Ca2+ signaling in rat hippocampus astrocytes. J. Neurosci. Res. 2000;61:409–420. doi: 10.1002/1097-4547(20000815)61:4<409::AID-JNR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kalderon N. Migration of Schwann cells and wrapping of neurites in vitro: a function of protease activity (plasmin) in the growth medium. Proc. Natl. Acad. Sci. USA. 1979;76:5992–5996. doi: 10.1073/pnas.76.11.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon N. Role of the plasmin-generating system in the developing nervous tissue: I. Proteolysis as a mitogenic signal for the glial cells. J. Neurosci. Res. 1982;8:509–519. doi: 10.1002/jnr.490080237. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann. NY Acad. Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Karp G. Cell and Molecular Biology. Concepts and Experiments. John Wiley & Sons, Inc.; New York, NY, USA: 2003. [Google Scholar]
- Kauppinen RA, Enkvist K, Holopainen I, Akerman KE. Glucose deprivation depolarizes plasma membrane of cultured astrocytes and collapses transmembrane potassium and glutamate gradients. Neuroscience. 1988;26:283–289. doi: 10.1016/0306-4522(88)90145-5. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem. Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kingston IB, Castro MJ, Anderson S. In vitro stimulation of tissue-type plasminogen activator by Alzheimer amyloid β-peptide analogues. Nat. Med. 1995;1:138–142. doi: 10.1038/nm0295-138. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J. Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi N, Tokushima Y, Oishi K, Takahashi Y, Shiojiri S, Nakamura S, Tanaka S, Kodaira R, Ito H. Determination of amyloid beta protein precursors harboring active form of proteinase inhibitor domains in cerebrospinal fluid of Alzheimer's disease patients by trypsin-antibody sandwich ELISA. Biochem. Biophys. Res. Commun. 1990;166:1453–1459. doi: 10.1016/0006-291x(90)91030-v. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Jr., Stetler-Stevenson WG. Structural biochemistry and activation of matrix metalloproteases. Curr. Opin. Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- Konakova M, Hucho F, Schleuning WD. Downstream targets of urokinase-type plasminogen-activator-mediated signal transduction. Eur. J. Biochem. 1998;253:421–429. doi: 10.1046/j.1432-1327.1998.2530421.x. [DOI] [PubMed] [Google Scholar]
- Konduri SD, Rao CN, Chandrasekar N, et al. A novel function of tissue factor pathway inhibitor-2 (TFPI-2) in human glioma invasion. Oncogene. 2001;20:6938–6945. doi: 10.1038/sj.onc.1204847. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Kovacs DM. α2-macroglobulin in late-onset Alzheimer's disease. Exp. Gerontol. 2000;35:473–479. doi: 10.1016/s0531-5565(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Bouma B, Kroon-Batenburg LM, Reijerkerk A, Wu YP, Voest EE, Gebbink MF. Tissue-type plasminogen activator is a multiligand cross-β structure receptor. Curr. Biol. 2002;12:1833–1839. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic. Biol. Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated α-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG. Brain plasmin enhances APP α-cleavage and Aβ degradation and is reduced in Alzheimer's disease brains. EMBO Rep. 2000;1:530–535. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TS, Hunt JB, Aveline LD, Jonscher KR, Louie DF, Yeh JM, Nahreini TS, Resing KA, Ahn NG. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol. Cell. 2000;6:1343–1354. doi: 10.1016/s1097-2765(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–4636. [PubMed] [Google Scholar]
- Lottenberg R, Broder CC, Boyle MD, Kain SJ, Schroeder BL, Curtiss R. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 1992;174:5204–5210. doi: 10.1128/jb.174.16.5204-5210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J. Neurosci. Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli JD. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi F, Van Nostrand WE, Schmaier AH. Protease nexin-2/amyloid β-protein precursor inhibits factor Xa in the prothrombinase complex. J. Biol. Chem. 1995;270:23468–23474. doi: 10.1074/jbc.270.40.23468. [DOI] [PubMed] [Google Scholar]
- Marcu KB, Bossone SA, Patel AJ. Myc function and regulation. Annu. Rev. Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch. Neurol. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch. Neurol. 2007;64:954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- McCarthy SA, Chen D, Yang BS, et al. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol. Cell. Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci. Lett. 2001;297:191–194. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-β (Aβ) degradation and inhibits Aβ-induced neurodegeneration. J. Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Gonzalez M, Perez-Pinera P, Martinez-Rivera M, Calatayud MT, Menes B. APP processing and the APP-KPI domain involvement in the amyloid cascade. Neurodegener. Dis. 2005;2:277–283. doi: 10.1159/000092315. [DOI] [PubMed] [Google Scholar]
- Mielke R, Schroder R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer's disease. Acta Neuropathol. 1996;91:174–179. doi: 10.1007/s004010050410. [DOI] [PubMed] [Google Scholar]
- Miles LA, Dahlberg CM, Levin EG, Plow EF. Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these fibrinolytic components to cells. Biochemistry. 1989;28:9337–9343. doi: 10.1021/bi00450a014. [DOI] [PubMed] [Google Scholar]
- Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: identification of α-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Iwamatsu A, Aki T, et al. ERK1/2 regulates intracellular ATP levels through α-enolase expression in cardiomyocytes exposed to ischemic hypoxia and reoxygenation. J. Biol. Chem. 2004;279:50120–50131. doi: 10.1074/jbc.M402299200. [DOI] [PubMed] [Google Scholar]
- Moir RD, Lynch T, Bush AI, et al. Relative increase in Alzheimer's disease of soluble forms of cerebral Aβ amyloid protein precursor containing the Kunitz protease inhibitory domain. J. Biol. Chem. 1998;273:5013–5019. doi: 10.1074/jbc.273.9.5013. [DOI] [PubMed] [Google Scholar]
- Moir RD, Tanzi RE. LRP-mediated clearance of Aβ is inhibited by KPI-containing isoforms of APP. Curr. Alzheimer Res. 2005;2:269–273. doi: 10.2174/1567205053585918. [DOI] [PubMed] [Google Scholar]
- Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Nagata K, Nakajima K, Kohsaka S. Plasminogen promotes the development of rat mesencephalic dopaminergic neurons in vitro. Brain Res. Dev. Brain Res. 1993;75:31–37. doi: 10.1016/0165-3806(93)90062-f. [DOI] [PubMed] [Google Scholar]
- Nagata K, Nakajima K, Takemoto N, Saito H, Kohsaka S. Microglia-derived plasminogen enhances neurite outgrowth from explant culture of rat brain. Int. J. Dev. Neurosci. 1992;11:227–237. doi: 10.1016/0736-5748(93)90081-n. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hatakeyama S, Ikesue A, Miyai H. Generation of interleukin-8 by plasmin from AVLPR-interleukin-8, the human fibroblast-derived neutrophil chemotactic factor. FEBS Lett. 1991;282:412–414. doi: 10.1016/0014-5793(91)80526-9. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Hamanoue M, Takemoto N, Hattori T, Kato K, Kohsaka S. Plasminogen binds specifically to α-enolase on rat neuronal plasma membrane. J. Neurochem. 1994;63:2048–2057. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Takemoto N, Kohsaka S. Retinoic acid enhances the secretion of plasminogen from cultured rat microglia. FEBS Lett. 1992a;314:167–170. doi: 10.1016/0014-5793(92)80966-k. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tsuzaki N, Nagata K, Takemoto N, Kohsaka S. Production and secretion of plasminogen in cultured rat brain microglia. FEBS Lett. 1992b;308:179–182. doi: 10.1016/0014-5793(92)81270-v. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tsuzaki N, Shimojo M, Hamanoue M, Kohsaka S. Microglia isolated from rat brain secrete a urokinase-type plasminogen activator. Brain Res. 1992c;577:285–292. doi: 10.1016/0006-8993(92)90285-h. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. W. H. Freeman & Co.; New York, NY: 2009. [Google Scholar]
- Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule, a proteomics approach. J. Neurosci. Res. 2007;85:1506–1514. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Onyango P, Lubyova B, Gardellin P, Kurzbauer R, Weith A. Molecular cloning and expression analysis of five novel genes in chromosome 1p36. Genomics. 1998;50:187–198. doi: 10.1006/geno.1997.5186. [DOI] [PubMed] [Google Scholar]
- Osthus RC, Shim H, Kim S, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Podlisny MB, Golde TE, et al. The beta amyloid protein precursor: mRNAs, membrane-associated forms, and soluble derivatives. Prog. Clin. Biol. Res. 1989a;317:971–984. [PubMed] [Google Scholar]
- Palmert MR, Podlisny MB, Witker DS, Oltersdorf T, Younkin LH, Selkoe DJ, Younkin SG. The β-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 1989b;86:6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V. Multifunctional α-enolase: its role in diseases. Cell. Mol. Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V, Fischetti VA. α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic Streptococci. J. Biol. Chem. 1998;273:14503–14515. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res. Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Palumbo B, Cardinali L, Loreti F, Chionne F, Cecchetti R, Senin U. Cerebrospinal fluid neuron-specific enolase in Alzheimer's disease and vascular dementia. Neurosci. Lett. 1995;183:43–45. doi: 10.1016/0304-3940(94)11110-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Sinha A, Singh MP. Identification of differentially expressed proteins in striatum of maneb-and paraquat-induced Parkinson's disease phenotype in mouse. Neurotoxicol. Teratol. 2007;29:578–585. doi: 10.1016/j.ntt.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Fai Poon H, Hensley K, Pierce WM, Klein JB, Calabrese V, De Marco C, Butterfield DA. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2005a;38:960–968. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Poon HF, Maragos W, Pierce WM, Klein JB, Calabrese V, Cini C, De Marco C, Butterfield DA. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: a model of Huntington disease. Mol. Cell. Proteomics. 2005b;4:1849–1861. doi: 10.1074/mcp.M500090-MCP200. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Sultana R, Cenini G, Domenico F, Memo M, Pierce WM, Coccia R, Butterfield DA. Redox proteomics identification of HNE-modified brain proteins in Alzheimer's disease: role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics Clin. Appl. 2009 doi: 10.1002/prca.200800161. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Calame K. Proteins binding to site C2 (μE3) in the immunoglobulin heavy-chain enhancer exist in multiple oligomeric forms. Mol. Cell. Biol. 1989;9:776–786. doi: 10.1128/mcb.9.2.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak J, Ivanek R, Toman O, Cmejla R, Cmejlova J, Vyoral D, Zivny J, Vulpe CD. Déjà vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics. 2008;8:1744–1749. doi: 10.1002/pmic.200700919. [DOI] [PubMed] [Google Scholar]
- Plow EF, Felez J, Miles LA. Cellular regulation of fibrinolysis. Thromb. Haemost. 1991;66:32–36. [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Ponte P, Gonzalez-DeWhitt P, Schilling J, et al. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988;331:525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P α-synuclein transgenic mice--a model of familial Parkinson's disease. Neurobiol. Dis. 2005;18:492–498. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Amyloid precursor protein mRNA levels in Alzheimer's disease brain. Brain Res. Mol. Brain Res. 2004;122:1–9. doi: 10.1016/j.molbrainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Borth W, Ye Z, Haass C, Teplow DB, Selkoe DJ. Degradation of amyloid β-protein by a serine protease-α2-macroglobulin complex. J. Biol. Chem. 1996;271:8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- Ray R, Miller DM. Cloning and characterization of a human c-myc promoter-binding protein. Mol Cell Biol. 1991a;11:2154–2161. doi: 10.1128/mcb.11.4.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Miller DM. Cloning and characterization of a human c-myc promoter-binding protein. Mol. Cell. Biol. 1991b;11:2154–2161. doi: 10.1128/mcb.11.4.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RB. Induction of cell death in murine fibroblasts by a c-myc promoter binding protein. Cell. Growth Differ. 1995;6:1089–1096. [PubMed] [Google Scholar]
- Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the α2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann. Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 1995;227:407–415. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol. Dis. 2008a;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Reed TT, Pierce WM, Jr., Turner DM, Markesbery WR, Butterfield DA. Proteomic identification of nitrated brain proteins in early Alzheimer's disease inferior parietal lobule. J. Cell. Mol. Med. 2008b doi: 10.1111/j.1582-4934.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1α (HIF-1α) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Rifkin DB, Moscatelli D, Bizik J, Quarto N, Blei F, Dennis P, Flaumenhaft R, Mignatti P. Growth factor control of extracellular proteolysis. Cell. Differ. Dev. 1990;32:313–318. doi: 10.1016/0922-3371(90)90045-x. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr. Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Saito F, Yanagisawa K, Miyatake T. Soluble derivatives of β/A4 amyloid protein precursor in human cerebrospinal fluid are both N- and O-glycosylated. Brain Res. Mol. Brain Res. 1993;19:171–174. doi: 10.1016/0169-328x(93)90164-k. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD. Extracellular proteolysis in the adult murine brain. J. Clin. Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin-1 and -2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Dahl LD, Rozemuller AJ, Roos RA, Wagner SL, Chung R, Nostrand WE. Protease nexin-2/amyloid-β protein precursor. A tight-binding inhibitor of coagulation factor IXa. J. Clin. Invest. 1993;92:2540–2545. doi: 10.1172/JCI116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedoris KC, Thomas SD, Miller DM. promoter binding protein regulates the cellular response to an altered glucose concentration. Biochemistry. 2007;46:8659–8668. doi: 10.1021/bi7003558. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- Shimokawa M, Nakamura K, Maruyama K, Tagawa K, Miyatake T, Sugita H, Ishiura S, Suzuki K. Inhibitory spectra of purified protease nexin-II and related proteins towards cellular proteinases. Biochimie. 1993;75:911–915. doi: 10.1016/0300-9084(93)90048-w. [DOI] [PubMed] [Google Scholar]
- Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel GJ, Albers RW, Brady ST, Price DL. Basic Neurochemistry. Molecular, Cellular and Medical Aspects. Elsivier Academic Press, Burlington, MA; USA: 2006. [Google Scholar]
- Silver IA, Erecinska M. Energetic demands of the Na+/K+-ATPase in mammalian astrocytes. Glia. 1997;21:35–45. doi: 10.1002/(sici)1098-1136(199709)21:1<35::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J. Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport. 2000;11:2759–2764. doi: 10.1097/00001756-200008210-00030. [DOI] [PubMed] [Google Scholar]
- Smith RP, Higuchi DA, Broze GJ., Jr. Platelet coagulation factor XIa-inhibitor, a form of Alzheimer amyloid precursor protein. Science. 1990;248:1126–1128. doi: 10.1126/science.2111585. [DOI] [PubMed] [Google Scholar]
- Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008;45:667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid-β peptide. Neuron. 2003;39:43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- Sousa LP, Silva BM, Brasil BS, Nogueira SV, Ferreira PC, Kroon EG, Kato K, Bonjardim CA. Plasminogen/plasmin regulates α-enolase expression through the MEK/ERK pathway. Biochem. Biophys. Res. Commun. 2005;337:1065–1071. doi: 10.1016/j.bbrc.2005.09.154. [DOI] [PubMed] [Google Scholar]
- Spencer CA, Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv. Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Stauber J, Lemaire R, Franck J, Bonnel D, Croix D, Day R, Wisztorski M, Fournier I, Salzet M. MALDI imaging of formalin-fixed paraffin-embedded tissues: application to model animals of Parkinson disease for biomarker hunting. J. Proteome Res. 2008;7:969–978. doi: 10.1021/pr070464x. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Miller DM. Structural analysis of α-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J. Biol. Chem. 2000;275:5958–5965. doi: 10.1074/jbc.275.8.5958. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer's disease hippocampal proteome. J. Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging. 2006a;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol. Dis. 2006b;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Newman SF, Pierce W, Cini C, Coccia R, Butterfield DA. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer's disease. Antioxidant Redox. Signal. 2009 doi: 10.1089/ars.2009.2810. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J. Neurochem. 1991;57:1178–1184. doi: 10.1111/j.1471-4159.1991.tb08277.x. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, McClatchey AI, Lamperti ED, Villa-Komaroff L, Gusella JF, Neve RL. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988;331:528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. Plasmin-induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J. Biol. Chem. 2002;277:33564–33570. doi: 10.1074/jbc.M205514200. [DOI] [PubMed] [Google Scholar]
- Thompson KS, Towle HC. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J. Biol. Chem. 1991;266:8679–8682. [PubMed] [Google Scholar]
- Toole-Simms W, Sun IL, Faulk WP, Low H, Lindgren A, Crane FL, Morre DJ. Inhibition of transplasma membrane electron transport by monoclonal antibodies to the transferrin receptor. Biochem. Biophys. Res. Commun. 1991;176:1437–1442. doi: 10.1016/0006-291x(91)90447-f. [DOI] [PubMed] [Google Scholar]
- Towle HC. Metabolic regulation of gene transcription in mammals. J. Biol. Chem. 1995;270:23235–23238. doi: 10.1074/jbc.270.40.23235. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Mawal-Dewan M, Schmidt ML, Martin J, Lee VM. Localization of the mitogen activated protein kinase ERK2 in Alzheimer's disease neurofibrillary tangles and senile plaque neurites. Brain Res. 1993;618:333–337. doi: 10.1016/0006-8993(93)91286-2. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S. Tissue plasminogen activator requires plasminogen to modulate amyloid-β neurotoxicity and deposition. J. Neurochem. 2000a;75:2172–2177. doi: 10.1046/j.1471-4159.2000.0752172.x. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko M, Caldwell JN, et al. The plasmin system is induced by and degrades amyloid-β aggregates. J. Neurosci. 2000b;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Porter M. Plasmin cleavage of the amyloid β-protein: alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry. 1999;38:11570–11576. doi: 10.1021/bi990610f. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Farrow JS, Cunningham DD. Immunopurification and protease inhibitory properties of protease nexin-2/amyloid β-protein precursor. J. Biol. Chem. 1990;265:9591–9594. [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid β-protein precursor. Nature. 1989;341:546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Varma H, Cheng R, Voisine C, Hart AC, Stockwell BR. Inhibitors of metabolism rescue cell death in Huntington's disease models. Proc. Natl. Acad. Sci. USA. 2007;104:14525–14530. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji MA, Vassalli JD, Estensen RD, Reich E. Plasminogen activator of islets of Langerhans: modulation by glucose and correlation with insulin production. Proc. Natl. Acad. Sci. USA. 1980;77:875–879. doi: 10.1073/pnas.77.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Liljestrom P, Mikus P, Andersson H, Ny T. The efficiency of the uncleaved secretion signal in the plasminogen activator inhibitor type 2 protein can be enhanced by point mutations that increase its hydrophobicity. J. Biol. Chem. 1991;266:15240–15243. [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993a;268:21513–21518. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA. 1993b;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein MJ, Doolittle RF. Differential specificities of the thrombin, plasmin and trypsin with regard to synthetic and natural substrates and inhibitors. Biochim. Biophys. Acta. 1972;258:577–590. doi: 10.1016/0005-2744(72)90250-1. [DOI] [PubMed] [Google Scholar]
- Willoughby DA, Rozovsky I, Lo AC, Finch CE. β-Amyloid precursor protein (APP) and APP-RNA are rapidly affected by glutamate in cultured neurons: selective increase of mRNAs encoding a Kunitz protease inhibitor domain. J. Mol. Neurosci. 1995;6:257–276. doi: 10.1007/BF02736785. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Dowjat WK, Buxbaum JD, et al. A novel Polish presenilin-1 mutation (P117L) is associated with familial Alzheimer's disease and leads to death as early as the age of 28 years. Neuroreport. 1998;9:217–221. doi: 10.1097/00001756-199801260-00008. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Lietman T, Williams LA, Stapel SO, De Jong WW, Horwitz J, Piatigorsky J. τ-Crystallin/α-enolase: one gene encodes both an enzyme and a lens structural protein. J. Cell Biol. 1988;107:2729–2736. doi: 10.1083/jcb.107.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnendt S, Wetzels I, Gunzler WA. Amyloid β peptides stimulate tissue-type plasminogen activator but not recombinant prourokinase. Thromb. Res. 1997;85:217–224. doi: 10.1016/s0049-3848(97)00006-6. [DOI] [PubMed] [Google Scholar]
- Xu F, Davis J, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Protease nexin-2/amyloid β-protein precursor limits cerebral thrombosis. Proc. Natl. Acad. Sci. USA. 2005;102:18135–18140. doi: 10.1073/pnas.0507798102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Previti ML, Nieman MT, Davis J, Schmaier AH, Van Nostrand WE. AβPP/APLP2 family of Kunitz serine proteinase inhibitors regulate cerebral thrombosis. J. Neurosci. 2009;29:5666–5670. doi: 10.1523/JNEUROSCI.0095-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ. Res. 1995;77:88–97. doi: 10.1161/01.res.77.1.88. [DOI] [PubMed] [Google Scholar]
- Yang YT, Ju TC, Yang DI. Induction of hypoxia inducible factor-1 attenuates metabolic insults induced by 3-nitropropionic acid in rat C6 glioma cells. J. Neurochem. 2005;93:513–525. doi: 10.1111/j.1471-4159.2005.03032.x. [DOI] [PubMed] [Google Scholar]
- Zhan SS, Sandbrink R, Beyreuther K, Schmitt HP. APP with Kunitz type protease inhibitor domain (KPI) correlates with neuritic plaque density but not with cortical synaptophysin immunoreactivity in Alzheimer's disease and non-demented aged subjects: a multifactorial analysis. Clin. Neuropathol. 1995;14:142–149. [PubMed] [Google Scholar]
- Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am. J. Pathol. 2002a;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002b;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.