Abstract
The α-α helix motif presents key recognition domains in protein-protein and protein-oligonucleotide binding, and is one of the most common super-secondary structures. Herein we describe the design, synthesis and structural characterization of an α-α hairpin analogue based on a tetra-coordinated Pd(II) bis-(iminoisoquinoline) complex as a template for the display of two α-helix mimics. This approach is exemplified by the attachment of two biphenyl peptidomimetics to reproduce the side-chains of the i and i+4 residues of two helices.
Keywords: structural mimicry; protein-protein interaction; alpha helix, side chain, GCN4
Introduction
The design of non-peptidic small-molecules to reproduce the recognition properties of therapeutically relevant secondary structural protein elements is now an established field.1 With a particular emphasis on the moderation of protein-protein interactions (PPIs), there are many examples of peptidomimetics of α-helices2,3,4 and a growing number that mimic some of the properties of β-strands and β-sheets.5,6 However, there are few non-peptidic mimics of more complex super-secondary structures.7 The majority of approaches are based on the modification of peptidic folding preferences via manipulation of the primary sequence.8,9 Examples include careful positioning of non-natural α,β-dehydrophenylalanine to promote hydrophobic interactions,10 or the incorporation of reactive natural amino acids, including cysteine,11 that are responsive to an oxidative stimulus.12 Strategies relying on the templating effect of cyclic peptides or synthetic molecules, such as norbornene, have also been explored.13,14
One of the most common super-secondary structures is the α-α hairpin, in which a sequence of amino acids links two α-helices, usually in an antiparallel arrangement.15,16 Others include the β-α-β motif,17,18 α-α corner,19 β-β corner,20 and β-β hairpin (Figure 1).21 Various models for α-helix packing emphasise the importance of helix complementarity,22,23,24 in which the nature of the side-chains determines the packing (or torsion) angle ω.25,26 For the α-α hairpin De Grado found a strong correlation between the number of linking residues and the inter-helical distance (the average of the orthogonal space between the axis of the two helices).27,28 Distances range between 5 Å for short linkers of one or two amino acids, with many examples of 10 Å where the turn is formed of several amino acids.
Figure 1.
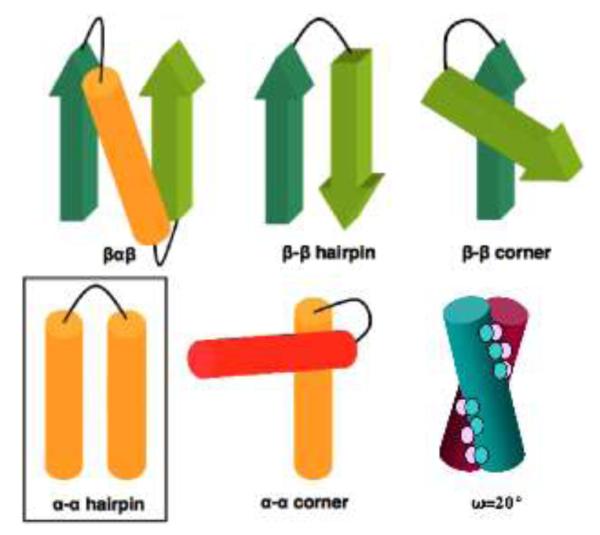
Common super-secondary structural motifs, with the focus of this work – the α-α hairpin, highlighted. Bottom right: an omega angle of 20° between two α-helices.
Results and discussion
Herein we report the design and synthesis of a tetra-coordinated Pd(II) bis- (iminoisoquinoline) complex that templates the display of a range of α-helical peptidomimetics to reproduce the structural characteristics of an α-α hairpin. To replicate the inter-helical loop angle ω, of approximately 0° for the parallel disposition and 180° for the anti-parallel hairpin, we sought a metal complex with a square-planar coordination geometry.29,30 Inspired by previously reported Pd(II)-2-imino-pyridine complexes,31,32 and other metals with a N4 coordination geometry,33 we designed and synthesized bis-(imino-isoquinoline) ligand 4 (Scheme 1).
Scheme 1.
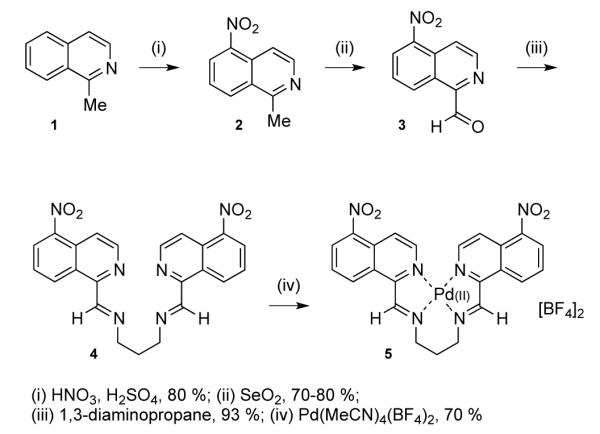
Synthesis of a model linker 5.
The synthesis commenced with nitration of 1-methyl-isoquinoline to give exclusively the 5-nitro regioisomer 2,34 followed by selenium dioxide-mediated oxidation of the methyl group to aldehyde 3.35 Condensation with 1,3-diaminopropane provided di-imine 4 as a reactive intermediate. Addition of commercially available tetrakis(acetonitrile)palladium(II) tetrafluoroborate gave complex 5, with an immediate chromatic change of the reaction mixture from yellow to dark orange-brown. Purification was achieved by precipitation from acetonitrile with chloroform (Scheme 1).
Upon chelation, the 1H-NMR signals corresponding to the methylenes adjacent to the imine nitrogens, and the central methylene of the propyl linker, show a change in multiplicity attributable to the introduction of cyclic rigidity. In addition, resonances corresponding to the aromatic and imine hydrogens undergo marked downfield shifts consistent with the withdrawal of electron density (Table 1, compound 5). Further characterization confirms the presence of the complex, with mass spectrometry showing ions consistent with the free di-cationic complex, and that of the mono-tetrafluoroborate anion. Elemental analysis is in good agreement with the hypothesized structure: found (calculated) C, 38.06 (38.24); H, 2.56 (2.51); N, 11.51 (11.63).
Table 1.
Selected 1H-NMR chemical shifts of the free ligand and the corresponding Pd(II) complex (5, 16 and 17). Values in ppm, multiplicities in brackets: (s) singlet, (d) doublet, (t) triplet, (qn) quintet, (m) multiplet, (br) broad singlet. [a] The chemical shift of this signal is overlapped with those of the biphenyl group.
| Compound | 1H Signal | Free Ligand | Complex |
|---|---|---|---|
| 5 | 3 | 8.70 (d) | 9.11 (d) |
| 5 | 4 | 8.24 (d) | 8.91 (m) |
| 5 | 6 | 8.38 (d) | 8.91 (m) |
| 5 | 7 | 7.62 (t) | 8.21 (m) |
| 5 | 8 | 9.88 (d) | 8.94 (d) |
| 5 | 9 | 8.77 (s) | 9.60 (br) |
| 5 | 11 | 4.02 (t) | 4.11 (m) |
| 5 | 12 | 2.37 (q) | 2.44 (m) |
| 16 | 3 | 8.27 (d) | 8.62 (d) |
| 16 | 4 | 7.36 (d) | 8.50 (d) |
| 16 | 6 | 7.48 (d) | 7.99 (d) |
| 16 | 7 | 7.09 (t) | 8.07 (m) |
| 16 | 8 | 7.84 (d) | 8.25 (d) |
| 16 | 9 | 8.72 (s) | 9.54 (s) |
| 16 | 11 | 4.00 (t) | 4.09 (m) |
| 16 | 12 | 2.40 (q) | 2.45 (m) |
| 16 | 13 | 7.80 (m) | 9.40 (s) |
| 17 | 3 | [a] | 8.65 (d) |
| 17 | 4 | [a] | 8.50 (d) |
| 17 | 6 | [a] | 8.21 (d) |
| 17 | 7 | [a] | 8.09 (m) |
| 17 | 8 | [a] | 8.46 (d) |
| 17 | 9 | 8.77 (br) | 9.54 (s) |
| 17 | 11 | 4.03 (m) | 4.11 (m) |
| 17 | 12 | 2.43 (m) | 2.44 (m) |
| 17 | 13 | [a] | 9.31 (s) |
Single-crystal X-ray diffraction was performed on samples grown at room temperature through slow evaporation of diethyl ether into a solution of complex 5 in acetonitrile.36 A slightly distorted square-planar geometry at palladium was observed with a mean planar deviation of 0.053 Å for the four Pd-N contacts. N-Pd-N cis- and trans-bite angles ranged from 79.67 to 105.16 and 174.91 to 172.94° respectively. The distance between the nitrogen atoms of the nitro groups, which provide attachment points for helix-mimetics, is 10.76 Å and thus congruent with the properties of common α-α hairpin motifs in proteins (Figure 2, see also supporting information Chapter 3).
Figure 2.
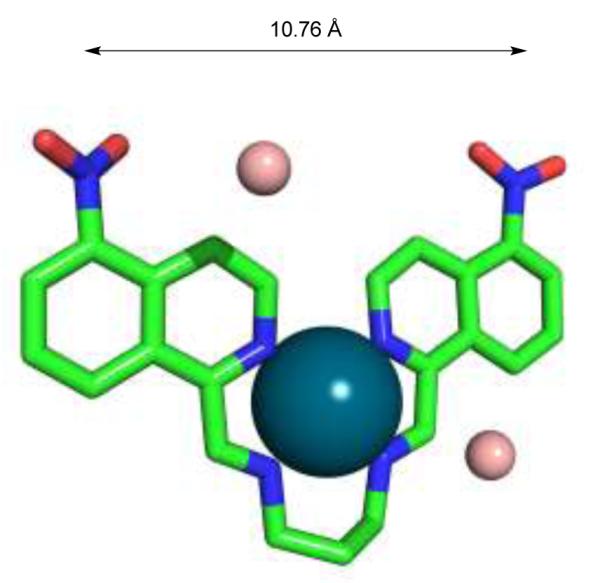
Single-crystal X-ray structure of Pd(II) bis(iminoisoquinoline) complex 5 (palladium grey, tetrafluoroborate brown). The position of the tetrafluoroborate ions is characterized by close intermolecular contacts: Pd…F 2.85 and 3.91 Å.
In order to adapt scaffold 5 for the display of helix mimetics, the synthesis required modification to allow nitro group reduction and coupling prior to imine formation and complexation. Accordingly, aldehyde 3 was protected as a dimethyl acetal and the nitro group reduced to give amine 7 in excellent yield (86 – 90 % over two steps). As proof of principle we chose commercially available 3-methyl-4-nitrobenzoic acid 8, as a mimic of the alanine side-chain and previously synthesised biphenyl carboxylic acid 937 as a mimic of two leucine residues in the i and i+4 positions. These acids were activated with thionyl chloride before coupling to give amides 10 and 11 respectively. The aldehyde was un-masked in good yield after treatment with a mixture of acetic- and hydrochloric acids. Di-imines were synthesized with 1,3-diaminopropane and used for complexation without further purification. Following the procedure for the synthesis of complex 5, precipitation gave two-amino acid mimetic 16 as a dark yellow powder in 60 % yield (Scheme 2).
Scheme 2.
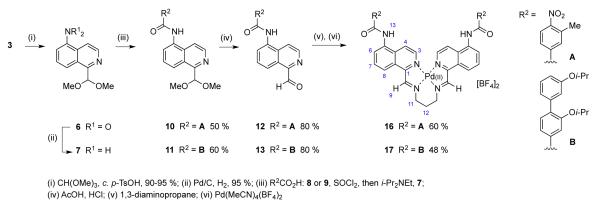
Synthesis of α-α hairpin mimetics bearing two 16, and four 17, side-chain groups. The methyl and iso-propoxy groups may be considered as mimics of alanine and leucine amino acid side-chains.
In accordance with model system 5, the 1H-NMR spectra of complexes 16 and 17 show a similar downfield shift of resonances when compared to those of the free ligands 14 and 15 (Table 1). Electrospray mass spectrometry and elemental analysis are also in close agreement with calculated isotope patterns (see supporting information).
Whilst we were unable to obtain diffraction-quality crystals of complexes 16 or 17, a computationally derived38,39 low energy conformation of four amino acid residue mimic 17 gave encouraging results when superimposed on the dimer conformation of the leucine zipper transcription factor GCN4.40 Given that coiled-coil α-helical transcription factors control the expression of myriad genes, it is an appealing strategy to use a synthetic agent to regulate transcription, and thus influence the translation of proteins implicated in human disease. The Cα positions of the peptide show good agreement with those of the oxygen atoms of the biphenyl helix (Figure 3).
Figure 3.
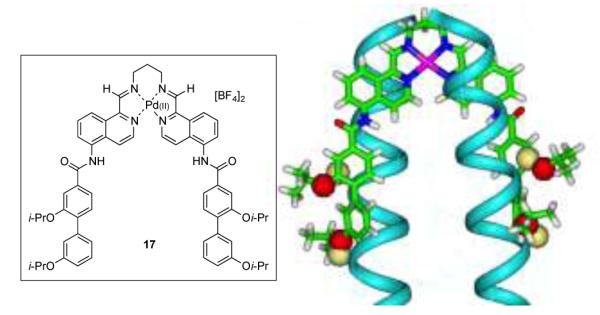
Superimposition of a low-energy conformation of four-residue bis-helix mimetic 17 (green sticks and red spheres) and the GCN4 dimer (pdb: 2ZTA; blue ribbon and yellow spheres).
Conclusions
We have designed, synthesized and structurally characterized a simple linker based on a Pd(II) bis-(iminoisoquinoline) complex that reproduces the geometric characteristics of a peptidic hairpin motif. Two amino groups allow for the attachment of a diverse array of peptidomimetics via amide bond formation. The approach was exemplified by the linking of α-helical mimics of the i and i+4 positions to replicate the structure of an α-α hairpin. However, the synthetic strategy is flexible and convergent, and in principle allows for the display of homo- or hetero-dimeric combinations of helical peptidomimetics to produce a wide range of super-secondary structural mimics. Improved aqueous solubility may be aided by the use of recently reported helix mimics that contain a greater number of heteroatoms in the scaffold, or the inclusion of side-chain mimics bearing polar groups.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (GM69850) and the University of Oxford for funding, Dr. Christopher Incarvito for X-ray analysis and Dr. Hayden Peacock (Oxford) for helpful discussions (originally omitted for peer review).
Footnotes
In celebration of the 70th birthday of Professor Rocco Ungaro
References
- (1).Adler MJ, Jamieson AG, Hamilton AD. Curr. Top. Microbiol. Immunol. 2011;348:1. doi: 10.1007/82_2010_91. [DOI] [PubMed] [Google Scholar]
- (2).Azzarito V, Long K, Murphy NS, Wilson AJ. Nat. Chem. 2013;5:161. doi: 10.1038/nchem.1568. [DOI] [PubMed] [Google Scholar]
- (3).Thompson S, Hamilton AD. Org. Biomol. Chem. 2012;10:5780. doi: 10.1039/c2ob25273b. [DOI] [PubMed] [Google Scholar]
- (4).Thompson S, Vallinayagam R, Adler MJ, Scott RTW, Hamilton AD. Tetrahedron. 2012;68:4501. [Google Scholar]
- (5).Sutherell CL, Thompson S, Scott RTW, Hamilton AD. Chem. Commun. 2012;48:9834. doi: 10.1039/c2cc34791a. [DOI] [PubMed] [Google Scholar]
- (6).Jamieson AG, Russell D, Hamilton AD. Chem. Commun. 2012;48:3709. doi: 10.1039/c2cc30295k. [DOI] [PubMed] [Google Scholar]
- (7).Kulikov OV, Thompson S, Xu H, Incarvito CD, Scott RTW, Saraogi I, Nevola L, Hamilton AD. Eur. J. Org. Chem. 2013:3433. [Google Scholar]
- (8).Ranganathan D, Haridas V, Nagaraj R, Karle IL. J. Org. Chem. 2000;65:4415. doi: 10.1021/jo0003807. [DOI] [PubMed] [Google Scholar]
- (9).Karle IL, Flippen-Anderson JL, Sukumar M, Uma K, Balaram P. J. Am. Chem. Soc. 1991;113:3952. [Google Scholar]
- (10).Ramagopal UA, Ramakumar S, Sahal D, Chauhan VS. Proc. Nat. Acad. Sci. USA. 2001;98:870. doi: 10.1073/pnas.98.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karle IL, Ranganathan D, Lakshmi C. Biopolymers. 2001;59:301. doi: 10.1002/1097-0282(20011015)59:5<301::AID-BIP1026>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- (12).Barthe P, Roumestand C, Rochette S, Vita C. Protein Sci. 2000;9:942. doi: 10.1110/ps.9.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ranganathan D, Haridas V, Kurur S, Nagaraj R, Bikshapathy E, Kunwar AC, Sarma AVS, Vairamani M. J. Org. Chem. 2000;65:365. doi: 10.1021/jo9912045. [DOI] [PubMed] [Google Scholar]
- (14).Ranganathan D, Kurur S, Karle IL. Biopolymers. 2000;54:249. doi: 10.1002/1097-0282(20001005)54:4<249::AID-BIP20>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- (15).Efimov AV. Protein Eng. 1991;4:245. doi: 10.1093/protein/4.3.245. [DOI] [PubMed] [Google Scholar]
- (16).Efimov AV. FEBS Lett. 1994;355:213. doi: 10.1016/0014-5793(94)01194-x. [DOI] [PubMed] [Google Scholar]
- (17).Rao ST, Rossmann MG. J. Mol. Biol. 1973;76:241. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- (18).Sternberg MJE, Thornton JM. J. Mol. Biol. 1976;105:367. doi: 10.1016/0022-2836(76)90099-1. [DOI] [PubMed] [Google Scholar]
- (19).Efimov AV. FEBS Lett. 1984;166:33. doi: 10.1016/0014-5793(84)80039-3. [DOI] [PubMed] [Google Scholar]
- (20).Efimov AV. FEBS Lett. 1991;284:288. doi: 10.1016/0014-5793(91)80706-9. [DOI] [PubMed] [Google Scholar]
- (21).Sun Z, Jiang BJ. Protein Chem. 1996;15:675. doi: 10.1007/BF01886750. [DOI] [PubMed] [Google Scholar]
- (22).Crick F. Acta Crystallogr. 1953;6:689. [Google Scholar]
- (23).Chothia C, Levitt M, Richardson D. Proc. Nat. Acad. Sci. USA. 1977;74:4130. doi: 10.1073/pnas.74.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chothia C, Levitt M, Richardson D. J. Mol. Biol. 1981;145:215. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- (25).Efimov AV. FEBS Lett. 1999;463:3. doi: 10.1016/s0014-5793(99)01507-0. [DOI] [PubMed] [Google Scholar]
- (26).Lee S, Chirikjian GS. Biophys. J. 2004;86:1105. doi: 10.1016/S0006-3495(04)74185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Engel DE, DeGrado WF. Proteins. 2005;61:325. doi: 10.1002/prot.20522. [DOI] [PubMed] [Google Scholar]
- (28).Walters RFS, DeGrado WF. Proc. Nat. Acad. Sci. USA. 2006;103:13658. doi: 10.1073/pnas.0605878103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yamada S. Coord. Chem. Rev. 1966;1:415. [Google Scholar]
- (30).Van Stein GC, Van Koten G, Vrieze K, Brevard C, Spek AL. J. Am. Chem. Soc. 1984;106:4486. [Google Scholar]
- (31).Zhang W, Sun W-H, Wu B, Zhang S, Ma H, Li Y, Chen J, Hao P. J. Organomet. Chem. 2006;691:4759. [Google Scholar]
- (32).Pelagatti P, Carcelli M, Costa M, Ianelli S, Pelizzi C, Rogolino D. J. Mol. Catal. A: Chem. 2005;226:107. [Google Scholar]
- (33).Hannon MJ, Moreno V, Prieto MJ, Moldrheim E, Sletten E, Meistermann I, Isaac CJ, Sanders KJ, Rodger A. Angew. Chem. Int. Ed. 2001;40:879. doi: 10.1002/1521-3773(20010302)40:5<879::AID-ANIE879>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- (34).Vanelle P, Rathelot P, Maldonado J, Crozet MP. Heterocycles. 1997;45:1519. [Google Scholar]
- (35).Tavares FX, Al-Barazanji KA, Bigham EC, Bishop MJ, Britt CS, Carlton DL, Feldman PL, Goetz AS, Grizzle MK, Guo YC, Handlon AL, Hertzog DL, Ignar DM, Lang DG, Ott RJ, Peat AJ, Zhou H-Q. J. Med. Chem. 2006;49:7095. doi: 10.1021/jm060572f. [DOI] [PubMed] [Google Scholar]
- (36).The Cambridge Crystallographic Data Centre: submission number 930541.
- (37).Rodriguez JM, Nevola L, Ross NT, Lee G, Hamilton AD. ChemBioChem. 2009;10:829. doi: 10.1002/cbic.200800715. [DOI] [PubMed] [Google Scholar]
- (38).Spartan (Amber forcefield); Wavefunction Inc, Irvine CA. Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O’Neill DP, DiStasio RA, Jr., Lochan RC, Wang T, Beran GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khalliulin RZ, Klunzinger P, Lee AM, Lee MS, Liang WZ, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee TM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock HL, III, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF, Kong J, Krylov AI, Gill PMW, Head-Gordon M. Phys. Chem. Chem. Phys. 2006;8:3172. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- (39).Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. J. Am. Chem. Soc. 1995;117:5179. [Google Scholar]
- (40).Oshea E, Klemm J, Kim P, Alber T. Science. 1991;254:539. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


