Abstract
Dementia is a growing public health issue. Activity, a positive therapeutic modality, has potential to enhance quality of life and reduce behavioral symptoms in persons with dementia—outcomes eluding pharmacological treatments. However, it is unclear how to effectively engage persons with dementia in activities for them to derive desired benefits. We present a systematic review of 28 studies involving 50 tests of different ways of modifying activities to enhance engagement and reduce behavioral and psychological symptoms for this group. Of 50 tests, 22 (44%) evaluated changes to objects and properties (e.g., introducing activities with intrinsic interest), 6 (12%) evaluated changes to space demands (e.g., lighting, noise levels), 8 (16%) evaluated changes to social demands (e.g., prompts, praise), and 14 (28%) combined two or more activity modifications. No modifications were made to the sequence and timing of activities. Although modifications to objects and properties were the most common, outcomes for engagement and behaviors were mixed. Modifications to space and social demands were less frequently tested, but consistently yielded positive outcomes. No modifications resulted in negative behavioral outcomes or decreased engagement. Methodological strengths of studies included direct observation of outcomes and fidelity assessments. Few studies however involved persons with dementia at home. Our review revealed a growing evidentiary base for different modifications to foster engagement in activities and reduce behavioral and psychological symptoms. Future studies should evaluate how contextual factors (e.g., physical environment, activity type) and caregiver ability to employ activity modifications affect engagement.
Keywords: activities, aging, behavioral interventions, dementia
Dementia, a worldwide epidemic, represents a significant burden to individuals and their families (World Health Organization & Alzheimer’s Disease International, 2012). With no cure in sight, developing and evaluating programs that sustain quality of life is critical. Of the more than 5 million persons with dementia in the United States, most live at home and are cared for by an estimated 15 million family members. Individuals with dementia are commonly found to spend most days doing little or not being engaged in meaningful activity (Ice, 2002; von Kuzleben, Schmid, Halek, Holle, & Bartholomeyczick, 2010). Persons with dementia and their family members cite engagement in meaningful activity as one of their most persistent and critical unmet needs (Miranda-Castillo, Woods, & Orrell, 2013). This lack of stimulation can have dire consequences including isolation, increased dependency, and decreased quality of life. Inactivity is also associated with behavioral and psychological symptoms such as aggression, agitation, depression, and apathy (Samus et al., 2005; Scherder, Bogen, Effermont, Hamers, & Swaab, 2010)—hallmark symptoms of dementia that are underrecognized and undertreated (Gitlin, Kales, & Lyketsos, 2012; Lyketsos et al., 2011).
A small but growing research corpus suggests that activity is a promising approach for people with dementia that may reduce behavioral disturbances (Aronstein, Olsen, & Schulman, 1996), increase positive emotions (Schreiner, Yamamoto, & Shiotani, 2005), and improve quality of life (Gitlin et al., 2009). Additionally, governments worldwide have recognized the importance of activities in dementia care, with some mandating their use as part of standard treatment (Omnibus Budget Reconciliation Act, 1987; United Kingdom’s National Collaborating Centre for Mental Health, 2007). Despite its promise, activity research for this clinical population is in a formative stage.
One important issue is how to optimally engage persons with dementia in activities. Cognitive impairments including deficits in memory, language, and spatial recognition present unique challenges to effectively engage this clinical population in activity. For activity to be a viable therapeutic modality in comprehensive dementia care, identifying strategies that best foster engagement, especially for those living at home, is essential.
To identify potential strategies that foster engagement, we draw on the American Occupational Therapy Association (Thomas, 2011) classificatory schema that identifies four types of possible modifications to activities. One modification concerns changes to “objects and property” (e.g., tools, materials, equipment) of activities. This might include offering activity that taps into previous and/or current habits, roles, and preferences or altering properties such as using oversized or enlarged playing cards. Another type of modification to an activity concerns changes to “space demands” (e.g., the physical environment), such as adjusting light or ambient noise levels. A third type of modification concerns changing the “social demands” of activities such as using specific prompts and praise statements. The fourth type of modification to an activity concerns changing the “sequence and timing,” such as simplifying or breaking down an activity into manageable, smaller steps or providing written or gestural cueing to help a person move through a multistep activity. It is unclear, however, as to which of these four types of modifications to activities effectively enhance engagement and reduce behavioral and psychological symptoms for people with dementia.
This article presents a systematic review of studies that evaluated one or more modifications to activities for the purpose of increasing engagement or decreasing behavioral and psychological symptoms in persons with dementia. Specifically, we sought to (a) determine which types of modifications have been evaluated, (b) identify the settings in which modifications have been tested, (d) evaluate the methodological rigor of studies, (d) determine if the type of modification used differed by the type of activity introduced, and (e) evaluate whether modifications resulted in enhanced engagement in activities and/or reduced behavioral and psychological symptoms.
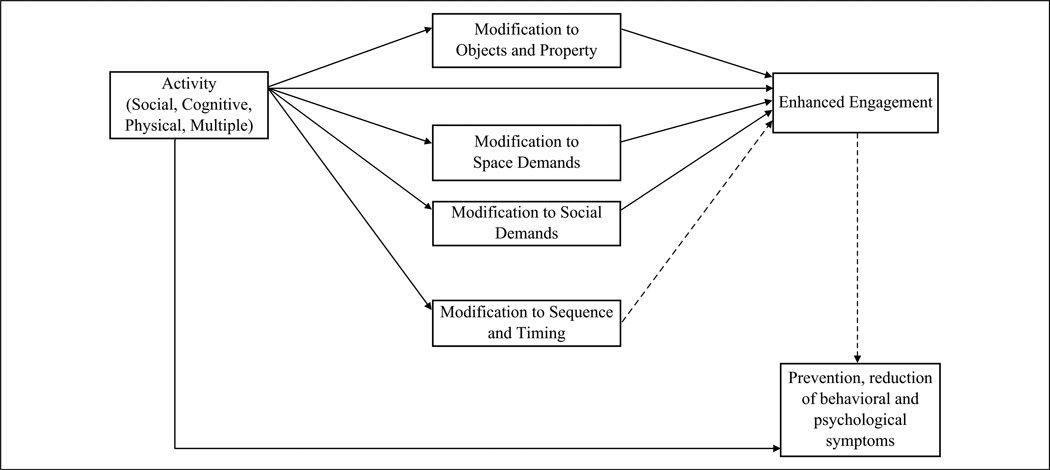
A modification can be viewed as a mediator between the activity itself and its outcome, that is, any one or combination of modifications may enhance or boost the effects of an activity on outcomes such as increased engagement and symptom reduction. Ultimately, we sought to understand whether modifications might reflect the underlying mechanism by which activities have their desired effects for persons with dementia (Figure 1).
Figure 1.
Mediation model of activity modification strategies to enhance engagement.
Note. Solid lines reflect relationships with evidence; dashed lines reflect relationships with inconclusive evidence.
Method
Search Strategy
A three-step search strategy was used: an initial search of PsychINFO to determine index terms; a full search of PsychoINFO, PubMED, and the Cochrane Library using all permutations of index terms (“cognitive impairment,” “dementia,” “Alzheimer,” or “neurodegenerative disease” paired with “activity” and “intervention” or “program”); and a search of reference lists in previous review articles on non-pharmacological interventions in dementia care. Titles of articles identified were first evaluated for their likelihood to meet inclusion criteria. The first two authors then independently reviewed abstracts of titles to determine which full articles to include.
Inclusion and Exclusion Criteria
Articles were selected for this review if they (a) purposely tested one of the four types of modifications to activity, (b) involved adults aged 60 years and older with any type of dementia or mild cognitive impairment, and who lived in the community or residential care setting, (c) involved an activity that was purposeful, goal-directed, or met a basic human need for enjoyment (e.g., this excluded self-care activities), (d) reported a measure of participant engagement, behavioral disturbance, psychological symptom, or another patient-oriented behavioral outcome, and (e) were published in English from January 2000 to December 2011. Studies using the full spectrum of experimental designs were included (e.g., randomized control trials, crossover, single-subject). Articles were excluded if repetitive cognitive or physical exercise training was the main activity as these interventions have been previously reviewed (Bahar-Fuchs, Clare, & Woods, 2013; Heyn, Abreu, & Ottenbacker, 2004).
Coding Scheme
We recorded the number of modifications evaluated in identified articles and categorized modifications as either changing objects and property, space demands, social demands, sequence and timing, or a combination. Next, we identified measurement strategies (observation, caregiver report) used, the behavioral topography investigated (e.g., agitation, engagement, affect), and if sufficient interrater reliability procedures were used. We defined reliability as sufficient if >25% of experimental observations were scored and kappa for interrater agreement exceeded 0.8. We also documented the type of research design and if treatment fidelity practices were used. We defined treatment fidelity as any methodological strategy introduced to enhance and monitor the reliability and validity of the activity intervention (e.g., use of intervention manuals; Bellg et al., 2004).
Additionally, modifications to activities were evaluated along other characteristics including expertise of interventionists, type of activity modified, and outcomes considered. Interventionist expertise was categorized as (a) specialists (e.g., professionals or experts in research implementation—authors, research therapists, graduate students), (b) formal caregivers (e.g., paid for services and trained in providing health care—nurses, nursing aides), (c) informal caregivers (e.g., family members (spouse, adult child) or friends who provide care usually without payment), or (d) other (e.g., adult peer). Activities used in studies were categorized as social if it involved another individual (e.g., painting fingernails, group sing-a-longs), as physical if it included body movement (e.g., gardening, dancing), and cognitive if it involved information processing (e.g., reading, music), and multiple if it involved a combination of the aforementioned.
Reliability of Coding
Interrater reliability of the coding of studies was established by comparing ratings between two readers (first two authors) who independently coded 11 dimensions (e.g., type of modification, measurement type) for 14 (28%) of the 50 tests of modifications. This yielded a total of 154 coded dimensions that were compared. Interrater reliability was then calculated by dividing the number of agreements (n = 153) by 154 coded dimensions, resulting in 99.4% agreement. For the 0.6% (n = 1) code not agreed upon, discussion led to consensus among authors.
Results
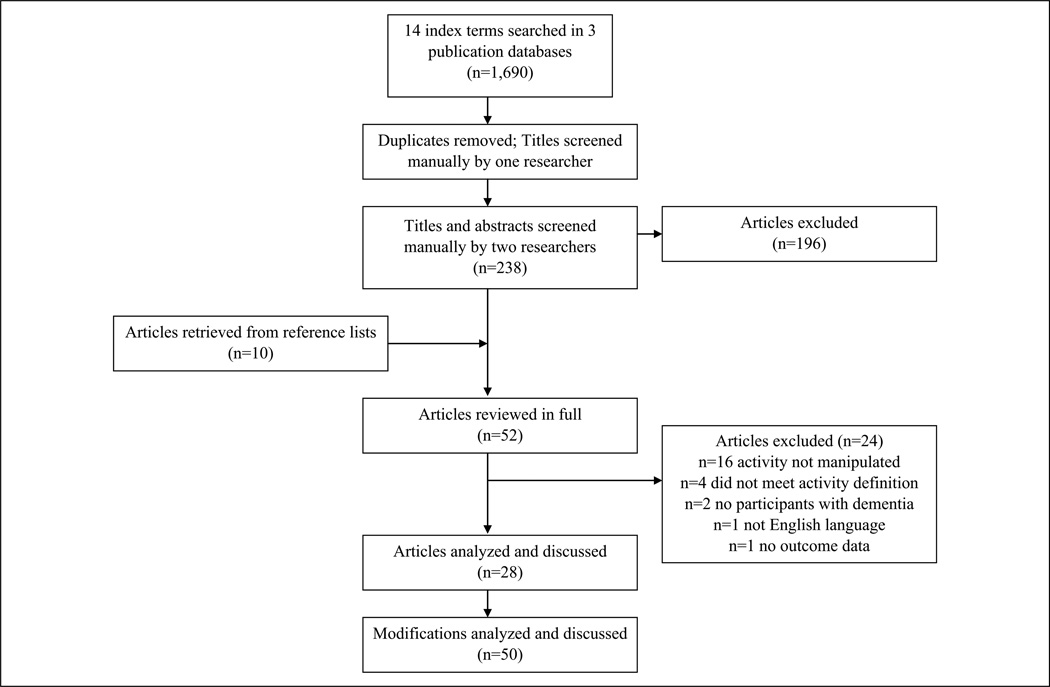
The search strategy identified 238 unduplicated citations (Figure 2). Title and abstract reviews resulted in the removal of 196 articles. A total of 52 articles were identified for full review and of these, 24 were subsequently eliminated for not meeting study inclusion criteria. Twenty-eight articles were consequently coded and analyzed. Of these, seven articles investigated more than one modification to activities, such that a total of 50 distinct modifications to activities were systematically evaluated across studies and analyzed in our review.
Figure 2.
Flowchart of included articles.
Types of Modifications
Table 1 provides a summary of the 50 modifications reviewed. Of these modifications, 22 (44%) reflected changes to objects and property, 14 (28%) made a combination of two or more modifications to the activity, 8 (16%) reflected changes to social demands, and 6 (12%) reflected changes to space demands. No modifications were made to the sequence and timing of activities.
Table 1.
Summary of Modification Characteristics.
| Overall | Objects and Property | Space Demand | Social Demand | Combinations | |
|---|---|---|---|---|---|
| Number of tests of modifications conducted | 50 | 22 (44%) | 6 (12%) | 8 (16%) | 14 (28%) |
| Setting | |||||
| Nursing home | 36 (72%) | 15 | 5 | 4 | 12 |
| Assisted living | 6 (12%) | 1 | 1 | 3 | 1 |
| Adult day center | 3 (6%) | 2 | 0 | 0 | 1 |
| In-home | 2 (4%) | 2 | 0 | 0 | 0 |
| Multiple settings | 3 (6%) | 2 | 0 | 1 | 0 |
| Measurement type | |||||
| Direct observation | 41 (82%) | 14 | 6 | 8 | 13 |
| Survey | 5 (10%) | 4 | 0 | 0 | 1 |
| Multiple types | 4 (8%) | 4 | 0 | 0 | 0 |
| Measured interrater reliabilitya | 9 (18%) | 3 | 0 | 5 | 1 |
| Design | |||||
| Crossover | 22 (44%) | 7 | 5 | 2 | 8 |
| Single-subject | 10 (20%) | 4 | 1 | 4 | 1 |
| Repeated measures | 9 (18%) | 6 | 0 | 1 | 2 |
| Pre–post | 8 (16%) | 3 | 0 | 2 | 3 |
| Randomized control | 3 (6%) | 3 | 0 | 0 | 0 |
| A measure of fidelity | 32 (64%) | 14 | 2 | 5 | 11 |
| Interventionist | |||||
| Specialist | 42 (84%) | 19 | 5 | 4 | 14 |
| Formal caregiver | 4 (8%) | 0 | 1 | 3 | 0 |
| Informal caregiver | 2 (4%) | 2 | 0 | 0 | 0 |
| Other | 1 (2%) | 0 | 0 | 1 | 0 |
| Multiple interventionists | 1 (2%) | 1 | 0 | 0 | 0 |
| Type of activity | |||||
| Social | 1 (2%) | 0 | 0 | 0 | 1 |
| Cognitive | 2 (4%) | 2 | 0 | 0 | 0 |
| Physical | 3 (6%) | 0 | 2 | 1 | 0 |
| Multiple | 41 (82%) | 18 | 4 | 6 | 13 |
| Unknown | 3 (6%) | 2 | 0 | 1 | 0 |
| Outcome measures | |||||
| Engagmentb | 26 (52%) | 6 | 6 | 7 | 7 |
| Behavioral symptomc | 12 (24%) | 9 | 0 | 0 | 3 |
| Psychological symptomd | 2 (4%) | 1 | 0 | 0 | 1 |
| Othere | 1 (2%) | 0 | 0 | 0 | 1 |
| Multiple measures | 9 (18%) | 6 | 0 | 1 | 2 |
| Outcome direction | |||||
| Desired direction | 40 (80%) | 15 | 6 | 8 | 11 |
| Undesired direction | 0 | 0 | 0 | 0 | 0 |
| No change | 5 (10%) | 3 | 0 | 0 | 2 |
| Multiple outcomes | 5 (10%) | 4 | 0 | 0 | 1 |
Defined as 25% or more of the intervention sessions with reliability measures, and the kappa for interrater agreement exceeded 0.8.
Engagement includes measures of engagement, participation, involvement, time on task, doing nothing, and attendance.
Behavioral symptoms include measures of agitation, hoarding, disorientation, problem behaviors, passivity, and sleep problems.
Psychological symptoms include measures of depression, affect, mood, and well-being.
Other includes measures of verbal communication, self-awareness, and identity.
Fourteen (63.4%) of the 22 modifications to objects and property involved manipulating activities based on participants’ preference, identity, and/or abilities (Kolanowski, Buettner, Costa, & Litaker, 2001; Kolanowski, Litaker, & Buettner,2005, Kolanowski, Litaker, Buettner, Moeller, & Costa, 2011), whereas 8 (36.4%) of the 22 modifications to objects and property did not. For example, Cohen-Mansfield, Thein, Dakheel-Ali, and Marx (2010b) and Cohen-Mansfield, Marx, Dakheel-Ali, et al. (2010) used different types of activities not dependent on person-identified preferences, including gender-specific activities (e.g., feminine activities included arranging flowers; neutral activities included sorting tasks), manipulative activities (e.g., a squeeze ball), or reading activities (Table 2).
Table 2.
Characteristics for Modifications Made Specifically When Social, Cognitive, Physical, Multiple, and Unknown Activity Types Were Used.
| Measurement Strategies |
Interventionist |
Type of Activity |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Participants and Setting |
Behavior Topography |
Type | Name | IRR | Design | TX Fid | Sp | FC | IC | O | Intervention | S | C | P | M | U | Outcomes |
| Objects and property (n = 22) | ||||||||||||||||||
| Gerdner (2000) |
n = 39, M age = 82.6, M MMSE = severe; NH |
Agitation | Survey | MCMAI | No | Crossover | × | Individualized music | × | ↓ Agitation° | ||||||||
| Baker, LeBlanc, Raetz, and Hilton (2011) |
n = 1; age = 80; MMSE = 4; DC |
Hoarding | DO | Frequency count |
Yes | Single-subject | ✓ | × | Sorting | × | ↓Hoarding | |||||||
| Cohen-Mansfield, Thein, Dakheel-Ali, and Marx (2010b) |
n = 193;M age 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Work-related activities (Social, cognitive, and physical) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield et al. (2010b) |
n = 193;M age 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Identity-based activities (Social, cognitive, and physical) |
× | ↑ Engagement° | |||||||
| LeBlanc, Cherup, Feliciano, and Sidener (2006) Exp 2 |
n = 4; M age = 80, M MMSE= 12.3; DC |
Engagement | DO | Time-sampling | Yes | Single-subject | ✓ | × | Preferred activities (Social, cognitive, and physical) |
× | ↑ Engagement | |||||||
| LeBlanc, Raetz, Baker, Strobel, and Feeney (2008) |
n = 9; M age = 79.9; M MMSE = 9.4; DC & NH |
Engagement | DO | Time-sampling | Yes | Single-subject | ✓ | × | Preferred activities (Social, cognitive, and physical) |
× | ↑ Engagement | |||||||
| Cohen-Mansfield et al. (2010b) |
n = 193;M age 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Gender-based activities (Social, cognitive, and physical) |
× | ≠ Engagement | |||||||
|
Cevasco and Grant (2003) Exp 2 |
n = 12;M MMSE = mild or mod; ALF |
Participation | DO | Time-sampling | No* | Crossover | ✓ | × | Instruments (cognitive) during physical activities |
× | ≠ Engagement | |||||||
| Cohen-Mansfield, Marx, Dakheel-Ali, et al. (2010) |
n = 111; M age = 85.4; M MMSE = 5.1;NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Work-related activities (Social, cognitive, and physical) |
× | ↓ Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali et al. (2010) |
n = 111; M age = 85.4; M MMSE = 5.1;NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Task-related activities (Social, cognitive, and physical) |
× | ↓Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali et al. (2010) |
n = 111; M age = 85.4; M MMSE = 5.1;NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Music | × | ↓Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali et al. (2010) |
n = 111; M age = 85.4; M MMSE = 5.1; NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Reading activities (cognitive, social) |
× | ↓ Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali et al. (2010) |
n= 111; M age = 85.4; M MMSE = 5.1; NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Identity-based activities (Social, cognitive, and physical) |
× | ↓Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali et al. (2010) |
n= 111; M age =85.4; M MMSE = 5.1; NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Manipulative activities (Social, cognitive, and physical) |
× | ≠ Agitation | ||||||||
| Cohen-Mansfield, Marx, Thein, and Dakheel-Ali (2011) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Affect | DO | LMBS | No | Pre–post | ✓ | × | Identity-based activities (Social, cognitive, and physical) |
× | ↑ Positive affect° | |||||||
| Fitzsimmons and Buettner (2002) |
n = 29; M age = 81.3, M MMSE= 12.9; Home |
Passivity, agitation |
survey | CMAI; PDS | No | Pre–post | × | Activities tailored to functioning, strengths, interests, and need (Social, cognitive, and physical) |
× | ↓Passivity° ↓Agitation° |
||||||||
| Cohen-Mansfield, Parpura-Gill, and Golander (2006) |
n = 93; M age = 87; M MMSE= 10.6; DC &NH |
Positive affect, self-awareness, disorientation, agitation, involvement |
survey, DO | LMBS; SIDQ; MOSES; ABMI |
No* | Randomized control trial |
✓ | × | Identity-based activities (Social, cognitive, and physical) |
× | ↑ Positive affect° ↑ Self-awareness° ↓Disorientation° ↓Agitation° ↑ Involvement° |
|||||||
| Kolanowski, Buettner, Costa, and Litaker (2001) |
n = 10; M age = 89.4, M MMSE = 10.2; NH |
Time on task positive affect, mood, problem behaviors |
Survey, DO | duration; PGCARS; DMPT; CMAI |
No | Crossover | ✓ | × | Activities tailored to style, interest, and skill level (Social, cognitive, and physical) |
× | ↑ Time on task° ↑ Positive affect° ≠ Mood° ≠ Problem behaviors° |
|||||||
| Kolanowski, Litaker, and Buettner (2005) |
n = 30, M age = 82.3, M MMSE = 8.6; NH |
Engagement, affect, mood, agitation |
Survey, DO | Duration; PGCARS; DMPT; CMAI, PDS |
No | Crossover | ✓ | × | Activities tailored to interest, cognition, and physical abilities (Social, cognitive, and physical) |
× | ↑ Engagement° ↑ Positive affect° ≠Mood ↓Agitation with any activity° |
|||||||
| Kolanowski, Litaker, Buettner, Moeller, and Costa (2011) |
n = 128; M ages = 85.3–87.2; M MMSE = 127–15.8; NH |
Engagement, agitation, passivity, mood |
Survey, DO | Duration; PGCARS; CMAI; PDS; DMPT |
No* | Randomized, double-blind clinical trial |
✓ | × | Activities tailored to functioning level and/ or interest (Social, cognitive, and physical) |
× | ↑ Engagement° ↓ Agitation° ↓ Passivity° ≠ Mood |
|||||||
| Feliciano, Steers, Elite-Marcandonatou, McLane, and Arean (2009) |
n = 1 1; M age =85.6; M MMSE = 7.1; NH |
Agitation, depression |
Survey | CMAI; CSDD | No* | Pre-post for depression; single-subject for agitation |
✓ | × | × | Preferred activities | × | ↓ Agitation ↓ Depression |
||||||
| Gitlin et al. (2008) |
n = 60; M age = 79.4, M MMSE = 11.6; Home |
Agitation, engagement, depression |
Survey | ABDS; study- developed tool; CSDD |
No | Prospective, two-group, randomized, controlled Pilot study |
✓ | × | Tailored activities | × | ↓ Agitation° ↑ Engagement° ≠ Depression |
|||||||
| Space demands (n = 6) | ||||||||||||||||||
|
Cevasco and Grant (2003) Exp 2 |
n = 12; M MMSE = mild or mod; ALF |
Participation | DO | Time-sampling | No* | Crossover | ✓ | × | Background music during exercise |
× | ↑ Participation with instrumental vs. vocal music |
|||||||
| Mathews, Clair, and Kosloski(200l) |
n = 18; M age = 85; M MMSE= 11; NH |
Participation | DO | Time-sampling | No | Single-subject | ✓ | × | Background music during exercise |
× | ↑ Participation° | |||||||
| Cohen-Mansfield et al. (2010a) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | × | Time of day (Social, cognitive, and physical activities) |
× | ↑ Engagement° in afternoon (2–5 p.m.) |
||||||||
| Cohen-Mansfield et al. (2010a) |
n= 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | × | Lighting (Social, cognitive, and physical activities) |
× | ↑ Engagement° when normal lighting |
||||||||
| Cohen-Mansfield et al. (2010a) |
n= 193; M age =86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | × | Sound level (Social, cognitive, and physical activities) |
× | ↑ Engagement° with moderate sound |
||||||||
| Cohen-Mansfield et al. (2010a) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | × | Number of people around (Social, cognitive, and physical activities) |
× | ↑ Engagement° with small group (4–9) |
||||||||
| Social demands (n = 8) | ||||||||||||||||||
|
Cevasco and Grant (2003) Exp 1 |
n = 14; ALF | Participation | DO | Time-sampling | No* | Crossover | ✓ | × | Continuous prompts | × | ↑ Participation° | |||||||
| Cohen-Mansfield et al. (2010a) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | × | Model prompts (Social, cognitive, and physical activities) |
× | ↑ Engagement° | ||||||||
| Brenske, Rudrud, Schulze, and Rapp (2008) | n = 6; age = 57–87; NH | Presence, engagement |
DO | Time-sampling | Yes | Single-subject | ✓ | × | Descriptive prompts | × | ↑ Presence ↑ Engagement |
|||||||
| Mathews, Clair, and Kosloski (2000) |
n = 16; M age = 86.7; M MMSE= 12.1; NH |
Engagement | DO | Time-sampling | Ys | Pre-post | × | Group music therapy (Cognitive, social) |
× | ↑ Engagement° | ||||||||
| Mathews et al. (2000) |
n = 16; M age = 86.7; M MMSE= 12.1; NH |
Engagement | DO | Time-sampling | Yes | Pre-post | × | One-to-one music therapy (Cognitive, social) |
× | ↑ Engagement° | ||||||||
| Polenick and Flora (2011) |
n = 3 (P 2,3,8); M age = 82; ALF |
Attendance | DO | Percentage activities attended |
Yes | Single-subject | ✓ | × | Descriptive prompts (Social, cognitive, and physical activities) |
× | ↑ Attendance° | |||||||
| Altus, Engelman, and Mathews (2002) |
n = 6; M age = 79; M MMSE = 6.3; ALF |
Engagement | DO | Time-sampling | Yes | Single-subject | ✓ | × | Staff record activities and receive feedback (Social, cognitive, and physical activities) |
× | ↑ Engagement | |||||||
| Skrajner and Camp (2010) |
n = 22; M age = 85.2, M MMSE= 15.7; DC &NH |
Engagement and affect |
DO | MPES | No | Repeated measures |
✓ | × | Peer-led activity (Cognitive, Social) |
× | ↑ Engagement° ↑ Affect° |
|||||||
| Combination of modifications (n = 14) | ||||||||||||||||||
|
Cevasco and Grant (2003) Exp 2 |
n = 12;M MMSE = mild or mod; ALF |
Participation | DO | Time-sampling | No* | Crossover | ✓ | × | Background music (Space Demands); Instruments (Cognitive) during exercise (Physical) |
× | ≠ Participation | |||||||
| Greer, Pustay, Zaun, and Coppens (2001) |
n = 6; M age = 87.8; M M MSE= 15; NH |
Verbal communication |
DO | Frequency counts |
Yes | Single-subject | ✓ | × | Change (O & P) to social, animal activities (Social demands) |
× | ↑ Verbal communication with live cats |
|||||||
| Cohen-Mansfield, Marx, Thein, and Dakheel-Ali (2010) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Preferred music (cognitive), art (cognitive), and pet (social) activities |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Thein, Dakheel-Ali, Regier, and Marx (2010) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Change (O & P) to social activities (Social Demands) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Thein, Dakeel-Ali, et al. (2010) |
n= 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Change (O & P) to realistic social activities (Social Demands) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Thein, Dakeel-Ali, et al. (2010) |
n= 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Change (O & P) to animated social activities (Social Demands) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Thein, Dakheel-Ali, et al. (2010) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Change (O & P) to human social activities (Social Demands) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Thein, Dakeel-Ali, et al. (2010) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Engagement | DO | OME | No* | Crossover | ✓ | × | Change (O & P) to alive social activities (Social Demands) |
× | ↑ Engagement° | |||||||
| Cohen-Mansfield, Marx, Dakheel-Ali, et al. (2010) |
n = 111; M age = 85.4; M MMSE = 5.1; NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Change (O & P) to live social activities (Social Demands) |
× | ↓Agitation° | ||||||||
| Cohen-Mansfield, Marx, Dakheel-Ali, et al. (2010) |
M = 111; M age = 85.4; M MMSE = 5.1; NH |
Agitation | DO | ABMI | No* | Repeated measures |
× | Change (O & P) to simulated social activities (Social Demands) |
× | ↓Agitation° | ||||||||
| Richards, Beck O’Sullivan, and Shue (2005) |
n = 147; Al age = 79, M MMSE = 8.7; NH |
Sleep/wake patterns |
Automated | Actigraphs | No | Pre-post | ✓ | × | Change (O & P) to individualized social activities (Social Demands) |
× | ↓Daytime sleep° | |||||||
| Cohen-Mansfield et al. (2011) |
n = 193; M age = 86; M MMSE = 7.2; NH |
Affect | DO | LMBS | No | Pre-post | ✓ | × | Change (O & P) to identity-based activities; Social activities (Social Demands) |
× | ↑ Positive affect° | |||||||
| Gigliotti, Jarrott, and Yorgason (2004) |
n = 14; M age = 83;M MMSE= 16.7; DC |
Affect, engagement, doing nothing |
DO | DCM; time- sampling |
No | Crossover | ✓ | × | Various horticulture activities (physical, cognitive, & social) |
× | ↑ Affect° ↑ Engagement ↓Doing nothing° |
|||||||
| Haslam et al. (2010) |
n = 73; age = 58–95, M MMSE= 16.2–17.3; NH |
Well-being, identity |
Survey | HADS; LIS, QoLCS; PIS, SGH |
No | Pre-post | × | Change in location (Space Demands); social stimuli (Social demands) |
× | ↑ Well-being° ≠Identity |
||||||||
Note. M = Mean; P = participant; MMSE = Mini-Mental State Examination; ALF = assisted living facility; DC = day center; NH = nursing home; DO = direct observation; ABDS = Agitated Behaviors in Dementia Scale; ABMI = Agitation Behavior Mapping Instrument; CMAI = Cohen-Mansfield Agitation Inventory; CSDD = Cornell Scale for Depression in Dementia; DMPT = Dementia Mood Picture Test; HADS = Hospital Anxiety and Depression Scale; LMBS = Lawtons Modified Behavior Stream; LIS = Life Improvement Scale; DCM = Dementia Care Mapping; MOSES = Multidimensional Observation Scale for Elderly Subjects; MPES = Menorah Park Engagement Scale; OME = Observational Measurement of Engagement; PDS = Passivity in Dementia Scale; PGCARS = Philadelphia Geriatric Center Affect Rating Scale; PIS = Personal Identity Strength; QoLCS = Quality of Life Change Scale; SIDQ = Self-Identity in Dementia Questionnaire; SGH = Social Group Homogeneity; TPA = Temporal Patterning Assessment; IRR = interrater reliabiltiy; * = some reliability collected, but less than 25% of sessions or kappa less than 0.8; HT = horticulture therapy; Sp = specialist; FC = formal caregiver; IC = informal caregiver; O = other; TX Fid = treatment fidelity; O&P = objects and property; Spc Dem = space demands; Soc Dem = social demands; S&T = sequence and timing; GR = group reminiscence; ↑ = increase; ↓ = decrease; ≠ = no change; ° = statistically significant results.
Of the eight modifications to social demands, four (50%) included use of prompting such as verbal (e.g., “complete a word search with me”) and nonverbal (e.g., gestural demonstrations) reminders to complete an activity. The other four (50%) modifications incorporated staff or peers to lead activities. For example, one modification evaluated the effects of an adult peer leader during a reading activity, whereas other modifications investigated the use of group versus one-on-one activities.
The six modifications to space demands involved testing the utility of background music during activity times, variation in time of day providing activities, overhead lighting and sound level changes during activities, and modifying the number of people within an activity space (Table 2).
There were 14 tests involving a combination of modifications. For example, Cohen-Mansfield, Marx, Thein, and Dakheel-Ali (2010) and Cohen-Mansfield, Thein, Dakheel-Ali, Regier, and Marx, (2010) investigated the use of different stimuli (i.e., changes to objects and property, such as including preferred activities) and changing social demands (i.e., activities involving other individuals).
Participants and Settings of Modifications
Nearly all tests of the modifications (n = 49/50, 98%) reported the gender of participants, which was predominantly female (n = 40/49, 82% reported that three quarters or more of participant population were women). All the 36 tests of modifications (72%) that reported race information largely encompassed a White patient population. No tests of modifications included a study sample of more than 25% non-White participants.
Most tests of the modifications (n = 36/50, 72%) were conducted with nursing home residents (Table 1). Six tests (12%) of modifications were evaluated with participants in assisted living facilities and three modifications (6%) were evaluated with participants at adult day centers. Only two (4%) modifications were evaluated in a home setting. Three of the modifications (6%) were evaluated at both nursing homes and adult day centers.
Robustness of Studies
Most modifications were evaluated using direct observational measurement strategies (n = 41/50, 82%; Table 1). The most frequently used measures included the Observational Measurement of Engagement (Cohen-Mansfield, Dakheel-Ali, & Marx, 2009), the Agitated Behavior Mapping Instrument (Cohen-Mansfield, Werner, & Marx, 1989), or involved frequency counts or time-sampling procedures (Table 2). Five of the 50 tests (10%) used outcome measures involving self-report by caregivers, and 4 tests (8%) included both direct observation and self-report by caregivers among outcome measures. Although most tests included direct observational measurement strategies, only 5 (10%) reported sufficient reliability checks.
As to fidelity of implementation, more than half of the evaluations of modifications (n = 32/50, 64%) included a treatment fidelity measurement. Most fidelity measures assessed the degree to which a participant demonstrated the ability to use the treatment skills such as engagement (e.g., treatment receipt).
Most evaluations of modifications involved crossover (n = 22, 44%), single-subject (n = 10, 20%), repeated measures (n = 9, 18%), and pre–post (n = 8, 16%) designs. Only three (6%) evaluations of modifications used randomized control designs. In addition, most studies involved specialists (e.g., trained research therapists, authors) as interventionists (n = 42, 84%), with only two (4%) of the modifications evaluated being introduced by informal caregivers (e.g., spouse, adult children). These interventionists also commonly performed the outcome assessments and were not blinded to procedures.
Modifications by Activity Types
Across 50 distinct modifications, most (n = 41, 82%) involved activities reflecting a combination of social, cognitive, and/or physical features (referred to as multiple in Tables 1 and 2). For example, Skrajner and Camp (2007) investigated the use of an adult peer reading activity for people with dementia that combined cognitive (e.g., reading game) and social (e.g., presence of peer) elements. Cohen-Mansfield et al. (2010a, 2010b) and Cohen-Mansfield, Marx, Thein, and Dakheel-Ali (2011) conducted a series of studies whereby interventionists provided participants with several different activities (e.g., reading magazines as cognitive, holding a real baby as social).
Only one (2%) modification was implemented using a social-type activity, two (4%) modifications were evaluated with cognitive-type activities, and three (6%) modifications were evaluated as physical-type activities. The activity type could not be determined for three tests (6%).
Outcomes of Modifications
Table 1 shows that most modifications were evaluated for effects on engagement (n = 26, 52%); 12 modifications (24%) were evaluated for impact on behavioral symptoms and 2 modifications (4%) were evaluated for impact on psychological symptoms. Nine modifications (18%) were evaluated on a combination of outcome measures (engagement, behavioral, and/or psychological symptoms). Nearly all outcome assessments were conducted during or immediately after the modification strategy was implemented. Because only four modifications included a measure of carry-forward benefits, only outcomes conducted during or immediately after the modification strategy are discussed below.
Overall, improvement was reported for 41 (82%) of the modifications evaluated, most of which were statistically significant. All modifications reflecting changes to space and social demands resulted in positive outcomes, and most that combined two or more modifications (n = 12/14, 85.7%) resulted in positive outcomes. Results for modifications to objects and property, however, were mixed. Of 14 modifications tailoring activities to preferences, skills, or abilities, 10 (71.4%) showed improvements for all outcome measures. Four (28.6%) tests reported mixed results whereby engagement increased, but psychological symptoms remained unchanged. For example, Gitlin et al. (2008) found reductions in behavioral symptoms but no statistically significant difference in depression measured by the Cornell Scale for Depression in Dementia (Alexopoulos, Abrams, Young, & Shamoian, 1988), between participants receiving a tailored activity intervention and a wait-list control group, although a trend in the right direction was noted. Similarly, Kolanowski et al. (2001, 2005, 2011) found no significant change in mood from an activity intervention. Of eight modifications to objects and property that did not involve tailoring, five (62.5%) resulted in positive outcomes for engagement and behavioral symptoms, whereas three (37.5%) did not.
Of note, no adverse events or undesired outcomes (e.g., decreased engagement, increase behavioral symptoms) were reported for any modification evaluated.
Discussion
As impairments in memory, cognition, and executive function characterize dementia, specific strategies may be required to effectively foster engagement for these individuals. How engagement can best be enhanced and for which types of activities are important empirical questions with practical clinical implications for formal and informal care-givers. Pinpointing specific strategies that enhance engagement can lead to more targeted and hence effective activity therapies.
We present one of the first comprehensive reviews to evaluate specific ways to modify activities with the goal of building an evidence base for enhancing engagement and decreasing behavioral and psychological symptoms in people with dementia. For 28 identified articles, a total of 50 modifications to activities were tested. The most common modification to an activity entailed changing its objects or property. This type of modification improved engagement and behavioral symptoms, but results were mixed for psychological symptoms, with some studies reporting benefits in this area and others not. These results may be due to limitations of self-report methods for this population and/or the progression of dementia on emotions. The decline experienced with dementia may affect negative emotions to a greater extent than positive emotions (Kolanowski, Hoffman, & Hofer, 2007). This may explain why positive affect measures improved while results for general measures of mood, which encompass both positive and negative affective items (e.g., Dementia Mood Picture Test), were inconsistent.
Overall, results from modifications made to objects and property were positive and support the person-centered care model (Zeman, 1999), which emphasizes that when a person’s information is matched to meaningful interventions, engagement increases. Results are also consistent with the unmet needs model (Algase et al., 1996) which suggests that when need for enrichment is addressed through activities tailored to personal preferences and needs, behavioral symptoms decrease.
The second most common modification tested involved changes to the social demands of activity, which resulted in positive outcomes for both engagement and psychological symptoms (e.g., affect). As tests did not investigate effects for behavioral symptoms, the impact of using modifications to social demands on this outcome remains unknown. Improvements in engagement derived from this form of modification, highlight the concept that social connectivity remains an enduring need throughout disease progression. Additionally, the success of prompting strategies to increase engagement support the notion that as a person’s senses become progressively impaired, the environment becomes less clear and more effort is needed to cue and stimulate engagement (Skinner, 1983).
Although less than a fifth of reviewed modifications involved changes to space demands (e.g., lighting, sound level), all resulted in facilitating engagement. However, these modifications were not evaluated for behavior or psychological symptom reduction.
No studies evaluated modifications to the sequence or timing of activity (e.g., changing the steps or order of an activity). Given the deficits in attention to detail and sequencing in multiple stepped activities, systematically evaluating the impact of this type of modification to an activity would be important to pursue. It is possible that modifications to sequence and timing were involved in the studies reviewed but were not adequately reported or identified as such. For example, it would seem likely that these types of modifications are integral to an approach tailored to a person’s abilities. This also may be a limitation of our coding schema as it may be difficult to differentiate tailoring from sequencing modifications in practice. Furthermore, studies may not make these distinctions. Alternately, it may be that this modification is most useful for individuals in milder stages of decline who may need verbal reminders or cueing to sequence a multistep activity. By moderate to severe stages, activities may be chosen that do not involve multiple steps and sequencing.
As to targeted populations and settings, most modifications were evaluated with residents of nursing homes. We are unable to discern whether modifications to activities in that setting are transferrable to home settings. Two of the 50 tested modifications were evaluated at home and 6 were in community day centers. This represents a major limitation of research to date, as most persons with dementia live at home alone or with family members. For activity intervention research to advance, future tests of modifications must be conducted in community and home settings. Of importance is to evaluate how contextual factors such as the physical environment and caregiver ability to manipulate activities affect desired outcomes.
Nevertheless, findings with nursing home residents may have relevance for community-dwelling persons. Participants included in these studies varied widely in age, cognitive abilities (i.e., mild, moderate, severe dementia), and physical functioning—similar to community-based populations. Additionally, the use of tailoring as a strategy to enhance engagement in activities has been shown to be effective in the home (Gitlin et al., 2008).
Strengths across studies included use of objective measures to evaluate outcomes and attention to treatment fidelity. Direct observation is a more reliable measurement strategy than reliance on self or proxy reports. However, this approach may be more feasible in residential settings than homes. The feasibility of using monitoring devices in the home setting to capture real-time and direct observations of activity warrants evaluation. Treatment fidelity, an indicator that an intervention is implemented as intended, indicates study robustness and appears to be minimally to adequately addressed in these studies.
Nevertheless, numerous study weaknesses are noted including small sample sizes, homogeneity of samples, restricted settings, lack of use of randomized trial designs, and dependence on specialists to deliver activities. Of particular concern is that studies included a predominately White and female patient population living in nursing homes. Only three evaluations of modifications involved randomized trial designs, whereas crossover, single-subject, repeated measures, and pre–post designs dominated this research corpus. Given that a double-blind, randomized control trial is the most rigorous clinical research design and that most of the research reviewed here did not include blinded procedures nor use randomization, the next generation of activity research should focus on applying more rigorous controls. This recommendation echoes previous calls for more robust research in activity programs for older adults with dementia (Carlson, 2011). Additionally, as most studies employed specialists as interventionists, the ability of families to use modifications needs to be evaluated as well as best approaches to instruct families. It is unclear whether one type of modification to activity would be easier than another for caregivers to implement.
Modification to an activity to enhance engagement and/or decrease behavioral and psychological symptoms of dementia can be conceptualized as a mediator (Figure 1). That is, a modification may mediate the relationship between the activity that is introduced and engagement and other outcomes from the activity. A mediation model provides a basis for understanding how activity may produce desired effects on engagement and behavioral or psychological symptoms. Our review revealed that with the exception of two modifications (gender-based activity modification in Cohen-Mansfield et al., 2010b; musical instruments during physical activities in Cevasco & Grant, 2003), changes made to objects and property, space demands, and social demands, regardless of type of activity, increased engagement. This provides preliminary evidence that these modifications mediate the relationship between activity and engagement. These results also indicate that engagement is a favorable and advantageous outcome measure for activity intervention research. It is unclear, however, if this relationship holds for behavioral and psychological symptoms. No conclusions can be drawn for modifications made to space and social demands given that only 1 of these 14 modifications included a behavioral or psychology outcome measure. Nevertheless, 15 of the 22 modifications made to objects and property included at least one behavioral measure (e.g., agitation, hoarding), and of those 15 evaluations, 13 resulted in positive changes to behavioral symptoms. Additionally, 7 of the 22 modifications to objects and property included one psychological measure (e.g., affect, mood) and in only 3 were positive changes found. There is no evidence of a meditational relationship for social and space demand modifications, some evidence for a meditational relationship for behavioral symptoms when modifications are made to objects and property, and inconclusive evidence of a meditational effect for psychological symptoms when modifications are made to objects and property. It may be that engagement is the mediator for these outcomes versus the type of modification used, a hypothesis that could be tested in future research.
Our review revealed that there is a growing and relatively robust body of research systematically evaluating ways to improve engagement of persons with dementia. Overall, we found that activities tailored to interest and abilities were more likely to decrease behavioral symptoms and that a supportive physical environment embodied features of normal lighting, moderate sound, and a small number of people. We also found that a socially supportive environment included descriptive prompts and appropriate cueing to meet the person’s needs. To improve care and activity services for older adults with dementia, future research is warranted. Most notably, there is a need for more studies to be conducted in the community and in homes, involving people across the long disease trajectory and with family caregivers. As modifications to sequence and timing of activities have not been systematically evaluated to date, studies determining whether such changes improve engagement are in order. Modifications in space demands should also be tested further. For example, activities may have a better effect on mood depending on the time of day they are introduced based on diurnal fluctuations. Finally, future studies need to include sufficient reliability checks of observational measures. By identifying methods to enhance activity engagement, we can improve the quality of life of persons with dementia—an expressed but unmet goal of comprehensive dementia care.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Trahan was supported by the National Institutes of Health Grant T32 AG000120; Dr. Kuo reports support for this article from National Institute of Aging Grant T32 AG000247; and Dr. Gitlin was supported by the Alzheimer’s Association Grant NPSAS-10–17426, and the National Institute of Aging Grant 1R01AG041781.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplement Issue Note
This article is part of an open access supplement “Fostering Engagement and Independence: Opportunities and Challenges for an Aging Society,” published in SOPHE’s Health Education & Behavior. This supplement was supported by funding provided by the Centers for Disease Control and Prevention’s (CDC) National Center for Chronic Disease Prevention and Health Promotion, Healthy Aging Program (Cooperative Agreement #U38HM000454) via the Association of State and Territorial Health Officials, and from a grant provided by the Retirement Research Foundation. Views presented herein do not represent the official views of the CDC.
References
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Algase DL, Beck C, Kolanowski A, Whall A, Berent S, Richards K, Beattie E. Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. American Journal of Alzheimer’s Disease & Other Dementias. 1996;11(6):10–18. [Google Scholar]
- Altus DE, Engelman KK, Mathews RM. Finding a practical method to increase engagement of residents on a dementia care unit. American Journal of Alzheimer’s Disease & Other Dementias. 2002;17:245–248. doi: 10.1177/153331750201700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronstein Z, Olsen R, Schulman E. The nursing assistants use of recreational interventions for behavioral management of residents with Alzheimer’s disease. American Journal of Alzheimer’s Disease & Other Dementias. 1996;11(3):26–31. [Google Scholar]
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database of Systematic Reviews. 2013;6:CD003260. doi: 10.1002/14651858.CD003260.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, LeBlanc L, Raetz PB, Hilton LC. Assessment and treatment of hoarding in an individual with dementia. Behavior Therapy. 2011;42:135–142. doi: 10.1016/j.beth.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH behavior change consortium. Health Psychology. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- Brenske S, Rudrud EH, Schulze KA, Rapp JT. Increasing activity attendance and engagement in individuals with dementia using descriptive prompts. Journal of Applied Behavior Analysis. 2008;41:273–277. doi: 10.1901/jaba.2008.41-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC. Introduction: A life course perspective on activity and neurocognitive health. Journal of the International Neuropsychological Society. 2011;17:970–974. doi: 10.1017/S1355617711001366. [DOI] [PubMed] [Google Scholar]
- Cevasco AM, Grant RE. Comparison of different methods for eliciting exercise-to-music for clients with Alzheimer’s disease. Journal of Music Therapy. 2003;40:41–56. doi: 10.1093/jmt/40.1.41. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Dakheel-Ali M, Marx MS. Engagement in persons with dementia: The concept and its measurement. American Journal of Geriatric Psychiatry. 2009;17:299–307. doi: 10.1097/JGP.0b013e31818f3a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli? Journal of the American Geriatrics Society. 2010;58:1459–1564. doi: 10.1111/j.1532-5415.2010.02951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging & Mental Health. 2010;14:67–73. doi: 10.1080/13607860902845574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of stimuli on affect in persons with dementia. Journal of Clinical Psychiatry. 2011;72:480–486. doi: 10.4088/JCP.09m05694oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Parpura-Gill A, Golander H. Utilization of self-identity roles for designing interventions for persons with dementia. Journals of Gerontology: Psychological Sciences. 2006;61:202–212. doi: 10.1093/geronb/61.4.p202. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Thein K, Dakheel-Ali M, Marx MS. Engaging nursing home residents with dementia in activities: The effects of modeling, presentation order, time of day, and setting characteristics. Aging & Mental Health. 2010a;14:471–480. doi: 10.1080/13607860903586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Thein K, Dakheel-Ali M, Marx MS. The underlying meaning of stimuli: Impact on engagement of persons with dementia. Psychiatry Research. 2010b;177:216–222. doi: 10.1016/j.psychres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Thein K, Dakheel-Ali M, Regier NG, Marx MS. The value of social attributes of stimuli for promoting engagement in persons with dementia. Journal of Nervous and Mental Disease. 2010;198:586–592. doi: 10.1097/NMD.0b013e3181e9dc76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Werner P, Marx MS. An observational study of agitation in agitated nursing home residents. International Psychogeriatrics. 1989;1:153–165. doi: 10.1017/s1041610289000165. [DOI] [PubMed] [Google Scholar]
- Feliciano L, Steers ME, Elite-Marcandonatou A, McLane M, Arean PA. Applications of preference assessment procedures in depression and agitation management in elders with dementia. Clinical Gerontologist. 2009;32:239–259. doi: 10.1080/07317110902895226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons S, Buettner LL. Therapeutic recreation interventions for need-driven dementia-compromised behaviors in community-dwelling elders. American Journal of Alzheimer’s Disease & Other Dementias. 2002;17:367–381. doi: 10.1177/153331750201700603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdner LA. Effects of individualized versus classical “relaxation” music on the frequency of agitation in elderly persons with Alzheimer’s disease and related disorders. International Psychogeriatrics. 2000;12:49–65. doi: 10.1017/s1041610200006190. [DOI] [PubMed] [Google Scholar]
- Gigliotti CM, Jarrott SE, Yorgason J. Harvesting health: Effects of three types of horticultural therapy activities for persons with dementia. Dementia. 2004;3:161–180. [Google Scholar]
- Gitlin LN, Kales HC, Lyketsos CC. Nonpharmacologic management of behavioral symptoms in dementia. Journal of the American Medical Association. 2012;19:2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsy-chiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. American Journal of Geriatric Psychiatry. 2008;16:229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Earlan TV, Herge EA, Chernett NL, Piersol CV, Burke JP. The Tailored Activity Program to reduce behavioral symptoms in individuals with dementia: Feasibility, acceptability, and replication potential. The Gerontologist. 2009;49:428–429. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer KL, Pustay KA, Zaun TC, Coppens P. A comparison of the effects of toys versus live animals on the communication of patients with dementia of the Alzheimer’s type. Clinical Gerontologist. 2001;22:157–182. [Google Scholar]
- Haslam C, Haslam SA, Jetten J, Bevins A, Ravenscroft S, Tonks J. The social treatment: The benefits of group interventions in residential care settings. Psychology and Aging. 2010;25:157–167. doi: 10.1037/a0018256. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacker KJ. The effects of exercise on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Psychical Medicine and Rehabilitation. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Ice G. Daily life in a nursing home: Has it changed in 25 years? Journal of Aging Studies. 2002;16:345–359. [Google Scholar]
- Kolanowski AM, Buettner L, Costa PT, Litaker MS. Capturing interest: Therapeutic recreation activities for persons with dementia. Therapeutic Recreation Journal. 2001;35:220–235. [Google Scholar]
- Kolanowski A, Hoffman L, Hofer SM. Concordance of self-report and information assessment of emotional well-being in nursing home residents with dementia. Journals of Gerontology: Psychology and Social Science. 2007;62:20–27. doi: 10.1093/geronb/62.1.p20. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Litaker M, Buettner L. Efficacy of theory-based activities for behavioral symptoms of dementia. Nursing Research. 2005;54:219–228. doi: 10.1097/00006199-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Litaker M, Buettner L, Moeller J, Costa PT. A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society. 2011;53:1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc LA, Cherup SM, Feliciano L, Sidener TM. Using choice-making opportunities to increase activity engagement in individuals with dementia. American Journal of Alzheimer’s Disease & Other Dementias. 2006;21:318–325. doi: 10.1177/1533317506292183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc LA, Raetz PB, Baker JC, Strobel MJ, Feeney BJ. Assessing preference in elders with dementia using multimedia and verbal pleasant event schedules. Behavioral Interventions. 2008;23:213–225. [Google Scholar]
- Lyketsos CG, Carrillo MC, Ryan M, Khachaturian AS, Trzepacz P, Amatniek J, Miller DS. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews RM, Clair AA, Kosloski K. Brief in-service training in music therapy for activity aides: Increasing engagement of persons with dementia in rhythm activities. Activities, Adaption, & Aging. 2000;24:41–49. [Google Scholar]
- Mathews RM, Clair AA, Kosloski K. Keeping the beat: Use of rhythmic music during exercise activities for the elderly with dementia. American Journal of Alzheimer’s Disease & Other Dementias. 2001;16:377–380. doi: 10.1177/153331750101600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Castillo C, Woods B, Orrell M. The needs of people with dementia living at home from user, caregiver and professional perspectives: A cross-sectional survey. BMC Health Services Research. 2013;13:43–52. doi: 10.1186/1472-6963-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omnibus Budget Reconciliation Act of. H.R. 3545, Pub. L. No. 100-203, U.S. Congress, 101 Stat. 1987:1330. [Google Scholar]
- Polenick CA, Flora SR. Increasing social activity attendance in assisted living residents using personalized prompts and positive social attention. Journal of Applied Gerontology. 2011;32:515–539. doi: 10.1177/0733464811427444. [DOI] [PubMed] [Google Scholar]
- Richards KC, Beck C, O’Sullivan PS, Shue VM. Effect of individualized social activity on sleep in nursing home residents with dementia. Journal of the American Geriatrics Society. 2005;53:1510–1517. doi: 10.1111/j.1532-5415.2005.53460.x. [DOI] [PubMed] [Google Scholar]
- Samus QM, Rosenblatt A, Steele C, Baker A, Harper M, Brandt J, Lyketsos CG. The association of neu-ropsychiatric symptoms and environmental characteristics with quality of life in assisted living residents with dementia. The Gerontologist. 2005;45:19–26. doi: 10.1093/geront/45.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- Scherder EJ, Bogen T, Effermont LH, Hamers JP, Swaab DF. The more physical inactivity, the more agitation in dementia. International Psychogeriatrics. 2010;22:1203–1208. doi: 10.1017/S1041610210001493. [DOI] [PubMed] [Google Scholar]
- Schreiner AS, Yamamoto E, Shiotani H. Positive affect among nursing home residents with Alzheimer’s dementia: The effect of recreational activity. Aging & Mental Health. 2005;9:129–134. doi: 10.1080/13607860412331336841. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Intellectual self-management in old age. American Psychology. 1983;38:239–244. doi: 10.1037//0003-066x.38.3.239. [DOI] [PubMed] [Google Scholar]
- Skrajner MJ, Camp CJ. Resident-Assisted Montessori Programming (RAMP): Use of small group reading activity run by persons with dementia in adult day health care and long term care settings. American Journal of Alzheimer’s disease & Other Dementias. 2007;22:27–36. doi: 10.1177/1533317506297895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. Occupation-based activity analysis. Thorofare, NJ: Slack; 2011. [Google Scholar]
- United Kingdom’s National Collaborating Centre for Mental Health. Dementia: A NICE-SCIE guideline on supporting people with dementia and their careers in health and social care. London, England: British Psychological Society and Gaskell; 2007. [PubMed] [Google Scholar]
- von Kuzleben M, Schmid W, Halek M, Holle B, Bartholomeyczick S. Community-dwelling persons with dementia: What do they need? What do they demand? What do they do? A systematic review of the subjective experiences of persons with dementia. Aging & Mental Health. 2010;16:378–390. doi: 10.1080/13607863.2011.614594. [DOI] [PubMed] [Google Scholar]
- World Health Organization & Alzheimer’s Disease International. Dementia: A public health priority. 2012 Retrieved from http://whqlibdoc.who.int/publications/2012/9789241564458_eng.pdf.
- Zeman S. Person-centered care for the patient with mid- and late stage dementia. American Journal of Alzheimer’s Disease & Other Dementias. 1999;14:308–310. [Google Scholar]