Abstract
High-frequency stimulation of the nucleus accumbens, also known as deep brain stimulation (DBS), is currently used to alleviate obsessive compulsive symptoms when pharmacotherapy is ineffective. However, the mechanism by which DBS achieves its therapeutic actions is not understood. Imaging studies and the actions of dopaminergic drugs in untreated patients suggest that the dopamine (DA) system likely plays a role in the pathophysiology of obsessive compulsive disorder. Therefore, we examined whether DBS would impact the DA system as a potential component of its therapeutic actions. The activity of DA neurons in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) were recorded in anesthetized rats under high-frequency stimulation. DA neuron activity was measured in terms of number of neurons firing, average firing rate and firing pattern. DBS of the nucleus accumbens core did not significantly affect VTA activity or discharge pattern. On the other hand, DBS caused a potent decrease in the number of SNc DA neurons firing spontaneously. Such an effect could contribute to the disruption of pathological habit formation in the SNc-dorsal striatal projection system that may have therapeutic implications for the treatment of obsessive compulsive disorder.
Keywords: Deep brain stimulation, dopamine neuron, extracellular single unit recording, nucleus accumbens, substantia nigra pars compacta
Introduction
High-frequency stimulation (HFS), also known as deep brain stimulation (DBS), is an invasive neuro-surgical intervention introduced in the 1990s to alleviate motor disorders in otherwise intractable patients (Benabid et al., 1991, 1994; Krack et al., 2003; Rehncrona et al., 2003; Deuschl et al., 2006). This approach has been extended into the realm of psychiatric disorders as an efficacious alternative to ablative neurosurgery for treatment-resistant patients. One disease area in which it has shown particular efficacy is in the treatment of intractable obsessive compulsive disorder (OCD; for review see Greenberg et al., 2010).
OCD is a psychiatric disorder characterized in the DSM IV (1994) by obsessions (intrusive recurrent thoughts) and compulsions (repetitive aberrant behavior). Current effective therapeutic intervention in patients suggests that dopaminergic and serotonergic systems are involved in the pathophysiology and treatment of compulsive behavior (Denys et al., 2004b; Koo et al., 2010). Serotonin reuptake inhibitors are the most common medication used in the clinic but do not work in every patient, and often dopamine (DA) antagonists are substituted or added (Koo et al., 2010). Furthermore, positrom emission tomography (PET) studies suggest the presence of an imbalanced dopaminergic system in OCD patients (Denys et al., 2004a; Perani et al., 2008; Wong et al., 2008; Olver et al., 2009), and dopamine transporter ligand binding is elevated in the striatum of unmedicated OCD patients (van der Wee et al., 2004). When pharmaco-therapeutic intervention fails to yield improvement in severity of the symptoms or if the side effects of the medication surpass the benefits, HFS of the ventral striatal region has been used as an alternative (Greenberg et al., 2010). However, the mechanisms by which HFS alleviates OCD symptoms are not fully understood.
Pre-clinical studies suggest that DBS of the nucleus accumbens core (NAc), analogous to the clinical DBS target, may alleviate OCD symptoms by reducing activity in subsets of orbitofrontal cortical neurons, potentially by inducing long-term potentiation in local recurrent inhibitory circuits via antidromic activation of corticostriatal axon collaterals (McCracken and Grace, 2007). Further work suggests that therapeutic efficacy may involve enhancement of rhythmicity and synchronous inhibition within and between afferent structures, thereby normalizing function of a neural circuit that shows aberrant activity in OCD (McCracken and Grace, 2009). In contrast, despite the implications of a dopaminergic involvement in OCD, the effect of NAc HFS on dopaminergic systems has never been specifically studied. In this study, we examined the direct effects of NAc HFS on the mesolimbic (ventral tegmental area, VTA) and nigrostriatal (substantia nigra pars compacta, SNc) dopaminergic systems.
Experimental procedures
Subjects
Forty male Sprague–Dawley rats obtained from Harlan Laboratories (USA) were housed in pairs on a 12-h light/dark cycle. The rats arrived weighing approximately 300 g and were housed directly in a regular light cycle room. All protocols are consistent with the guidelines outlined in the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh, USA.
Extracellular recordings from dopamine neurons
Male Sprague–Dawley rats (300–450 g) were anaesthetized with chloral hydrate (400 mg/kg i.p.) and placed in a stereotaxic apparatus. Chloral hydrate was used for all recordings as under this anesthetic, the evoked cortical activity is relatively unaltered (Angel and Gratton, 1982) and DA neuron activity states more closely resemble that observed in freely moving rats (Hyland et al., 2002). Anesthesia was maintained by supplemental administration of chloral hydrate i.p. as required to maintain suppression of the hindlimb compression withdrawal reflex. A core body temperature of 37 °C was maintained by a thermostatically controlled heating pad (TR-200; Fine Science Tools, USA).
Stimulation of the nucleus accumbens bilaterally (A/P= +1.2, M/L=±2 from bregma and ventral 6.9 mm from the skull) was performed with bipolar concentric stimulating electrodes (NEX100) (David Kopf Instruments, USA) set at 130 Hz, 200 μA and 100 μs pulse width. The coordinates of the nucleus accumbens core and the parameters of stimulation were chosen based on our previous study (McCracken and Grace, 2007). Glass extracellular micro-electrodes were constructed from 2.0 mm Omegadot tubing (World Precision Instrument, Inc., USA), pulled on a Narishige PE-2 puller, broken back under microscopic control to an impedance of 6–14 MΩ, and filled with Chicago Sky Blue dye dissolved in saline (Sigma-Aldricht, USA). DA neurons were recorded either in the VTA or the SNc. A burr hole was drilled in the skull overlying the VTA (A/P=−5.3 mm, M/L=+0.6 mm from bregma and 6.5 to 9.0 mm ventral of brain surface) or the SNc (A/P=+2.8 mm, M/L=+2 mm from lambda and 6.5 to 9.0 mm ventral of brain surface), the dura resected, and the electrode lowered into the respective regions using a hydraulic microdrive (model 640; David Kopf Instruments, USA). The activity of the population of DA neurons was determined by counting the number of spontaneously active DA neurons encountered while making 6–9 vertical passes or tracks, separated by 200 μM, in a predetermined pattern to sample equivalent regions of the SNc and VTA, and recording the spontaneous firing rate and discharge pattern for each DA neuron encountered. The array of tracks was designed as a square with a side of three possible tracks centered (track 5) on the most central part of VTA or the SNc, which allowed the most antero-posterior or medio-lateral tracks to remain within the boundary of the region of interest according to an atlas (Paxinos and Watson, 1998). When a recording electrode’s resistance significantly changed (i.e. broken or clogged), the track was discarded from the analysis. The number of analyzed tracks per animal was not significantly different across groups. Spontaneously active dopamine neurons were identified with open filter settings (high pass: 3 Hz, low pass: 30 kHz) using previously established electrophysiological criteria (Grace and Bunney, 1983) including an action potential duration greater than 2 ms, an irregular (variable interspike interval) or bursting firing pattern (at least two 3-spike bursts occurring during a 500 spike epoch), and a firing rate between 0.5 to 12 Hz. These criteria were found to be sufficient to accurately identify the vast majority of neurons as dopaminergic (Ungless and Grace, 2012). Once a stable dopamine neuron was identified (signal-to-noise typically greater than 3:1), high pass filter settings were increased (to 10 or 100 Hz) to optimize data acquisition and subsequent analysis. After isolating the first spontaneously active DA neuron the stimulation was turned on (130 Hz, 0.1–0.2 mA and 0.1 ms pulse duration) and remained active until the end of the recording session. For sham groups the stimulation was never turned on. Each neuron was recorded for a minimum period of 6 min and the analysis was performed on a block of 3 min. DA neurons were subdivided into bursting (at least two 3-spike bursts occurring during a 500 spike epoch) or non-bursting, as previously defined (Grace and Bunney, 1984). A burst was defined as at least two spikes with an interspike interval equal to or less than 80 ms, with burst termination defined as a subsequent interspike interval greater than 160 ms (Grace and Bunney, 1984). The general activity of the VTA was assessed with the number of spontaneously active DA neurons per track (or cells per track, CPT), their respective firing rate (FR) and the proportion of spikes occurring in bursts (%SIB). Then the bursting neuron population was calculated and the bursting neuron ratio (BNR) was derived from this analysis, and is defined as the number of bursting DA neurons divided by the total number of active DA neurons recorded.
Stimulation of the NAc electrode could potentially result in antidromic spikes recorded in SNc or VTA DA neurons that provide direct input to the NAc core (Groenewegen et al., 1999). This is not likely given that the pulse duration used was substantially less than that typically required to antidromically activate the thin, non-myelinated axons of DA neurons (Guyenet and Aghajanian, 1978; Grace and Bunney, 1983). Moreover, none of the DA neuron spikes recorded demonstrated fixed latency or high-frequency following, which would be expected for antidromic activation (Maurice et al., 2003).
Histology
Following the cessation of each electrophysiological experiment, the recording site was marked using electrophoretic ejection of Chicago Sky Blue dye (−20 μA constant current: 20–30 min). Rats were euthanized with an overdose of chloral hydrate, decapitated and the brains removed. The brains were submerged in 8% para-formaldehyde (in PB) for fixation for a period of at least 48 h. The brains were then transferred to a 25% sucrose solution (in PB) for cryoprotection before being frozen and sectioned on a cryostat in the coronal plane (thickness: 60 μM). Sections were placed on gelatin-chromalum-coated slides and stained using Cresyl Violet and Neutral Red stain for histochemical verification of electrode placement with reference to a stereotaxic atlas (Paxinos and Watson, 1998).
Data analysis
The electrophysiological recording data collected were processed using LabChart Pro (AD Instrument Inc., USA) and Neuroexplorer 4 (Nex Technologie, USA). As the neuron spike duration was much longer than the stimulation artifact, the spikes could be accurately discriminated. Similar observation was reported during extracellular recordings of dopamine neuron (Hu et al., 2011). Dopamine neurons recorded from the SNc or the VTA, despite sharing some electrophysiological characteristics, are regulated by different sets input and output projections and cannot be considered identical (Watabe-Uchida et al., 2012). In that regard, the respective controls were compared with stimulated animals using Student’s t test, the equality of variance was tested beforehand using a F-test. No differences of variances were found across the entire set of data we collected, and no corrections were applied for the subsequent Student’s t tests. When possible 3 min of the baseline of the first DA neuron recorded in stimulated animals before the onset of stimulation was compared with the first 3 min of high-frequency stimulation of the NAc core in their respective recorded target for firing rate and proportion of spikes occurring into bursts. In that context a paired Student’s t test was used after the equality of variance was assessed using an F-test.
For all tests, a p value less than 0.05 was considered to be significantly different between the tested groups.
Results
Histology showed that three rats stimulated in the NAc core had misplaced electrodes and in one rat the recording site was not in the SNc; these data were excluded from the analysis (Fig. 1). The final groups are as follow: 19 sham-stimulated rats (10 recorded in the VTA and 9 in the SNc) and 17 stimulated rats (9 recorded in the VTA and 8 in the SNc). The total number of tracks recorded was consistent between groups and showed no statistical differences (VTA: control=6.40±0.16 and DBS=6.77±0.22, t17 =1.389, n.s.; SNc: control=7.22±0.32 and DBS=7.62±0.53, t15 =0.663, n.s.).
Fig. 1.
Schematic localization of the stimulating electrodes in the nucleus accumbens core (NAc) (a), recording electrodes in the ventral tegmental area (VTA) (b), and the substantia nigra pars compacta (SNc) (c). Animals recorded in the VTA are represented by circles and animals recorded on the SNc by triangles. Filled symbols indicate that the rats were stimulated and open symbols indicate that the rat were not stimulated (sham). The stimulating electrodes were implanted bilaterally, but for simplicity purposes only the localization of the right electrode in shown for the stimulated animals and the left electrodes for the sham animals. Three rats stimulating electrodes were located outside of the boundaries of the NAc, their respective recording sites were not reported in panels B and C as they were discarded from the study.
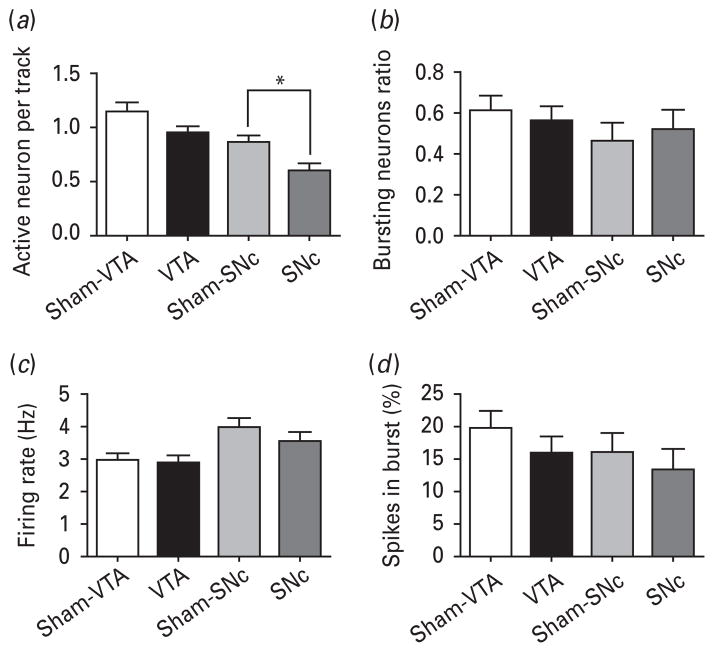
HFS of the NAc core significantly decreased the DA neuron population activity in the SNc as compared with controls (i.e. number of DA neurons firing; control=0.86 ±0.05; DBS=0.59±0.07; t15 =2.995, p =0.009; Fig. 2a), but DA neuron activity recorded in the VTA remained unaffected (control=1.13±0.08; DBS=0.95±0.06; t17 =1.753; n.s.). No other differences were found between sham and stimulated animals in the VTA or SNc and their controls with respect to ratio of bursting DA neurons (t<0.5, n.s.; Fig. 2b), or the average firing rate (t<1, n.s.; Fig. 2c), or percentage of spikes in bursts (t<1.1, n.s.; Fig. 2d). Therefore, HFS of the NAc core reduces the overall output of the SNc DA neurons.
Fig. 2.
High-frequency stimulation of the nucleus accumbens core (NAc) reduces the overall output of the substantia nigra pars compacta (SNc) dopamine neurons (DA) but does not impact the ventral tegmental area (VTA) DA system. (a) High-frequency stimulation (black fill) did not affect the number of DA neurons firing in the VTA, but significantly decreased the number firing in the SNc by 30% when compared with sham controls (light gray fill). In contrast, there was no effect of stimulation on the number of bursting neurons (b), neuron firing rate (c) or the percentage of spikes fired in bursts (d) in any stimulation condition (see related text). Measures are presented as mean±S.E.M., * indicates significant difference (p<0.05).
In three stimulated rats, two of which were recorded in the SNc and one in the VTA, we were not able to maintain the recording of the first DA neuron after the onset of high-frequency stimulation. The analysis of the immediate effect of HFS is based on six stimulated animals in which we recorded in the SNc and eight in the VTA. No difference was found in the SNc in terms of firing rate or proportion of spikes in bursts (firing rate: DBS off=4.01±0.87 and DBS on=3.10±0.82, t5 =1.536, n.s.; percentage of spikes in bursts: DBS off=9.19±7.34 and DBS on=8.34±3.89, t5 =0.143, n.s.); nor in the VTA (firing rate: DBS off=2.38±0.45 and DBS on=2.28 ±0.45, t7 =0.286, n.s.; percentage of spikes in bursts: DBS off=11.86±4.22 and DBS on=11.80±6.68, t7 =0.009, n.s.).
Discussion
In this study, we examined the impact of DBS applied bilaterally to the NAc core region on the activity of the mesolimbic and nigrostriatal DA neurons. Surprisingly, the stimulation did not impact the mesolimbic, reward-related or salience-related (Sesack and Grace, 2009) DA neuron population, which is in line with recently reported experiments (Hu et al., 2011). In contrast, the stimulation was found to significantly reduce the number of active DA neurons in the SNc, whereas the number of bursting dopamine neurons, the average firing rate and the percentage of spikes occurring in bursts in this structure were otherwise not affected. A decrease in the number of DA neurons firing would functionally attenuate the influence of the DA system on this circuit. NAc core DBS has been reported to decrease the level of DA and its metabolites in medial pre-frontal cortex, which was associated with lower tyrosine hydroxylase expression (Sesia et al., 2010; Falowski et al., 2011).
What is the impact of a decrease in SNc dopamine neuron population activity? Only spontaneously firing DA neurons can be recruited to fire in phasic bursts (Floresco et al., 2003). Furthermore, we have shown in a subsequent study that the number of DA neurons firing spontaneously determines the amplitude of the phasic DA response to stimuli (Lodge and Grace, 2006). Therefore by decreasing the number of DA neurons active, phasic events would have less influence over the striatum and other targeted structures such as the prefrontal cortex. A decrease in the activity of SNc DA neurons projecting to regions involved in maintenance of habits could be a therapeutically relevant action in OCD, either through direct inhibition of formed habits or alternately by enabling the system to reconfigure in the absence of a dopaminergic influence. Given the delayed onset of therapeutic action of DBS in OCD (Greenberg et al., 2006), the latter explanation is more likely.
The fact that we observed no effect on the VTA but a decrease in general activity of the SNc suggests that HFS actions may be mediated via either the striatofugal fibers targeting the SNc or the corticofugal fibers innervating the striatum (Groenewegen et al., 1999). Whether this is attributable to alterations in cortical interneuron function, as proposed by McCracken and Grace (2007), inhibition of the ventral pallidum (Hu et al., 2011) leading to alterations via a multisynaptic circuit, or via direct pathway activation, is not clear at this point. Our data, however, do not suggest antidromic inhibition and provide little support for direct inhibition. Indeed the DA neurons recorded while the stimulation was initiated showed no changes in activity. Nonetheless, the finding that the average firing rate and firing pattern did not change does not conclusively mean that individual neurons did not change in different directions (activation vs. inhibition), or even that the neurons which activities were silenced during stimulation were not compensated for by changes in other neuron firing characteristics, which failed to show a significant difference when averaged across the population. Thus, by sampling population activity, we can assess changes in the overall output of SN DA neuron activity, but cannot speculate on how it affects all of the individual neurons.
There are several pathways that could account for the impact of DBS on the dopamine system. Dopamine fibers reaching the nucleus accumbens core originate in the lateral half of the VTA and medial part of the substantia nigra pars compacta (SNc) (Heimer et al., 1997; Ikemoto, 2007). Reciprocally, there is a direct striatonigral projection from the NAc to the lateral part of VTA and throughout much of the SNc (Somogyi et al., 1981; Zahm and Heimer, 1993; Watabe-Uchida et al., 2012). Outputs of the NAc parallel the dorsal striatal projections by sending fibers to the dorsolateral portion of the sub-commissural ventral pallidum and both output nuclei of the basal ganglia, the entopeduncular nucleus and substantia nigra pars reticulata (Haber et al., 1990; Zahm and Heimer, 1990; Heimer et al., 1991; Deniau et al., 1994; Groenewegen et al., 1999). The ventral pallidum projects as well to the entopeduncular nucleus, both with direct inhibitory projections and indirectly through the excitatory subthalamic nucleus (Groenewegen et al., 1993). Importantly, the entopeduncular nucleus sends much denser inhibitory projections to the SNc than to the VTA (Watabe-Uchida et al., 2012). The ventral striatal input to the SNc is significant as it potentially allows ventral striatal modulation of the dorsal striatum (Nauta et al., 1978; Haber et al., 2000; Everitt et al., 2008) and could therefore account for the ventral striatal DBS actions on SNc dopamine neuron activity.
The fact that DBS affected primarily the nigrostriatal DA system has potential relevance for OCD: in OCD there is an inability to disengage from a task. Although the mesolimbic DA system has projections to ventral striatal regions involved in reward and stimulus salience (Schultz, 2010; Lodge and Grace, 2011), the SNc projections to the dorsal striatum are considered to have more involvement in habit formation (Everitt et al., 2008).
Similar DBS protocols (comparable parameters of stimulation between 30 min to a few hours) have been reported to have a potential therapeutic effect on addiction to various substance of abuse (Liu et al., 2008; Knapp et al., 2009; Henderson et al., 2010; Guo et al., 2013), impulsive behavior (Sesia et al., 2008) and compulsive-like behavior (van Kuyck et al., 2003; Mundt et al., 2009). In some instances the effect was shown to be specific to the time of stimulation (van Kuyck et al., 2003, 2008; Sesia et al., 2008, 2010; Mundt et al., 2009), suggesting a short onset of action with no long-term effects. Given that each of these involve habit formation and dorsal striatal function (Robbins et al., 2012), a modulation via SNc dopamine neuron activity could be a common therapeutic response.
In conclusion, high-frequency stimulation of the nucleus accumbens core inhibits the activity of the SN pars compacta DA neurons but does not affect VTA DA neuron activity. Thus, HFS of the NAc may influence the dorsal striatum activity and exert control over expression of habits.
Acknowledgments
The authors thank Niki MacMurdo for technical support and Sarah Schreiber for participation in this research. This research was funded by NIH (MH086400).
Footnotes
Statement of Interest
During the past years, Anthony A. Grace has received consulting honoraria from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Eli Lilly, and Roche.
References
- Angel A, Gratton DA. The effect of anaesthetic agents on cerebral cortical responses in the rat. Br J Pharmacol. 1982;76:541–549. doi: 10.1111/j.1476-5381.1982.tb09252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Thierry AM. Indirect nucleus accumbens input to the prefrontal cortex via the substantia nigra pars reticulata: a combined anatomical and electrophysiological study in the rat. Neuroscience. 1994;61:533–545. doi: 10.1016/0306-4522(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. 2004a;55:1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Denys D, Zohar J, Westenberg HG. The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence. J Clin Psychiatry. 2004b;65(Suppl 14):11–17. [PubMed] [Google Scholar]
- Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- DSMIV. American Psychiatric Association, Committee on Nomenclature and Statistics: Diagnostic and Statistical Manual of Mental Disorders 1994 [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc B: Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falowski S, Sharan A, Reyes B, Sikkema C, Szot P, Van Bockstaele E. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69:1281–1290. doi: 10.1227/NEU.0b013e3182237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S, West A, Ash B, Moore H, Grace A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B, Malone D, Friehs G, Rezai A, Kubu C, Malloy P, Salloway S, Okun M, Goodman W, Rasmussen S. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, Tang S, Liu H, Jiang J. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Dependence. 2013;129:70–81. doi: 10.1016/j.drugalcdep.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Guyenet P, Aghajanian G. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Wolfe DP, Groenewegen HJ. The relationship between ventral striatal efferent fibers and the distribution of peptide-positive woolly fibers in the forebrain of the rhesus monkey. Neuroscience. 1990;39:323–338. doi: 10.1016/0306-4522(90)90271-5. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Henderson M, Green A, Bradford P, Chau D, Roberts D, Leiter J. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29 doi: 10.3171/2010.4.FOCUS10105. [DOI] [PubMed] [Google Scholar]
- Hu W-H, Bi Y-F, Zhang K, Meng F-G, Zhang J-G. High-frequency electrical stimulation in the nucleus accumbens of morphine-treated rats suppresses neuronal firing in reward-related brain regions. Med Sci Monit. 2011;17:60. doi: 10.12659/MSM.881802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C, Tozier L, Pak A, Ciraulo D, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2009;92:474–479. doi: 10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo MS, Kim EJ, Roh D, Kim CH. Role of dopamine in the pathophysiology and treatment of obsessive-compulsive disorder. Expert Rev Neurother. 2010;10:275–290. doi: 10.1586/ern.09.148. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Liu H-Y, Jin J, Tang J-S, Sun W-X, Jia H, Yang X-P, Cui J-M, Wang C-G. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13:40–46. doi: 10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- Lodge D, Grace A. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Thierry AM, Glowinski J, Deniau JM. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high frequency stimulation of the subthalamic nucleus. J Neurosci. 2003;223:9929–9936. doi: 10.1523/JNEUROSCI.23-30-09929.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt A, Klein J, Joel D, Heinz A, Djodari-Irani A, Harnack D, Kupsch A, Orawa H, Juckel G, Morgenstern R, Winter C. High-frequency stimulation of the nucleus accumbens core and shell reduces quinpirole-induced compulsive checking in rats. Eur J Neurosci. 2009;29:2401–2412. doi: 10.1111/j.1460-9568.2009.06777.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Olver JS, O’Keefe G, Jones GR, Burrows GD, Tochon-Danguy HJ, Ackermann U, Scott A, Norman TR. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J Affect Disord. 2009;114:321–326. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT2A serotonin and D2 dopamine dysfunction in drug-naive obsessive-compulsive disorder. NeuroImage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2009;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesia T, Temel Y, Lim L, Blokland A, Steinbusch H, Visser-Vandewalle V. Deep brain stimulation of the nucleus accumbens core and shell: opposite effects on impulsive action. Exp Neurol. 2008;214:135–139. doi: 10.1016/j.expneurol.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Sesia T, Bulthuis V, Tan S, Lim L, Vlamings R, Blokland A, Steinbusch H, Sharp T, Visser-Vandewalle V, Temel Y. Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Exp Neurol. 2010;225:302–309. doi: 10.1016/j.expneurol.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Totterdell S, Smith AD. Monosynaptic input from the nucleus accumbens–ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wee NJ, Stevens H, Hardeman JA, Mandl RC, Denys DA, van Megen HJ, Kahn RS, Westenberg HM. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am J Psychiatry. 2004;161:2201–2206. doi: 10.1176/appi.ajp.161.12.2201. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Demeulemeester H, Feys H, De Weerdt W, Dewil M, Tousseyn T, De Sutter P, Gybels J, Bogaerts K, Dom R, Nuttin B. Effects of electrical stimulation or lesion in nucleus accumbens on the behaviour of rats in a T-maze after administration of 8-OH-DPAT or vehicle. Behav Brain Res. 2003;140:165–173. doi: 10.1016/s0166-4328(02)00295-4. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Brak K, Das J, Rizopoulos D, Nuttin B. Comparative study of the effects of electrical stimulation in the nucleus accumbens, the mediodorsal thalamic nucleus and the bed nucleus of the stria terminalis in rats with schedule-induced polydipsia. Brain Res. 2008;1201:93–99. doi: 10.1016/j.brainres.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, Rousset O, Kumar A, Szabo Z, Gjedde A, Grace AA. Mechanisms of dopaminergic and serotonergic neuro-transmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm D, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]