Abstract
Background
The prevalence of short sleep duration in adolescence and the relevance of early risk factors to cardiovascular disease in adulthood suggest that adolescence is an opportune time to evaluate links between sleep duration and cardiovascular disease risk. We examined associations among actigraphy-assessed sleep duration and sleep debt with elevated C-Reactive Protein (CRP), a known risk factor for cardiovascular disease.
Methods
Participants were 244 (137 Blacks, 116 males) healthy high school students, each of whom wore wrist actigraphs for one week and provided a fasting blood draw. CRP was examined as both a continuous and categorical outcome, with CRP > 3 mg/L identifying a High Risk Group.
Results
Sleep duration and sleep debt were significantly associated with CRP High Risk Group in covariate-adjusted analyses. Shorter sleep duration on school nights was associated with a greater likelihood of being in the High Risk CRP Group. Likelihood of being in the High Risk CRP Group was doubled in students who obtained an average of two or more hours of “catch up” sleep on weekend nights.
Conclusions
Reduced weekday sleep duration and sleep debt were both associated with CRP Risk Group in adolescence. That these relationships may be observed prior to the onset of clinical disease suggests that adolescence may provide an opportune period for disease prevention.
Keywords: Sleep duration, Sleep debt, C-reactive protein, adolescence, sex, race
Introduction
Short sleep duration, which is highly prevalent in industrialized nations, is a significant risk factor for cardiovascular disease (CVD) [1;2]. Chronic low-grade inflammation, including elevated circulating levels of C-reactive protein (CRP), represents one pathway through which short sleep duration may influence both the pathogenesis and clinical course of cardiovascular disease [3]. C-reactive protein contributes to multiple aspects of atherogenesis and plaque vulnerability across the lifespan and elevated CRP has been prospectively associated with cardiovascular disease [4;5]. For instance, individuals with CRP values > 3 mg/L are at increased risk for cardiovascular events [6].
Several epidemiological studies have reported that short sleep duration is associated with increased circulating levels of plasma CRP in adults, although discrepant findings have been reported by some [7–12]. Important to questions of causality, a number of studies have reported that CRP levels increase in response to experimental sleep restriction and sleep deprivation in healthy young adults, although some have reported null effects [13–16]. Discrepant findings in the published literature may be related to methodological differences across studies and/or differences in important effect modifiers such as sex, race, medication use, co-morbidities including undiagnosed cardiovascular disease, and survivor bias.
Several factors suggest that adolescence is an opportune time to evaluate links between short sleep duration and cardiovascular disease risk, including markers of inflammation. First, sleep restriction is endemic in high school students due, in part, to a constellation of biological and social factors that favor later bedtimes in spite of societally-mandated early school start times [17;18]. Numerous studies, including our own, have reported that most high school students do not obtain the recommended 8 to 9 hours of sleep suggested by the Centers for Disease Control (CDC) [19–21]. For instance, nearly 40% of high school students who completed the 2007 CDC National Youth Behavior Survey reported sleeping 6 hours or less on school nights [22]. Although high school students sleep an average of two hours longer on weekend nights, most studies suggest their weekly sleep duration averages are still generally below 8 hours [19–21]. Second, longitudinal studies have established that risk factors for cardiovascular disease, including chronic low-grade inflammation, can be identified in adolescents, decades before the onset of clinical disease [23–27]. Among identified early risk factors, elevated CRP in adolescents has been linked to the structural and functional vascular abnormalities as well as the metabolic syndrome [28–30]. Finally, adolescence allows examination of the association between short sleep duration and elevated CRP in the relative absence of underlying disease and without the confounding of survivor bias present in older samples.
Modest inverse correlations between sleep duration and circulating CRP levels have been observed in high school students in the United States and Spain [31;32]. Both studies averaged sleep duration values across the week, despite evidence that many high school students sleep an average of 2 hours longer on weekend compared to week nights [33]. Although neither study evaluated the influence of race, others have reported increased CRP in Black short sleeper adults [34;35]. Results for sex as an effect modifier in adult samples have been mixed [8;32;34;36;37].
The present study evaluated relationships among actigraphy-assessed sleep duration and fasting high-sensitivity CRP (hsCRP) in White and Black high school students, about half of whom were female. These data extend the published literature on sleep duration and CRP in adolescents by examining weekday and weekend sleep duration independently and by evaluating the possible influence of race and sex on these associations. These issues are critical to both risk stratification and development of evidence-based interventions to reduce cardiovascular risk early in the development of this prevalent and costly disease.
Methods
Participants
Participants were 250 adolescents between the ages of 14 and 19 enrolled in a public high school that serves a racially diverse (42% Black, 56% White, 2% other), lower to middle socioeconomic status community near Pittsburgh, PA. Recruitment and study procedures took place between November 2008 and May 2011, except for summers and school vacations. The study was described to potential participants during regularly scheduled high school physical/health education classes as concerning the relationships among stress, sleep, and cardiovascular risk factors. Students interested in participation contacted the research team to learn more about the study and determine eligibility. Approval of the research project was obtained from the superintendent of the school district, the principal of the high school, and the University of Pittsburgh Institutional Review Board. Participants or legal guardians of students under the age of 18 provided written informed consent prior to commencing the study. A total of 16 students expressed interest in the study but were deemed ineligible due to one or more of the following exclusions: medication use for emotional or psychological disorders, use of diabetes or blood pressure medication, and use of any medication known to affect the cardiovascular system or sleep. Symptoms of sleep apnea including snoring, pauses in breathing, and snorting and gasping during sleep were assessed by parent report. Only one student’s parent reported modest symptoms in their child. The parent was subsequently interviewed by a pediatrician and sleep medicine expert, and it was determined that the student did not have sufficient sleep problems to warrant exclusion. The present report includes 244 participants who had complete data available for sleep assessments and CRP.
Measures
Sleep
Sleep measures were derived from rest-activity patterns recorded by wrist actigraphs (Actiwatch-16, Philips Respironics, Bend, OR) worn continuously by participants for a 7-day period during the academic school year. Data were not collected during the summer and over school vacations. Wrist actigraphy has been validated against polysomnography for measuring sleep duration in adolescents [38]. Nocturnal sleep intervals were identified as previously described [39]. Sleep duration was operationalized as the total minutes of sleep between initial sleep onset and final sleep offset, excluding periods of actigraphy-assessed wakefulness during the night. Average sleep duration was calculated separately for week nights (Sun – Thurs nights) and weekend nights (Fri-Sat nights). For comparison to published papers, average weekly sleep duration was calculated over the week-long study period. A fourth variable, sleep debt, was used to evaluate whether the need for “catch up” sleep on weekend nights was associated with CRP. We first subtracted average weeknight sleep duration from average weekend sleep duration. Sleep debt was then labeled as “present” in participants who slept at least 2 hours longer on weekend compared to week nights.[40;41]
C-Reactive Protein
Fasting blood samples were obtained on school days that coincided with study assessments. Blood draws were rescheduled to a later date if participants endorsed any sign of illness (e.g., upper respiratory infection, taking antibiotics, etc.) within the past three days. Once collected, serum was separated by refrigerated centrifuge, aliquoted, and stored at −80 C until assay in the Heinz Lipid Laboratory at the Graduate School of Public Health at the University of Pittsburgh. Each high sensitivity assay (hsCRP) run included duplicate samples, standards and control sera. CRP was measured turbidimetrically by measuring increased absorbance when the CRP in the sample reacts with anti-CRP antibodies. The intra-assay coefficient of variation was 5.5% and inter-assay coefficient of variation was 3.0%. We evaluated hsCRP as both a continuous and categorical outcome, given the prognostic value of evaluating clinically-significant cut-off scores. The Low to Moderate Risk Group included participants with CRP values ≤ 3 mg/L (n=211; 86.5%) and the High Risk Group included participants with CRP values > 3 mg/L (n=33; 13.5%).
Covariates
Age, sex, and race/ethnicity were determined by participant self-report. Height was measured using a stadiometer, and weight was measured on a Tanita digital scale to the 1/10 of a pound. Body mass index (BMI) was calculated using the NHLBI on-line calculator: www.nhlbi.nih.gov/guidelines/obesity/BMI/bmicalc.htm. Parental education was used to characterize household socioeconomic status. Greatest educational attainment by the participant’s mother or father in the household was dichotomized as a high school degree/GED (n=113) or less (n=29) versus college-educated, including some college (n=70) or a college degree (n=32). Daytime sleepiness., which may be viewed as an indicator for likely sleep apnea and/or a mechanism to cope with short nighttime sleep, was measured by a ten-item scale focused on situations in which they may have struggled to stay awake or fallen asleep in the past 2 weeks, e.g. attending a performance, in class at school, driving a car. Each item was rated on a four-point scale from no, struggled to stay awake, fallen asleep, or both struggled to stay awake and fallen asleep, and were totaled. Average daytime nap duration was calculated from daily self-reported sleep diaries and recorded as average minutes per day (weekday and weekend). Finally, season was defined as fall (September through November), winter (December through February) and spring (March through June).
Statistical Analysis
Descriptive statistics were used to examine sample characteristics, check for outliers, and evaluate normality. A natural log transformation was used to normalize hsCRP levels. We used Chi2 tests and analysis of variance (ANOVA) to characterize categorical and continuous sample characteristics as a function of sex and race. In the primary analyses, multivariate linear regression and binary logistic regression were used to evaluate associations among sleep duration and hsCRP levels and CRP Risk Group, respectively. Covariates included in all analyses were sex, race, BMI and parental education. Age, daytime sleepiness, naps and season were not included as covariates as they were unrelated to CRP outcomes in the present sample (p>.20). Actigraphy-assessed weekday and weekend sleep duration were included in the same model as these variables were uncorrelated (r=0.10, p>.05). Separate models were used for average weekly sleep duration and for sleep debt, which was derived from and collinear with weekday (r=−0.41, p<.001) and weekend sleep duration (r=−0.61, p<.001). In secondary analyses we examined the extent to which associations among sleep duration and CRP outcomes differed as a function of sex or race, based on the literature in adults [8;34;35]. We evaluated interactions using multivariate models with centering of sleep variables and stratified multivariate analyses explored associations among sleep duration and CRP outcomes by sex and race. Fit statistics (Hosmer & Lemeshow Test for logistic regression and F Test for linear regression) suggested good fit for all models with the exception of interactions and exploratory stratified analyses of effect modifiers. Finally, sensitivity analyses excluding participants with CRP levels > 10 mg/L (n=9) were used to examine possible confounding by undetected acute illness at the time of the blood draw. Results in the reduced sample were unchanged from those in the full sample (sensitivity analyses not shown).
Results
As shown in Table 1, 52.5% of the sample was female, 56.1% were Black, and average BMI was above the 75th percentile for national age and sex norms. Wrist actigraphy revealed that participants obtained just under 6 hours of sleep on school nights and about 7 ½ hours of sleep on the weekends. Actigraphy also indicated that over 1/3 of the sample experienced sleep debt, defined as sleeping at least 2 hours longer on weekend relative to week nights. On average, participants napped over 30 minutes per day during the week and over 15 minutes per day on weekends. As previously reported [20], sleep duration was an average of 30 minutes shorter in males compared to females on school nights (p<.05), and sleep duration was nearly 40 minutes longer on weeknights and weekend nights in Whites compared to Blacks (p<.001 and p<.05, respectively). Other sample characteristics including age, hsCRP levels, BMI, sleep debt and daytime sleepiness were similar for males and females as well as for Whites and Blacks.
Table 1.
Sample characteristics
| All (n=244) | Sex | Race | |||
|---|---|---|---|---|---|
| Female (n=128) | Male (n=116) | White (n=107) | Black (n=137) | ||
| Age, years | |||||
| mean (SD) | 15.71 (1.31) | 15.69 (1.29) | 15.74 (1.33) | 15.65 (1.30) | 15.76 (1.31) |
| range | 14 – 19 | 14 – 19 | 14 – 19 | 14 – 19 | 14 – 19 |
| BMI | |||||
| Mean (SD) | 26.08 (5.86) | 26.11 (6.12) | 26.05 (5.60) | 26.05 (6.34) | 26.11 (5.48) |
| range | 16.44–46.89 | 16.44–43.48 | 16.67–46.89 | 16.44–46.89 | 17.75–43.48 |
| BMI Percentile (sex & age) | |||||
| mean (SD) | 78.64 (22.64) | 77.41 (23.41) | 79.98 (21.79) | 76.09 (25.93) | 80.62 (19.57) |
| range | 27 – 100 | 3 – 100 | 6 – 100 | 3 – 100 | 10 – 100 |
| Parental Education1 | |||||
| No. (%) ≤ high school | 142 (58,2%) | 82 (64.1%) | 6 (51.7%) | 73 (68.2%) | 69 (50.4%) |
| No. (%) ≥ some college | 102 (41.2%) | 46 (35.9%) | 56 (48.3%) | 34 (31.8%) | 68 (49.6%) |
| hsCRP Levels (mg/L)2 | |||||
| Mean (SD) | 1.71 (3.16) | 1.98 (3.63) | 1.42 (2.53) | 1.87 (3.21) | 1.59 (3.13) |
| Range | 0.03 – 23.10 | 0.03 – 23.10 | 0.05 – 15.70 | 0.03 – 20.20 | 0.03 – 23.10 |
| Median | 0.64 | 0.65 | 0.58 | 0.75 | 0.50 |
| Interquartile Range | 0.24 – 1.67 | 0.25 – 2.26 | 0.23 – 1.47 | 0.32 – 1.64 | 0.22 – 1.72 |
| High Risk CRP Group | |||||
| No. (%) No | 211 (86.5%) | 107 (83.6%) | 104 (89.7%) | 88 (82.2%) | 123 (89.8%) |
| No. (%) Yes | 33 (13.5%) | 21 (16.4%) | 12 (10.3%) | 19 (17.8%) | 14 (10.2%) |
| Sleep Duration (hours) | 5.94 (.89) | 6.07 (.91) | 5.80 (.84) | ||
| Mean (SD) Weekday3 | 7.42 (1.23) | 7.55 (1.29) | 7.27 (1.14) | 6.18 (.94) | 5.76 (.80) |
| Mean (SD) Weekend4 | 6.44 (.79) | 6.57 (.82) | 6.30 (.74) | 7.62 (1.25) | 7.26 (1.19) |
| Mean (SD) Full Week5 | 6.70 (.84) | 6.24 (.70) | |||
| Sleep Debt | |||||
| No. (%) No | 159 (65.2%) | 79 (61.7%) | 80 (69.0%) | 71 (66.4%) | 88 (64.2%) |
| No. (%) Yes | 85 (34.8%) | 49 (38.3%) | 36 (31.0%) | 36 (33.6%) | 49 (35.8%) |
| Daytime Sleepiness | |||||
| Mean (SD) | 16.0 (3.67) | 16.33 (3.51) | 15.63 (3.83) | 15.83 (3.56) | 16.13 (3.77) |
| range | 10 – 29 | 10 – 28 | 10 – 29 | 10 – 26 | 10 – 29 |
p<.05 for sex comparison and p<.01 for race comparison;
Raw values are shown in table; hsCRP levels were natural log transformed prior to analyses;
p<.05 for sex and p<.001 for race comparisons;
p<.05 for race comparison;
p<.01 for sex and p<.001 for race comparisons
Participants in the High Risk CRP Group had significantly higher BMIs (88.09% ± 18.9) compared to participants in the Low to Moderate Risk CRP Group (77.16% ± 22.86; p<.01). In univariate linear analyses, BMI accounted for 21% of the variance in hsCRP levels; higher BMIs were associated with higher hsCRP levels (p<.001). There was a trend for higher parental education in the Low to Moderate Risk CRP Group (44.1% with some college or college degree) compared to 27.3% with education beyond high school in the High Risk CRP Group (Chi2=3.31, p<.07). Age, daytime sleepiness, naps and season in which the study was conducted did not differ as a function of CRP Risk Group.
Sleep duration and sleep debt were unrelated to hsCRP levels (see Table 2). In contrast, both weekday sleep duration and sleep debt were significantly associated with CRP Risk Group. Multivariate logistic regression revealed that shorter weekday sleep duration was a significant correlate of CRP High Risk Group, independent of weekend sleep duration and other model covariates including sex, race, parental education, and BMI. As shown in Figure 1, students who slept longer on school nights were less likely to be in the High Risk CRP Group (Odds Ratio (OR) = 0.62, 95% Confidence Interval (CI) = 0.39 – 0.98. In contrast, weekend sleep duration was unrelated to CRP Risk Group (OR = 0.96, 95% CI = 0.69 – 1.34). Average sleep duration over the 7-day study period was unrelated to CRP Risk Group. The sleep debt model revealed that students who slept at least 2 hours longer on weekend compared to weeknights were nearly two and a half times more likely to be in the High Risk CRP Group compared to those without sleep debt (OR = 2.33, 95% CI = 1.01 – 4.89).
Table 2.
Associations among sleep duration and sleep debt with hsCRP levels1
| Beta | P value | |
|---|---|---|
| Weeknight Sleep Duration | −0.04 | 0.51 |
| Weekend Sleep Duration | −0.01 | 0.86 |
| Average Sleep Duration | −0.04 | 0.54 |
| Sleep Debt | 0.03 | 0.62 |
hsCRP level was natural log transformed prior to analyses
Figure 1.
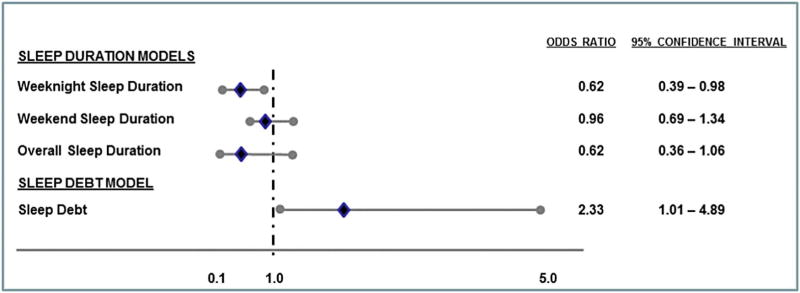
Associations among sleep duration and sleep debt with CRP Risk Groups. Diamonds depict adjusted odds ratios for the High Risk CRP group and circles represent lower and upper confidence limits. Odds ratios are adjusted for sex, race, highest parental education and BMI.
Although race-by-sleep duration and gender-by-sleep duration interactions were not significant, exploratory analyses suggest possible differences. In exploratory analyses stratified by race, the association between short weekday sleep duration and CRP Risk Group was observed in Whites (OR = 0.51, 95% CI 0.28 – 0.93) but not in Blacks (OR = 0.83, 95% CI = 0.37 – 1.86). Exploratory analyses stratified by sex revealed that short weekday sleep duration was associated with the High Risk CRP Group in females (OR = 0.43, 95% CI = 0.22 – 0.84) but not in males (OR = 0.88, 95% CI = 0.39 – 1.98).
Discussion
The prevalence of short sleep duration in adolescence and the relevance of early risk factors to cardiovascular disease in adulthood suggest that adolescence is an opportune time to evaluate links between short sleep duration and cardiovascular disease risk. The limited data in adolescents is striking, given that cardiovascular disease develops over years and decades, starting during youth [23–27]. To our knowledge, this study is the first to concurrently examine associations among both weeknight and weekend sleep duration with CRP in adolescents. Students with shorter actigraphy-assessed sleep durations on school nights were more likely to be in the High Risk CRP Group, compared to their classmates who obtained more sleep on school nights. Critically, this association was independent of weekend sleep duration and other factors known to influence circulating CRP levels in healthy adolescents. While weekend sleep duration was not uniquely associated with CRP, likelihood of being in the High Risk CRP Group was doubled in students who obtained an average of two or more hours of “catch up” sleep on weekend compared to weekend nights. We know of no study that has evaluated sleep debt in relation to CRP, although sleep debt in adults has been associated with other adverse cardiometabolic outcomes including increased inflammation (IL-1beta, IL-6) and poor glycemic control [42;43].
Two previous studies in adolescents reported modest inverse linear associations between circulating hsCRP levels and sleep duration averaged across a 7-day period [31;32]. In contrast, we observed a threshold effect in our sample. These divergent results may be a function of sample characteristics. For instance, average sleep duration in our sample across the 7-day study period was 1.4 to 2.2 hours shorter than values observed by Larkin and colleagues and Martinez-Gomez and colleagues, respectively. Given that only three participants in our sample obtained 8 or more hours of sleep on school nights, the sleep duration range may have been too restricted to observe a linear trend between sleep duration and CRP. In addition, our sample was more racially diverse than the previous studies in adolescents. Associations among weekday sleep duration and sleep debt with CRP Risk Group were independent of BMI despite its strong link with study outcomes and the striking number of overweight/obese adolescents in the sample. The average sample BMI was above the 75th age- and sex-normed percentile. Taken as a whole, these studies suggest that sleep duration is a significant correlate of high hsCRP in healthy adolescents, although the nature of this association may differ as a function of sample characteristics.
Although formal tests for interaction effects were not significant, stratified analyses revealed different associations among sleep duration and CRP Risk Group for males and females and for Whites and Blacks. The probability of being in the High Risk CRP Group was increased in females and Whites with shorter weekday sleep durations while sleep duration and CRP Risk Group were unrelated in males and Blacks. Pirkola and colleagues reported similar results in sex-stratified analyses of 16-year-old students in Finland [37]. Girls who reported sleeping less than 8 hours per day on average were more likely to be in a combined High Risk CRP and Low Leukocyte Count group than those who reported habitual sleep durations of 8 or more hours (OR=1.39, 95% CI 1.02 – 1.90); sleep duration was unrelated to hsCRP levels in males. Results have been mixed in adults, where curvilinear associations among sleep duration and adverse health outcomes are more common due, in part, to increased medical and psychiatric morbidity in long-sleeping adults (e.g., sleep durations > 9 h) [44]. One study observed significant associations between short sleep duration (<7 h) and hsCRP levels in adult women only [45], while another found significant associations only in adult men [34]. Although none of the previous studies in adolescents evaluated race as a modifier of the sleep duration-CRP relationship, two studies in adults reported significant associations among short sleep duration and hsCRP levelsin Blacks but not in Whites [46;47]. It is difficult to determine whether sex- and race-specific effects in the present study reflect true differences or are a result of sample characteristics or chance and certainly the absence of significant interaction effects suggest we were not powered to fully examine these possible effect modifiers. Although our sample was roughly comprised of equal numbers of females and males and Blacks and Whites, sleep durations profiles were markedly different – actigraphy-assessed sleep durations of more than 7 hours were observed in 15% of females compared to less than 5% of males and in 30% of Whites compared to 14% of Blacks. Although these results should be interpreted cautiously, the collection of additional data in future studies appears warranted to determine whether risk stratification schemes for sleep duration and cardiovascular risk in adolescents should account for the individuals’ sex and race.
Several plausible biological, behavioral, and psychological pathways may lead to increased systemic inflammation, including CRP, in response to insufficient sleep. Converging evidence suggests that sleep restriction leads to weight gain via alterations in systemic and cellular metabolism as well as behaviors associated with craving and food choices [48–50]. Release of IL-6 from adipocytes, in turn, stimulates CRP release by the liver. Sleep restriction has also been associated with increased autonomic arousal which, similarly, increases circulating CRP levels [51–54]. Psychological pathways including increased interpersonal conflict, psychological stress, and emotional lability are, similarly, plausible pathways through which sleep duration may influence inflammation. Short sleep duration and experimental sleep deprivation have been associated with decreased emotion regulation and increased reactivity to stress [55–57]. In a separate literature, experimental manipulation of stress has been associated with increased circulating CRP levels [58]. While these “pathway” studies have been conducted in adults, these mechanisms are equally plausible for adolescent samples. Certainly, additional research is needed to probe the biological, behavioral and psychological pathways through which short sleep duration may influence inflammation, including CRP in order to identify plausible targets for intervention in adolescents.
Interpretation of the present data should take into consideration the following limitations. First, the cross-sectional nature of our data precludes causal attributions. Although several studies have reported increased circulating CRP levels in response to experimental sleep restriction and deprivation in healthy adults, results have not always been consistent [13–16]. Moreover, marked differences in sleep profiles in adolescents and adults suggest that associations among sleep duration and inflammation may differ as a function of age. Second, we did not assess sleep apnea, which has been associated with inflammation, including increased CRP [59;60]. Adjustment for BMI in our statistical analyses may have attenuated the influence of sleep apnea in our models as overweight/obesity is a leading risk factor for sleep apnea in adolescents [61]. Although we evaluated daytime sleepiness as a proxy for sleep apnea, it was unrelated to CRP in our sample. Third, data cannot be generalized to adolescents with different sleep duration and inflammation profiles as a function of race/ethnicity or existing medical/psychiatric co-morbidities. Notable study strengths include behavioral assessment of sleep duration via wrist actigraphy, examination of the independent associations among weeknight and weekend sleep duration with CRP, evaluation of sleep debt, statistical adjustment for possible confounders including BMI, and the absence of health confounds such as diabetes, hypertension and kidney disease. Fourth, although formal tests of interactions were not significant, analyses stratified by race suggest that race and sex may modify associations among weekday sleep duration and CRP risk. Larger studies will be needed to more fully evaluate these relationships.
These data extend the limited literature on sleep duration and CRP in adolescents by evaluating weekday and weekend nights separately. Sleep duration differences across weekday and weekend nights are especially relevant in adolescents who operate under conditions of chronic sleep restriction during the school year due to the constraints imposed by biological and social drives for later sleep start times despite societally-mandated early school start times [17;18]. Prospective studies are needed to evaluate the impact of sleep restriction and sleep debt in otherwise healthy adolescents to chronic inflammation and to the pathogenesis and clinical course of cardiovascular disease. Identification of important effect modifiers and the modifiable biological, behavioral and psychological pathways through which short sleep duration in adolescence influences inflammation and its downstream consequences to cardiovascular risk will be critical to risk stratification and intervention. That these relationships may be observed prior to the onset of clinical or even subclinical disease suggests that adolescence may provide opportunities for disease prevention.
Highlights.
Short sleep duration on school nights was associated with CRP values > 3 mg/L.
Sleep debt was also associated CRP values > 3 mg/L.
Associations among sleep and CRP in adolescence may differ as by sex and race.
Findings were independent of known risk factors for elevated CRP.
Acknowledgments
This work was supported by the National Institutes of Health (HL025767, HL104607). The authors would like to thank Drs. Christopher Kline, Matthew Cribbet and Fanyin He for their assistance in the preparation of this manuscript.
Footnotes
Conflict of Interest: Authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 3.Solarz DE, Mullington JM, Meier-Ewert HK. Sleep, inflammation and cardiovascular disease. Front Biosci (Elite Ed) 2012;4:2490–501. doi: 10.2741/e560. [DOI] [PubMed] [Google Scholar]
- 4.Grad E, Danenberg HD. C-reactive protein and atherothrombosis: Cause or effect? Blood Rev. 2013;27:23–9. doi: 10.1016/j.blre.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67:970–6. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HE, Cho GY, Chun EJ, Choi SI, Lee SP, Kim HK, et al. Can C-reactive protein predict cardiovascular events in asymptomatic patients? Analysis based on plaque characterization. Atherosclerosis. 2012;224:201–7. doi: 10.1016/j.atherosclerosis.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178:956–61. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–40. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 13.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 16.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carskadon MA. Maturation of processes regulating sleep in adolescents. In: Marcus C, editor. Sleep in Children: Developmental Changes in Sleep Patterns. New York: Informa Healthcare; 2008. pp. 95–14. [Google Scholar]
- 18.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Matthews KA, Hall M, Dahl RE. Sleep duration, fragmentation and sleep quality among black and white adolescents: Should pediatricians be concerned? Pediatrics. 1 A.D In press. [Google Scholar]
- 21.Olds T, Blunden S, Petkov J, Forchino F. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010;14:371–8. doi: 10.1016/j.smrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53:271–3. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Metabolic syndrome variables at low levels in childhood are beneficially associated with adulthood cardiovascular risk: the Bogalusa Heart Study. Diabetes Care. 2005;28:126–31. doi: 10.2337/diacare.28.1.126. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the bogalusa heart study. Hypertension. 2004;43:541–6. doi: 10.1161/01.HYP.0000115922.98155.23. [DOI] [PubMed] [Google Scholar]
- 27.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 28.Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, et al. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–69. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 29.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29:379–86. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zieske AW, Tracy RP, McMahan CA, Herderick EE, Homma S, Malcom GT, et al. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25:1237–43. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 31.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.National Sleep Foundation poll task force. Teens and Sleep. 2006 Sleep in America poll. 2006 [Google Scholar]
- 34.Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–779E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women’s Health across the Nation sleep study. Sleep. 2010;33:1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liukkonen T, Rasanen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 37.Pirkola J, Vaarasmaki M, Ala-Korpela M, Bloigu A, Canoy D, Hartikainen AL, et al. Low-grade, systemic inflammation in adolescents: association with early-life factors, gender, and lifestyle. Am J Epidemiol. 2010;171:72–82. doi: 10.1093/aje/kwp320. [DOI] [PubMed] [Google Scholar]
- 38.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–52. doi: 10.1161/HYPERTENSIONAHA.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mak KK, Lee SL, Ho SY, Lo WS, Lam TH. Sleep and academic performance in Hong Kong adolescents. J Sch Health. 2012;82:522–7. doi: 10.1111/j.1746-1561.2012.00732.x. [DOI] [PubMed] [Google Scholar]
- 41.Leger D, Beck F, Richard JB, Godeau E. Total sleep time severely drops during adolescence. PLoS One. 2012;7:e45204. doi: 10.1371/journal.pone.0045204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 43.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12–7. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 45.Miller MA, Kandala NB, Kumari M, Marmot MG, Cappuccio FP. Relationships between sleep duration and von Willebrand factor, factor VII, and fibrinogen: Whitehall II study. Arterioscler Thromb Vasc Biol. 2010;30:2032–8. doi: 10.1161/ATVBAHA.110.206987. [DOI] [PubMed] [Google Scholar]
- 46.Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–779E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthews KA, Zheng H, Kravitz HM, Sowers MF, Bromberger JT, Buysse DJ, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in midlife women? Study of women’s health across the nation sleep study. Sleep. 2010;33:1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol. 2012;24:361–71. doi: 10.1002/ajhb.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 51.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC, et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol. 2012;113:232–6. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 52.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31:197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Takase B, Akima T, Satomura K, Ohsuzu F, Mastui T, Ishihara M, et al. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomed Pharmacother. 2004;58 (Suppl 1):S35–S39. doi: 10.1016/s0753-3322(04)80007-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol (1985) 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 55.Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–82. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mezick EJ, Matthews KA, Hall MH, Richard JJ, Kamarck TW. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology. 2014;51:88–96. doi: 10.1111/psyp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Hamer M, Gibson EL, Vuononvirta R, Williams E, Steptoe A. Inflammatory and hemostatic responses to repeated mental stress: individual stability and habituation over time. Brain Behav Immun. 2006;20:456–9. doi: 10.1016/j.bbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-reactive protein and obstructive sleep apnea syndrome in children. Front Biosci (Elite Ed) 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 60.Phillips BG, Somers VK. Sleep disordered breathing and risk factors for cardiovascular disease. Curr Opin Pulm Med. 2002;8:516–20. doi: 10.1097/00063198-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Verhulst SL, Franckx H, Van GL, De BW, Desager K. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity (Silver Spring) 2009;17:1178–83. doi: 10.1038/oby.2008.673. [DOI] [PubMed] [Google Scholar]


