Abstract
The hyperphagia, low sympathetic nervous system tone, and decreased circulating concentrations of bioactive thyroid hormones that are common to states of congenital leptin deficiency and hypoleptinemia following and during weight loss suggest that the major physiological function of leptin is to signal states of negative energy balance and decreased energy stores. In weight-reduced humans, these phenotypes together with pronounced hypometabolism and increased parasympathetic nervous system tone create the optimal circumstance for weight regain. Based on the weight loss induced by leptin administration in states of leptin deficiency (obese) and observed similarity of phenotypes in states of congenital and dietary-induced states of hypoleptinemia (reduced obese), it has been suggested that exogenous leptin could potentially be useful in initiating, promoting, and sustaining weight reduction. However, the responses of human beings to exogenous leptin administration are dependent not only on extant energy stores but also on energy balance. Leptin administration to humans at usual weight has little, if any, effect on body weight while leptin administration during weight loss mitigates hunger, especially if given in supraphysiological doses during severe caloric restriction. Leptin repletion is most effective following weight loss by dietary restriction. In this state of weight stability but reduced energy stores, leptin at least partially reverses many of the metabolic, autonomic, neuroendocrine, and behavioral adaptations that favor weight regain. The major physiological function of leptin is to signal states of negative energy balance and decreased energy stores. Leptin, and pharmacotherapies affecting leptin signaling pathways, is likely to be most useful in sustaining weight loss.
Keywords: leptin, metabolism, energy, obesity
Background
Coleman (1973) reported that the parabiosis of obese (ob/ob, later Lepob) mice with control mice or with diabetes (db/db, later Leprdb) mice resulted in hypophagia and starvation of the ob/ob mice while not affecting the phenotypes of either the control or db/db mice. With characteristic understatement, Coleman wrote, ‘it is postulated that the obese mouse is able to produce sufficient satiety factor to regulate its food consumption, whereas the diabetes mouse produces satiety factor, but cannot respond to it’ (Coleman 1973).
In the early 1980s, Ravussin et al. (1982) found no significant differences in 24 h or resting energy expenditure (REE) between lean and obese individuals corrected for body composition. Leibel & Hirsch (1984) and others (Welle et al. 1984) found that maintenance of a reduced body weight was accompanied by a decline in energy expenditure that was disproportionate to the changes in body weight and composition, and resembled the hypometabolic state of the ob/ob and db/db mice. The observation of persistent hypometabolism following weight loss while in a state of energy balance was consistent with the so-called ‘lipostatic’ theory of body weight maintenance, which posited a ‘signal’ reflecting adipose tissue mass and affecting hypothalamic neural circuitry regulating energy intake and expenditure.
The centricity of the hypothalamus in regulating this lipostat was based initially on a work by Hetheringon & Ranson in the early 1940s reporting that electrolytic lesioning of the ventromedial hypothalamus (VMH) in rats resulted in animals that defended higher levels of body fat against both over-feeding and weight-reduction (Hetherington & Ranson 1940, 1942). This work was replicated by Anand & Brobeck (1951), who also noted that rats and cats with lesions of the lateral hypothalamus (LH) defended a lower body weight. Kennedy (1953) suggested that ‘metabolites’ resulted in lipostasis of fat depots by virtue of their effects in the hypothalamus, thus energizing speculation as to the nature of these metabolic markers of energy stores.
Mayer (1955) proposed that energy homeostasis and body energy stores were largely regulated by the interactions of ‘glucostatic’ and ‘lipostatic’ signals reflecting, respectively, short-term and long-term energy stores and balance. Based on their studies of energy metabolism in rodents with lesions of the lateral or VMH, Keesey & Corbett (1984) proposed a hypothalamically regulated individualized ‘set-point’. This set-point was defined as the particular body weight at which daily energy expenditure is congruent with the value predicted by the best-fit function describing the body mass–expenditure relationship for that species and which could be perturbed upwards by VMH lesions and downwards by LH lesions.
The identification of four genetically distinct but phenotypically similar obesity syndromes in rodents (the autosomal dominant Ay and the recessive ob, db, fat, and tub (Coleman 1978)) suggested that each of these mutations subserved integrated pathways regulating energy output and intake (Leibel 1990, Leibel et al. 1990) that were possibly mediated by the satiety factor hypothesized by Coleman (1973). Efforts to identify the ob and db gene products then began in earnest (Leibel et al. 1993).
Coleman’s ‘satiety factor’ of course proved to be leptin (Zhang et al. 1994). Leptin repletion of congenitally leptin-deficient rodents and humans reversed the obesity and many of the associated comorbid phenotypes (Halaas et al. 1995, 1997, Barash et al. 1996, Montague et al. 1997, Farooqi et al. 1999). The effects of leptin on energy homeostasis in humans without such mutations are heterogeneous and are influenced by extant nutritional stores (usual weight or reduced weight) and energy balance (dynamic weight loss vs static weight maintenance). More specifically, exogenous leptin administration exerts effects in weight-stable and weight-reduced individuals, which mostly resembles those seen in states of congenital leptin deficiency and at least partially reverses many of the neuroendocrine, autonomic nervous system (ANS), appetitive, and thermogenic changes that favor weight regain following otherwise successful weight loss (Leibel & Rosenbaum 2010). As discussed below, the exogenous hormone is less effective in promoting weight loss during dietary restriction and least effective in initiating weight loss on its own. Thus, the effects of exogenous leptin are affected by both energy stores (and their perturbation from usual) and energy balance.
Body weight regulation
Studies by our group and others (Ravussin et al. 1985, Weyer et al. 2000, Civitarese et al. 2007, Leibel & Rosenbaum 2010, Myers et al. 2010) demonstrate that the process of dynamic weight loss and the sustaining of weight loss in a weight-stable state invoke similar but not identical phenotypes involving energy expenditure (declines that are disproportionate to changes in body composition and weight and largely mediated by skeletal muscle), the ANS (increased parasympathetic tone and decreased sympathetic tone), neuroendocrine function (decreased circulating concentrations of leptin and bio-active thyroid hormones), and energy intake behavior (delayed satiation, decreased perception of how much food has been eaten, increased food reward value, and decreased food restraint). These responses act coordinately to promote regain of lost weight (see Table 1). In-patient and out-patient studies of individuals successful at sustaining weight loss for longer periods of time (>1 year) indicate that this metabolic opposition to keeping weight off does not abate over time (McGuire et al. 1999, Wing & Hill 2001, DelParigi et al. 2004, Wing & Phelan 2005, Rosenbaum et al. 2008a, Phelan et al. 2011, Sumithran et al. 2011).
Table 1.
Differences in metabolic and behavioral responses to dynamic weight loss vs maintenance of reduced weight (Leibel et al. 1995, Fontana et al. 2006, Martin et al. 2007, Redman et al. 2009, Leibel & Rosenbaum 2010)
| Weight loss | Maintenance of reduced body weight | |
|---|---|---|
| Energy expenditure | ↓↓REE ~300 kcal < reduced weight maintenance | ↓REE |
| ↓NREE | ↓NREEa | |
| Burn more fat at rest (↓RER) | Resting RER=pre-loss | |
| Neuroendocrine axes | ↓↓T3, ↓↓T4, ↓↓TSH | ↓T3a, ↓T4a, ↓TSH |
| ↓↓Leptin/FM by 40–50%a | ↓Leptin/FM by ~10%a | |
| ↑Cortisol | Cortisol within the normal range | |
| ↑GH | No change or small ↑GH | |
| Autonomics | ↑↑PNS and ↓↓SNS | ↑PNS and ↓SNSa |
| Energy intake | ↓↓Satiation | Less↓↓Satiationa |
| ↑Hungera | Less↑Hungera |
REE, resting energy expenditure; NREE, non-resting energy expenditure; RER, respiratory exchange ratio; T3, triiodothyronine; T4, thyroxin; TSH, thyroid-stimulating hormone; GH, growth hormone; FM, fat mass; PNS, parasympathetic nervous system tone; SNS, sympathetic nervous system tone.
At least partially reversed by leptin repletion.
Skeletal muscle is the primary effector organ for the disproportionate decline in energy expenditure that occurs in subjects maintaining a reduced body weight. The approximate 20% increase in skeletal muscle work efficiency during low level exercise (pedaling a bicycle to generate 10–25 W) that occurs following 10% weight loss by hypocaloric diet is of sufficient magnitude to account for ~75% of the decline in non-resting energy expenditure (NREE) (Rosenbaum et al. 2003, Goldsmith et al. 2010). The increased skeletal muscle chemomechanical contractile efficiency is most probably due to an increase in the expression of the more efficient molecular isotypes for myosin heavy chain I (MHCI (MYH1)) and sarcoplasmic/endoplasmic reticulum Ca++-dependent ATPase 2 (SERCA2 (ATP2A2)) in muscle (Baldwin et al. 2011). These changes in muscle may be due, in part, to the declines in sympathetic nervous system (SNS) tone and circulating concentrations of bioactive thyroid hormones discussed above (Li & Larsson 1997, Canepari et al. 1998, Kardos et al. 2000, Simonides et al. 2001, Rosenbaum et al. 2003, Baldwin et al. 2011).
Systems regulating energy intake are also altered during reduced weight maintenance. Dietary weight-reduced and weight-stable subjects are hungry and show decreased perception of the amount of food eaten and delayed satiation (Kissileff et al. 2012) despite being in a state of energy balance. Functional magnetic resonance imaging studies of these subjects show increased activity in response to seeing food (vs non-food items) in brain areas related to the emotional and cognitive response to food (predominantly the orbitofrontal cortex) and decreased activity in brain areas related to emotional and cognitive control (restraint) in response to food (predominantly the prefrontal cortex), as well as decreased activity in the hypothalamus (Rosenbaum et al. 2008b).
The potency of this regulatory physiology – combining coordinate effects on both energy expenditure and drive to eat – is apparent in weight loss studies. Clinically, attempts to lose weight and keep it off are depressingly unsuccessful. In most studies, weight loss achieved by lifestyle intervention (Look AHEAD Research Group 2007, Foster et al. 2010, Wadden et al. 2011), pharmacotherapy (Franz et al. 2007, Ryan et al. 2010, Johansson et al. 2014), or bariatric surgery (Sjostrom 2013) persists for an average of ~6–8 months and is then usually followed by gradual weight regain with substantial treatment-related variability in the degree of weight loss (surgery>pharmacotherapy>lifestyle intervention) and the proportion of the weight regained (surgery<pharmacotherapy<lifestyle intervention).
Leptin provides a signal to the brain regarding the ‘status’ (both the mass and the stability) of somatic fat stores. The intensity of neuronal signaling is proportional to the ambient leptin concentration (Myers et al. 2008) and, as discussed below, is also influenced by the nutritional state of the organism. Signals originating in leptin-sensitive brain regions influence neuroendocrine functions, autonomic efferents, and food-related behaviors (Korner et al. 1999, 2001, Korner & Aronne 2003). Leptin-mediated signals are central to a nexus of neural circuitry that mediates what is physiologically apparent as the regulation of body weight via the integration of short- (e.g., gut-derived hormones and glucose) and long- (e.g., leptin, insulin, and free fatty acids) term signals related to energy homeostasis (Korner et al. 1999, 2001, Schwartz et al. 2000, Korner & Aronne 2003). Among the most critical roles of this system is the protection of the organism from reductions in fat mass that could threaten reproductive capacity/fertility and/or survival (Rosenbaum & Leibel 1998). Hence, leptin has a strong functional bias in favor of the preservation of body fat stores vs their reduction.
Leptin administration before, during, and after weight loss
Leptin administration before weight loss: state of leptin sufficiency
Exogenous leptin administration to rodents fed ad libitum in doses sufficient to raise circulating leptin concentrations by ~30–50% above baseline results in a transient (<1 week) anorexiant effect and a persistently lower weight and body fat content (Halaas et al. 1995, 1997, Satoh et al. 1997, Boozer et al. 2001, Ravussin et al. 2014; Table 2). Transgenic mice overexpressing leptin show persistently lower food intake, as well as lower weight and body fat content, than WT mice (Ogawa et al. 1999, Yura et al. 2000). The persistence of reduced weight, with and without persistence of decreased food intake, suggests that hyperleptinemia in mice affects both energy intake and expenditure.
Table 2.
Effects of leptin administration to subjects at usual weight
| References | Subjects | Leptin
|
Mean (S.D.) leptin concentration in plasma (ng/ml)a | Mean (S.D.) leptin effect | ||
|---|---|---|---|---|---|---|
| Type | Duration | Dose (administered s.c.) | ||||
| Moon et al. (2011) | Outpatient obese subjects with type 2 diabetes controlled by diet | r-met hu leptin | 16 weeks | Placebo | 36.8 (28.3) | BMI: −0.5 (0.8) kg/m2 |
| 10 mg bid | 987.1 (343.5) | BMI: −0.7 (0.7) kg/m2 | ||||
| Heymsfield et al. (1999) | Outpatient obese subjects | r-met hu leptin | 24 weeks | Placebo | 25.0 (39.6) | −1.0 (3.8) kg weight loss |
| 0.01 mg/kg per day | 28.3 (20.2) | −0.7 (4.6) kg weight loss | ||||
| 0.03 mg/kg per day | 115.5 (99.9) | −1.4 (4.1) kg weight loss | ||||
| 0.10 mg/kg per day | 271.7 (322.4) | −2.1 (5.0) kg weight loss | ||||
| 0.30 mg/kg per day | 480.3 (522.0) | −3.3 (6.7) kg weight loss | ||||
| Mittendorfer et al. (2011) | Outpatient obese subjects with newly diagnosed type 2 diabetes untreated | r-met hu leptin | 2 weeks | Placebo | 25 (11) | No significant change in body composition or glucose homeostasis |
| 15 mg bid | 76 (42) | |||||
| 40 mg bid | 5024 (1115) | |||||
| Mackintosh & Hirsch (2001) | In-patient never-obese subjects | r-met hu leptin | 1 week | 0.3 mg/kg per day | Not reported (see Heymsfield et al. (1999) for data on similar dose) | No significant effect on weight, resting energy expenditure, or ANS tone |
Leptin levels are expressed as mean (S.D.) maximum levels detected at the end of the study period, while subjects were receiving leptin or placebo.
The limited data regarding leptin dosing, circulating concentrations of leptin, and effects of leptin administration to human subjects at their usual body weight are summarized in Table 2. Heymsfield et al. (1999) administered leptin in doses ranging from 0 (placebo) to 0.30 mg/kg per day (producing blood levels up to 20-fold above normal physiological range) to 54 lean and 73 obese subjects for 4 weeks and to 47 obese subjects for 24 weeks. Obese subjects were prescribed diets constituting an ~500 kcal/day caloric deficit, but dietary compliance was not assessed and there was no significant weight loss in the placebo group. After 4 weeks of leptin treatment, there was a significant correlation of leptin dose and weight decrease across all subjects (though lean subjects did not show a significant dose–response curve when analyzed as a single group). However, overall weight reduction in leptin-treated lean or obese subjects was not different from placebo-treated subjects. In obese subjects receiving exogenous leptin for a period of 24 weeks, there was similarly a significant correlation between leptin dose and weight loss; but there was significant weight loss (2.3 kg more than placebo) only in subjects receiving the highest doses of leptin (see Table 2). Those subjects receiving higher doses of leptin (0.1 and 0.3 mg/kg per day with circulating leptin concentrations ten- to 20-fold above initial concentration) reported a small but statistically insignificant decrease in daily energy intake. The high circulating leptin concentrations and low levels of weight loss in obese subjects following exogenous leptin administration have been interpreted to indicate that obese individuals are ‘leptin resistant’ (Friedman & Halaas 1998, Kaira 2001, Lee et al. 2001, Scarpace & Zhang 2007). However, it was obese, and not lean, individuals who showed a dose–response relationship of weight decrease (modest as it was) to leptin.
A more recent study by Moon et al. (2011) has examined the effects of 16 weeks of leptin administration (10 mg s.c. bid) to 71 weight-stable (followed for at least 4 weeks before enrollment) obese subjects with type 2 diabetes managed by diet alone. Peak plasma leptin concentrations were raised to ~25 times the level of subjects receiving placebo; but no significant changes in body weight were noted in either leptin-treated or placebo groups. Similarly, Mittendorfer et al. (2011) found no effects of 14 days of high (40 mg s.c. bid) or lower (15 mg s.c. bid) dose leptin on either body composition or glucose homeostasis in 18 subjects who were newly diagnosed with type 2 diabetes and not receiving any anti-diabetic medications. Mackintosh and Hirsch reported no effects of high-dose (0.3 mg/kg per day s.c.) leptin administration –the highest dose used by Heymsfield et al. (1999) – on ANS tone in weight-stable lean subjects. This finding is in sharp contrast to the potent effects of low-dose leptin ‘repletion’ to decrease SNS tone in weight-reduced subjects (Rosenbaum et al. 2005). Taken together, these studies indicate that leptin administration to obese or lean humans at their usual body weights has little effect on energy homeostasis. In this sense, both lean and obese subjects at their usual body weight are ‘resistant’ to the effects of leptin on energy balance. As discussed below, to use the term ‘resistant’ in this context misrepresents the biology of leptin in the regulation of body fat stores.
Leptin administration during and following weight loss: states of leptin depletion
The hypometabolic/hyperphagic state that accompanies and follows weight loss is similar to that observed in individuals with congenital leptin deficiency (Farooqi et al. 1999, 2002, 2003, Ozata et al. 1999; Tables 3 and 4). Data regarding leptin dosing, circulating concentrations of leptin, and effects of leptin administration to subjects receiving leptin during dynamic weight loss are summarized in Table 3. During dynamic weight loss, circulating concentrations of leptin are significantly reduced relative to fat mass (Rosenbaum et al. 1997), primarily due to decreased leptin gene expression in adipose tissue (Siklova-Vitkova et al. 2012), as well as increased leptin clearance rates (particularly in males) (Chan et al. 2008). Thus, leptin gene expression and circulating leptin concentrations reflect not only energy stores (adipose tissue mass) but also the status of energy balance.
Table 3.
Effects of leptin administration to subjects during weight loss
| Reference | Subjects | Leptin (s.c. administration)
|
Mean (S.D.) plasma leptin concentration (ng/ml)a | Leptin effect | ||
|---|---|---|---|---|---|---|
| Type | Duration | Dose | ||||
| Fogteloo et al. (2003) | Outpatient obese subjects with diet-controlled type 2 diabetes and receiving a hypocaloric (500 kcal/day deficit) diet | r-met hu leptin begun after 6 weeks of weight loss | 12 weeks | Pre-loss | 20.1 (12.9) | |
| Placebo | 18.0 (13.1) | −2.8 (3.0) kg weight loss | ||||
| 10 mg daily | 133.2 (119.4) | −5.2 (3.4) kg weight loss | ||||
| 10 mg bid | 217.6 (127.9) | −7.9 (3.1) kg weight loss | ||||
| No effect on REE | ||||||
| Shetty et al. (2011) | Outpatient obese subjects receiving a hypocaloric (500 kcal/day deficit) diet | r-met hu leptin | 6 months | Placebo 5 mg bid | Leptin levels approximately threefold greater in treated groupb | No significant treatment effect on weight loss, thyroid function, or somatotrope function |
| Hukshorn et al. (2000), and Westerterp-Plantenga et al. (2001) | Outpatient obese men receiving a hypocaloric (500 kcal/day deficit) diet | PEG-OB | 12 weeks | Pre-loss | 21.2 (12.4) | Decreased appetite in treated group. No significant effect on weight loss or body composition changes |
| Placebo | 14.6 (14.8) | |||||
| 20 mg/week | 24.0 (13.9) | |||||
| Hukshorn et al. (2002) | Outpatient obese men receiving a hypocaloric (750 kcal/day deficit) diet | PEG-OB started after 6 weeks of LCD | 12 weeks | Pre-loss | 18.4 | No significant effect on weight loss or body composition changes |
| Placebo | 13.6 (25.4) | |||||
| 20 mg/week | 25.7 (21.7) | |||||
| Hukshorn et al. (2003) and Lejeune et al. (2003) | Outpatient overweight men receiving a hypocaloric (500 kcal/day) diet | PEG-OB started after 6 weeks of LCD | 6.5 weeks | Pre-loss | 7.3 (3.6) | Treated group lost an average of 2.8 kg more than placebo without a significant treatment effect on body composition, energy expenditure, or appetite |
| Placebo | 2.0 (0.6) | |||||
| 80 mg/week | 3980 (609) | |||||
| Zelissen et al. (2005) | Outpatient obese subjects receiving a hypocaloric (500 kcal/day) diet | r-met hu leptin | 12 weeks | Placebo | Leptin levels approximately fivefold greater in daily treated groups and tenfold greater in the bid treated groupb | −2.6 (3.1) kg weight loss |
| 10 mg qAM | −2.8 (3.8) kg weight loss | |||||
| 10 mg qPM | −2.7 (3.8) kg weight loss | |||||
| 10 mg bid | −3.4 (3.4) kg weight loss | |||||
Leptin levels are expressed as mean (S.D.) maximum levels detected at the end of the study period, while subjects were receiving leptin or placebo unless otherwise indicated. Note that doses of r-met human leptin (r-met hu lep) in the range of 0.03–0.05 mg/kg per day in weight-reduced subjects have been reported to result in through leptin levels similar to those observed in subjects before weight loss (Rosenbaum et al. 2005, 2008b, Baldwin et al. 2011, Conroy et al. 2011, Kissileff et al. 2012) and doses of pegylated leptin (PEG-OB) in the range of 10–20 mg/week in subjects undergoing weight loss results in steady-state leptin levels similar to those observed in the same subjects before weight loss (Saris et al. 2000, Westerterp-Plantenga et al. 2001, Fogteloo et al. 2003, Zelissen et al. 2005).
Exact data not reported or presented in graphic form only.
Table 4.
Effects of leptin administration to subjects stable at reduced body weight
| Reference | Subjects | Leptin (s.c. administration)
|
Mean (S.D.) leptin plasma concentration (ng/ml)a | Leptin effect | ||
|---|---|---|---|---|---|---|
| Type | Duration | Dose | ||||
| Rosenbaum et al. (2005, 2008b), Baldwin et al. (2011), Conroy et al. (2011) and Kissileff et al. (2012) | In-patient crossover design of lean and obese subjects on a liquid formula diet, who have previously undergone a 10% in-patient weight loss | r-met hu leptin | 5 weeks | Placebo | 36.3 (31.5) | −2.1 (3.1) kg weight loss |
| Leptin (doses titrated to 0800 h levels before weight loss) | 57.6 (41.5) | −1.0 (2.2) kg fat loss | ||||
| (pre-weight loss leptin: 48.2 (44.3)) | −1.1 (2.6) kg FFM loss | |||||
| Significant reversal of declines in energy expenditure, thyroid hormones, and satiation and of increases in muscle efficiency, hunger, and SNS tone that occur as a result of weight loss. No effect on TSH or PNS tone | ||||||
| Korner et al. (1999, 2001, 2013) | Out-patient crossover design of obese women who had undergone Roux-en-Y gastric bypass surgery and were weight stable within 3% over 4–6 weeks | r-met hu leptin | Placebo | 26 (8) | Trend toward loss of fat mass during leptin treatment (P=0.06). No significant effect on thyroid function, resting energy expenditure, or cortisol | |
| Leptin | 258 (386) | |||||
| Brinkoetter et al. (2011), Chou et al. (2011) and Sienkiewicz et al. (2011) | Outpatient women with hypothalamic amenorrhea due to low fat mass | r-met hu leptin | 36 weeks | Placebo | 2.3 (3.7) | Significantly greater loss of fat with treatment (~−4% body fat with placebo vs ~−28% with leptin) with borderline significant differences in weight loss (~−1% with placebo vs ~−5% with leptin) b Menses and normal gonadotropin function restored |
| 0.04 mg/kg bid, increased to 0.06 mg/kg bid if subject amenorrheic at 12 weeks | 59.3 (46.4) | |||||
| Javor et al. (2005) | Out-patient women with generalized lipodystrophy | r-met hu leptin | 12 months | Baseline | 1.6 (0.7) | Weight decreased from 61.8 (13.5) to 57.4 (12.7) and % fat from 7.9 (1.9) to 6.7 (1.3) with significant reductions in 24 h energy expenditure and energy intake |
| 0.03–0.04 mg/kg bid | 21.1 (18.0) | |||||
| Lee et al. (2006) | Out-patients with HAART-induced lipodystrophy | r-met hu leptin | Placebo | 1.3 (0.5) | Weight decreased from 71.0 (12.1) to 69.2 (12.8) and fat mass from 9.3 (3.8) to 8.7 (3.7) with significant reductions in 24 h energy expenditure and energy intake | |
| 0.04 mg/kg bid | Not reportedb | |||||
Leptin values are expressed as mean (S.D.) maximum levels detected at the end of the study period, while subjects were receiving leptin or placebo unless otherwise indicated. Note that doses of r-met hu leptin in the range of 0.03–0.05 mg/kg per day in weight-reduced subjects have been reported to result in trough leptin levels similar to those observed in subjects before weight loss (Rosenbaum et al. 2005, 2008b, Baldwin et al. 2011, Conroy et al. 2011, Kissileff et al. 2012) and doses of pegylated leptin (PEG-OB) in the range of 10–20 mg/week in subjects undergoing weight loss results in steady-state leptin levels similar to those observed in the same subjects before weight loss (Saris et al. 2000, Westerterp-Plantenga et al. 2001, Fogteloo et al. 2003, Zelissen et al. 2005).
Exact data not reported or presented in graphic form only.
Treatment of food-restricted mice with leptin prevents the decrease in energy expenditure that normally occurs during reduced energy intake (Ahima et al. 1996, Halaas et al. 1997). Teleologically, this acute decline constitutes a ‘signal’ regarding imminent threats to somatic body energy stores (Ahima et al. 1996) and, therefore, reproductive capacity and survival (Rosenbaum & Leibel 1999, Rosenbaum et al. 2005, 2008b). Compared with subjects at stable reduced body weight, circulating leptin concentrations are lower while changes in metabolic, behavioral, autonomic, and neuroendocrine systems favoring weight regain are more activated in calorie-restricted individuals losing weight. However, leptin administration during caloric restriction produces a less complete ‘reversal’ of the metabolic, behavioral, neuroendocrine, and autonomic effects of weight loss than that observed in subjects stable at a reduced body weight.
In subjects ingesting a diet restricted by 500 kcal/day below usual intake (Fogteloo et al. (2003) reported that 10 mg leptin administered subcutaneously daily (n=15) or twice a day (n=6) for 12 weeks promoted significantly greater weight loss than placebo (n=9). However, they found no significant between-group differences in energy intake or expenditure (see Table 3). These results differ from other studies using a similar design. In an out-patient study by Shetty et al. (2011), leptin (10 mg s.c. daily) was administered to 18 obese or overweight subjects while placebo was administered to six similar subjects. All subjects were prescribed a hypocaloric diet designed to reduce energy intake by 500 kcal/day for 6 months. There were no significant differences between placebo and leptin-treated subjects in weight change or neuroendocrine function (IGF1, IGFBP1, IGFBP3, or bioactive thyroid hormones). Similarly, neither administration of s.c. leptin at doses of 10–20 mg/day (resulting in circulating leptin concentrations that are ~3–5 times the pre-weight loss levels) nor administration of pegylated leptin at a dose of 20 mg/week (Hukshorn et al. 2000, Zelissen et al. 2005) (resulting in leptin concentrations similar to baseline concentrations) induced significant changes in body weight changes, resting metabolic rate, or hunger compared with placebo in subjects prescribed a 500 kcal/day caloric deficit. However, with more severe caloric restriction of a very low energy diet (VLED, total intake of 500 kcal/day) and the same dose of leptin, Westerterp-Plantenga et al. (2001) found a significant decline in hunger without changes in body composition, weight, or energy expenditure over 6 weeks. When the dose of pegylated leptin was increased to 80 mg/week (Hukshorn et al. 2003, Lejeune et al. 2003), the VLED leptin-treated subjects lost significantly more weight and exhibited significantly more dietary restraint (measured by standardized questionnaires) than controls. However, there were no significant between-group differences in the composition of lost weight (absolute and relative amounts of fat or fat-free mass (FFM)), REE, or respiratory quotient.
Taken together, these data suggest that the effects of leptin ‘repletion’ in calorically restricted subjects are primarily a modest reduction in energy intake and perhaps better compliance with caloric restriction. If leptin is administered in higher doses (achieving blood levels up to 100-fold above baseline) and the diet is more restricted (500 vs 500 kcal/day caloric deficit), there appears to be an increased weight loss, again mostly due to decreased energy intake (see Table 3). As discussed below, the efficacy of leptin in diminishing the hyperphagia and hypometabolism that occur during weight loss is much less than the potent effects of leptin repletion observed in subjects attempting to sustain a reduced weight.
Data regarding leptin dosing, circulating concentrations of leptin, and effects of leptin administration to subjects receiving leptin during maintenance of reduced body weights are summarized in Table 4. There are fewer studies examining the effects of leptin administration/repletion following weight loss, but exogenous leptin appears to be substantially more potent when it is administered to individuals who are relatively hypoleptinemic by virtue of maintaining a 10% or greater reduction in body weight. The doses required to achieve these effects are low, being sufficient only to restore circulating leptin concentrations to their pre-weight loss levels. Leptin repletion under these circumstances results in further body weight reduction and largely reverses the physiological and behavioral responses to weight loss – including increased skeletal muscle work efficiency, circulating concentrations of triiodothyronine and thyroxine, SNS tone, feeding behavior, and brain fMRI patterns (Rosenbaum & Leibel 1998, Rosenbaum et al. 2005, 2008b; see Table 4).
In a longer-term study of subjects sustaining weight loss, Brinkoetter et al. (2011) and Sienkiewicz et al. (2011) examined the effects of 9 months of exogenous leptin administration (0.04–0.12 mg/kg per day to hypoleptinemic (as a result of reduced fat mass) female athletes with hypothalamic amenorrhea). Though weight and fat mass declined throughout the period of leptin administration, normal hypothalamic–pituitary–gonadal function was restored (see Table 4). Similarly, leptin administration to individuals with moderate to severe hypoleptinemia by virtue of congenital or HAART-induced lipodystrophy also results in weight loss (see Table 4). The potent effects of both short- and long-term leptin repletion (i.e. to within the normal pre-weight loss range) to ‘reverse’ some of the metabolic, behavioral, and neuroendocrine consequences of weight loss if given following weight reduction as opposed to losing weight or at their usual weight suggests that leptin ‘repletion’ and/or leptin-sensitizing agents may be helpful in maintaining a reduced body weight (Ravussin et al. 2009).
Implications for the role of leptin in human energy homeostasis
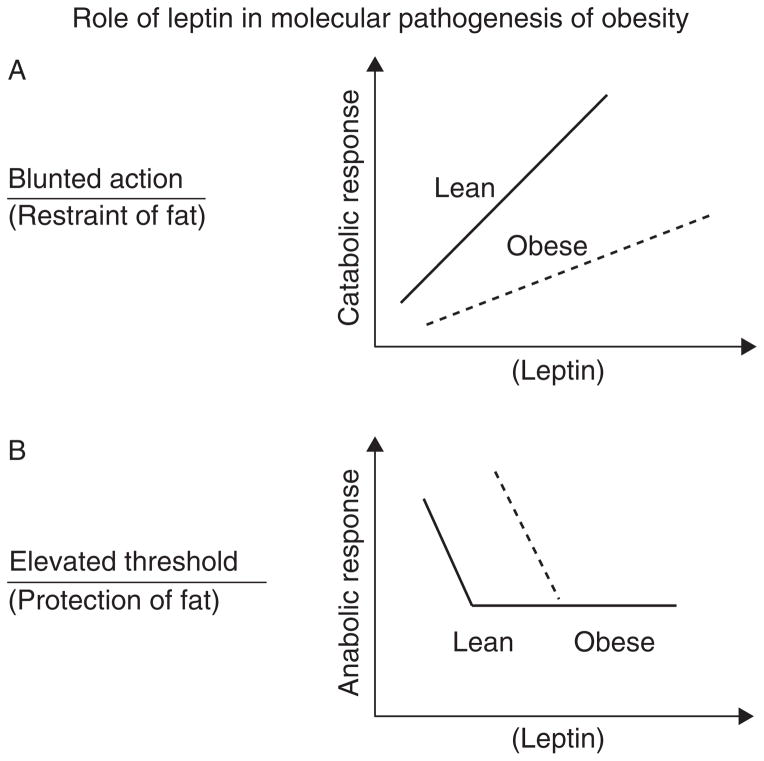
As schematized in Fig. 1, biological responses to hormones and metabolites are not necessarily linear. Symmetric, essentially linear responses are illustrated by testosterone, the exogenous administration of which results in dose-dependent increases in anabolism. Asymmetric physiology is illustrated by the tachycardia, diaphoresis, confusion, and poly-hormonal response that can be induced by a 30–40 mg/dl decline in blood glucose level vs the lack of symptomatology associated with a comparable elevation in glucose level in an otherwise healthy individual.
Figure 1.
Two models of leptin endocrine action in the context of obesity, which illustrate possible answers to the question as to whether leptin’s primary homeostatic role is to restrain adiposity by provoking catabolic responses (reduced energy intake and increased energy expenditure), or to protect body fat in service of reproductive integrity and survival in adverse environmental circumstances? This distinction is critical to integrating the large literature on the biology of leptin and to formulating therapeutic approaches to obesity. 1A depicts a classical linear ‘symmetrical’ catabolic endocrine response (e.g., to thyroid hormone), in which leptin sensitivity is depicted as congenitally reduced in individuals predisposed to obesity. 1B depicts a very different model in which leptin’s primary role is to provoke anabolic responses when its circulating concentration reaches a critical minimum (‘threshold’), which is determined by genetic, developmental, and intercurrent metabolic circumstances. The threshold is higher in individuals predisposed to obesity; hence their acquisition and defense of a higher level of body fat. As discussed in the text, physiological and pharmacological studies support the 1B model. Of note is that the leptin concentration ‘thresholds’ for specific physiological processes (e.g., gonadal axis, insulin homeostasis, immune function, and energy intake/expenditure) are not identical, and brain regions and cells, somatic cell types, and signaling mechanisms (e.g., JAK–STAT) also differ (Myers et al. 2008, Villanueva & Myers 2008).
As indicated by the varied effects of exogenous leptin administration depending upon both the status of energy stores and energy balance, physiological/behavioral responses to ambient leptin are highly asymmetrical. Changes in energy expenditure and intake in relatively hyperleptinemic states due to either obesity or exogenous administration are minimal. In this sense, both lean and obese subjects at their ‘usual’ weight are ‘resistant’ to the effects of leptin on energy homeostasis. On the other hand, reductions in ambient leptin – signaling a deficiency of somatic energy stores and/or negative energy balance –are met with metabolic and behavioral responses designed to protect those stores. These observations have been variously formulated as ‘set point’ and ‘settling point’ models of the molecular physiology of the regulation of body weight (Speakman et al. 2011).
The asymmetric physiological responses to leptin described above (weak for increases and strong for decreases) have led us (Rosenbaum & Leibel 1999, Myers et al. 2010), and others (Ahima et al. 1996, 1999), to propose a ‘threshold model’ for leptin signaling (see Fig. 1). This model posits a threshold for leptin (and possibly other centrally active hormones such as insulin, ghrelin, and Peptide YY) action that is determined by genetics, structural development, and ambient metabolites. The amount of leptin required to signal through relevant central sites of action is determined by the aggregate effects of these components on the sensitivity of the molecular circuitry. Individuals with higher thresholds require higher ambient/CNS leptin levels (hence more somatic fat) to activate the circuitry resulting in reduced energy intake and increased energy expenditure. Ambient levels below this concentration trigger hunger and energy conservation in response to centrally perceived critical diminution of body fat. Elevations above the threshold provoke little metabolic or behavioral response. The mechanism is designed to conserve body fat, as in evolutionary terms loss of fat has been a constant threat to fertility and survival. The threshold determines the minimum level of body fat tolerated by the individual. Below this level, ‘reported’ as reduced circulating leptin concentration, homeostatic responses are invoked to restore the fat. In obese individuals, the threshold is set higher than that in lean individuals. The responses of both lean and obese individuals to reductions below these different thresholds are similar if not identical. The threshold is not lowered by chronic maintenance of a reduced body weight (Leibel & Hirsch 1984, Rosenbaum et al. 2008a), but may be raised – in mice – by chronic maintenance of an elevated body weight (Ravussin et al. 2011). This effect does not appear to be conveyed by elevations of leptin per se as mice do not become obese following cessation of a chronic (16 weeks) leptin infusion (Ravussin et al. 2014). The model predicts that even large doses of leptin would not have much impact on body weight in the leptin-sufficient individual (usual weight), whereas low ‘replacement’ doses could normalize energy expenditure and food intake in weight-reduced individuals whose circulating leptin concentration is below their threshold concentration.
The extent to which a threshold model for leptin action is applicable to leptin effects on other systems (immunological, bone metabolism, glucose homeostasis, etc.) is yet to be established. It should be noted that this threshold model does not stipulate that all physiological and behavioral systems will necessarily have the same threshold concentration, or even have a leptin threshold (Chan et al. 2006). For example, in humans, leptin-reversible infertility occurs only at levels of body fatness that are well below the 10% or greater weight loss, which is sufficient or produces a hypometabolic hyperphagic state (Lev-Ran 1974, Frisch 1980, Chou et al. 2011). It should also be clear that leptin does not reverse all of the metabolic and behavioral adaptations that characterize the weight-reduced state. Leptin repletion following weight loss reduces most, but not all, of the hypometabolism and hyperphagia. It does not affect the decline in thyroid-stimulating hormone (TSH) or the increase in parasympathetic nervous system tone, nor does it reverse all of the changes in muscle gene expression and contractile efficiency (Rosenbaum et al. 2005, Baldwin et al. 2011, Kissileff et al. 2012, Hinkle et al. 2013). Further evidence that leptin is not the sole mediator of the metabolic responses to weight loss comes from studies of congenitally leptin-resistant fa/fa rats that demonstrate similar adaptations to caloric restriction in arcuate nucleus expression of NPY, AGRP, and CART (CARTPT), as those observed in WT mice (Chiba et al. 2009).
Based on observations that both lean and obese humans at usual body weight are functionally ‘resistant’ to the effects of leptin on energy homeostasis, it is probably more accurate to indicate that the biology is simply not designed to respond very effectively to what would constitute hyperleptinemia for a given individual (see Table 2). Such asymmetry of responses would be consistent with the evolutionary circumstances in which this regulatory system arose, defense against caloric insufficiency being a much more frequent and severe problem than dealing with presumably rare access to excessive food supplies. Leptin administration during weight loss in humans, especially in states of severe caloric restriction and supraphysiological doses of leptin, will decrease appetite, but there is no substantive evidence that it will affect energy expenditure or neuroendocrine function in humans (unlike rodent studies in which leptin administration during starvation blunts the activation of the hypothalamic–pituitary–adrenal (HPA) axis as well as the reductions in hypothalamic expression of POMC and CART (Legradi et al. 1997, Ahima et al. 2000)) (see Table 3). Following moderate weight loss, leptin repletion at doses designed to restore circulating leptin concentrations to their pre-weight loss range will reverse many, but not all, of the metabolic consequences of weight loss. Following more severe reductions in body weight leading to profound hypoleptinemia, as in lipodystrophy and hypo-thalamic amenorrhea, leptin repletion still promotes weight loss and increases hypothalamic–pituitary–thyroid (HPT) and hypothalamic–pituitary–gonadal (HPG) axis activity (Mantzoros et al. 2011) (see Table 4). In a study of leptin administration to bariatric surgery patients, Korner et al. (2013) noted a non-significant increase in weight loss following leptin repletion (n=14) vs controls (n=13), again indicating the contextual nature of responses to exogenous leptin.
A key point is that the response of systems regulating energy homeostasis to exogenous leptin is influenced by the metabolic status of energy balance as well as the magnitude of energy stores (Mantzoros et al. 2011). Hence, the subtle differences in metabolic and behavioral responses to leptin of individuals who are actively losing weight and those who are weight stable at that same reduced weight do exist. In addition, though it seems that metabolic and behavioral opposition to circulating leptin concentrations below an individualized threshold is invoked whether leptin levels are low due to negative energy balance or simply due to loss of fat mass, the same cannot be said for leptin repletion. The effects of leptin repletion during energy restriction are very much constrained compared with the effect observed during reduced-weight maintenance.
The mechanism(s) underlying the effects of both energy stores and energy balance on leptin responsiveness is unclear. Specifically, why is responsiveness to exogenous leptin in low leptin states accompanying dynamic weight loss less than that observed in the less severe hypoleptinemia of subjects maintaining a reduced weight or more severe hypoleptinemia of subjects with exercise-induced fat loss or lipodystrophy? The observation that the co-administration of the nominal leptin-sensitizing agent, amylin, with exogenous leptin synergistically enhanced weight loss in out-patients prescribed a 20% calorically restricted diet (Ravussin et al. 2009, Trevaskis et al. 2010) suggests that there is a primary decline in leptin sensitivity during caloric restriction. In addition, during caloric restriction, leptin clearance is increased (Chan et al. 2008), and it is also possible that alterations in leptin transport into the brain or inhibition of leptin action by molecules such as SOCS3, PTP1B, or SH2 may diminish sensitivity to exogenous leptin during caloric restriction (Flier 1998, Ahima et al. 1999, Morrison 2009), or in euleptinemic or hyperleptinemic states (Morrison 2009). Teleologically, such reductions in sensitivity to leptin would prevent life-threatening anorexia in circumstances of reduced availability of food. Ahima et al. (1999, 2000) also suggested that the observed lack of efficacy of leptin administration during negative energy balance or at usual weight compared with following weight loss may reflect downstream changes in the leptin signaling pathway that is mediated by other molecules, such as glucocorticoids or insulin, which are altered as a consequence of energy stores or balance.
Concluding remarks
Based on the predicted consequences of the evolutionary pressures discussed above, as well as the asymmetry of responsiveness to exogenous leptin, the major function of leptin in human physiology is to signal inadequate energy stores or balance rather than an overabundance of fat. These findings have important implications for the potential role of exogenous leptin in the treatment of obesity. Though there is substantial inter-individual variation in response, it seems clear that leptin administration is not likely to be very effective in inducing weight loss as a stand-alone intervention. The limited efficacy of high-dose leptin in promoting weight loss in subjects during caloric restriction suggests that leptin could potentially assist in either prolonging the period of weight loss or increasing the amount of weight lost within a certain period of time, if given along with a ‘leptin-sensitizing agent’ that might overcome the effects of negative energy balance on leptin responsiveness. Leptin is most likely to be most effective, however, as a weight maintenance therapy in individuals who have previously lost weight. Repletion of leptin to pre-weight loss levels following weight loss results in potent reduction of the metabolic and behavioral opposition to sustained weight loss – by signaling nominal adequacy of energy stores to CNS tracts regulating energy homeostasis. These same contextual differences in response to leptin may well pertain to the pharmacological agents designed to assist in the medical management of obesity; their greatest efficacy may be in the weight-reduced state (Lehmann et al. 2014, Skowronski et al. 2014).
Acknowledgments
Funding
These studies were supported in part by NIH Grants # DK30583, DK26687, DK37948, DK64773, DKP30 26687, RR00102, RR00645, RR024156 and UL1 TR00040, and the Russell Berrie Foundation. For work done by the authors, recombinant human leptin was provided by Amgen Inc (Thousand Oaks, CAm USA) and Amylin Pharmaceuticals Inc (San Diego, CA, USA). The authors would also like to acknowledge a large number of indispensable collaborators who have contributed significantly to our ongoing work with leptin, much of which is presented in this Thematic Review. These collaborators include: Drs Jules Hirsch, Louis Aronne and Karen Segal; the nursing and nutritional staffs at Rockefeller University Hospital; the staff at Irving Center for Clinical and Translational Research at Columbia University Medical Center; and Drs Daniel Bloomfield, Dympna Gallagher, Rochelle Goldsmith, Steven Heymsfield, Joy Hirsch, Anthony Magnano, Laurel Mayer, Richard Smiley, Sharon Wardlaw, and Louis Weimer at Columbia University Medical Centre.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
- Ahima R, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Ahima R, Kelly J, Elmquist J, Flier J. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- Ahima R, Saper C, Flier J, Elmquist J. Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology. 2000;21:263–207. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Anand B, Brobeck J. Hypothalamic control of food intake in rats and cats. Yale Journal of Biology and Medicine. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- Baldwin K, Joanisse D, Haddad F, Goldsmith R, Gallagher D, Pavlovich K, Shamoon E, Leibel R, Rosenbaum M. Effects of weight loss and leptin on skeletal muscle in human subjects. American Journal of Physiology. Endocrinology and Metabolism. 2011;301:R1259–R1266. doi: 10.1152/ajpregu.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash I, Cheung C, Weigle D, Ren H, Kramer J, Fallon M, Kabigting E, Kujiper J, Clifton D, Steiner R. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- Boozer C, Love R, Cha M, Leibel R, Aronne L. Synergy of leptin and sibutramine in treatment of diet-induced obesity in rats. Metabolism. 2001;50:889–893. doi: 10.1053/meta.2001.24917. [DOI] [PubMed] [Google Scholar]
- Brinkoetter M, Magkos F, Vamviini M, Mantzoros C. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin–follistatin–activin axis in lean hypoleptinemic women. American Journal of Physiology. Endocrinology and Metabolism. 2011;301:E99–104. doi: 10.1152/ajpendo.00146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Cappelli V, Pellegrino M, Zanardi M, Reggiani C. Thyroid hormone regulation of MHC isoform composition and myofibrillar ATPase activity in rat skeletal muscles. Archives of Physiology and Biochemistry. 1998;106:308–315. doi: 10.1076/apab.106.4.308.4373. [DOI] [PubMed] [Google Scholar]
- Chan J, Matarese G, Shetty G, Raciti P, Klesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantozoros C. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. PNAS. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Wong S, Mantzoros C. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clinical Pharmacokinetics. 2008;47:753–764. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Komatsu T, Nakayama M, Adachi T, Tamashiro Y, Hayashi H, Yamaza H, Higami Y, Shmokawa I. Similar metabolic response to calorie restriction in lean and obese Zucker rats. Molecular and Cellular Endocrinology. 2009;309:17–25. doi: 10.1016/j.mce.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Chou S, Chamberland J, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter M, Gong H, Arampatzi K, Mantzoros C. Leptin is an effective treatment for hypothalamic amenorrhea. PNAS. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese A, Smith S, Ravussin E. Diet, energy expenditure, and mitochondrial biogenesis. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10:679–687. doi: 10.1097/MCO.0b013e3282f0ecd2. [DOI] [PubMed] [Google Scholar]
- Coleman D. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Coleman D. Obesity and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Conroy R, Girotra M, Shane E, McMahon C, Pavlovich K, Leibel R, Rosenbaum M, Korner J. Leptin administration does not prevent the bone mineral metabolism changes induced by weight loss. Metabolism. 2011;60:1222–1226. doi: 10.1016/j.metabol.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe A, Hill J, Wing R, Reiman E, Tataranni P. Persistence of abnormal neural response to a meal in postobese individuals. International Journal of Obesity. 2004;28:370–307. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Farooqi I, Jebb S, Langmack G, Lawrence E, Cheetham C, Prentice A, Hughes I, McCamish M, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. New England Journal of Medicine. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi I, Matarese G, Lord G, Keogh J, Lawrence E, Agwu C, Sanna V, Jebb S, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity. T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. Journal of Clinical Investigation. 2002;110:1093–1103. doi: 10.1172/JCI0215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I, Keogh J, Yeo G, Lank E, Ceetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New England Journal of Medicine. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Flier J. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. Journal of Clinical Endocrinology and Metabolism. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- Fogteloo A, Pijl H, Frolich M, McCamish M, Meinders A. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutrition & Metabolism. 2003;16:109–114. [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy J, Premachandra B. Effect of long-term caloric restriction with adequate protein and micronutrients on thyroid hormones. Journal of Clinical Endocrinology and Metabolism. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Foster G, Wyatt H, Hill J, Makris A, Rosenbaum D, Brill C, Stein R, Mohammed B, Miller B, Rader D, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Annals of Internal Medicine. 2010;153:147–157. doi: 10.7326/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M, VanWormer J, Crain A, Boucher J, Histon R, Caplan W, Bowman J, Pronk N. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with minimum 1-year follow-up. Journal of the American Diabetic Association. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Friedman J, Halaas J. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Frisch R. Delayed menarche and amenorrhea in ballet dancers. New England Journal of Medicine. 1980;303:17–19. doi: 10.1056/NEJM198007033030105. [DOI] [PubMed] [Google Scholar]
- Goldsmith R, Joanisse D, Gallagher D, Pavlovich K, Shamoon E, Leibel R, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. American Journal of Physiology. 2010;298:R79–R88. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J, Gajiwala K, Maffei M, Cohen S, Chait B, Rabinowitz D, Lallone R, Burley S, Friedman J. Weight reducing effects of the plasma protein encoded by the ob gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Halaas J, Boozer C, Blair-West J, Fidahusein N, Denton D, Friedman J. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. PNAS. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington A, Ranson S. Hypothalamic lesions and adiposity in the rat. Anatomical Record. 1940;78:149–172. doi: 10.1002/ar.1090780203. [DOI] [Google Scholar]
- Hetherington A, Ranson S. The spontaneous activity and food intake of rats with hypothalamic lesions. American Journal of Physiology. 1942;136:609–617. [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Journal of the American Medical Association. 1999;292:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS ONE. 2013;8:e59114. doi: 10.1371/journal.pone.0059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukshorn C, Saris CHW, Westerterp-Plantenga M, Farid A, Smith F, Campfield L. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. Journal of Clinical Endocrinology and Metabolism. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, van Dielen FM, Berman WA, Westerp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. International Journal of Obesity. 2002;26:504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- Hukshorn C, Westerterp-Plantenga M, Saris W. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted overweight men. American Journal of Clinical Nutrition. 2003;77:771–776. doi: 10.1093/ajcn/77.4.771. [DOI] [PubMed] [Google Scholar]
- Javor E, Cochran E, Musso C, Young J, Depaoli A, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- Johansson K, Neovius M, Hemingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2014;99:14–23. doi: 10.3945/ajcn.113.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira S. Circumventing leptin resistance for weight control. PNAS. 2001;98:4279–4281. doi: 10.1073/pnas.091101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos A, Taylor D, Thompson C, Styles P, Hands L, Collin J, Casadei B. Sympathetic denervation of the upper limb improves forearm exercise performance and skeletal muscle bioenergetics. Circulation. 2000;13:2716–2720. doi: 10.1161/01.CIR.101.23.2716. [DOI] [PubMed] [Google Scholar]
- Keesey R, Corbett S. Metabolic defense of the body weight set-point. Research Publications – Association for Research in Nervous and Mental Disease. 1984;62:87–96. [PubMed] [Google Scholar]
- Kennedy G. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1953;140:578–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kissileff H, Thornton M, Torres M, Pavlovich K, Leibel R, Rosenbaum M. Maintenance of reduced body weight in humans is associated with leptin-reversible declines in satiation. American Journal of Clinical Nutrition. 2012;95:309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Aronne L. The emerging science of body weight regulation and its impact on obesity treatment. Journal of Clinical Investigation. 2003;111:565–570. doi: 10.1172/JCI17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Chua S, WIlliams J, Leibel R, Wardlaw S. Regulation of hypothalamic pro-opiomalanocortin by lean and obese rats. Neuroendocrinology. 1999;70:377–383. doi: 10.1159/000054499. [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua S, Leibel R, Wardlaw S. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. Journal of Neuroendocrinology. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Korner J, Conroy R, Febres G, McMahon D, Cornwell I, Karmally W, Aronne L. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity. 2013;21:951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Reed D, Price R. Leptin resistance is associated with extreme obesity and aggregates in families. International Journal of Obesity. 2001;25:1471–1473. doi: 10.1038/sj.ijo.0801736. [DOI] [PubMed] [Google Scholar]
- Lee J, Chan J, Sourlas E, Raptopoulos V, Mantzoros C. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. Journal of Clinical Endocrinology and Metabolism. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson C, Ahima R, Flier J, Lechan R. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2596–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Bauer U, Jhorth S, Rosenbaum M, Leibel R. Emerging pharmacological approaches to the management of obesity through appetite suppression. In: Dickson S, Mercer J, editors. Neuroendocrinology of Appetite, International Neuroscience Federation Master Class Series. Gothenberg; Sweden: 2014. [Google Scholar]
- Leibel R. Is obesity due to a heritable difference in “set-point” for adiposity? Western Journal of Medicine. 1990;153:429–431. [PMC free article] [PubMed] [Google Scholar]
- Leibel R, Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33:164–170. doi: 10.1016/0026-0495(84)90130-6. [DOI] [PubMed] [Google Scholar]
- Leibel R, Rosenbaum M. Metabolic response to weight perturbation. In: Clément K, editor. Novel Insights into Adipose Cell Functions, Research and Perspectives in Endocrine Interactions. Heidelberg, Germany: Springer-Verlag; 2010. pp. 121–133. [Google Scholar]
- Leibel RL, Bahary N, Friedman JM. Genetic variation and nutrition in obesity: approaches to the molecular genetics of obesity. In: Simopoulos AP, Childs B, editors. Genetic Variation and Nutrition. Basel, Switzerland: Karger; 1990. pp. 90–101. World Review of Nutrition and Dietetics series. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Bahary N, Friedman JM. Strategies for the molecular genetic analysis of obesity in humans. Critical Reviews in Food Science and Nutrition. 1993;33:351–358. doi: 10.1080/10408399309527632. [DOI] [PubMed] [Google Scholar]
- Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Lejeune M, Hukshorn C, Saris W, Westerterp-Plantenga M. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. International Journal of Obesity. 2003;27:1494–1499. doi: 10.1038/sj.ijo.0802431. [DOI] [PubMed] [Google Scholar]
- Lev-Ran A. Secondary amenorrhea resulting from uncontrolled weight reduction diets. Fertility and Sterility. 1974;25:459–462. doi: 10.1016/s0015-0282(16)40398-5. [DOI] [PubMed] [Google Scholar]
- Li X, Larsson L. Contractility and myosin isoform compositions of skeletal muscles and muscle cells from rats treated with thyroid hormone for 0, 4 and 8 weeks. Journal of Muscle Research and Cell Motility. 1997;18:335–344. doi: 10.1023/A:1018674126229. [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group . Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh R, Hirsch J. The effects of leptin administration in non-obese human subjects. Obesity Research. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- Mantzoros C, Magkos F, Brinkoetter M, Sienkiewica E, Dardeno T, Hamnvik O, Koniaris A. Leptin in human physiology and pathophysiology. American Journal of Physiology. Endocrinology and Metabolism. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Heilbronn L, de Jonge L, DeLany J, Volaufova J, Anton S, Redman L, Smith S, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007;15:2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Annals of the New York Academy of Sciences. 1955;63:15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- McGuire M, Wing R, Klem M, Hill J. Behavioral strategies of individuals who have maintained long-term weight losses. Obesity Research. 1999;7:334–341. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Horowitz J, DePaoli A, McCamish M, Patterson B, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Swewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Moon H, Matarese G, Brennan A, Chamberland J, Liu X, Fiorenza C, Mylvaganam G, Abanni L, Carbone F, Williams C, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. Leptin resistance and the response to positive energy balance. Physiology & Behavior. 2009;94:660–663. doi: 10.1016/j.physbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Cowley M, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual Review of Physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Leibel R, Seeley R, Schwartz M. Obesity and leptin resistance: distinguishing cause from effect. Trends in Endocrinology and Metabolism. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, et al. Increased glucose metabolism and insulin sensitivity transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. doi: 10.2337/diabetes.48.9.1822. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir I, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. Journal of Clinical Endocrinology and Metabolism. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Phelan S, Hassenstab J, McCaffery J, Sweet L, Raynor H, Cohen R, Wing R. Cognitive interference from food cues in weight loss maintainers, normal weight, and obese individuals. Obesity. 2011;19:69–73. doi: 10.1038/oby.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. American Journal of Clinical Nutrition. 1982;35:566–573. doi: 10.1093/ajcn/35.3.566. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Burnand B, Schutz Y. Energy expenditure before and during energy restriction in obese patients. American Journal of Clinical Nutrition. 1985;41:753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Smith S, Mitchell J, Shringarpure R, Shan K, Maier H, Koda J, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B, Lehmann A, Leduc C, Rosenbaum M, Horvath T, et al. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. American Journal of Physiology. Endocrinology and Metabolism. 2011;300:R1352–R1362. doi: 10.1152/ajpregu.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, LeDuc C, Watanabe K, Mueller B, Skowronski A, Rosenbaum M, Leibel R. Effects of chronic leptin infusion on subsequent body weight and composition in male mice: can body weight set-point be reset? Molecular Metabolism. 2014;3:432–440. doi: 10.1016/j.molmet.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman L, Heilbronn L, Martin C, de Jonge L, Williamson D, Delany J, Ravussin E Pennington CALERIE Team. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel R. Leptin: a molecule integrating somatic energy stores, energy expenditure, and fertility. Trends in Endocrinology and Metabolism. 1998;9:117–123. doi: 10.1016/S1043-2760(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel R. The role of leptin in human physiology. New England Journal of Medicine. 1999;341:913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel R. Effects of weight change on plasma leptin concentrations and energy expenditure. Journal of Clinical Endocrinology and Metabolism. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J, Heymsfield S, Joanisse D, Hirsch J, Murphy E, Matthews D, Segal K, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel R. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. Journal of Clinical Investigation. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Hirsch J, Gallagher D, Leibel R. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. American Journal of Clinical Nutrition. 2008a;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Sy M, Pavlovich K, Leibel R, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. Journal of Clinical Investigation. 2008b;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, Johnson W, Nyers V, Prather T, McGlone M, Rood J, Brantley P, Bray G, Gupta A, Brousard A, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Archives of Internal Medicine. 2010;170:146–154. doi: 10.1001/archinternmed.2009.508. [DOI] [PubMed] [Google Scholar]
- Saris CHW, Westerterp-Plantenga M, Farid A, Smith F, Campfield L. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. Journal of Clinical Endocrinology and Metabolism. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G, Tsuji T, Masuzaki H, Hiraoka J, Okazaki T, Tamaki M, Hayase M, Yoshimasa Y, et al. Pathophysiological significance of the obese gene product, leptin, in ventromedial hypothalamus (VMH)-lesioned rats: evidence for loss of its satiety effect in VMH-lesioned rats. Endocrinology. 1997;138:947–954. doi: 10.1210/endo.138.3.4989. [DOI] [PubMed] [Google Scholar]
- Scarpace P, Zhang Y. Elevated leptin: consequence or cause of obesity. Frontiers in Bioscience. 2007;12:3531–3544. doi: 10.2741/2332. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Woods S, Porte D, Seeley R, Baskin D. Central nervous system control of food intake. Nature. 2000;404:661–670. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shetty G, Matarese G, Magkos F, Moon H, Brennan A, Mylvganam G, Sykoutri D, Depaoli A, Mantzoros C. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. European Journal of Endocrinology. 2011;165:249–254. doi: 10.1530/EJE-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz E, Magkos F, Aronis K, Brinkoetter M, Chamberland J, Chou S, Arampatzi K, Gao C, Koniaris A, Mantozoros C. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Siklova-Vitkova M, Klimcakova E, Polak J, Kovacova Z, Tencerova M, Rossmeislove L, Bajzova M, Langin D, Stich V. Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1176–E1182. doi: 10.1210/jc.2011-2380. [DOI] [PubMed] [Google Scholar]
- Simonides W, Thelon M, VanderLinden C, Larsen P, Hardeveld C. Mechanism of thyroid-hormone regulated expression of SERCA genes in skeletal muscle: implications for thermogenesis. Bioscience Reports. 2001;90:139–154. doi: 10.1023/A:1013692023449. [DOI] [PubMed] [Google Scholar]
- Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. Journal of Internal Medicine. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- Skowronski A, Morabito M, Mueller B, Lee S, Hjorth S, Lehmann A, Watanabe K, Zeltser L, Ravussin Y, Rosenbaum M, et al. Effects of a novel MC4R agonist on maintenance of reduced body weight in diet-induced obese mice. Obesity. 2014;22:1287–1295. doi: 10.1002/oby.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J, Levitsky D, Allison D, Bray M, de Castro J, Clegg D, Clapman J, Dulloo A, Gruer L, Haw S, et al. Set points, settling points, and some alternative models: theoretic options to understand how genes and environments comebine to regulate body adiposity. Disease Models & Mechanisms. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumithran P, Prendergast L, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistance of hormonal adaptations to weight loss. New England Journal of Medicine. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- Trevaskis J, Parkes D, Roth J. Insights into amylin–leptin synergy. Trends in Endocrinology and Metabolism. 2010;21:473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Villanueva E, Myers M., Jr Leptin receptor signaling and the regulation of mammalian physiology. International Journal of Obesity. 2008;32:S8–12. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden T, Neiberg R, Wing R, Clark J, Delahanty L, Hill J, Krakoff J, Otto A, Ryan D, Vitolins M, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Amatruda J, Forbes G, Lockwood D. Resting metabolic rates of obese women after rapid weight loss. Journal of Clinical Endocrinology and Metabolism. 1984;59:41–44. doi: 10.1210/jcem-59-1-41. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M, Saris W, Hukshorn C, Campfield L. Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. American Journal of Clinical Nutrition. 2001;74:426–434. doi: 10.1093/ajcn/74.4.426. [DOI] [PubMed] [Google Scholar]
- Weyer C, Walford R, Harper I, Milner M, MacCallum T, Tataranni P, Ravussin E. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. American Journal of Clinical Nutrition. 2000;72:946–953. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- Wing R, Hill J. Successful weight loss maintenance. Annual Review of Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- Wing R, Phelan S. Long-term weight maintenance. American Journal of Clinical Nutrition. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypo-thalamic hypogonadism in female transgenic skinny mice overexpres-sing leptin. Journal of Clinical Investigation. 2000;105:749–755. doi: 10.1172/JCI8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelissen P, Stenlof K, Lean M, Fogteloo J, Keulen E, Wilding J, Finer N, Rossner S, Lawrence E, Fletcher C, et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes, Obesity & Metabolism. 2005;7:755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]