Abstract
Objective
The significance of indeterminate pulmonary nodules (IPNs) in patients undergoing resection of pancreatic ductal adenocarcinoma (PDAC) is unknown. We sought to define the prevalence and impact of IPN in such patients.
Methods
We studied all patients who underwent surgical resection of PDAC between 1980 and 2013. IPN was defined as ≥1 well-defined lung nodule(s) less than 3 cm in diameter. Survival was assessed using univariate and multivariate Cox models.
Results
Of the 2306 resected patients, 374 (16.2 %) had a preoperative chest computed tomography (CT) scan. Of these patients, 183 (49 %) had ≥1 IPN. Demographic and clinicopathological characteristics were similar among patients with or without IPN (all P>0.05). Median survival was comparable among patients who did (15.6 months) or did not (18.0 months) have IPN (P=0.66). Of the 183 patients with IPN, 29 (16 %) progressed to clinically recognizable metastatic lung disease compared to 13 % without IPN (P=0.38). The presence of >1 IPN was associated with the development of lung metastasis (relative risk 1.58, 95 % CI 1.03–2.4; P=0.05). However, lung metastasis was not associated with survival (P=0.24).
Conclusions
An IPN proved to be a lung metastasis in only one of six patients with PDAC undergoing surgical resection in this study. Survival was not impacted, even among patients who developed lung metastasis. Patients with PDAC who have IPN should not be precluded from surgical consideration.
Keywords: Pancreatic adenocarcinoma, Indeterminate pulmonary nodule
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the USA.1 As the majority of patients are asymptomatic or present with non-specific signs and symptoms such as weight loss, jaundice, and abdominal pain, they are often not diagnosed until late in the course of the disease when regional or distant spread has occurred.2 This late presentation contributes to an overall 5-year survival of 6 %.1 The best chance for survival is early detection when the tumor can be treated with surgical resection.3 Studies have shown that patients who undergo pancreatic resection have increased survival compared with those patients without any cancer directed treatment.4 However, surgery is limited to only patients with technically resectable disease in the absence of distant metastasis, making it imperative that a patient is accurately staged prior to any surgical intervention.
Currently, all patients with newly diagnosed pancreatic adenocarcinoma undergo preoperative staging with computed tomography (CT) of the abdomen and pelvis. This is based on National Comprehensive Cancer Network (NCCN) guidelines which recommend defined pancreas protocol imaging involving thin cuts (3 mm or less) through the abdomen by CT or, alternatively, MRI.5 This allows for assessment of the tumor's resectability and relationship to vascular structures and can be used to identify distant metastases in the abdominal cavity. However, it is not routine for patients to undergo chest imaging to identify distant disease in the chest. While the 2014 NCCN guidelines have been updated to recommend chest imaging for metastatic workup, there are currently no recommendations noted as to the specific type of imaging that should be undertaken. In addition, when CT scans of the chest are performed, the findings are often confounded by the presence of small subcentimeter nodules for which metastasis cannot be excluded.
Indeterminate pulmonary nodules (IPNs) are defined as well-defined, non-calcified, nodules in the lung less than 3 cm in size.6 By definition, these nodules are completely surrounded by parenchyma and are without associated adenopathy.6 These indeterminate nodules are clinically challenging because they could represent malignancy, especially metastases in patients already known to have a primary tumor with high metastatic potential. Several recent studies in patients with colorectal cancer have shown that between 4 and 26 % of patients who undergo preoperative imaging have at least one IPN; approximately 10–11 % of these nodules turn out to be metastatic colorectal cancer at follow-up.7–9 Yet, despite these findings, colorectal cancer patients undergoing hepatic resection who have IPN do not seem to have significantly worse survival, even when these IPN turn out to be metastatic disease.10 At this time, no such study has been done for pancreatic cancer. This creates an urgent need to determine the significance of these nodules in patients about to undergo pancreatic resection. We sought to define the prevalence and clinical impact of IPN in a large cohort of patients undergoing resection of PDAC.
Materials and Methods
Study Participants
Between January 1, 1980, and December 31, 2013, 2306 patients underwent resection of PDAC at the Johns Hopkins Hospital by pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy. Of these 2306 patients, 374 were identified as having a preoperative chest CT scan available for evaluation. A retrospective chart review was performed on these 374 patients with preoperative chest CT. All charts were reviewed, and information on patient demographics, tumor characteristics, perioperative characteristics, and chemoradiation therapy were collected. The pathology of the resected cancers was reviewed, including analysis of tumor stage, grade, margin status, perineural invasion, microvascular invasion, and nodal status. IPN was defined as ≥1 well-defined lung nodule less than 3 cm in diameter based on published guidelines, and radiologic characteristics assessed and collected included number and size.
Standard protocol is to follow patients postoperatively with routine clinic visits every 3–6 months in addition to imaging that include CT of the chest, abdomen, and pelvis to assess for recurrence. Patients were determined to have clinically recognizable metastatic disease in the lung based primarily on radiographic changes including increase in number and/or size of nodules felt to be consistent with metastasis upon review by a radiologist, medical oncologist, or surgeon. In addition, a subset of patients underwent biopsy and/or video-assisted thoracic surgery (VATS) in which pathologic analysis of tissue was determined to be consistent with metastatic pancreatic cancer.
During the course of the study, the CT scans were performed on a range of scanners but currently are performed on a Siemens Somatom Dual Source Scanners (Siemens Medical Solutions, Malvern, PA). Scan protocols are 3-mm thickness and include subsecond scanning, 120 kVp, 180–230 mAs and single phase acquisition. All chest CT scans were reviewed by a board certified radiologist at the time of patient imaging.
Statistical Methods
Summary statistics for the study population were presented as frequencies and percentages for categorical variables and as mean values with ranges for continuous variables. Statistical analysis to assess overall survival estimates in patients was performed using Kaplan-Meier method calculated from the date of surgery to the date of last follow-up or death and univariate Cox proportional hazards models. The differences in survival were examined with the log-rank test. All statistical analyses were carried out using StatView, version 5.0.1 (SAS Institute, Inc.) and STATA version 12.0 (StataCorp, College Station, TX). All tests were two-sided and a P value <0.05 was considered statistically significant.
IRB Approval
All studies were carried out with the approval of the Johns Hopkins Hospital Institutional Review Board.
Results
Demographics, Clinical, and Pathologic Data of Patients Undergoing PDAC Resection
During the defined study period, 2306 patients underwent resection of a pancreatic adenocarcinoma, of which 374 had a preoperative chest CT scan available for review. Of the 374, 51 % were female and 49 % male, and they had an average age of 67.8 years (range 38.3–94.1 years). The majority of the 374 patients did not have diabetes (n=277, 74 %). The majority of the carcinomas were located in the head of the pancreas (n=303, 81 %), and the majority were of moderate histologic grade (n=201, 54 %). Two hundred ninety-four (79 %) patients were treated with pancreaticoduodenectomy (either classic or pylorus-sparing), while 64 underwent distal pancreatectomy with splenectomy, and 18 had a total pancreatectomy. Postoperative margins were positive on 98 (26 %) patients (defined as either an R1 or R2 resection). A majority of patients (n=255, 68 %) had metastatic lymph nodes. Preoperatively, 99 (26 %) patients received neoadjuvant chemotherapy and 44 (12 %) received neoadjuvant radiation therapy while the majority of patients (n=274, 73 %) received no chemoradiation prior to surgery. While 240 (64 %) patients underwent adjuvant chemotherapy with 125 (33 %) undergoing postoperative radiation treatment, 132 (35 %) patients did not receive postoperative chemotherapy or radiation. Only 98 (26 %) did not receive neoadjuvant or adjuvant chemoradiation therapy. A prior history of malignancy was noted in 89 (23.8 %) patients, with the most common type being breast cancer (n=20). Eight (2.1 %) patients had a history of prior lung malignancy.
IPN and Progression to Clinically Recognizable Metastatic Disease in PDAC Patients
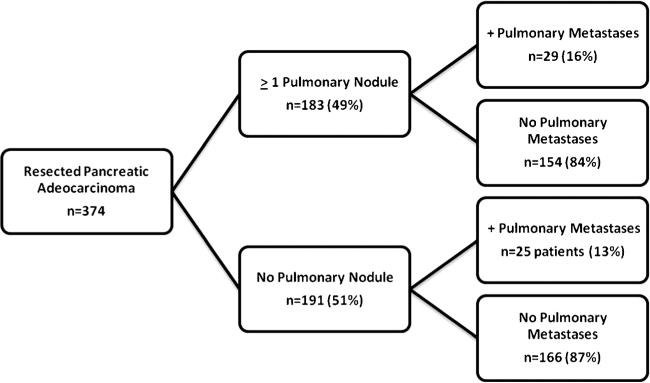
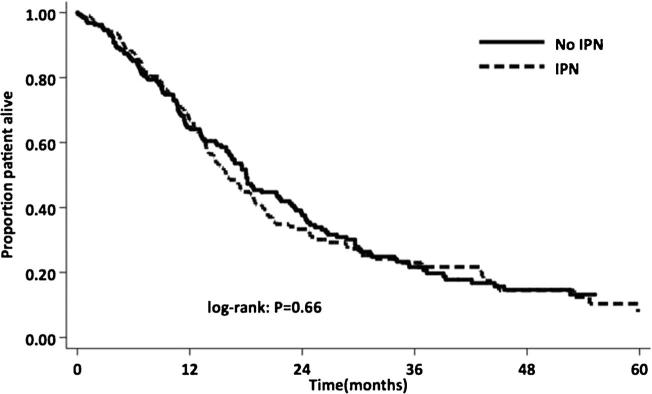
Among the 374 patients with a preresection chest CT, 183 patients (49 %) had ≥1 IPN on imaging (Fig. 1). No significant differences in demographics were observed in patients with and without IPN (Table 1). The majority of patients with IPN had only one (n=86, 46.9 %) and 36 had two IPNs (19.6 %). The mean size of IPN in all patients was 0.6 cm (range 0.2–2.7 cm). The majority of patients had nodules ≤1 cm (n=159, 86 %), and only five patients had nodules ≥2 cm but <3 cm. The median survival of all patients in this study was 17.4 months. Overall median survival was comparable among patients who did (15.6 months) or did not (18.0 months) have IPN (P=0.66) (Fig. 2). In addition, overall median survival was comparable for patients with an IPN at least 1 cm in size (15.5 months) compared to those with a subcentimeter IPN (15.8 months) or without an IPN (18.0 months) (P=0.58).
Fig. 1.
Development of pulmonary metastases in patients with and without pulmonary nodules
Table 1.
Comparative demographics for patients with and without IPN
| Clinical factors | Split by | Patients with IPN (n = 183) | Patients without IPN (n = 191) | P value |
|---|---|---|---|---|
| Average age (range) | 68 (46-94) | 68 (38-90) | 0.99 | |
| Gender | Male | 92 (50 %) | 92 (48 %) | 0.75 |
| Female | 91 (50 %) | 99 (52 %) | ||
| History of smoking | 78 (43 %) | 74 (39 %) | 0.46 | |
| History of prior malignancy | 44 (24 %) | 45 (24 %) | 0.99 | |
| History of prior lung malignancy | 4 (2 %) | 4 (2 %) | 0.99 | |
| Surgical resection | Whipple | 138 (75 %) | 156 (82 %) | 0.15 |
| Distal Pancreatectomy | 33 (18 %) | 29 (15 %) | ||
| Total Pancreatectomy | 12 (7 %) | 6 (3 %) | ||
| Tumor location | Head | 145 (80 %) | 158 (83 %) | 0.13 |
| Body | 19 (10 %) | 11 (6 %) | ||
| Tail | 19 (10 %) | 22 (11 %) | ||
| Average size pancreatic tumor (range) | 3.0 (0-11.0) | 3.3 (0-11.5) | 0.06 | |
| Pathology | Well | 9 (5 %) | 6 (3 %) | 0.43 |
| Moderate | 95 (52 %) | 106 (56 %) | ||
| Poor | 78 (42 %) | 77 (40 %) | ||
| Unknown | 2 (1 %) | 2 (1 %) | ||
| Positive nodes | 125 (68 %) | 130 (68 %) | 0.83 | |
| Positive margin | 46 (25 %) | 52 (27 %) | 0.72 | |
| Margin status | R0 | 137 (75 %) | 139 (73 %) | 0.28 |
| R1 | 45 (24 %) | 47 (25 %) | ||
| R2 | 1 (1 %) | 5 (2 %) |
Fig. 2.
Survival for pancreatic adenocarcinoma patients undergoing resection with and without IPN. IPN indeterminate pulmonary nodule
Mean clinical follow-up for patients in this study was 17.7 months (0.5–85 months). The majority of patients had at least 1 year of clinical follow-up (206 patients, 55 %), and 94 patients (25 %) had follow-up of greater than 2 years at the time of this study. Of the 183 patients with IPN, 29 (16 %) subsequently progressed to have clinically recognizable metastatic lung disease at the location of the prior IPN based on radiological assessment. Ten (34 %) of these 29 patients underwent lung biopsy which showed proven malignancy, and one patient underwent a biopsy which was found to be benign. Four patients (14 %) underwent resection of their lung disease while 16 (55 %) were treated with chemotherapy. Among patients without IPN (n=191), a similar proportion (13 %) developed clinically recognizable lung metastasis (P=0.38). In 61 patients (16 %), there was no adequate follow-up to determine if pulmonary metastases occurred.
The average size of IPN in patients who progressed to lung metastases was 0.69 cm (0.2–2.0 cm) compared to 0.60 cm (0.2–2.7 cm) in those without (P=0.29). While the majority of patients with subcentimeter IPNs tended to be less likely to have progression to lung metastases (P=0.055), there was no size cutoff at which lung metastases were more likely. In addition, the majority of patients with an IPN that progressed to lung metastases were less than 1 cm in size. There was no difference in the number of patients who progressed to clinically recognizable lung metastases between those with a history of smoking (n=25) and non-smokers (n=20) (P=0.74). Of the five patients with nodules ≥2 cm, only one went on to develop clinically recognizable lung metastases. While the presence of a solitary IPN was not associated with risk of subsequent lung metastasis, the presence of >1 IPN was associated with ensuing lung metastasis (relative risk 1.58, 95 % CI 1.03–2.4; P=0.05).
Survival Analysis of Patients Undergoing Pancreatic Resection for PDAC
The median length of survival for all patients in this study was 17.4 months. Stage, tumor grade, positive margins, and the presence of positive lymph nodes were all associated with survival (all P<0.05) (Table 2). These factors all remained significant by multivariate analysis. Age, sex, type of surgical resection, and a history of previous malignancy were not associated with survival. In addition, the development of clinically recognizable lung metastasis was not associated with a difference in survival (no lung metastasis 18.4 months vs lung metastasis 17.4 months; P=0.24). In a subgroup analysis of those with a preoperative IPN, there was no statistical difference in survival for those who went on to develop clinically recognizable lung metastases (P=0.59).
Table 2.
Univariate analysis of survival in all patients undergoing pancreatic resection
| Characteristic | Stratification | Median (months) | P value |
|---|---|---|---|
| Age | Continuous | – | 0.14 |
| Gender | Female | 17.6 | 0.52 |
| Male | 15.9 | ||
| Diabetes | Y | 17.6 | 0.43 |
| N | 17.1 | ||
| Smoking | Current | 16.5 | 0.82 |
| Past | 17.4 | ||
| Never | 15.8 | ||
| Unknown | 18.1 | ||
| Stage | I | 36.4 | 0.002 |
| II | 16.2 | ||
| III | 15.6 | ||
| Unknown | – | ||
| Grade | Well | 39.4 | <0.001 |
| Moderate | 18.4 | ||
| Poor | 13.7 | ||
| Surgical resection | Whipple | 17.5 | 0.79 |
| Distal pancreatectomy | 16.8 | ||
| Total pancreatectomy | 7.9 | ||
| Positive margins | Y | 13.7 | 0.01 |
| N | 18.1 | ||
| Positive nodes | Y | 14.8 | <0.001 |
| N | 31.4 | ||
| IPN pre-op | Y | 15.8 | 0.67 |
| N | 18.0 | ||
| Lung metastasis | Y | 18.1 | 0.24 |
| N | 17.2 | ||
| Number ofSPN | 1 | 15.1 | 0.50 |
| 2 or more | 17.0 | ||
| History of prior malignancy | Y | 17.7 | 0.36 |
| N | 17.0 |
Discussion
The best chance for survival for pancreatic cancer is early detection when the carcinoma can be treated with surgical resection. At this time, surgery is limited to those with resectable local disease without distant metastasis, making it imperative that a patient is accurately staged prior to any surgical intervention. Based on NCCN guidelines, patients with newly diagnosed pancreatic adenocarcinoma currently undergo preoperative staging with a defined pancreas protocol CT of the abdomen and pelvis with thin cuts to assess for tumor resectability in addition to regional or distant metastases in the abdomen. While the current NCCN guidelines recommend chest imaging as part of the metastatic workup, there are no current recommendations for the specific type of imaging to obtain. Thus, while the majority of physicians would likely pursue chest CT for workup, it is conceivable that some would only obtain a chest X-ray. We found that almost half of all patients who underwent preoperative chest CT had at least one IPN, making IPNs a relatively common finding on chest CT. Therefore, it is important to know how to best manage these patients and to determine the significance of these nodules patients about to undergo resection, especially as they relate to risk of metastases.
The reported prevalence of pulmonary nodules noted on CT scans in the general population varies, with a prevalence believed to be as high as 69 % in smokers or patients at risk for lung cancer.11–13 In patients with non-pulmonary cancers, a similar prevalence has been noted as well. A study of patients with colorectal cancer and known liver metastases found IPNs in 16 % while another which evaluated colorectal cancer patients without metastases found a rate of only 4.1 %.7, 10 A similar study of patients with extrapulmonary malignancy or sarcoma showed a rate of 75 % with at least one IPN, later classified as benign, malignant, or indeterminate.14 Studies of patients with a known malignancy and IPN demonstrated that the primary tumor's histology, the size of the nodule, and a history of smoking increased the likelihood the nodule was malignant.15, 16Our study shows a similar prevalence of IPN in patients in comparison to other studies, which could be related to environmental exposures or the advanced age of patients in our cohort. However, patients with a history of smoking were not more likely to have an IPN. Further evaluation may be needed to determine the underlying reason for such a large number of patients having this incidental finding.
There was no difference in survival between patients with or without at least one IPN on preoperative CT. This may be attributed to the relatively low percentage of patients with an IPN that progressed to clinically recognizable pulmonary metastasis. In addition, an equal percentage of patients with and without IPN developed clinically recognizable pulmonary metastases, and it would be believed that any potential survival disadvantage would be equivalent between both groups. Thus, IPN does not appear to be a contraindication to pancreatic resection. However, patients with increasing numbers of IPN had a higher association of clinically recognizable pulmonary metastasis. Therefore, close follow-up postoperatively should be initiated to monitor patients for development of lung metastases, especially those patients with two or more IPN.
An interesting finding from this study was that there was no significant difference in survival for patients with and without the development of clinically recognizable pulmonary metastases. This was true even in those patients with a preoperative IPN, as those who did go on to develop lung metastases did not have worse survival. This is unlikely to be related to the resection of clinically recognizable lung metastases, as only 6 (11 %) of the total 53 patients with lung metastases underwent lung resection. The majority of patients who progressed to clinically recognizable lung metastases were treated with adjuvant chemotherapy and had similar survival to patients who did not develop pulmonary metastases. Therefore, it is possible that the eventual development of lung metastases may not impact overall survival. Instead, other factors related the primary tumor or disease recurrence patterns may be more important. Further research will be needed to determine if there is a difference in survival based on the location of metastases.
As a retrospective chart review, this study has several limitations. Patients were chosen for surgical resection based upon tumor characteristics and the absence of any suspicion of metastatic disease. We therefore do not have data on patients who did not undergo surgical resection. Additionally, it was the decision of each individual physician whether to obtain preoperative chest imaging and, if so, whether this was a chest X-ray or CT, contributing to the large number of patients who did not undergo preoperative chest CT. Secondly, some patients were lost to follow-up based on their preference to receive adjuvant treatment at outside institutions. This led to a group of patients in which it was not possible to determine if the disease progressed to lung metastases. In addition, the development of lung metastases was determined in many cases by radiographic findings such as increase in size or number of nodules. Only a third of patients underwent surgical resection or biopsy allowing for tissue diagnosis to confirm metastatic disease. Thus, there is the possibility for either overestimating or underestimating the number of patients with lung metastases in this study. Finally, the majority of patients with pancreatic adenocarcinoma, including those in this study, ultimately went on to develop locally recurrent or metastatic disease. The development of widespread metastases in different organs can make it difficult to adequately determine the primary etiology of death and is a potential confounder of survival that is difficult to control for in each individual patient.
This is the first study of its kind examining the management of IPN in patients with pancreatic adenocarcinoma amenable to surgical resection. While IPN is a common finding in patients with pancreatic cancer, IPN should not be a contraindication to surgical resection of their disease. An IPN developed into clinically recognizable lung metastasis in only one of six patients with PDAC undergoing surgical resection in this study, and further research will be needed to determine the best course of follow-up in these patients postoperatively. Survival was not impacted in pancreatic cancer patients with IPN, even among patients who did eventually develop clinically recognizable lung metastasis. Patients with pancreatic adenocarcinoma who have IPN should not be precluded from surgical consideration.
Conclusion
Among our cohort of patients with preoperative chest CT, at least one IPN was found in almost half of all patients (n=183, 49 %). There was no difference between the presence of an IPN between smokers and non-smokers. Survival was not impacted among patients who had an IPN. Furthermore, an IPN developed into clinically recognizable lung metastases in only one of six patients with PDAC undergoing surgical resection in this study. While the presence of a solitary IPN was not associated with risk of subsequent lung metastasis, the presence of ≥1 IPN was associated with ensuing clinically recognizable lung metastasis. Survival in patients who developed lung metastases was not impacted. Patients with PDAC who have IPN should not be precluded from surgical consideration.
Acknowledgments
The authors of this manuscript do not have any financial or material support to acknowledge.
Contributor Information
Katherine E. Poruk, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
Yuhree Kim, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA.
John L. Cameron, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
Jin He, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA.
Frederic E. Eckhauser, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
Neda Rezaee, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA.
Joseph Herman, Department of Radiation Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Daniel Laheru, Department of Medical Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Lei Zheng, Department of Medical Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Elliot K. Fishman, Department of Radiology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Ralph H. Hruban, Department of Surgery Pathology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Timothy M. Pawlik, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
Christopher L. Wolfgang, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
Matthew J. Weiss, Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Halsted 608, 600 N. Wolfe Street, Baltimore, MD 21287, USA
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7(5):189–97. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 3.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318–48. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014. J Natl Compr Canc Netw. 2014;12(8):1083–93. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 7.Varol Y, Varol U, Karaca B, et al. The frequency and significance of radiologically detected indeterminate pulmonary nodules in patients with colorectal cancer. Med Princ Pract. 2012;21(5):457–61. doi: 10.1159/000337426. [DOI] [PubMed] [Google Scholar]
- 8.Nordholm-Carstensen A, Wille-Jorgensen PA, Jorgensen LN, et al. Indeterminate pulmonary nodules at colorectal cancer staging: a systematic review of predictive parameters for malignancy. Ann Surg Oncol. 2013;20(12):4022–30. doi: 10.1245/s10434-013-3062-y. [DOI] [PubMed] [Google Scholar]
- 9.Baek SJ, Kim SH, Kwak JM, et al. Indeterminate pulmonary nodules in rectal cancer: a recommendation for follow-up guidelines. J Surg Oncol. 2012;106(4):481–5. doi: 10.1002/jso.23106. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D, Kamali D, Dunn WK, et al. Outcomes in patients with indeterminate pulmonary nodules undergoing resection for colorectal liver metastases. HPB (Oxford) 2012;14(7):448–54. doi: 10.1111/j.1477-2574.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest. 2004;126(1):114–21. doi: 10.1378/chest.126.1.114. [DOI] [PubMed] [Google Scholar]
- 12.Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226(3):756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 13.Furtado CD, Aguirre DA, Sirlin CB, et al. Whole-body CTscreening: spectrum of findings and recommendations in 1192 patients. Radiology. 2005;237(2):385–94. doi: 10.1148/radiol.2372041741. [DOI] [PubMed] [Google Scholar]
- 14.Hanamiya M, Aoki T, Yamashita Y, et al. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol. 2012;81(1):152–7. doi: 10.1016/j.ejrad.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Khokhar S, Vickers A, Moore MS, et al. Significance of non-calcified pulmonary nodules in patients with extrapulmonary cancers. Thorax. 2006;61(4):331–6. doi: 10.1136/thx.2005.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology. 2000;217(1):257–61. doi: 10.1148/radiology.217.1.r00oc20257. [DOI] [PubMed] [Google Scholar]