Abstract
Objective
To use 18F-fluorodeoxyglucose (FDG) and PET to investigate changes in regional metabolism associated with mild cognitive impairment (MCI) in Parkinson disease (PD). Cognitive abnormalities are common in PD. However, little is known about the functional abnormalities that underlie the manifestations of MCI in this disorder.
Methods
We used FDG PET to measure regional glucose metabolism in patients with PD with multiple-domain MCI (MD-MCI; n = 18), with single-domain MCI (SD-MCI; n = 15), and without MCI (N-MCI; n = 18). These patients were matched for age, education, disease duration, and motor disability. Maps of regional metabolism in the three groups were compared using statistical parametric mapping (SPM). We also computed the expression of a previously validated cognition-related spatial covariance pattern (PDCP) in the patient groups and in an age-matched healthy control cohort (n = 15). PDCP expression was compared across groups using analysis of variance.
Results
SPM revealed decreased prefrontal and parietal metabolism (p < 0.001) in MD-MCI relative to N-MCI, as well as an increase in brainstem/cerebellar metabolism (p < 0.001) in this group. In these regions, SD-MCI occupied an intermediate position between the two other groups. PDCP expression was abnormally elevated in the N-, SD-, and MD-MCI groups (p < 0.05), increasing stepwise with worsening cognitive impairment (p < 0.01).
Conclusions
Early cognitive decline in Parkinson disease as defined by mild cognitive impairment is associated with discrete regional changes and abnormal metabolic network activity. The quantification of these alterations with 18F-fluorodeoxyglucose PET may allow for the objective assessment of the progression and treatment of this disease manifestation.
Cognitive decline in Parkinson disease (PD) constitutes a well-defined behavioral syndrome characterized by difficulties in executive and visuospatial functions, as well as deficits in memory and verbal fluency.1 These changes in cognitive functioning can be identified early in the course of the disease.2 PD patients with quantifiable cognitive deficits, but who do not meet criteria for dementia, can be considered to have mild cognitive impairment (MCI), which is conceptualized as a transitional stage between normal cognition and dementia, during which a person is not demented but has measurable cognitive deficits in some form.3 MCI can be clinically divided into subtypes in which patients with deficits in a single cognitive domain (SD-MCI) are differentiated from those with involvement of more than one domain (multiple domain, MD-MCI).4
The use of MCI criteria in patients already diagnosed with PD has been shown to have some prognostic value in that 64% of patients with MCI converted to dementia over a 4-year follow-up period as compared with only 20% of those without MCI.5 Contrary to prodromal Alzheimer disease, PD patients with SD-MCI without memory impairment, as well as those with MD-MCI, appear more likely to progress to dementia.3 However, the clinical characterization of MCI in PD has not been validated and the underlying pathology is not known.
Metabolic imaging with 18F-fluorodeoxyglucose (FDG) PET, an in vivo assay of synaptic activity in the brain, can potentially be used to identify regional changes in brain function that differentiate PD patients with and without cognitive dysfunction. The presence of specific metabolic abnormalities in patients with PD fulfilling diagnostic criteria for MCI can be used to validate this syndrome as a distinct diagnostic entity. Moreover, these scans can be used to quantify the activity of a distinct spatial covariance pattern associated with cognitive functioning in PD patients without dementia.6 This PD-related cognitive pattern (PDCP) is characterized by metabolic reductions in frontal and parietal association areas associated with relative increases in the cerebellar vermis and dentate nuclei. We have found that the expression of this pattern correlates with neuropsychological tests of memory and executive functioning in prospectively evaluated PD patients. Additionally, quantitative measures of PDCP activity exhibit excellent test–retest reproducibility and are not altered by routine antiparkinsonian treatment.6,7
In this study, we examined the hypothesis that PDCP expression is elevated in patients satisfying MCI criteria relative to their counterparts without cognitive abnormalities, and that pattern scores are relatively greater in MD-MCI relative to SDMCI. To test these hypotheses, we employed resting state FDG PET to measure differences in regional metabolism in 33 patients with PD satisfying the criteria for MCI (MD-MCI, n = 18; SD-MCI, n = 15) and those with a similar degree of motor disability but without these cognitive abnormalities (N-MCI, n = 18). We additionally quantified PDCP expression in each scan and contrasted these values across the MCI subgroups.
METHODS
Subjects
Fifty-one PD patients without dementia (Mini-Mental State Examination [MMSE] > 24) (19 women, 32 men; age: 61.0 ± 8.1 years [mean ± SD]; mean duration: 9.0 ± 3.1 years; Unified Parkinson’s Disease Rating Scale [UPDRS] off-state motor ratings 32.3 ± 16.2) underwent FDG PET imaging and completed a neuropsychological battery. A diagnosis of PD was made if the patients had pure parkinsonism without a history of known causative factors such as encephalitis or neuroleptic treatment, and did not have dementia, supranuclear gaze abnormalities, or ataxia. All patients had a clear-cut (>20% change in motor UPDRS ratings) response to levodopa, dopamine agonist medications, or both. Based on neuropsychological assessment (see below), 18 of the patients were classified as MD-MCI, 15 as SD-MCI (6 amnestic, 9 nonamnestic), and 18 as N-MCI. The N-MCI, SD-MCI, and MD-MCI subgroups were matched for age, education, disease duration, and off-state motor UPDRS ratings (table 1). Limited metabolic and neuropsychological data from a portion of the current sample were previously reported.6
Table 1.
Clinical characteristics of the study sample
| N-MCI | SD-MCI | MD-MCI | p Value* | |
|---|---|---|---|---|
|
| ||||
| No. of subjects | 18 | 15 | 18 | — |
|
| ||||
| Age, y | 59.0 ± 9.3 | 62.1 ± 5.2 | 62.4 ± 8.7 | 0.4 |
|
| ||||
| F/M | 6/12 | 6/9 | 7/11 | 0.9 |
|
| ||||
| Education, y | 15.1 ± 2.3 | 14.7 ± 2.6 | 14.2 ± 3.1 | 0.6 |
|
| ||||
| Disease duration, y | 9.5 ± 1.0 | 8.5 ± 1.0 | 9.2 ± 1.4 | 0.8 |
|
| ||||
| Hoehn &Yahr stage | 3.1 ± 1.1 | 3.2 ± 1.0 | 3.6 ± 0.6 | 0.3 |
|
| ||||
| UPDRS (motor)† | 29.2 ± 16.9 | 32.8 ± 15.3 | 34.9 ± 16.7 | 0.6 |
Analysis of variance with the exception of chi-square for gender.
Off-state motor ratings according to the UPDRS.
N-MCI = without mild cognitive impairment; MD-MCI = multiple domain MCI; SD-MCI = single domain MCI; UPDRS = Unified Parkinson’s Disease Rating Scale.
Neuropsychological tests
All patients underwent neuropsychological examination while on their routine medications. Cognitive functioning was assessed in five domains. The MMSE8 was utilized to screen for dementia. Attention and executive function were assessed utilizing the Stroop Tests,9 Wisconsin Card Sorting Test (WCST),10 Symbol Digit Modality Test (SDMT),11 and Trail Making Test.12 Visuospatial functioning was assessed utilizing Hooper Visual Organization Test (HVOT).13 Language was assessed utilizing Boston Naming Test (BNT).14 Memory was assessed by the California Verbal Learning Test (CVLT).15 Affective status was evaluated utilizing Beck Depression Inventory.16
A diagnosis of MCI was made according to consensus criteria.4 Specifically, the diagnosis of MCI was made if at least one of the four cognitive domains assessed was 1.5 SD below an age-corrected normative sample. Executive function was assessed with WCST, number of categories achieved. Language was assessed with BNT. Visuospatial function was assessed with HVOT. Memory was assessed with CVLT, delayed recall.
In accordance with MCI consensus criteria,4 we further categorized the patients by the number of cognitive domains found to be impaired. Thus, the patients were separated into discrete groups based upon the severity of cognitive impairment. Patients having no test scores below the normative samples were termed non-MCI (N-MCI). Single domain MCI (SD-MCI) was utilized for those subjects with only one test score significantly below the normative sample. (Because of sample size limitations, we did not directly compare the SD-MCI subgroup with and without amnestic features.) Multiple domain MCI (MD-MCI) was utilized to characterize subjects who had two or more test scores that fell significantly below the normative sample.
Positron emission tomography
All subjects were scanned with FDG PET within 3 months of neuropsychological evaluation. The average interval between evaluation and PET imaging was 15 days. The subjects fasted overnight before imaging; all antiparkinsonian medications were discontinued at least 12 hours before the PET scan was conducted. PET imaging was performed in three-dimensional mode using a GE Advance tomograph (General Electric; Milwaukee, WI). The 18-ring bismuth germanate scanner provides 35 image planes with an axial field of view of 14.5 cm and an intrinsic resolution of 4.2 mm of full width at half maximum (FWHM) in all directions. All the subjects were set in a dimly lit room with minimal auditory stimulation with eyes open. Patients were positioned in the scanner using a stereoadapter with three-dimensional laser alignment with reference to the orbitomeatal line. Images were constructed into a 128 X 128 X 35 matrix with a voxel size of 2 X 2 X 4, and a 6 mm Hanning filter. This gave an effective three-dimensional image resolution of 8 mm FWHM.
Ethical permission for this study was obtained from the Institutional Review Board of North Shore University Hospital. Written consent was obtained from each subject with detailed explanation of the procedures.
Data analysis
FDG image preprocessing was performed using SPM5 (Wellcome Department of Cognitive Neurology, University College, London) running on Matlab 6.5 (Mathworks Inc., Natick, MA). Scans were spatially normalized to a Talairach-based PET template using a 12-parameter affine transformation, non-linear transformation, and trilinear interpolation. The normalized scans were then smoothed using a Gaussian kernel at FWHM = 10 mm. The SPM{t} maps were obtained at a height threshold of p = 0.001.
Group comparisons among MD-MCI, SD-MCI, and N-MCI were done by the full factorial model, analysis of variance, with motor UPDRS as a nuisance variable. Contrasts of MD-MCI vs N-MCI, MD-MCI vs SD-MCI, and SD-MCI vs N-MCI were defined to examine both positive and negative differences in brain metabolism between groups. Significance was set at p < 0.05, corrected for multiple comparisons. Coordinates were reported in the standard anatomic space developed at the Montreal Neurological Institute. The cytoarchitectonic localization of each reported cluster was confirmed using the Talairach space utility (available at http://www.ihb.spb.ru/~pet_lab/TSU/TSUMain.html). For each significant cluster, we performed a post hoc analysis within a spherical (radius = 4 mm) volume of interest (VOI) centered on the peak voxel.17 Each regional value was ratio normalized by the global metabolic rate and compared with values for the same VOIs measured in 15 age-matched healthy volunteer subjects (7 women, 8 men; age: 56.7 ± 12.3 years [mean ± SD]).
In each subject, we also measured the expression of a previously validated cognition-related spatial covariance pattern.6 This PDCP is characterized by reductions in prefrontal and parietal metabolism associated with relative increases in the cerebellum and dentate nuclei. In all subjects, the expression of the PDCP network was quantified using a fully automated voxel-based algorithm18,19 (software available at http://feinsteinneuroscience.org/software.html). These network computations were performed blind to subject, cognitive status (MD-, SD-, or N-MCI), and disease severity (UPDRS motor ratings). Reference values for PDCP expression were computed in the same group of 15 healthy control subjects used for the post hoc analysis of the SPM results (see above). Subject scores for the entire cohort (patients with PD and healthy controls) were z-transformed and offset so that the control mean was zero.
Group comparisons of VOI-based regional metabolism and PDCP expression among MD-MCI, SD-MCI, and N-MCI were assessed by one-way analysis of variance followed by post hoc Scheffé tests. The values of each MCI subgroup were separately compared with those from the age-matched control cohort using Student t tests.
RESULTS
Neuropsychology and behavior
The results of neuropsychological testing are presented in table 2. Of the tests of attention and executive functioning, the three patient groups were statistically distinct only with regard to the SDMT, with patients with MD-MCI performing worse than patients with SD-MCI and patients with N-MCI, and patients with SD-MCI performing worse than N-MCI. Patients with MD-MCI performed worse than patients with SD-MCI and patients with N-MCI on Trails A and WCST categories achieved, and performed worse than the N-MCI group on Trails B. For our memory measure (CVLT), the patients with MD-MCI and patients with SD-MCI performed worse than the N-MCI group on multiple subscales (sum 1 to 5, shortdelay free recall, short-delay cued recall, longdelay free recall, and long-delay cued recall). Patients with MD-MCI also made a greater number of false positive errors on recognition than N-MCI subjects. For our language measure (BNT), the MD-MCI group performed worse than both SD-MCI and N-MCI groups. All three patient groups were statistically distinct on our visuospatial measure (HVOT), with patients with MD-MCI performing worse than both patients with SD-MCI and patients with N-MCI, and patients with SD-MCI performing worse than N-MCI. The groups did not differ in their endorsement of depressive symptoms (BDI).
Table 2.
Neuropsychological testing in Parkinson disease patients with mild cognitive impairment (MCI)
| Cognitive test | Reference value* |
N-MCI | SD-MCI | MD-MCI | p Value | Post hoc significance |
|---|---|---|---|---|---|---|
|
| ||||||
| MMSE | >25 | 28.2 ± 1.4 | 28.2 ± 1.6 | 27.1 ± 1.9 | — | — |
|
| ||||||
| Stroop Interference Test | ||||||
|
| ||||||
| Word | >78 | 104.1 ± 11.0 | 99.1 ± 16.3 | 98.9 ± 20.6 | — | — |
|
| ||||||
| Color | >57.5 | 68.8 ± 11.3 | 63.8 ± 7.9 | 59.2 ± 11.1 | 0.049 | — |
|
| ||||||
| Color/Word | >30 | 44.6 ± 10.0 | 36.9 ± 10.4 | 36.8 ± 9.7 | 0.061 | — |
|
| ||||||
| Trail Making Test (sec) | ||||||
|
| ||||||
| A | <42 | 37.6 ± 11.9 | 42.1 ± 14.1 | 65.6 ± 35.8 | 0.003 | [M>N†] [M>S‡] |
|
| ||||||
| B | <93 | 91.0 ± 41.1 | 134.0 ± 63.2 | 155.5 ± 62.8 | 0.009 | [M>N‡] |
|
| ||||||
| SDMT | >54 | 45.8 ± 8.6 | 36.1 ± 9.9 | 27.0 ± 11.0 | <0.001 | [M<N§] [S<N‡] [M<S‡] |
|
| ||||||
| WCST | ||||||
|
| ||||||
| Categories | >3 | 2.8 ± 1.4 | 1.6 ± 1.1 | 0.8 ± 1.1 | <0.001 | [M<N§] [S<N‡] |
|
| ||||||
| Perseverative Responses |
<48 | 9.6 ± 8.7 | 14.8 ± 8.9 | 21.4 ± 13.4 | 0.057 | — |
|
| ||||||
| Failure Maintain Test | <2 | 0.5 ± 1.0 | 0.1 ± 0.4 | 0.6 ± 0.5 | — | — |
|
| ||||||
| BNT | >50 | 56.2 ± 2.9 | 52.6 ± 3.5 | 46.7 ± 10.1 | <0.001 | [M<S‡] [M<S‡] |
|
| ||||||
| HVOT | >19 | 23.5 ± 2.8 | 19.8 ± 4.4 | 14.8 ± 4.0 | <0.001 | [M<N§] [S<N‡] [M<S†] |
|
| ||||||
| CVLT | ||||||
|
| ||||||
| Sum 1 to 5 | >37 | 44.7 ± 7.9 | 34.6 ± 9.5 | 31.2 ± 8.4 | <0.001 | [M<N§] [S<N‡] |
|
| ||||||
| List B | >3 | 5.4 ± 1.9 | 4.5 ± 1.4 | 4.6 ± 1.6 | — | — |
|
| ||||||
| Short-Delay Free Recall | >5 | 8.9 ± 3.0 | 6.1 ± 2.8 | 4.9 ± 3.0 | 0.001 | [M<S§] [S<N‡] |
|
| ||||||
| Short-Delay Cued Recall | >9 | 10.3 ± 1.7 | 7.8 ± 2.9 | 6.0 ± 2.5 | <0.001 | [M<N§] [S<N‡] |
|
| ||||||
| Long-Delay Free Recall | >5 | 9.4 ± 2.2 | 6.9 ± 3.1 | 5.4 ± 2.2 | <0.001 | [M<N§] [S<N‡] |
|
| ||||||
| Long-Delay Cued Recall | >7 | 10.7 ± 2.3 | 7.5 ± 2.4 | 5.7 ± 1.9 | <0.001 | [M<N§] [S<N‡] |
|
| ||||||
| Hits | >11 | 14.3 ± 1.9 | 12.9 ± 2.5 | 12.2 ± 3.0 | 0.056 | — |
|
| ||||||
| False Positives | <5 | 1.0 ± 1.4 | 3.7 ± 2.7 | 5.2 ± 4.4 | 0.002 | [M>N†] |
|
| ||||||
| Beck Depression Inventory |
<16 | 9.1 ± 4.0 | 9.5 ± 5.6 | 12.8 ± 7.5 | — | — |
p Value represents the significance level of the analysis of variance performed for each test across the three groups.
For each test, the reference value was estimated at the 16th percentile or 1.5 SD below the standard mean for a 61-year-old man with 14 years of education.8-11,13,15,26,27 For Beck Depression Inventory, we used the score for mild depression as a cutoff.16
p > 0.01,
p > 0.05,
p > 0.001.
N-MCI = without mild cognitive impairment; SD-MCI = single domain MCI; MD-MCI = multiple domain MCI; MMSE = Mini-Mental State Examination; SDMT = Symbol Digit Modality Test; WCST = Wisconsin Card Sorting Test; BNT = Boston Naming Test; HVOT = Hooper Visual Organization Test; CVLT = California Verbal Learning; M = MD-MCI; S = SD-MCI; N = N-MCI.
Regional differences
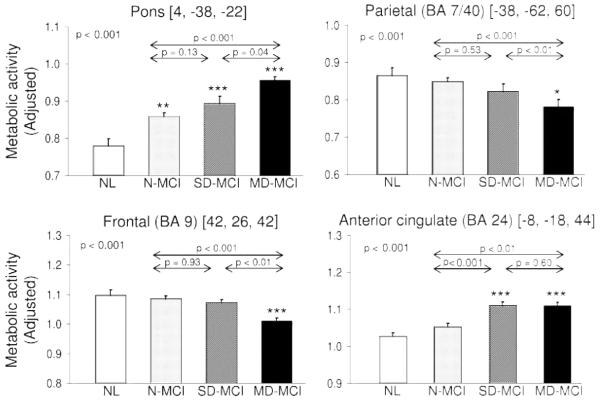
Regions with significant differences across the MD-MCI, SD-MCI, and N-MCI groups are presented in figures 1 and 2 and table 3. Relative to N-MCI, patients with MD-MCI exhibited significant metabolic reductions in the inferior parietal lobule (BA 7/40) and in the middle frontal gyrus (BA 9/46) (p < 0.001). In these regions, metabolism in the MD-MCI group was lower than in SD-MCI (p < 0.01), although the latter group did not differ significantly from N-MCI. Regional metabolism of parietal and frontal lobe was reduced below the normal mean (p < 0.01) in the MD-MCI group, but not in the SD- or N-MCI groups.
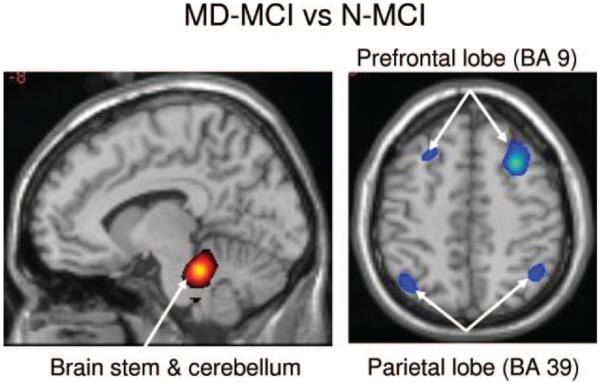
Figure 1.
Group comparison of regional metabolic changes between multiple domain mild cognitive impairment (MD-MCI) and without mild cognitive impairment (N-MCI) utilizing voxel-based statistical parametric mapping analysis
MD-MCI had significant metabolic decreases in middle frontal lobe and inferior parietal lobe, associated with increases in pons and cerebellum as compared to N-MCI. Metabolic increases are displayed using a red-yellow scale and declines are displayed using a blue-purple scale. Both displays were superimposed on a single-subject MRI brain template and thresholded at t = 3.27, p = 0.001 (peak voxel, uncorrected).
Figure 2.
Plots of means, standard errors, and significances of the metabolic rates with 4 mm sphere centered on the significant peaks acquired from voxel-based statistical parametric mapping analysis in multiple domain mild cognitive impairment (MCI), single domain MCI, without mild cognitive impairment, and 15 age-matched controls
*Represents the significance of each group compared to the controls. *p < 0.05, **p <= 0.005, ***p < 0.001.
Table 3.
Peak locations and structures of significant metabolic changes of mild cognitive impairment (MCI) in Parkinson disease
| Coordinates* |
|||||
|---|---|---|---|---|---|
|
| |||||
| Group comparison | Structure | Z score | x | y | z |
|
| |||||
| MD-MCI vs N-MCI | |||||
|
| |||||
| MD-MCI < N-MCI | Left inferior parietal lobulus (BA 7/40) | 4.28† | −38 | −62 | 60 |
|
| |||||
| Supramarginal gyrus (BA 40) | 3.60 | −52 | −58 | 34 | |
|
| |||||
| Right middle frontal gyrus (BA 8) | 4.52‡ | 30 | 18 | 48 | |
|
| |||||
| Middle frontal gyrus (BA 9/46) | 4.16 | 40 | 26 | 42 | |
|
| |||||
| Middle frontal gyrus (BA 46) | 4.04 | 46 | 26 | 32 | |
|
| |||||
| Left middle frontal gyrus (BA 8/9) | 3.60§ | −30 | 24 | 46 | |
|
| |||||
| MD-MCI > N-MCI | Left pons/cerebellum | 4.36¶ | −8 | −34 | −24 |
|
| |||||
| Right pons/cerebellum | 4.33 | 6 | −38 | −22 | |
|
| |||||
| SD-MCI vs N-MCI | |||||
|
| |||||
| SD-MCI > N-MCI | Cingulate gyrus (BA 24/31) | 3.94§ | −10 | −18 | 44 |
|
| |||||
| MD-MCI vs SD-MCI | |||||
|
| |||||
| MD-MCI < SD-MCI | Right middle frontal gyrus (BA 9) | 4.11† | 44 | 26 | 32 |
|
| |||||
| Middle frontal gyrus (BA 9) | 3.95 | 42 | 32 | 40 | |
|
| |||||
| Left inferior parietal lobulus (BA 40) | 4.22§ | 54 | −30 | 56 | |
|
| |||||
| MD-MCI > SD-MCI | NA | NA | NA | NA | NA |
Indented areas are submaxima of preceding cluster.
Montreal Neurological Institute standard space.
p < 0.05,
p < 0.01,
p < 0.001, Cluster corrected.
p < 0.05, Corrected for area.
MD-MCI=multiple domain MCI; N-MCI=without mild cognitive impairment; BA=Brodmann area; SD-MCI=single domain MCI.
Patients with MD-MCI exhibited significant metabolic increases relative to N-MCI in a large subcortical region that included the dorsal pons and neighboring cerebellum and dentate nuclei (p < 0.001). In this region, MD-MCI values were higher (p < 0.05) than for SD-MCI, but there was no difference between SD-MCI and N-MCI. Relative to the normal mean, metabolic values in this region were elevated for all three patient groups (p < 0.01). Significant increases in cingulate (BA 24/31) relative to both N-MCI and normal controls were also present in MD-MCI (p < 0.01) and SD-MCI (p < 0.001), with no difference in metabolic activity between the latter two groups.
Comparison of network scores
Prospectively computed PDCP scores for the N-, SD-, and MD-MCI PD groups, and for the healthy control cohort, are displayed in figure 3. PDCP expression was elevated above the normal mean in all three patient groups (p < 0.05, p < 0.01, and p < 0.001 for the N-, SD-, and MD-MCI groups, respectively). Network values increased stepwise across the three PD groups (F2,48 = 5.08, p < 0.01, analysis of variance). The significance level of this finding did not change with the inclusion of the UPDRS motor ratings in the model. Post hoc analysis revealed a significant increase in the MD-MCI group relative to the N-MCI group (p < 0.01), but not between SD-MCI and either the MD-MCI or N-MCI. Comparison of PDCP expression in patients with SD-MCI with and without amnestic features did not suggest a difference between these subgroups (amnestic: 1.8 ± 2.1; non-amnestic: 1.4 ± 0.9, p = 0.6).
Figure 3.
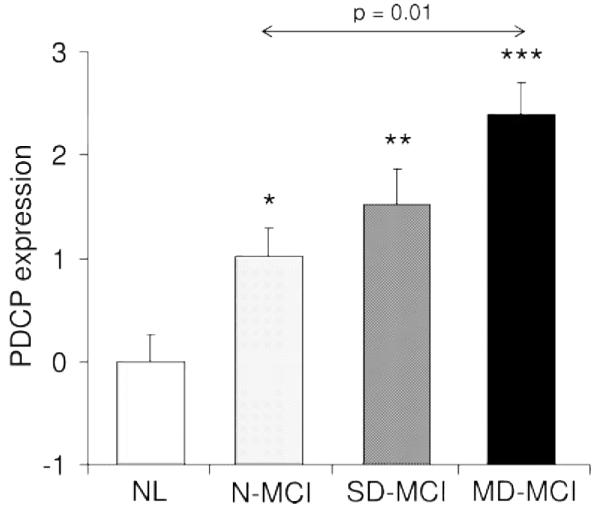
Plot of means, standard errors, and significances of the individual values for Parkinson disease-related cognitive pattern expression in multiple domain mild cognitive impairment (MD-MCI), single domain MCI (SD-MCI), without mild cognitive impairment (N-MCI), and age-matched controls
*Represents the significance of each group compared to the controls. *p < 0.05, **p < 0.005, ***p < 0.001.
DISCUSSION
The current study demonstrates that the clinical criteria for MCI possess a metabolic basis in PD. This supports a prior study5 that indicated that these criteria have predictive value for the development of dementia in patients with PD. In our sample of patients with moderately advanced PD, there was a clear distinction in several brain regions that differentiated PD patients with MD-MCI from those PD patients with normal cognition (N-MCI). Moreover, these findings are unlikely to reflect differences in motoric status or other factors such as age, education, or duration of illness, as these variables were controlled across the study populations.
Given that our subgroups were defined based on performance on neuropsychological measures, it is not surprising that there was a strong relationship between neuropsychological testing and MCI status. However, it is noteworthy that the relationship was not restricted to those measures utilized to assign subjects to the MCI groups. Although there were several measures that displayed a stepwise decline in performance from N-MCI to SD-MCI, and finally to MD-MCI, this relationship was not present in all tasks. Indeed, as in the imaging data, we found a consistent differentiation between MD-MCI and N-MCI, with SD-MCI performing more like MD-MCI in some situations and like N-MCI in others.
FDG PET imaging revealed metabolic reductions in the inferior parietal lobule (BA 7/40) and middle frontal gyrus (BA 8/9/46) of patients with MD-MCI compared to N-MCI and healthy control subjects. In general, these findings are consistent with previous studies demonstrating an association between cognitive performance in PD and frontal and parietal neural activity.6 In both of these cortical regions, the N-MCI and SD-MCI groups were not significantly different from healthy control values, suggesting that the reduction in cortical metabolism is specific to those patients with more significant cognitive impairment.
In addition to the cortical hypometabolism, we also found increased metabolism in the pons/cerebellum region. However, unlike the changes noted in the cerebral cortex, all three PD groups were found to exhibit abnormally elevated metabolic activity in this subcortical area, with stepwise increases in parallel with MCI severity. Increased metabolic activity in the cerebellum/dentate regions may represent a compensatory response to the loss of dopaminergic input to the striatum.6,20 Interestingly, in this study we found that these regions are abnormal even in patients with N-MCI, suggesting that the compensatory process may already be active prior to the onset of an identifiable cognitive impairment.
Increases in metabolic activity were also identified in the cingulate region. By contrast to the cerebellar/dentate metabolic increases, hypermetabolism in the cingulate region was not present in N-MCI, and was relatively constant across the SD- and MD-MCI groups. This region, known to be involved in monitoring errors,21 may also play a compensatory role in PD patients with cognitive dysfunction. Given that metabolic elevations in this region are similar for SD- and MD-MCI, it is likely that any compensation for cognitive decline provided by this region is insufficiently dynamic to cope with clinically advanced cognitive impairment. In other words, the cingulate reaches a ceiling past which it cannot further rise to compensate for advancing cognitive decline associated with the MD-MCI state.
The regions that metabolically differentiate the MCI patient groups are key elements of the previously validated PDCP metabolic network.6 However, unlike the individual frontal and parietal regions in which significant metabolic reductions were present only in patients with MD-MCI, elevated PDCP expression was evident in all PD groups, even in N-MCI subjects. Interestingly, in a recent longitudinal FDG PET study of patients with early stage PD,17 we found that abnormal PDCP expression was attained only at approximately 6 years following diagnosis, with values approaching those observed in the current SD-MCI group. While patients with PDCP expression in this range likely occupy an intermediate position between the N-MCI and MD-MCI groups, they do not necessarily represent a discrete diagnostic category from a metabolic standpoint. Indeed, the SD-MCI group appears to be both clinically and metabolically heterogeneous.
It has recently been demonstrated that among PD patients with SD-MCI, only those with nonamnestic features were likely to progress to dementia, at a rate comparable to that of MD-MCI.5 We found that patients with SD-MCI performing poorly on tests of memory (n = 6, mean PDCP score = 1.77) and visuospatial (n = 4, mean PDCP score = 1.77) functioning had scores closer to those of the MD-MCI group. By contrast, those with poor language (n = 3, mean PDCP score = 0.87) and executive (n = 2, mean PDCP score = 1.26) functioning had scores closer to the mean of the N-MCI group. Further studies of larger SD-MCI cohorts, with long-term clinical follow-up, will be needed to understand the potential implications of these findings.
The pathologic basis for the metabolic findings observed in PD patients with MCI is not fully understood. Recent studies suggest a relationship between the presence of cortical Lewy bodies in PD and the manifestation of dementia in this disorder.22 The cortical regions found to differentiate our MCI populations, i.e., the inferior parietal lobule and middle frontal gyrus, are not typically affected until Braak stage 5.23 Thus, the presence of significant metabolic reductions in these regions may be interpreted as indicated at a later stage of deterioration in patients with MCI. Alternatively, the relationship of cortical dysfunction to cognitive impairment may be explained by other mechanisms, including dysfunction of nondopaminergic neurotransmitter systems.6,24 For example, a multitracer PET imaging study of PD patients with dementia revealed cholinergic dysfunction in the frontal and temporo-parietal cortical regions similar to those identified in the current study.25 The combination of FDG PET with other imaging agents will be helpful in determining the basis for the observed metabolic abnormalities in PD patients with and without MCI.
The current study provides support for the use of MCI clinical criteria in PD. Given that PD patients with MCI are at increased risk to progress to actual dementia,5 the current findings suggest that PDCP expression may be a useful biomarker of prodromal dementia in this disorder. Several factors support the use in this manner. First, we found that PDCP is expressed even without the presence of a clinically defined cognitive disorder, suggesting its potential sensitivity to the earliest stages of this aspect of the neurodegenerative process. Further, PDCP expression is known to progress with advancing disease.17 In addition to being dissociable from the motor features of PD,6,17 PDCP expression is not altered by routine therapy for these disease manifestations.
ACKNOWLEDGMENT
The authors thank Aaron Edelstein and Shivani Rachakonda for assistance in data management and analysis and Toni Flanagan for editorial assistance and manuscript preparation.
Supported by NIH National Institute of Neurological Disorders and Stroke R01 35069 (D.E.) and the General Clinical Research Center at the North Shore-Long Island Jewish Health System (NIH RR MO1 018535).
GLOSSARY
- BA
Brodmann area
- BNT
Boston Naming Test
- CVLT
California Verbal Learning Test
- FDG
18F-fluorodeoxyglucose
- FWHM
full width at half maximum
- HVOT
Hooper Visual Organization Test
- MCI
mild cognitive impairment
- MD-MCI
multiple domain MCI
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- PDCP
PD-related cognitive pattern
- SD-MCI
single domain MCI
- SDMT
Symbol Digit Modality Test
- SPM
statistical parametric mapping
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VOI
volume of interest
- WCST
Wisconsin Card Sorting Test
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm. 2004;111:1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 2.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK: The CamPaIGN study. Brain. 2004;127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert T, Tang C, Eidelberg D. Assessing the progression of Parkinson’s disease: A metabolic network approach. Lancet Neurol. 2007;6:926–932. doi: 10.1016/S1474-4422(07)70245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Goldon C. Stoelting Company; Chicago: 1978. Stroop Color and Word Test. [Google Scholar]
- 10.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Psychological Assessment Resources, Inc.; Odessa: 1993. Wisconsin Card Sorting Test Manual: Revised and Expanded. [Google Scholar]
- 11.Smith A. Western Psychological Services; Los Angeles: 1982. Symbol Digit Modalities Test (SDMT) Manual (revised) [Google Scholar]
- 12.Army Individual Test Battery . War Department, Adjutant General’s Office; Washington, DC: 1944. Manual of directions and scoring. [Google Scholar]
- 13.Hooper HE. Western Psychological Services; Los Angeles: 1983. Hooper visual orientation test (VOT) [Google Scholar]
- 14.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test; Boston: 1978. [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. The Psychological Corporation; San Antonio: 1987. California Verbal Learning Test: adult version. [Google Scholar]
- 16.Beck A. Psychological Corporation; San Antonio, TX: 1987. Beck Depression Inventory: manual. [Google Scholar]
- 17.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spetsieris P, Ma Y, Dhawan V, Moeller JR, Eidelberg D. Highly automated computer-aided diagnosis of neurological disorders using functional brain imaging. Proc SPIE Medical Imaging. 2006;6144:61445M1–12. [Google Scholar]
- 19.Ma Y, Tang C, Spetsieres P, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: Test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mentis MJ, Dhawan V, Nakamura T, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003;60:612–619. doi: 10.1212/01.wnl.0000044154.92143.dc. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell RL. Anterior cingulate activity and level of cognitive conflict: explicit comparisons. Behav Neurosci. 2006;120:1395–1401. doi: 10.1037/0735-7044.120.6.1395. [DOI] [PubMed] [Google Scholar]
- 22.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson’s disease: a prospective, community-based study. Ann Neurol. 2005;58:773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 27.Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston Naming Test. Clin Neuropsychol. 2007;21:617–637. doi: 10.1080/13854040701339356. [DOI] [PubMed] [Google Scholar]