Abstract
The tumor-tropic properties of neural stem cells (NSCs) have been shown to serve as a novel strategy to deliver therapeutic genes to tumors. Recently, we have reported that the cardiac glycoside lanatoside C (Lan C) sensitizes glioma cells to the anticancer agent tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Here, we engineered an FDA-approved human NSC line to synthesize and secrete TRAIL and the Gaussia luciferase (Gluc) blood reporter. We showed that upon systemic injection, these cells selectively migrate toward tumors in the mice brain across the blood-brain barrier, target invasive glioma stem-like cells, and induce tumor regression when combined with Lan C. Gluc blood assay revealed that 30% of NSCs survived 1 day postsystemic injection and around 0.5% of these cells remained viable after 5 weeks in glioma-bearing mice. This study demonstrates the potential of systemic injection of NSCs to deliver anticancer agents, such as TRAIL, which yields glioma regression when combined with Lan C.
Keywords: Glioblastoma, Neural stem cells, Tumor-tropic, Tumor necrosis factor-related apoptosis-inducing ligand, Cardiac glycoside
Introduction
Glioblastoma (GBM, also known as grade IV astrocytoma) is the most common and most lethal malignant primary brain tumor in humans. Current multimodal treatments, including maximal safe surgical resection followed by radiation and chemotherapy, have been shown to provide modest clinical benefits. However, in less than 1 year, many patients continue to suffer from recurrent disease. The prognosis of patients remains dismal and GBM remains virtually incurable [1, 2]. A number of factors, especially the intrinsic and/or acquired resistance against conventional treatment, account for the poor response to therapy. It is now believed that more than one gene/protein is involved and multiple signaling pathways are implicated in the observed resistance. For example, the ability to recognize and repair DNA adducts, selection of pre-existent resistant cells in the paternal tumor, remodeling of mitochondrial electron transport in response to genotoxic stresses, upregulation of glucose transporter and drug metabolizing enzymes, loss of p53 function, and activation of the Akt pathway have all been reported to be responsible for the resistance to therapy in GBM [3–5].
One of the impediments to the treatment of brain tumors has been the degree to which they expand and infiltrate the normal brain, usually rendering them elusive to effective resection and therapies. Recently, many studies have demonstrated the unique inherent tumor-tropic properties of neural stem cells (NSCs) [6, 7]. These cells have multipotent capacity to differentiate into three major types of neural cell lineages: neurons, astrocytes, and oligodendrocytes. NSCs have been tested extensively to treat neurodegenerative diseases as a platform for cell replacement in animal experiments [8–10]. When NSCs are transplanted locally into animal models of brain neoplasia, these cells could be found near disseminated tumor beds far from their site of original transplantation [6, 11]. These observations galvanized a novel adjuvant strategy of NSC-based targeted delivery of anticancer agents, namely modifying NSC to stably express various therapeutic genes and to carry these products selectively to brain tumor foci [7, 12]. Promising preclinical results using this strategy have led to the first FDA-approved NSC-based therapy for the treatment of brain tumors. Most studies targeting gliomas delivered NSCs through intratumoral or intracerebral route [8, 13]. Recently, systemic injection such as intravenous (i.v.) route, which is minimally invasive and can be applied repeatedly allowing injected cells to home in on multiple tumors foci, has been explored for the delivery of stem cells to brain tumors [12, 14–16].
With a sequence homology to tumor necrosis factor, the death ligand TNF-related apoptosis-inducing ligand (TRAIL) has recently emerged as a promising anticancer agent because it preferentially induces apoptosis in a variety of transformed or malignant cells while sparing normal cells [17]. TRAIL is a transmembrane protein processed by cysteine protease to generate a soluble ligand that binds to death receptors (DR) and activating the extrinsic apoptotic pathway. Prior work has demonstrated that TRAIL has antitumor effect in both localized and disseminated glioma xenograft models [18, 19]. Recombinant human TRAIL and agonistic antibodies against DR4 or DR5 are being tested in preclinical studies and phase I or II clinical trials [20]. However, in vivo use of TRAIL is limited due to the rapidly developed tumor-resistance and its extremely short half-life in plasma. Repeated administration of large quantities of TRAIL to achieve clinical-relevant efficacy yields a significant systemic toxicity especially to the liver [20]. Recently, we have reported that the cardiac glycoside lanatoside C (Lan C) has the ability to restore the sensitivity to TRAIL-induced apoptosis in GBM cells [21, 22]. In this study, we evaluated the efficiency of systemic injection of the first FDA-approved NSC line (HB1.F3.CD) to target infiltrating glioma cells and deliver sustained active form of TRAIL, in combination with Lan C, using an invasive orthotopic GBM xenograft animal model.
Materials and Methods
Cell Culture, Transfection, and Reagents
U87, U251, LNZ308, HS683, and Gli36 human GBM cell lines, and HB1.F3.CD cell line (a human neural progenitor cell line derived from the ventricular zone of a fetal brain at 15 weeks of gestation and immortalized by an amphotropic, replication-incompetent retroviral vector containing v-myc) [6, 23], were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U of penicillin, and 0.1 mg/ml streptomycin at 37°C in a 5% CO2 humidified incubator. GBM stem-like cells (GSCs) used in this study were derived from surgical specimens obtained from GBM patients undergoing treatment at the Massachusetts General Hospital in accordance with the appropriate Institutional Review Board approval, and have been previously characterized [24, 25]. GSCs were grown as neurospheres in neurobasal medium supplemented with 3 mM L-glutamine, 1:100 N2, 1:50 B27, and 50 μg/ml primocin, 2 μg/ml heparin (all from Life Technologies, Carlsbad, CA, www.lifetechnologies.com), 20 ng/ml human recombinant epidermal growth factor (R & D Systems, Minneapolis, MN, www.rndsystems.com), and 20 ng/ml human recombinant basic fibroblast growth factor-2 (Peprotech, Rocky Hill, NJ, www.peprotech.com) (NBM E/F20). Spheroids were dissociated using NeuroCult chemical dissociation kit (Stem Cell Technologies, Vancouver, Canada, www.stemcell.com). Human astrocytes (HA) were obtained from ScienCell (Carlsbad, CA, www.sciencellonline.com). NSCs were transduced with the CSCW-Gluc-IRES-GFP lentivirus vector carrying an expression cassette for both Gaussia luciferase (Gluc) and the green fluorescent protein (GFP) separated by an internal ribosomal entry site, under the control of the cytomegalovirus promoter [26]. When expression of Firefly luciferase (Fluc) is desired, cells were transduced with the CSCW-Fluc-IRES-mCherry lentivirus vector to express Fluc and mCherry fluorescent protein [26]. Expression of secreted soluble TRAIL in NSCs was achieved by transducing these cells with the adeno-associated viral vector AAV2-sTRAIL carrying a transgene cassette for secreted soluble TRAIL consisting of amino acid 1–150 from human Flt3L, an isoleucine zipper domain, and the extracellular domain (amino acid 114–281) of the human TRAIL, under the control of cytomegalovirus/chicken beta actin promoter as we previously described (NSC-sTRAIL) [27]. As a negative control, NSCs were transduced with a similar vector expressing the secreted Vargula luciferase reporter (NSC-Vluc) [27]. AAV2 vectors were generated as described [27]. Lan C (Sigma-Aldrich, St. Louis, MO, www.sigmaaldrich.com) was reconstituted at 10 mg/ml in dimethyl sulfoxide (DMSO). Z-VAD-FMK and staurosporine were purchased from EMD Millipore (Billerica, MA).
Gluc Assay
Cells were plated in a 96-well clear bottom, black plates at a density of 3,000–5,000 cells/well. To monitor cell growth, aliquots of cell-free conditioned medium (10 μl) were transferred to a white 96-well plate. Gluc activity was determined using FlexStation3 microplate reader (Molecular Device, Sunnyvale, CA, www.moleculardevices.com) under “Flex” mode after adding 100 μl 40 μM of coelenterazine (Nanolight, Pinetop, AZ, www.nanolight.com), which is dissolved in acidified methanol and further diluted in phosphate buffered saline (PBS). To prepare blood samples, 5 μl of blood was withdrawn using a pipette tip from a small incision at the tail tip of conscious mice and immediately mixed with 1 μl of 20 mM EDTA. Gluc activity was then measured using a plate luminometer (Dynex, Richfield, MN, www.dynexproducts.com) after injecting 100 μl of 100 μM coelenterazine and acquiring signal over 10 seconds [28].
Coculture and In Vitro Migration Assay
To test the efficacy of NSC-sTRAIL, human GBM cells expressing Gluc were plated in the bottom well of the 96-well Transwell cell permeable supports plates with pore size of 0.4 μm (Corning, Corning, NY, www.corning.com). NSC-sTRAIL cells were seeded in the upper wells of the transwell plate. Tumor cell growth was monitored by assaying Gluc activity in the conditioned medium of the bottom wells.
The directed migration ability of NSCs to GBM spheres was determined using a modified transwell migration assay with pore size of 8.0 μm (Corning). NSCs expressing Gluc and GFP in NBM E/F20 were seeded in the upper well of the transwell plates at increasing amounts (100:1, 10:1, 1:1, and 1:10) with respect to tumor cells. 293T fibroblast cells were plated as a negative control. All experiments were conducted in quadruplicates.
Cell Cycle and Apoptosis Assays
Cells were washed, fixed in 70% ethanol before incubation in PBS containing 40 μg/ml propidium iodide (PI) (Sigma-Aldrich), and 200 μg/ml RNase A (New England Biolabs, Beverly, MA, www.neb.com). DNA content was analyzed by flow cytometry. Single-cell suspensions were stained with Annexin V-Cy5 (Biovision Inc., Milpitas, CA, www.biovision.com) and PI. Cells were then analyzed using BD LSR II flow cytometer (BD biosciences, San Jose, CA, www.bdbiosciences.com) as previously described [29]. Flow cytometry data were analyzed using Flowjo 7 (Flowjo, Ashland, OR, www.flowjo.com). Caspase 3, 7, and 8 activities were determined using caspase 3/7-Glo and caspase 8-Glo (Promega, Madison, WI, www.promega.com) as recommended by the manufacturer.
ELISA Assay for TRAIL
The concentration of TRAIL in cocultured conditioned medium released by NSC-TRAIL or in the blood from mice was measured using Quantikine Human TRAIL/TNFSF 10 Immunoassay Kit (R&D Systems) and the FlexStation3 microplate reader according to the manufacturer’s instructions.
In Vivo Tumor Model
All animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care following guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. To assess the migratory ability and therapeutic potential of NSC-sTRAIL in combination with Lan C in vivo, nude female mice were anesthetized (100 mg/kg ketamine and 5 mg/kg xylazine) and stereotactically implanted with 400 small spheres (~2 × 104 cells) of GBM8 cells expressing Fluc and mCherry in 2 μl PBS via a 30-gauge Hamilton syringe into the left forebrain (2.5 mm lateral and 0.5 mm anterior to bregma, at a 2.5 mm depth from the skull surface) to generate orthotopic xenografts. One–two weeks later, mice were randomized into four groups of 10 mice/group. NSC-Vluc cells (2 × 105) expressing Gluc and GFP (NSC-Vluc/Gluc) in 100 μl of PBS were injected intravenously through the tail vein of Group 1 on day 0 and day 14. These animals also received intraperitoneal (i.p.) injection of 100 μl 1% DMSO three times/week for 4 weeks. Group 2 received i.v. injection of 2 × 105 NSC-sTRAIL cells expressing Gluc and GFP (NSC-sTRAIL/Gluc) as well as i.p. dose of 100 μl 1% DMSO at similar time points. Group 3 received i.v. injection of 2 × 105 NSC-Vluc/Gluc and i.p. dose of 1 mg/kg b.wt. Lan C. Group 4 received i.v. injection of 2 × 105 NSC-sTRAIL/Gluc cells and i.p. dose of 1 mg/kg b.wt. Lan C. DMSO or Lan C injection was initiated 1 day post-NSCs injection (at day 1). NSCs injection was repeated 2 weeks post-first injection. Two days after injection of NSCs, two animals from each group were perfused with 4% paraformaldehyde under deep anesthesia. The brains were harvested, embedded in Microm Neg-50 frozen section medium (Thermo, Waltham, MA, www.fishersci.com), and stored at −80°C. Brains were cryosectioned coronally (15 μm) using a Microm HM-500M CryoStat (GMI Inc., Ramsey, MN, www.gmi-inc.com), mounted on slides. Tissues were stained with Hematoxylin-Eosin (H&E) per standard protocol. Intracranial distribution of mCherry-labeled GBM cells and GFP-labeled NSCs was assessed with fluorescent microscopy.
Bioluminescence Imaging
Bioluminescence imaging was performed using the Xenogen IVIS 200 Imaging System (PerkinElmer, Waltham, MA, www.perkinelmer.com). The system is composed of an imaging chamber, gas anesthesia system which is connected to an oxygen cylinder and isoflurane tank, and a highly sensitive cryogenically cooled charge-coupled device camera. Fresh luciferin (GOLD Biotechnology, St. Louis, MO, www.goldbio.com) solution is prepared by dissolving luciferin powder (25 mg/ml) in phosphate-buffered saline. Mice were injected intraperitoneally with 150 μg of luciferin per gram body weight and transferred into the image chamber. Imaging was acquired 10 minutes post-luciferin injection and the image intensity was quantitated using the Living Image software 3.0 from PerkinElmer as previously described [30].
Statistics
Comparisons of data in cell survival and viral yield assays were performed using a two-tailed Student’s t test (unpaired), ANOVA, and Tukey’s post hoc test as indicated. Survival analysis was conducted by Kaplan-Meier curves method using Graphpad Prism 5 (Graphpad software, La Jolla, CA, www.graphpad.com), and their comparison was determined by log rank test. p values of <.05 were considered significant.
Results
Expression of Soluble TRAIL by NSCs and Its Effect on GBM Cells
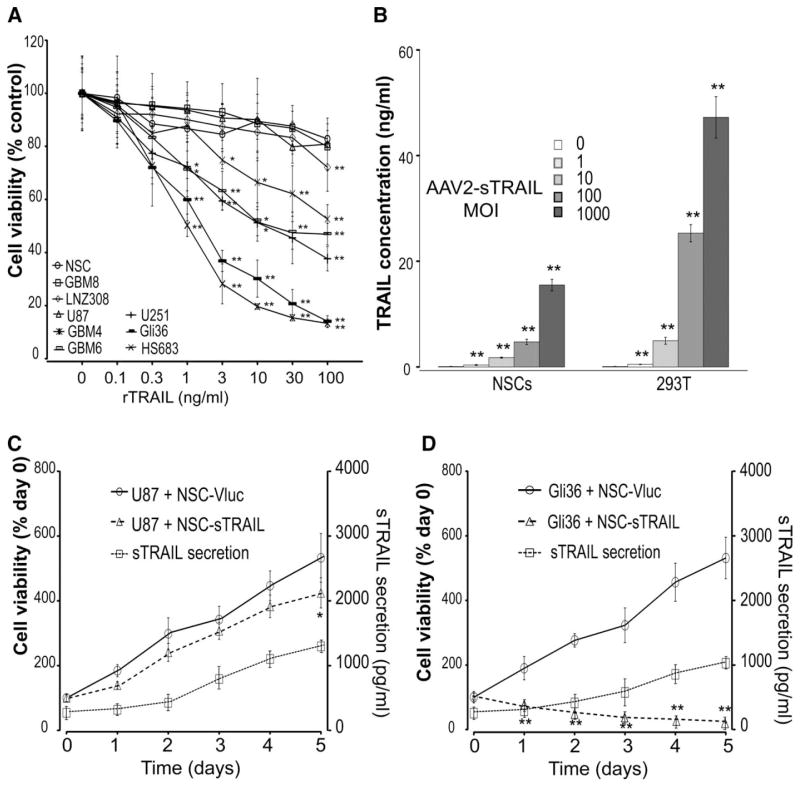
We first tested the effect of TRAIL on a panel of five glioma cell lines, U87, U251, LNZ308, HS683, and Gli36, as well as three primary GSC cultured as neurospheres (GBM4, GBM6, and GBM8), and HB1.F3.CD NSCs. All cells were first infected with a lentivirus vector to stably express the naturally secreted Gluc as a viability marker and GFP. Cells were treated with 0.1–100 ng/ml of recombinant human TRAIL and viability was measured by assaying an aliquot of the conditioned medium for Gluc activity over time. As previously described for some of these cell lines, Gli36 and HS683 were sensitive to sTRAIL with an IC50 around 2 ng/ml. U251, GBM4, and GBM6 revealed moderate sensitivity (IC50 ~ 10 ng/ml), whereas LNZ308, U87, and GBM8 cells were somewhat resistant to TRAIL [19, 21] (Fig. 1A). We then infected NSCs and 293T fibroblast cells with an AAV2 vector carrying the expression cassette of either secreted soluble TRAIL or secreted Vargula luciferase (as a control) using different multiplicity of infection (1, 10, 100, or 1,000). NSCs were resistant to sTRAIL that is secreted by themselves, and continuously released high concentration of sTRAIL into the culture media (1–15.5 ng/ml) in a dose-dependent manner as revealed by ELISA (Fig. 1B). We then tested the effect of NSC-sTRAIL or NSC-Vluc on two selected GBM cell lines-expressing Gluc using a trans-well system. In wells containing TRAIL-resistant U87 cells, sTRAIL production increased from 290 pg/ml to 1,028 pg/ml in 5 days; U87 cell viability steadily increased during this culture period (4.2-fold increase), which was slightly slower compared to U87 cells cocultured with NSC-Vluc control cells (5.3-fold increase; Fig. 1C). Conversely, the TRAIL-sensitive Gli36 cells cocultured with NSC-sTRAIL revealed a similar increase in sTRAIL production, but a 78.7% reduction in cell viability over 5 days (Fig. 1D); Gli36 cells cocultured with NSC-Vluc control showed a 5.3-fold increase in viability over the same time period. No effect was observed on GBM cells cocultured with NSC-Vluc. These results confirm that sTRAIL is being secreted by NSCs in an active form.
Figure 1.
Expression of soluble TRAIL in NSCs and its effect on glioblastoma cells. (A): 3 × 103 U87, U251, LNZ308, HS683, and Gli36 human glioma cell lines, HB1.F3.CD normal NSCs, as well as GBM4, GBM6, and GBM8 primary glioblastoma (GBM) stem-like cells were treated with 0–100 ng/ml of recombinant TRAIL (rTRAIL). Cell viability was monitored after 3 days by assaying Gluc activity in the medium. Data presented as the percentage cell viability ± SD (n = 8; *, p <.05; **, p <.01 vs. no treatment by two-way ANOVA and Tukey’s post hoc test) in which the control DMSO group is set at 100%. (B): NSCs or 293T cells were infected with AAV2-sTRAIL vector at different MOI. Three days later, conditioned medium were assayed for TRAIL secretion using ELISA. Data presented as mean TRAIL concentration (ng/ml) ± SD (n = 4; **, p <.01 vs. cells transfected with Vluc). (C, D): 3 × 103 U87 (C) or Gli36 (D) glioma cells expressing Gluc were seeded in the lower wells of a 96-well 0.4 μm transwell plate. 103 NSC-sTRAIL or NSC-Vluc (control) cells were seeded in the upper wells. Aliquots of conditioned medium were assayed daily for TRAIL secretion using ELISA. GBM cell viability was also monitored by assaying Gluc activity in the conditioned medium over time. Left y-axis: Normalized Gluc RLU as cell viability (day 0 set at 100) ± SD (n = 8; *, p <.05; **, p <.01 cells cocultured with NSC-Vluc vs. cells cocultured with NSC-sTRAIL by two-way ANOVA). Right y-axis: TRAIL concentrations in the medium presented as mean sTRAIL concentration (pg/ml) ± SD. Abbreviations: GBM, glioblastoma; MOI, multiplicity of infection; NSC, neural stem cell; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Effect of Lan C on Glioma Cells Cocultured with NSC-sTRAIL
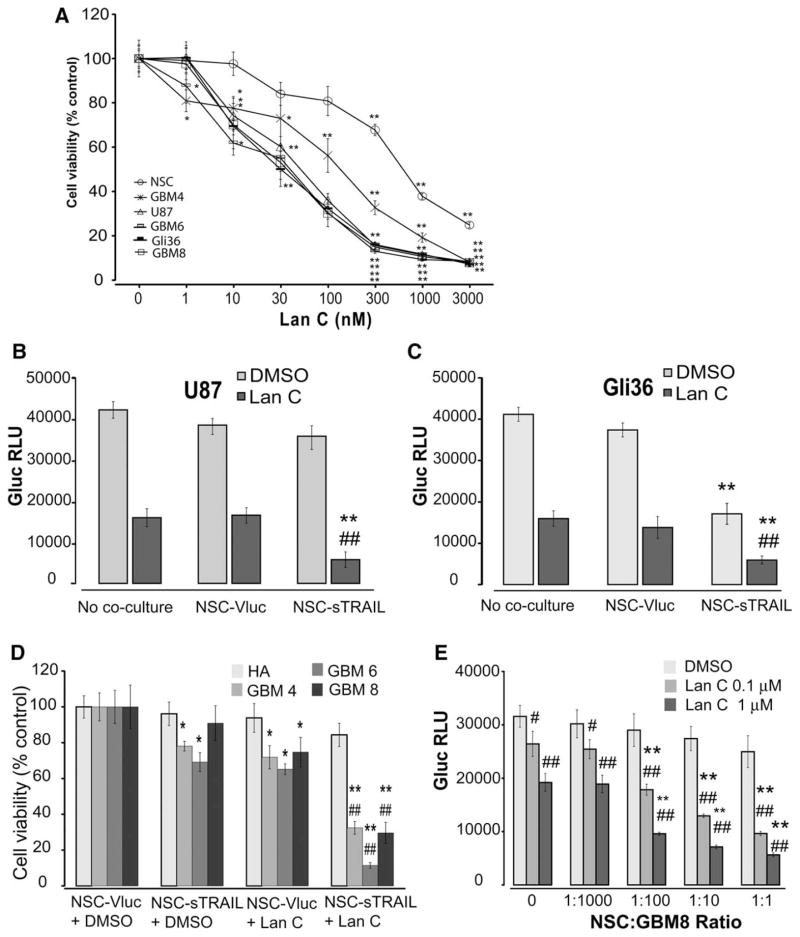
To investigate the potential of Lan C in sensitizing glioma cells to sTRAIL secreted by NSCs, we first tested the sensitivity of Lan C on a panel of two glioma cell lines (U87 and Gli36) and three primary GSCs cultured as neurospheres (GBM4, GBM6, and GBM8) as well as NSCs. Lan C significantly inhibited cell growth in all cancer cells with an IC50 around 50–150 nM, compared to an IC50 of 474 nM in NSCs at 48 hours post-treatment (Fig. 2A). In both TRAIL-resistant U87 cells and TRAIL-sensitive Gli36 cells, Lan C (0.25 μM) alone decreased cell viability by around 60% over 48 hours, as compared to cells treated with DMSO control (Fig. 2B). When Lan C (0.25 μM) was applied to U87 cells cocultured with NSC-sTRAIL (1:10 ratio of NSC/GBM cells), a significant cytotoxic effect was observed as compared to NSC-sTRAIL monotherapy (81% cell death vs. 9.3%; Fig. 2B). Conversely, NSC-sTRAIL-induced cytotoxicity on Gli36 cells was further enhanced by combination of Lan C (Fig. 2C). These results suggest that Lan C may sensitize the resistant GBM cells to TRAIL therapy.
Figure 2.
Effect of NSC-sTRAIL in combination with Lan C on GBM cells and stem-like cells. (A): 3 × 103 NSCs, U87, Gli36, GBM4, GBM6, or GBM8 GSCs expressing Gluc were treated with 0–3,000 nM of Lan C. Cell viability was monitored 3 days later by assaying Gluc activity in the medium. Data presented as the mean Gluc RLU/ml ± SD (n = 8; *, p <.05; **, p <.01 vs. no treatment by two-way ANOVA). (B, C): 3 × 103 U87-Guc cells (B) or Gli36-Gluc cells (C) were cocultured with 300 NSC-sTRAIL cells and treated with Lan C (0.25 μM) or DMSO. GBM cell viability was monitored using Gluc assay 48 hours later (**, p <.01 vs. control; ##, p <.01 vs. Lan C alone using ANOVA and Tukey’s post hoc test). (D): 3 × 103 GBM4, GBM6, or GBM8 GSCs (~60 small neurospheres), or HA expressing Gluc were seeded were cocultured with 300 NSC-sTRAIL cells and/or 0.1 μM Lan C. Cell viability was monitored by measuring Gluc activity in the medium 48 hours post-treatment. Data presented as the mean relative Gluc RLU ± SD (n = 8) in which cells cocultured with NSC-Vluc control is set at 100 (*, p <.05 and **, p <.01 vs. control; ##, p <.01 vs. Lan C alone using ANOVA and Tukey’s post hoc test). (E): 3 × 103 GBM8-Gluc cells (~60 small neurospheres) were seeded in lower well of a 96-well 0.4 μm Transwell plate in NBM E/F20. Different amounts (3, 30, 300, or 3,000) of NSC-sTRAIL cells were seeded in the upper wells. After 12 hours, GBM cells were treated with DMSO (control), 0.1, or 1 μM of Lan C and viability was monitored 48 hours later using the Gluc assay. Data presented as the mean Gluc RLU/ml ± SD (n = 8; **, p <.01 vs. no Lan C; #, p <.05; ##, p <.01 vs. no coculture with NSC cells). Abbreviations: DMSO, dimethyl sulfoxide; GBM, glioblastoma; HA, human astrocytes; NSC, neural stem cell; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
To confirm the specificity of NSC-sTRAIL and Lan C to tumor cells, we compared their effect on three primary GSCs cultured as neurospheres and normal HA. Cocultures of NSC-sTRAIL for 72 hours had a moderate effect on GBM4 and GBM6 cells (22.9% and 31.8% cell death, respectively) and minimal effect on GBM8 cells (Fig. 2D). Lan C (0.25 μM) alone induced 25%–35% decrease in cell viability in all three GSC cultures. Conversely, the combination of Lan C with NSC-sTRAIL eliminated >70% of all three GSC cultures. Importantly, none of the mono or combined therapy had a significant effect on normal astrocytes, suggesting that Lan C acts selectively against tumor cells (Fig. 2D).
We then evaluated different amounts of Lan C on GBM8 cells cocultured with different ratios of NSC-sTRAIL. Lan C resulted in a dose-dependent decrease in viability with median effective concentration of approximately 0.1 μM. Interestingly, low sTRAIL (1:100 of NSC-sTRAIL/GBM8 ratio) and high sTRAIL (1:10 ratio) amounts in combination with Lan C had a similar effect on GBM8 cell viability (Fig. 2E). These results reveal that a very low dose of Lan C is sufficient to overcome TRAIL resistance, which also synergizes with low amounts of sTRAIL.
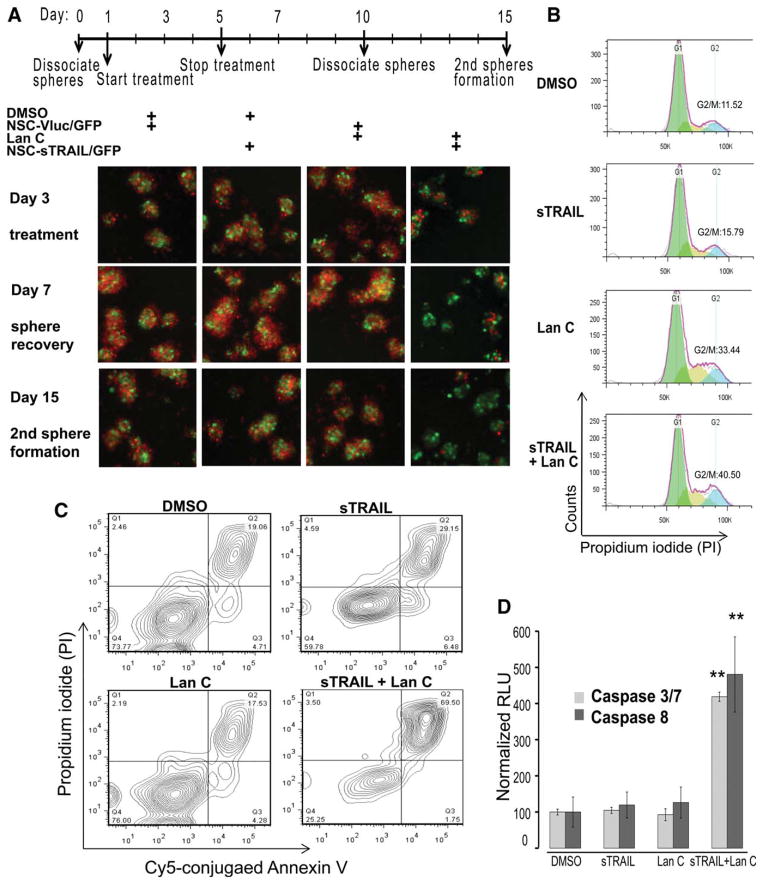
We also assessed the migratory potential of NSCs to GBM8 cells as compared to human embryonic kidney 293T cells in vitro. GBM8 cells expressing Fluc and mCherry fluorescent protein grown as neurospheres were seeded in the lower well of an 8 μm transwell plate and NSCs or fibroblast cells expressing either Vluc and GFP (NSC-Vluc/GFP) control, or both sTRAIL and GFP (NSC-sTRAIL/GFP) were seeded in the upper well in NBM E/F20. In contrast to 293T cells, which remained localized to the area of initial seeding (Supporting Information Fig. S1), NSCs penetrated through the membrane rapidly and interspersed throughout the glioma neurospheres (Fig. 3A). These cells were then treated with DMSO (control) or 0.1 μM of Lan C (day 1) and lower wells were imaged on day 3 for mCherry and GFP expression. Treatment was stopped by refreshing the culture medium and neurosphere recovery was imaged at day 7. Only wells in which NSC-sTRAIL/GFP have migrated to GBM8 neurospheres and treated with Lan C showed toxic effect as observed by a decrease in mCherry fluorescence (Fig. 3A). To evaluate GBM8 cells renewal, neurospheres from lower well were dissociated into single cells and replated in a new well to measure secondary neurosphere formation. In contrast to NSC-sTRAIL/GFP or Lan C monotreatment, coculture with NSC-sTRAIL/GFP and treatment with Lan C strongly inhibited neurosphere formation with characteristic apoptotic features including cell detachment, shrinkage, and generation of apoptotic bodies (Fig. 3A).
Figure 3.
NSC-sTRAIL in combination with Lan C induce apoptosis and inhibit neurosphere recovery in primary glioblastoma stem-like cells. (A): 103 GBM8 cells (~20 small neurospheres) expressing Fluc and mCherry fluorescent protein were seeded in each lower well of 8 μm Transwell plate in NBM E/F20. 103 NSC-Vluc or NSC-sTRAIL cells expressing Gluc and GFP were seeded in the upper well. After 12 hours (Day 1), GBM cells were treated with DMSO or 0.1 μM Lan C. The lower wells were imaged on day 3 for mCherry and GFP expression using fluorescence microscopy. At day 5, treatment was stopped and at day 7, sphere recovery was imaged. Neurospheres were then dissociated to single cells at day 10 and replated to monitor secondary neurosphere formation at day 15. Shown are representative overlay images of GBM8 cells (red), and NSC-Vluc or NSC-sTRAIL cells (green). The side length of a square = 500 μm. (B): 103 GBM8 cells were treated with conditioned medium-containing sTRAIL, Lan C, or both. Three days later, cells were fixed, stained with PI, and analyzed by flow cytometry. Fractions of cells in G1 (green), S (brown), and G2/M (blue) phases are determined using Flowjo; the percentage of cells in G2/M phase is shown. (C): GBM8 cells were treated with Lan C, sTRAIL, or both. Seventy-two hours later, cells were stained with Cy5-conjugated Annexin-V and PI and analyzed using flow cytometry. Numbers in the right upper quadrants indicate the percentage of advanced apoptotic/necrotic cells. (D): 3 × 103 GBM8 cells were cocultured with 300 NSC-Vluc or NSC-sTRAIL cells and/or treated with 0.1% DMSO or 0.1 μM Lan C. Activities of caspase 3/7 and 8 were measured 48 hours later. Caspase activities in cells cocultured with NSC-Vluc and treated with 0.1% DMSO were arbitrarily set at 100 (**, p <.01 vs. control). Data shown in (A)–(C) are representative of three independent experiments. Abbreviations: DMSO, dimethyl sulfoxide; NSC, neural stem cell; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
To evaluate the effect of combined sTRAIL and Lan C therapy on GSCs cell arrest, we examined cell cycle phase distribution using PI staining on ethanol-fixed cells. Treatment of GBM8 GSCs with conditioned medium from 293T-sTRAIL cells did not significantly alter cell phase distribution. GSCs treated with 0.25 μM Lan C induced a substantial G2/M arrest (33%), whereas the combined therapy showed a slight further increase in cell arrest (40%, Fig. 3B). Uptake of PI by the cells reflects the plasma membrane integrity and indicates necrosis when observed in the absence of evidence for apoptosis such as externalization of phosphatidylserine which can be detected by Annesin V staining. Double staining of live cells with Cy5-conjugated Annexin V and PI revealed that PI uptake was not significant in cells treated with Lan C, whereas sTRAIL alone caused a slight increase in double-stained cells (29%). Conversely, the majority of cells treated with the combination of sTRAIL and Lan C became necrotic or late apoptotic (70%, Fig. 3C). These results suggest that treatment with Lan C makes GSCs more susceptible to TRAIL-induced apoptosis through G2/M arrest.
To confirm that the reduction in cell viability in response to the combined sTRAIL and Lan C therapy is mediated by apoptosis, we assessed caspase-3/7 and 8 activities 24 hours post-treatment of GSCs with low dose of Lan C (0.25 μM) and conditioned medium from 293T-sTRAIL cells. Very low-to-no caspase activation was observed in GBM8 cells treated with sTRAIL or Lan C alone. However, around fivefold increase in caspase 3/7 and caspase 8 activities was observed in GBM8 cells treated with sTRAIL and Lan C (Fig. 3D). These results confirm that Lan C is sensitizing GBM cells to sTRAIL in an apoptosis-mediated manner.
Intravenous Injection of NSC-sTRAIL in Combination with Lan C Induces Invasive Glioma Regression In Vivo
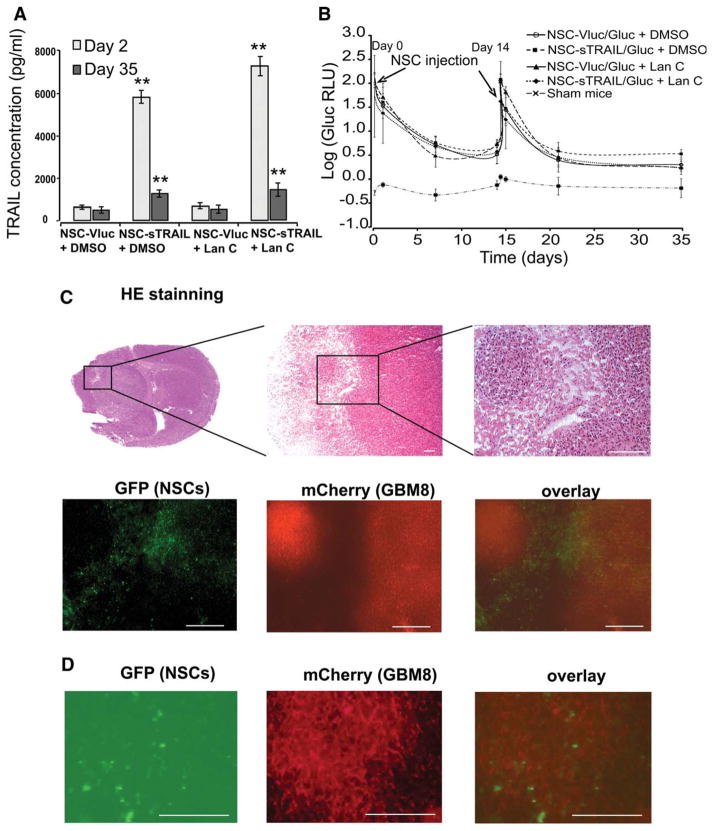
To determine the behavior of NSCs upon i.v. administration and whether these cells are capable of targeting brain tumors and deliver active form of TRAIL, invasive glioma model was first established in mice by intracranial injection of GBM8 neurospheres expressing Fluc and the mCherry fluorescent protein in the left forebrain. One week postimplantation, mice were divided randomly into four different groups (n = 10) which received either NSC-Vluc/Gluc + DMSO, NSC-sTRAIL/Gluc + DMSO, NSC-Vluc/Gluc + Lan C, or NSC-sTRAIL/Gluc + Lan C. Intraperitoneal injection of DMSO (100 μl of 1% DMSO) or Lan C (1 mg/kg b.wt.) was initiated 1 day post-NSCs injection and was repeated three times/week for 4 weeks. At different time points, blood was withdrawn and analyzed for Gluc level to track survival of circulating NSCs and for TRAIL secretion. One day post-NSCs injection, mice in both NSC-sTRAIL/Gluc + DMSO or NSC-sTRAIL/Gluc + Lan C groups had around 6 ng/ml of TRAIL blood levels. NSCs injection was repeated 14 days post-first NSCs injection. Survived NSCs continued to release relatively high amount of TRAIL into the blood even after 5 weeks postinjection (around 1.2 ng/ml; Fig. 4A). Similarly, in all groups (except the sham group), the Gluc blood level had similar trend which was very high 1 hour post-NSCs injection and dropped by 30% over 24 hours, reaching 0.5% of its original value at day 7, after which it remained constant over 5 weeks. These results show that around 0.5% of NSCs survived and are circulating (or presumably at the tumor site) but not multiplying (Fig. 4B).
Figure 4.
NSCs injected systemically migrate and distribute throughout infiltrating glioblastoma tumors across the blood-brain barrier. Athymic nude mice were stereotactically implanted with 2 × 105 GBM8 cells (~4,000 neurospheres) expressing Fluc and mCherry into the left forebrain. One week later, mice received intravenous injection with 2 × 105 cells of either NSC-sTRAIL/Gluc or NSC-Vluc/Gluc (day 0). NSCs injection was repeated 2 weeks later (day 14). Each group of mice was then divided into two subgroups receiving an i.p. injection (three times per week for 4 weeks) of either 100 μl 1% DMSO or Lan C (1 mg/kg b.wt.). DMSO or Lan C injection was initiated 1 day post-first NSCs injection (day 1). (A): sTRAIL concentration in blood was quantified using ELISA 1 day and 5 weeks after the first injection of NSCs. Data presented as the mean sTRAIL concentration (pg/ml) ± SD (n = 4). **, p <.01 versus control (NSC-Vluc). (B): The fate of injected NSCs was monitored using the Gluc blood assay twice per week. Data presented as the mean value of log (Gluc RLU) ± SD (n = 6). (C): H&E staining showing the invasive foci of glioblastoma cells and fluorescence photomicrograph of GFP (NSC-sTRAIL cells) and mCherry (GBM8 cells) confirms the extensive migration and distribution of NSCs throughout the infiltrating glioblastoma. (D): Higher magnification fluorescence of GFP (NSC-sTRAIL cells) and mCherry (GBM8 cells) confirming the colocalization of NSCs throughout the infiltrating glioblastoma. In (C) and (D), scale bar = 100 μm. Abbreviations: DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; NSC, neural stem cell; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
H&E staining and fluorescence mCherry microscopy on brain sections demonstrated that GBM8 neurospheres invaded the normal brain and migrated invasively away from the injection site (Fig. 4C). GFP fluorescence showed that NSCs migrated toward glioma tumors as well as infiltrating tumor cells upon i.v. injection. NSCs were distributed throughout the intracerebral tumor mass (Fig. 4D), but were not found in surrounding normal-appearing brain tissue, elsewhere in the brain, or in brains of control animals (NSC-injected mice without intracerebral gliomas or tumor-bearing mice in the absence of NSC-injection; data not shown).
It has been demonstrated that following administration, Lan C is transformed into digoxin and its metabolites by deglucosylation [31]. Digoxin has been shown to penetrate across the blood-brain barrier [32]. In the left forebrains (where GBM tumor was formed) of animals treated with Lan C, a trace amount of Lan C as well as digoxin-like breakdown products was detected using high performance liquid chromatography/mass spectrometry showing that Lan C penetrated the brain tumor (Supporting Information Fig. S2).
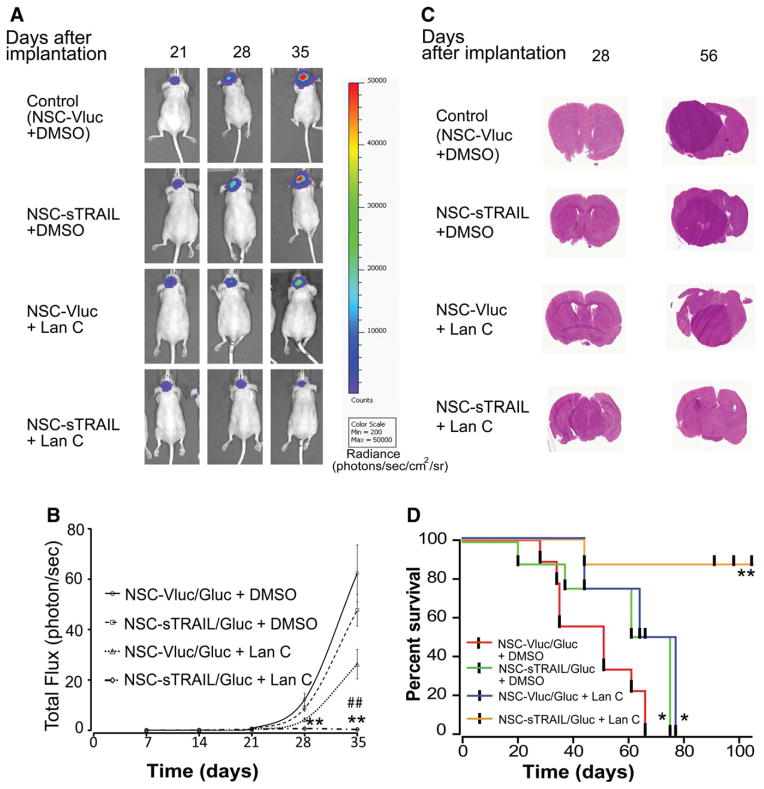
In vivo Fluc bioluminescence imaging was used to monitor tumor volume once per week and animals were sacrificed upon showing 20% loss of body weight. The NSC-Vluc/Gluc + Lan C and NSC-sTRAIL/Gluc + DMSO groups had a modest therapeutic effect as compared to NSC-Vluc/Gluc + DMSO control (Fig. 5A). Conversely, mice injected with NSC-sTRAIL/Gluc and treated with Lan C resulted in a significant tumor regression (Fig. 5A). Tumor-associated Fluc signal (total flux) 5 weeks post-treatment in NSC-Vluc/Gluc + DMSO control group increased by 62,322% ± 11,275%; NSC-sTRAIL/Gluc + DMSO or NSC-Vluc/Gluc + Lan C groups increased by 47,719% ± 6,365% and 26,251% ± 5,861%, respectively; however, the NSC-sTRAIL/Gluc + Lan C increased only 369% ± 127% (Fig. 5B). These results were confirmed by H&E staining of the corresponding brain sections (Fig. 5C). Kaplan-Meier survival analysis revealed that the combined treatment of NSC-sTRAIL/Gluc + Lan C significantly prolonged mouse survival (median survival time >105 days, p value = .0075 by Log-rank [Mantel-Cox] test), compared to the group receiving control NSC-Vluc/Gluc + DMSO (median survival time = 51 days), and groups receiving NSC-sTRAIL/Gluc + DMSO (median survival time = 61 days, p value = .0414 by Log-rank test) or NSC-Vluc/Gluc + Lan C (median survival time = 68 days; p value = .0198 by Log-rank test) (Fig. 5D). These data collectively suggest that NSCs exhibit tropism to disseminated glioma tumor sites upon i.v. injection and are detected as far out as 35 days postinjection. The in vitro and in vivo studies clearly show the ability of these NSCs expressing sTRAIL to target GBM and induce tumor regression upon injection of the cardiac glycoside Lan C.
Figure 5.
Systemic injection of NSC-sTRAIL in combination with Lan C induces invasive glioblastoma (GBM) tumor regression in vivo. Mice were implanted with 2 × 104 GBM8 cells (~400 neurospheres) expressing Fluc and mCherry into the left forebrain. One and three weeks post-tumor cells implantation, mice received intravenous injection with 2 × 105 cells of either NSC-sTRAIL or NSC-Vluc (control) expressing Gluc. Each group of mice was divided into two subgroups which received an i.p. injection (three times per week over 4 weeks) of either DMSO or Lan C (1 mg/kg b.wt.). (A): In vivo Fluc bioluminescence imaging was performed once/week to monitor tumor growth. Representative images at 21, 28, and 35 days post-GBM cells implantation are shown. Pseudocolor represents radiance intensity of the tumors (photons/second per cm2 per surface radiance). (B): Tumor-associated photon counts were quantified using an IVIS imaging system software. Data presented as the mean of total flux of Fluc (photons/second) ± SD (n = 10; **, p <.01 vs. control; ##, p <.01 vs. Lan C alone by ANOVA and Tukey’s post hoc test). (C): H&E staining of representative brain sections the different treatment groups. (D): Kaplan-Meier survival curves of orthotopic GBM8 bearing athymic nude mice with different treatment strategies. *, p <.05 versus NSC-Vluc/Gluc + DMSO; **, p <.01, NSC-sTRAIL/Gluc + Lan C versus NSC-Vluc/Gluc + DMSO, NSC-Vluc/Gluc + Lan C, or NSC-sTRAIL/Gluc + DMSO. Abbreviations: DMSO, dimethyl sulfoxide; NSC, neural stem cell; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Discussion
With current treatment, tumor recurrence is highly probable in GBM patients. Experimental TRAIL therapy was severely hurdled by its short in vivo half-life, systemic toxic effects, and quickly developed resistance. Substantial changes in treatment strategies are required to overcome these problems [20, 33]. Using highly malignant and invasive human glioma model with correlative neuropathology and using real-time imaging techniques, we have demonstrated that NSCs injected i.v. are able to cross the blood-brain barrier, target invasive gliomas, and secrete soluble TRAIL at the tumor site. When combined with Lan C, these sTRAIL-expressing cells produce a remarkable antitumor effect in vivo, overcoming resistance of this anticancer agent.
The ideal cell-based delivery vehicle for cancer therapy must exhibit four important characteristics: (a) tumor-selective migratory capacity; (b) being receptive to in vitro genetic manipulation; (c) being able to carry the therapeutic agent while protecting it from the host immune system; (d) the resistance of the cell used to cancerous transformation. NSCs are good candidates targeting CNS malignancies due to their inherent ability to migrate toward sites of pathology, including tumors in the brain [10]. However, fast growing or immortalized stem cells with a higher degree of multipotency tumor formation increases the probability of tumor formation; the incorporation of the cytosine deaminase gene into the NSCs could potentially alleviate this problem by eradication of these cells with 5-fluorocytosine in case of tumor formation [6]. The precise mechanism governing the tumor-tropic properties of NSCs is not fully understood. One hypothesis is that these cells migrate to the tumor site to repair damaged tissue. It is also possible that gradients of agents such as chemokines, cytokines, and proangiogenic growth factors produced in the tumor microenvironment may act as chemoattractants for NSCs [7]. Similar to ischemia, hypoxia is a critical factor in gliomas resulting in HIF-1α-mediated upregulation of numerous proangiogenic factors and chemoattractants [34].
A number of therapeutic systems have been evaluated in preclinical studies using NSCs, including prodrug-activating enzymes, immunomodulatory cytokines, and proteins with antiangiogenic activity [7, 11, 13]. We chose to use the human HB1.F3.CD line since it is well-characterized and the only FDA-approved NSCs for brain tumor therapy [8, 13]. No signs of local or systemic toxicity in NSCs-treated mice have been reported [11, 13]. Our interest is to determine the fate and migratory potential of NSCs to widely expanding and advanced invasive tumors in the brain upon intravenous injection. We showed that the engineered NSCs are resistant to the cytokine TRAIL that they produce themselves, migrate efficiently to glioma tumors and invasive cells across the blood-brain barrier, and provide a robust antiglioma response when combined with the cardiac glycoside Lan C. Recently, crosstalk between the membrane and intracellular pathways has been observed as an amplification loop to overcome the acquired resistance to anticancer agents [35]. We have previously shown that Lan C on its own induces an alternative tumor cell death (not by apoptosis) as well as upregulation of DR5 protein on tumor cell surface; thus, the combined caspase-independent cell death (Lan C) and induced apoptotic cell death (TRAIL) may help achieve a maximal therapeutic benefit [21].
Although the initial discovery and pharmacological development of cardiac glycosides are unrelated to oncology and are widely used, several clinical studies have suggested that cardiac glycosides may affect the clinical course of cancer [36]. Furthermore, these drugs have shown to provide neuroprotection against ischemic stroke in preclinical studies [37, 38], and have been confirmed in humans in a study of 28 years that showed a low mortality of stroke in 1,150 cardiac patients taking these drugs (available at: http://www.medical-newstoday.com/releases/47200.php; and http://www.infarct-combat.org/heartnews-16.html; accessed on April 21, 2014). Altogether, these data suggest that cardiac glycosides penetrate the brain efficiently and therefore the use of Lan C in combination with NSC-sTRAIL could be ideal for the treatment of brain tumors.
Conclusions
In summary, here we report that the cardiac glycoside Lan C sensitizes GBM cells to TRAIL-induced apoptosis in an invasive mouse orthotopic GSCs model. Our results show the potential of NSCs injected systemically as an effective delivery system to target and disseminate therapeutic agents to brain tumors. However, secreted TRAIL alone is not sufficient to reduce tumor burden but combination of NSC-sTRAIL with Lan C can effectually treat human GBM. We believe that this study provides a potential for clinical translation since the different components including the NSC line used, TRAIL, and the cardiac glycoside have been tested in clinical trials separately. This approach offers an opportunity to achieve brain tumor recession, and is likely applicable for the treatment of other cancers.
Acknowledgments
This work was supported by grant from NIH/NINDS 1R01NS064983 (BAT). We would like to acknowledge the MGH Neuroscience Image Analysis Core (for confocal microscopy), and the MGH Vector Core (for producing the viral vector) supported by NIH/NINDS P30NS04776 as well as 1S10RR025504 Shared Instrumentation grant for the IVIS imaging system that was used to acquire imaging data. We thank Drs. Samuel Rabkin and Hiroaki Wakimoto for providing the different GSCs used in this study and Mark de Goojier for technical assistance.
Footnotes
Author Contributions
J.T.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; S.H.: collection and/or assembly of data, manuscript writing, and final approval of manuscript; C.E.B.: provision of study material or patients, data analysis and interpretation, manuscript writing, and final approval of manuscript; B.A.T.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support, and administrative support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Rinne ML, Wykosky J, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: Multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14:92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squatrito M, Holland EC. DNA damage response and growth factor signaling pathways in gliomagenesis and therapeutic resistance. Cancer Res. 2011;71:5945–5949. doi: 10.1158/0008-5472.CAN-11-1245. [DOI] [PubMed] [Google Scholar]
- 6.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bovenberg MS, Degeling MH, Tannous BA. Advances in stem cell therapy against gliomas. Trends Mol Med. 2013;19:281–291. doi: 10.1016/j.molmed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Aboody K, Capela A, Niazi N, et al. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.De Feo D, Merlini A, Laterza C, et al. Neural stem cell transplantation in central nervous system disorders: From cell replacement to neuroprotection. Curr Opin Neurol. 2012;25:322–333. doi: 10.1097/WCO.0b013e328352ec45. [DOI] [PubMed] [Google Scholar]
- 10.Noble M. Can neural stem cells be used to track down and destroy migratory brain tumor cells while also providing a means of repairing tumor-associated damage? Proc Natl Acad Sci USA. 2000;97:12393–12395. doi: 10.1073/pnas.97.23.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK, Kim SU, Park IH, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 12.Kim SM, Oh JH, Park SA, et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28:2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 13.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: Preclinical studies. Sci Transl Med. 2013;5:184ra159. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kono TM, Sims EK, Moss DR, et al. Human adipose derived stromal/stem cells (hASCs) protect against STZ-induced hyperglycemia; analysis of hASC-derived paracrine effectors. Stem Cells. 2014 doi: 10.1002/stem.1676. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz MZ, Gutova M, Lacey SF, et al. Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: Implications for clinical use. Stem Cells Transl Med. 2013;2:983–992. doi: 10.5966/sctm.2012-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Coranguez M, Segovia J, Lopez-Ornelas A, et al. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS One. 2013;8:e60655. doi: 10.1371/journal.pone.0060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 18.Nagane M, Pan G, Weddle JJ, et al. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 19.Shah K, Tang Y, Breakefield X, et al. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22:6865–6872. doi: 10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
- 20.Stuckey DW, Shah K. TRAIL on trial: Pre-clinical advances in cancer therapy. Trends Mol Med. 2012;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badr CE, Wurdinger T, Nilsson J, et al. Lanatoside C sensitizes glioblastoma cells to tumor necrosis factor-related apoptosis-inducing ligand and induces an alternative cell death pathway. Neuro Oncol. 2011;13:1213–1224. doi: 10.1093/neuonc/nor067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badr CE, Wurdinger T, Tannous BA. Functional drug screening assay reveals potential glioma therapeutics. Assay Drug Dev Technol. 2011;9:281–289. doi: 10.1089/adt.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flax JD, Aurora S, Yang C, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 24.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: Establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14:132–144. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wurdinger T, Badr C, Pike L, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire CA, Bovenberg MS, Crommentuijn MH, et al. Triple bioluminescence imaging for in vivo monitoring of cellular processes. Mol Ther Nucleic Acids. 2013;2:e99. doi: 10.1038/mtna.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr CE, Van Hoppe S, Dumbuya H, et al. Targeting cancer cells with the natural compound obtusaquinone. J Natl Cancer Inst. 2013;105:643–653. doi: 10.1093/jnci/djt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire CA, Balaj L, Sivaraman S, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2009;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldous S, Thomas R. Absorption and metabolism of lanatoside C. II. Fate after oral administration. Clin Pharmacol Ther. 1977;21:647–658. doi: 10.1002/cpt1977216647. [DOI] [PubMed] [Google Scholar]
- 32.Batrakova EV, Miller DW, Li S, et al. Pluronic P85 enhances the delivery of digoxin to the brain: In vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296:551–557. [PubMed] [Google Scholar]
- 33.Ding L, Yuan C, Wei F, et al. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Cancer Invest. 2011;29:511–520. doi: 10.3109/07357907.2011.605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu YL, DeLay M, Jahangiri A, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773–1783. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagci-Onder T, Wakimoto H, Anderegg M, et al. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 36.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 37.Wang JK, Portbury S, Thomas MB, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: Discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA. 2006;103:10461–10466. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn DE, He DN, Yang P, et al. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J Neurochem. 2011;119:805–814. doi: 10.1111/j.1471-4159.2011.07439.x. [DOI] [PubMed] [Google Scholar]