Abstract
We have found that motor sequence learning and related brain activation is impaired in non-manifesting (nm) carriers of the DYT1deletion for dystonia. In the present study we used a trial-and-error sequence-learning task in conjunction with an equiperformance study design to identify the neural substrates that support sequence learning in nmDYT1 mutation carriers. Six nmDYT1 mutation carriers and six control subjects were scanned with H215OPET during the performance of a trial-and-error guided, kinematically controlled motor sequence learning task and a matched motor execution task. Controls were matched for age and performance. PET data analysis was performed using statistical parametric mapping (SPM99). Although performing at matched levels, nmDYT1 mutation carriers over activated the lateral cerebellum and the right inferotemporal cortex relative to age-matched controls (P < 0.001). In contrast, they showed relative activation deficits in the dorsolateral prefrontal cortex bilaterally, as well as in the left anterior cingulate and the dorsal premotor cortex (P < 0.001). Prominent compensatory involvement of the cerebellum during target learning is consistent with our prior sequence-learning experiments in nmDYT1 mutation carriers. Contrasting to mutation carriers, normals used bilateral cerebellar activation in conjunction with a prominent prefrontal bilateralization only when confronted with a much higher task difficulty. nmDYT1 mutation carriers lack recruitment of these prefrontal regions that depend on modulation within the cortico-striato-pallido-thalamocortical (CSPTC) loops. Instead, they compensate solely using cerebellar activation. This observation is in keeping with recent evidence of impaired structure/function relationships within CSPTC networks in dystonia perhaps occurring on a neurodevelopmental basis. The inability to recruit the appropriate set of neocortical areas because of altered fronto-striatal connectivity may have led to the shift to cerebellar processing.
Keywords: sequence-learning, brain activation, PET, DYT1 dystonia
Introduction
Dystonia syndromes are characterized by excessive and sustained muscle contractions resulting in abnormal postures and involuntary movements. These syndromes are generally attributed to functional abnormalities in the cortico-striato-pallido-thalamocortical (CSPTC) loops and related pathways (Berardelli et al., 1998; Vitek, 2002). The most frequent genetic cause of primary dystonia is a heterozygous GAG deletion in the gene, DYT1, which results in the loss of a glutamic acid residue in the encoded protein, torsinA (Ozelius et al., 1997). As only 30% of mutation carriers clinically manifest dystonia (Bressman et al., 1994), studying non-manifesting DYT1 mutation carriers (nmDYT1) provides a unique opportunity to assess the effects of the genotype, without the confound of motor behavioural abnormalities associated with the phenotype (Eidelberg, 1998).
In previous positron emission tomography (PET) studies conducted in the resting state, we identified a consistent pattern of regional metabolic abnormalities that was present in manifesting as well as non-manifesting carriers of the DYT1 mutation (Eidelberg, 1998; Trošt et al., 2002). Mutation carriers exhibited relative increases in resting state glucose utilization in the putamen/globus pallidus, the supplementary motor area (SMA) and the lateral cerebellum (Eidelberg, 1998; Trošt et al., 2002). Because this genotype-related spatial covariance pattern included brain regions involved in motor planning and in the processing of sequential information, we proposed that sequence learning might be defective in carriers of the DYT1 deletion (Ghilardi et al., 2003). Indeed, we found that despite normal motor functioning, nmDYT1 mutation carriers demonstrated impaired sequence-learning performance. A comparable learning deficit has recently been reproduced in a DYT1 mutant mouse model in which age-dependent motor learning deficits were noted but only subtle signs of dystonia (Sharma et al., 2005). PET recording of brain activation responses in non-manifesting human carriers of the DYT1 deletion performing a serial reaction time sequence-learning task revealed abnormally increased activation of the left ventral prefrontal cortex, and of the right pre-SMA, occipital association cortex, and lateral cerebellum (Ghilardi et al., 2003). However, the relationship of these overactive regions to learning performance was not addressed in that study. Moreover, the presence of a sequence-learning deficit in nmDYT1 was limited to the experimental setting in which target presentation was continuous throughout the learning epoch.
In the present study we addressed these issues by utilizing a different sequence-learning task design to identify the neural substrates that support sequence learning in nmDYT1 mutation carriers. In contrast to the earlier activation study, in the current paradigm the sequence order was not revealed, and subjects had to discover the elements of the sequence by trial-and-error. This error-based learning design emphasizes the role of the cerebellum (Doya, 2000). Additionally, to identify the neural substrates that support sequence learning in nmDYT1 mutation carriers, we used an equiperformance learning design (Mentis et al., 2003b). Control subject scans were chosen to match the nmDYT1 group performance level, thus facilitating the detection of compensatory brain activation. In this design, overactive areas do not account for performance differences, but illustrate the additional recruitment of neuronal resources during the learning process.
Methods
Subjects
Six right-handed nmDYT1 mutation carriers [five men and one woman; age 43.3 ± 9.5 years (mean ± SD); range: 33–57 years] were scanned with 15O-labelled water (H215O) and PET during the performance of a trial-and-error guided, motor sequence-learning task and a kinematically similar motor execution task (Ghilardi et al., 2000; Mentis et al., 2003b). These subjects were recruited, genotyped and neurologically examined through the Dystonia Clinical Research Center at Beth Israel Hospital in New York. They were all normal on neurological examination, did not show signs of dystonia, and did not have a history of dystonia.
We also studied six age-matched right-handed healthy volunteer subjects (three men and three women; age 42.7 ± 11.3 years; range: 29–57 years) who served as controls for the equiperformance comparisons with the mutation carriers. These subjects were selected from a larger group of 12 healthy volunteer subjects who performed these tasks during H215O PET imaging (Ghilardi et al., 2000). Those subjects selected as controls for the current study were matched for age with the nmDYT1 mutation carriers. In these age-matched control subjects, sequence-learning scans were selected from a total of 36 scans such that performance during each run was matched to that for each mutation carrier (Mentis et al., 2003b). Motor baseline scans for each control subject were chosen corresponding to the learning scans that were selected. Inclusion/exclusion criteria for mutation carriers and controls were otherwise as presented previously (Ghilardi et al., 2003). Written informed consent was obtained from all participants under protocols approved by the institutional review boards of the participating institutions.
Motor tasks
During scan acquisition, all subjects (nmDYT1 and controls) performed two runs of a trial-and-error-guided motor sequence-learning task (TSEQ) and a matched motor baseline task (CCW) with the dominant right hand. Both tasks have been described in detail previously (Ghilardi et al., 2000). In both tasks, subjects observed a computer screen, where radially arranged targets were displayed and had to be reached with the cursor of a hand-held mouse moving on a digitizing tablet. Similarly, in both tasks a tone at 1Hz intervals was presented to pace the reaching movements.
For TSEQ a new sequence of five or six targets had to be learned in each run. The sequence to be learned was not revealed to the subjects, but had to be guessed initially. In order to do so, subjects moved the cursor in self-initiated reaching movements to the target of their choice. Upon hitting the correct target subjects received feedback (targets that were correctly hit turned gray, while they remained transparent during incorrect hits). Via this explicit feedback subjects gradually uncovered and memorized the sequence. In each novel sequence the number of targets equalled the number of targets on the screen. Each target was only visited once during the sequence and the sequence cycled through its veridical order throughout the duration of the scan.
In controls as well as in nmDYT1 mutation carriers, two subjects performed the task with five targets while four performed the task with six targets to be learned. The average rate of correctly hit targets was 58.3 ± 21.7 in the nmDYT1 and 58.2 ± 22.1 in the controls.
During the baseline motor task (CCW) subjects moved the cursor on the digitizing tablet with their right hand out and back from a central starting position to one of eight radial targets that were displayed in a predictable counterclockwise order. Subjects had to anticipate target appearance and to initiate the movement before the tone with a temporal window of ±100 ms. (A videoclip of this task can be seen at http://www.neuroscience-nslij.org.) Testing was done in separate trial blocks of 90 s. All subjects learned to perform the task in a training session conducted 1 or 2 days before testing. In the nmDYT1 group, the training session was also used to individually determine the longest sequence, which each DYT1 mutation carrier was able to learn in TSEQ. In the course of the training session, the number of targets per sequence was gradually increased from three to seven. The number of targets selected for testing during the PET recording was based upon each subject’s performance during the last training session: this number was increased until subjects discovered and remembered the correct order of all targets within the first 60 s of the 90 s trial block.
During the scanning session each nmDYT1 mutation carrier performed TSEQ at this highest individual level. In contrast, controls were scanned while learning four, five and six targets in different runs. For group comparison, we then selected only those scans where normal performance matched nmDYT1 levels at the same number of targets.
To characterize performance during CCW and TSEQ, we computed several kinematic measurements for each movement as described previously (Ghilardi et al., 2000, 2003; Mentis et al., 2003b). To characterize performance in CCW, we calculated movement time, spatial error and the percentage of correct movements per trial. To characterize performance in TSEQ and CCW, we calculated the percentage of correct movements per learning block. A movement was categorized as correct when the directional error towards the target had <22° on either side of the appropriate target midpoint, and when the movement reversal occurred during the appropriate time window (100 ms around the pacing tone). Further details of the study design (Ghilardi et al., 2003) and the behavioural tasks (Ghilardi et al., 2000; Mentis et al., 2003b) have also been published previously.
Performance matching
For nmDYT1, the percentage of correct movements during TSEQ was 58.3 ± 21.7% (mean ± SE). For group comparison at equiperformance, a set of 12 control scans was selected from the healthy volunteer cohort matching the performance level of the DYT1 mutation carriers. The mean percentage of correct movements in the selected control group was 58.2 ± 22.1%.
During CCW, the mean percentage of correct movements was 97.8 ± 1.2% for nmDYT1 and 98.3 ± 0.9% for controls (Student’s t-test; P > 0.5). Movement times during CCW were 389.3 ± 38.5 ms in nmDYT1 and 410.5 ± 48.7 ms in controls (P > 0.3). Spatial errors were 0.26 ± 0.09 cm in nmDYT1 and 0.27 ± 0.03 cm in controls (P > 0.5). Thus both groups were not only matched for learning performance, but also for motor performance levels.
Positron emission tomography
All subjects were scanned with H215O PET. They fasted overnight prior to scanning. PET imaging was performed using the GE Advance tomograph in 3D mode (Dhawan et al., 1998).
The details of the imaging procedures have been presented previously. In each run, relative regional cerebral blood flow (rCBF) was estimated using a modification of the slow bolus method (Silbersweig et al., 1993). Using this injection protocol, there was a time delay of ~17 s before onset of brain radioactivity, and the time from onset to peak count rate was 45–50 s. The timing of task initiation was individually adjusted so that the arrival of radioactivity occurred ~10 s after the start of each task. PET data acquisition began at the time of radioactivity arrival in the brain and continued for 80 s. The end of task thus coincided with the end of data acquisition. In this slow bolus H215O PET method, images reflect rCBF during the rising phase of the brain radioactivity, corresponding to the 2nd–8th cycles in our tasks. The interval between successive H215O administrations was 10 min to allow for the decay of radioactivity.
Image analysis
Scan preprocessing was performed using SPM 99 (Wellcome Department Cognitive Neurology, London, UK) implemented in Matlab (Mathworks, Sherborn, MA). The H215O PET scans from each subject were realigned using the mean image as a reference. All images were proportionally rescaled to a global CBF of 50 ml/min/dl and stereotaxically normalized into a standard anatomical space developed at the Montreal Neurological Institute (Collins et al., 1994). The images were smoothed with an isotropic Gaussian kernel (FWHM 10mm for all directions) to allow for interindividual gyral variation and to improve the signal-to-noise ratio.
The specific regions activated during TSEQ in the carriers of the DYT1 deletion and controls were identified using the multi-group, conditions and covariate option in SPM, with TSEQ and CCW representing the two conditions per subject and nmDYT1 and controls the two groups. Comparison of rCBF during the different conditions was performed by generating SPM{t} maps and defining the appropriate contrasts.
In all analyses, activations and regional correlations were considered significant at a threshold of P ≤ 0.05, corrected for multiple comparisons. Additionally, we reported activations at P ≤ 0.001 at peak voxel uncorrected (T = 3.5; for >80 contiguous voxels). Coordinates were reported in the standard anatomical space developed at the Montreal Neurological Institute.
All calculations were performed on PCs running Windows 2000. All additional statistical analyses were performed using JMP software (SAS Institute Inc., Cary, NC) for PC.
Results
Within-group analyses
Learning-related brain activation in controls
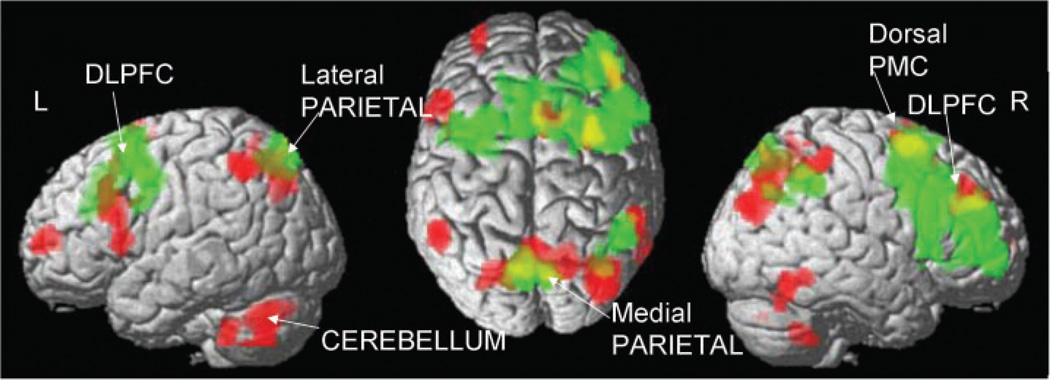
Analysis of learning-related brain activation (TSEQ>CCW) in controls revealed a large prefrontal cluster involving both hemispheres (Table 1, Fig. 1; green overlay). The activated region spanned the dorsal premotor cortex (dPMC), dorsolateral prefrontal cortex (DLPFC) (right>left) and anterior cingulate bilaterally. On the right side, this cluster extended into the ventral prefrontal cortex and into the pre-SMA. Additional task-specific activation was localized to the precuneus bilaterally, with left-sided predominance and to the right inferior parietal lobule.
Table 1.
Brain regions with significant increases in TSEQ related brain activation (TSEQ > CCW) in controls (see Figure 1)
| Brain Region | Coordinatesa | Cluster extent ke |
|||
|---|---|---|---|---|---|
| Zmax | x | y | z | ||
| R DLPFC (BA 46) | 5.2 | 30 | 40 | 18 | 8233 |
| R DLPFC (BA 9) | 4.2 | 44 | 2 | 46 | |
| L DLPFC (BA 9) | 4.0 | −34 | −2 | 40 | |
| R dPMC (BA 6) | 4.1 | 46 | 10 | 34 | |
| L dPMC (BA 6) | 4.2 | −26 | 14 | 64 | |
| R preSMA (BA 6) | 4.0 | 12 | 8 | 60 | |
| R frontal eye field (BA 8) | 4.0 | 48 | 22 | 52 | |
| R ventral PFC (BA 45) | 3.9 | 64 | 18 | 2 | |
| R ACC (BA 32) | 3.5 | 10 | 26 | 40 | |
| L ACC (BA 32) | 3.8 | −2 | 26 | 36 | |
| R precuneus (BA 7) | 4.9 | 2 | −78 | 58 | 805 |
| L precuneus (BA 7) | 4.8 | −10 | −70 | 54 | |
| R inferior parietal lobule (BA 40) | 4.1 | 50 | −46 | 40 | 430 |
For large clusters (>500 voxels) the regional distribution is illustrated using the localization of the peak voxel (left-most entry to the left column) and the localization of the submaxima of the respective cluster (right-most entry to the left column) (Significant at P < 0.05 corrected for multiple comparisons).
DLPFC = dorsolateral prefrontal cortex; BA = Brodmann Area; dPMC = dorsal premotor cortex; ACC = anterior cingulate cortex.
Montreal Neurological Institute (MNI) standard space.
Fig. 1.
Activation responses during the trial-and-error-based sequence-learning task in nmDYT1 mutation carriers and controls (see text; Tables1 and 2).The surface rendering of the statistical map (SPM99 canonical template) reflects sequence-learning-related activation (TSEQ>CCW) specific to the six non-manifesting DYT1 mutation carriers (red) and specific to the six controls (green) as well as areas of overlapping activation (yellow). Sequence-learning activation responses in both groups were localized to the dorsolateral prefrontal cortex (DLPFC), dorsal premotor cortex (PMC) and to the medial and lateral parietal association cortices. Significant cerebellar activation responses were present in theDYT1group only.
Learning-related brain activation in nmDYT1mutation carriers
Analysis of learning-related brain activation (TSEQ>CCW) in nmDYT1 mutation carriers revealed bilateral activation of the dorsolateral prefrontal cortex (DLPFC), and of the right dPMC, extending into the anterior cingulate area (Table 2, Fig. 1; red overlay). Additional contributions were present bilaterally in parietal association regions and the cerebellum, and in right occipital and inferotemporal areas. A smaller cluster was also noted in the left ventral prefrontal cortex.
Table 2.
Brain regions with significant increases in TSEQ related brain activation (TSEQ > CCW) in nmDYT1 (see Figure 1)
| Brain region | Coordinatesa | Cluster extent ke |
|||
|---|---|---|---|---|---|
| Zmax | x | y | z | ||
| R lateral cerebellar cortex** | 3.6 | 40 | −50 | −46 | 84 |
| L lateral cerebellar cortex*, lobulus VI | 4.8 | −36 | −64 | −34 | 984 |
| L lateral cerebellar cortex, crus I | 4.0 | −48 | −60 | −50 | |
| R inferotemporal (BA 37)* | 4.7 | 66 | −62 | −16 | 251 |
| L ventral prefrontal (BA 10)** | 3.6 | −32 | 58 | 10 | 132 |
| L DLPFC (BA 9, 44–46)* | 4.0 | −56 | 22 | 22 | 330 |
| R DLPFC (BA 9/46)* | 3.9 | 44 | 34 | 28 | 240 |
| R angular (BA 39)* | 5.1 | 32 | −74 | 30 | 802 |
| R occipital (BA 19) | 4.6 | 38 | −78 | 26 | |
| R superior parietal (BA 7) | 4.5 | 36 | −72 | 42 | |
| L precuneus (BA 7)* | 4.8 | −14 | −74 | 44 | 1153 |
| R precuneus (BA 7) | 4.1 | 8 | −66 | 50 | |
| L inferior parietal lobule (BA 40)** | 4.7 | −56 | −52 | 50 | 206 |
| R inferior parietal lobule (BA 40)* | 4.3 | 60 | −42 | 54 | 257 |
| R dorsal PMC (BA 6)* | 4.8 | 30 | 8 | 62 | 232 |
| R pre-SMA (BA 6)* | 3.9 | 4 | 12 | 62 | 291 |
DLPFC = dorsolateral prefrontal cortex; BA = Brodmann Area; PMC = premotor cortex; SMA = supplementary motor area.
Montreal Neurological Institute (MNI) standard space.
P < 0.05 corrected for multiple comparisons,
P < 0.001 uncorrected.
Between-group analysis
Decreased learning-related brain activation in nmDYT1mutation carriers compared to controls
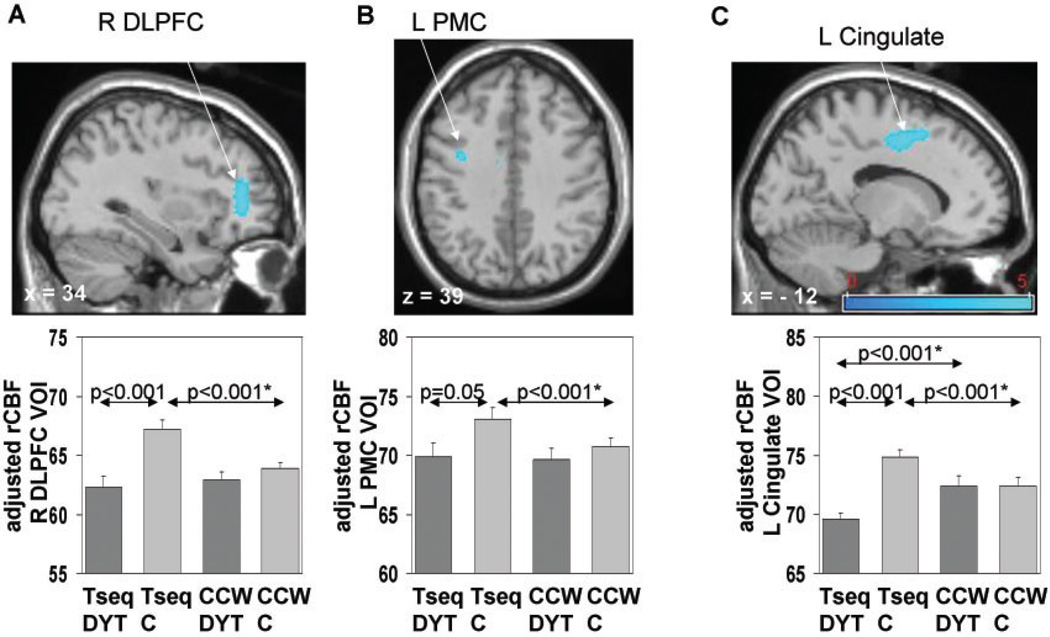
Compared to controls, nmDYT1 mutation carriers showed markedly decreased activation of the bilateral DLPFC and the left dPMC (Table 3, Fig. 2A). Moreover, nmDYT1 mutation carriers deactivated the left cingulate cortex while this region was activated by controls during learning (Table 3, Fig. 2B).
Table 3.
Brain regions with relatively decreased activation in TSEQ in nmDYT1 carriers compared to controls
| Brain region | Coordinatesa | Cluster extent ke |
|||
|---|---|---|---|---|---|
| Zmax | x | y | z | ||
| R DLPFC (BA 10/46)* | 4.0 | 34 | 40 | 2 | 315 |
| L DLPFC (BA 9/32)** | 3.8 | −18 | 38 | 30 | 84 |
| L PMC (BA 6)** | 3.8 | −34 | −2 | 40 | 81 |
| L dorsal anterior cingulate (BA 32)* | 4.0 | −14 | 0 | 46 | 490 |
DLPFC = dorsolateral prefrontal cortex; BA = Brodmann Area; PMC = premotor cortex.
Montreal Neurological Institute (MNI) standard space.
P < 0.05 corrected for multiple comparisons,
P < 0.001 uncorrected.
Fig. 2.
Brain regions in which learning-related activation responses during trial-and-error-based sequence-learning were reduced in nmDYT1 carriers relative to controls (Table 3, see text): SPM{t} maps (top panel) were superimposed on a single-subject MRI T1 template (x, y, z coordinates in MNI space indicate the position of the slice). Bar diagrams (bottom) illustrate adjusted regional cerebral blood flow (rCBF) during sequence learning (TSEQ) and during the motor reference task (CCW) in the respective cluster/VOI (mean ± SE) in the DYT1mutation carriers (dark gray) and controls (light gray). Decreased learning-related activation was present in the DLPFC (A) as well as in the left dPMC (B) with significant activation in controls, but not in carriers of the DYT1mutation. Additionally, nmDYT1s deactivated the anterior cingulate cortex (B), while controls activated this region. Significant contrasts of post hoc VOI comparisons are displayed (P-values of paired T-tests marked with asterisk). Brain activation during the baseline motor task was comparable across groups in all displayed regions (P > 0.2). (The colour stripe represents Tvalues thresholded at 3.5, P < 0.001.)
Increased learning-related brain activation in nmDYT1 mutation carriers compared to controls
Increased task-specific activation relative to controls was detected in the left lateral cerebellum (Table 4, Fig. 3A). These increases were lateralized to the left at the specified thresholds for significance. However at slightly lower thresholds (P < 0.005), they were bilateral (Fig. 3A). Additionally, nmDYT1 mutation carriers activated the right inferotemporal cortex, while the controls deactivated this area (Table 4, Fig. 3B).
Table 4.
Brain regions with relatively increased activation in TSEQ in nmDYT1 carriers
| Brain region | Coordinatesa | Cluster extent ke |
|||
|---|---|---|---|---|---|
| Zmax | x | y | z | ||
| L lateral cerebellar cortex, lobulus VI | 3.5 | −34 | −44 | −42 | 97 |
| R inferotemporal area (BA 37) | 3.9 | 58 | −52 | −8 | 87 |
BA = Brodmann Area.
(P < 0.001, uncorrected).
Montreal Neurological Institute (MNI) standard space.
Fig. 3.
Brain regions in which learning-related activation responses during trial-and-error-based sequence-learning were increased in DYT1 carriers relative to controls (Table 4; see text): SPM{t}maps (top panel) were superimposed on a single-subject MRI T1 template (x, y, z coordinates in MNI space indicate the position of the slice). Bar diagrams (bottom) illustrate adjusted regional cerebral blood flow (rCBF) during sequence learning (TSEQ) and during the motor reference task (CCW) in the respective cluster/VOI (mean ± SE) in the DYT1 mutation carriers (dark gray) and controls (light gray). Increased learning-related activation was present in the left cerebellar cortex and to a lesser degree in the right-sided homologue (A) as well as in the right inferotemporal cortex (B). Brain activation during the baseline motor task was comparable across groups in all displayed regions (P > 0.2). P-values refer to post hoc VOI testing using unpaired and paired (asterisk) T-tests. (The colour stripe for the left and right column represents T values thresholded at 3.5, P < 0.001, T values for the middle column were thresholded at 3.0, P < 0.005.)
Discussion
In the present study we used an equiperformance design to identify compensatory brain activation during sequence learning in clinically unaffected DYT1 mutation carriers. Our results indicate that nmDYT1 mutation carriers utilize cerebellar activation to achieve successful sequence-learning at a moderate level of difficulty. The general pattern of TSEQ-related brain activation identified in controls and nmDYT1 includes the dPMC, pre-SMA, DLPFC, the cingulate and parietal areas. These areas have been previously implied in similar sequence-learning paradigms in healthy subjects (Sakai et al., 2002; Mentis et al., 2003a, b; Carbon et al., 2003; Halsband and Lange, 2006). With increasing task difficulty, healthy subjects displayed prominent bilateralization of prefrontal and parietal learning-related activation, as well as activation of the cerebellum (Mentis et al., 2003a). In contrast, nmDYT1 did not exhibit the former activation responses during learning, but only activated the lateral cerebellum. Surprisingly, despite performing at only a moderate level of difficulty, and being matched to equally performing controls, nmDYT1 showed deficient activation of the bilateral DLPFC and left PMC. Moreover, in contrast to controls, nmDYT1 deactivated the cingulate region.
Our data support the notion that cerebellar activation in nmDYT1 is a compensatory mechanism to improve learning performance. This is consistent with our prior sequence-learning experiments in nmDYT1 (Ghilardi et al., 2003). However, since in our prior experiments nmDYT1 and healthy controls were not matched for performance, the functional role of the cerebellum could not be specified. Overactivation of the cerebellum in our earlier study could have been either compensatory or pathological. Resolving this question is important because the cerebellum has been implicated as a key structure involved in expression of dystonia (LeDoux et al., 1998; LeDoux and Brady, 2003; Raike et al., 2005; Jinnah and Hess, 2006). Moreover, cerebellar hypermetabolism is part of the abnormal metabolic brain network associated with the DYT1 genotype (Eidelberg, 1998; Trošt et al., 2002; Carbon et al., 2004b). The cerebellum and the basal ganglia are thought to have complementary roles in sequence-learning (Hikosaka et al., 1999; Doya, 2000; Ohyama et al., 2003). Cerebellar learning results from error-based feed-forward control (Ohyama et al., 2003), while the basal ganglia are specialized in reinforcement-based learning involving positive feedback (Graybiel, 2005). Additionally, the cerebellum is engaged in the earliest phases of sequence learning, with the cortex being activated before the dentate nucleus, while the striatum supports memory storage of sequences (Doyon et al., 2003). Our short trial-and-error-based task design emphasized the cerebellar role in the learning process. Nevertheless, striatal activation has been demonstrated in controls, possibly due to the presence of feedback acting as implicit reward (Carbon et al., 2003). In the nmDYT1 mutation carriers, resting state metabolic abnormalities in the basal ganglia (Eidelberg, 1998; Trošt et al., 2002), may have interfered with learning-related activation in this region, necessitating compensatory shifts to the cerebellum. Although DYT1 mutation carriers also display abnormal resting metabolism (Trošt et al., 2002) and abnormal motor activation (Ghilardi et al., 2003) in the lateral cerebellum, the functional shift that was observed during sequence learning was to cerebellar regions without metabolic abnormalities in the resting state or during simple motor execution.
Contrary to PD patients, who are likely to use physiological pathways throughout the disease (Carbon et al., 2003), nmDYT1 mutation carriers lack recruitment of prefrontal regions and compensate solely using cerebellar activation. This observation is in keeping with recent evidence of impaired structure/function relationships within CSPTC networks in dystonia (Vitek, 2002; Carbon et al., 2004a, d) perhaps occurring on a neurodevelopmental basis (Carbon et al., 2004d; Goodchild et al., 2005; Bahn et al., 2006; Hewett et al., 2006; Vasudevan et al., 2006). The inability to recruit the appropriate set of neocortical areas (see below) because of altered frontostriatal connectivity may have led to the shift to cerebellar processing. We note that in this case, compensatory brain activation occurs in a region that may also mediate the clinical manifestations of disease (LeDoux et al., 1998; LeDoux and Brady, 2003; Raike et al., 2005; Jinnah and Hess, 2006). Indeed, compensatory brain activation can be viewed as a normal sign of adaptive cerebral plasticity (Johansen-Berg et al., 2002; Ptito et al., 2005; Desmurget et al., 2007). However, an association between abnormal neuroplasticity and symptoms has long been recognized in focal dystonia (Byl et al., 1996; Quartarone et al., 2006; Weise et al., 2006). If cerebellar overactivation is viewed as a maladaptive neuroplastic response in nmDYT1 carriers, a similar abnormal relationship may underlie the appearance of symptoms in affecteds with DYT1 dystonia.
Surprisingly, we also found that nmDYT1 mutation carriers activated the right inferotemporal area, while controls de-activated this area. The latter observation is in line with earlier findings in healthy controls performing a similar sequence-learning task (Sakai et al., 2002). Although a role of the ventral visual stream in motor control appears contradictory to the distinct functions of the dorsal and ventral visual stream (Goodale and Milner, 1992), Fogassi and Luppino (2005) have recently emphasized the role of parietoinferotemporal connections in motor control. The inferotemporal cortex receives strong input from the inferior parietal lobule in the primate (Tanaka, 1996; Zhong and Rockland, 2003). This conjoined input is implied in retrieving the semantic properties of objects during a motor act (Fogassi and Luppino, 2005). It is possible that activation of the inferotemporal cortex during sequence learning in nmDYT1 mutation carriers indicated the use of alternate learning strategies that rely on visual object properties (rather than on or in addition to sensory properties). Although the motor baseline task and the learning task are matched for sensory input during target presentation, subtle differences in the sensory feedback that are not important in controls may necessitate the use of such an alternate learning strategy in the face of sensory processing deficits in the DYT1 mutation carrier state (Sakai et al., 2002; Fiorio et al., 2007).
It is striking that all areas showing reduced learning-related activation in nmDYT1 (DLPFC, anterior cingulate and dPMC) are cortical elements of the major CSPTC loops. DLPFC in conjunction with the anterior cingulate is typically activated when healthy subjects learn new motor sequences (Jenkins et al., 1994; Jueptner et al., 1997a, b; Ghilardi et al., 2000; Nakamura et al., 2001; Carbon et al., 2003; Mentis et al., 2003a; for review see Ashe et al., 2006). This activation with right-sided predominance is typically attributed to the aspect of spatial working memory (Wager and Smith, 2003; Curtis, 2006; Halsband and Lange, 2006; Muller and Knight, 2006) as well as to the aspect of attention to action (Jueptner et al., 1997a, b). Surprisingly, relatively decreased DLPFC activation in nmDYT1 was not found in our earlier study (Ghilardi et al., 2003). This discrepancy may have resulted from the increased working memory load associated with trial-and-error-based target detection as compared with the earlier serial reaction time task, especially given that DLPFC activation is typically related to working memory load (Rypma and D’Esposito, 1999; Rypma et al., 2002; Lewis et al., 2004; Leung et al., 2007). It is possible that the inadequate prefrontal response to task demands in the nmDYT1 mutation carriers is caused by an impairment of frontostriatal connectivity. Striatal modulation of the cortical response is critical for task performance (Carbon et al., 2004c) and may be impaired in nmDYT1 carriers, who display abnormal resting striatal metabolism (Eidelberg, 1998; Trošt et al., 2002) and reduced D2-receptor binding (Asanuma et al., 2005).
Consistent with our previous study (Ghilardi et al., 2003), we found that nmDYT1 carriers de-activated the dorsal anterior cingulate (BA 32) during sequence learning, while healthy controls activated this area. Anterior cingulate (ACC) activation in conjunction with lateral prefrontal activation has been repeatedly observed during sequence learning in health (Jueptner et al., 1997a, b; Ghilardi et al., 2000; Nakamura et al., 2001; Sakai et al., 2002; Carbon et al., 2003). However, ACC activation is not specific to sequence learning, but occurs during a wide array of cognitive tasks. Therefore caudal ACC activation has been proposed to reflect action monitoring either via response conflict monitoring (Barch et al., 2001; Gehring and Fencsik, 2001; di Pellegrino et al., 2007) or via error detection (Gehring and Knight, 2000; Swick and Turken, 2002; Mars et al., 2005; van Veen and Carter, 2006). In particular, it has been proposed that intact prefrontal-ACC interaction is crucial for proper action monitoring (Gehring and Knight, 2000; Swick and Turken, 2002; Markela-Lerenc et al., 2004; Dias et al., 2006). Abnormal activation was present in both of these areas in the DYT1 mutation carriers, suggesting the possibility of disturbed action monitoring processes.
The attenuation of learning-related activations in the PMC may relate to the previously observed overactivation of this region in DYT1 mutation carriers performing the motor execution task without learning (Ghilardi et al., 2003). Nonetheless, in this region there was no group difference in activation during the performance of the motor task. It is conceivable that a ceiling in the local brain activation may have been reached during movement, thus not permitting further activation in response to the additional challenge of motor sequence-learning. Alternatively, impaired PMC activation during learning may be secondary to functional abnormalities in the basal ganglia. Because of the strong anatomical relationship between these structures (Nakamura et al., 2001; Carbon et al., 2003), the results may also be consistent with a disruption of cortico-striatal functional connectivity in carriers of the DYT1 deletion.
We recognize that the number of subjects in the DYT1 carrier and control groups is small and that the results may not necessarily be generalized to other cohorts. That said, there was no evidence of a difference between the learning-related activation responses observed in the six healthy volunteers used for equiperformance comparison with the nmDYT1 carriers and those recorded in the remaining six normal subjects who comprised our original cohort (Ghilardi et al., 2000). Likewise, additionally the gender distribution was not strictly matched across the carrier and control groups (P = 0.09, chi-square test). We note that in the original TSEQ study of 12 healthy volunteers, learning-related activation in the precuneus region was significantly greater (P < 0.001) for male versus female subjects. Nonetheless, this region was not among those that discriminated DYT1 carriers from controls during learning. Moreover, there were no identifiable gender differences in any of the regions that were found to distinguish the two groups at equiperformance. Future studies will help determine whether similar changes in regional brain function are evident in affected DYT1 carriers performing this and related sequence-learning tasks.
Conclusion
The presence of a stable different learning topography in non-manifesting DYT1 mutation carriers raises the possibility of functional reorganization of fronto-striatal pathways in these subjects, perhaps on a genetic or developmental basis. Indeed, metabolic changes in the basal ganglia may lead to the shift from striatal to cerebellar processing as a feature of the DYT1 mutation carrier state. The clinical presentation of dystonia in DYT1 mutation carriers may reflect a limitation of the compensatory increased activation responses related to motor control that occur with this genotype.
Acknowledgements
This work was supported by the National Institutes of Health (NIH RO1 NS 047668 (D.E.)). The authors would like to thank Dr Thomas Chaly for radiochemistry support, Loreta Quartarolo and Deborah Raymond for support in study coordination and patient recruitment, and Nathaniel Brown and Toni Flanagan for editorial assistance.
Abbreviations
- CSPTC
cortico-striato-pallido-thalamocortical
- SMA
supplementary motor area
References
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol. 2006;16:213–221. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Bahn E, Siegert S, Pfander T, Kramer ML, Schulz-Schaeffer WJ, Hewett JW, et al. TorsinB expression in the developing human brain. Brain Res. 2006;1116:112–119. doi: 10.1016/j.brainres.2006.07.102. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell J, Hallett M, Thompson P, Manfredi M, Marsden C. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Bressman SB, de Leon D, Kramer PL, Ozelius LJ, Brin MF, Greene PE, et al. Dystonia in Ashkenazi Jews: clinical characterization of a founder mutation. Ann Neurol. 1994;36:771–777. doi: 10.1002/ana.410360514. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Jenkins WM. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- Carbon M, Trost M, Ghilardi MF, Eidelberg D. Abnormal brain networks in primary torsion dystonia. Adv Neurol. 2004a;94:155–161. [PubMed] [Google Scholar]
- Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004b;62:1384–1390. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, et al. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004c;21:1497–1507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004d;56:283–286. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, Mentis MJ, et al. Learning networks in health and Parkinson’s disease: reproducibility and treatment effects. Hum Brain Mapp. 2003;19:197–211. doi: 10.1002/hbm.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, et al. Quantitative Brain PET. Comparison of 2D and 3D Acquisitions on the GE Advance Scanner. Clin Positron Imaging. 1998;1:135–144. doi: 10.1016/s1095-0397(98)00009-0. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Ladavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. 2007;19:275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res. 2006;174:279–291. doi: 10.1007/s00221-006-0444-4. [DOI] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the corticostriatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Functional brain networks in movement disorders. Curr Opin Neurol. 1998;11:319–326. doi: 10.1097/00019052-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Gambarin M, Valente EM, Liberini P, Loi M, Cossu G, et al. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain. 2007;130:134–142. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Disease. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, et al. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. Neuroscience. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol. 1997a;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997b;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86:533–545. doi: 10.1016/s0306-4522(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Leung H-C, Oh H, Ferri J, Yi Y. Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage. 2007;35:368–377. doi: 10.1016/j.neuroimage.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Wing AM, Pope PA, Praamstra P, Miall RC. Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia. 2004;42:1301–1312. doi: 10.1016/j.neuropsychologia.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Markela-Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M. Prefrontal-cingulate activation during executive control: which comes first? Brain Res Cogn Brain Res. 2004;18:278–287. doi: 10.1016/j.cogbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mars RB, Coles MG, Grol MJ, Holroyd CB, Nieuwenhuis S, Hulstijn W, et al. Neural dynamics of error processing in medial frontal cortex. Neuroimage. 2005;28:1007–1013. doi: 10.1016/j.neuroimage.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Feigin A, Delalot D, Zgaljardic D, Edwards C, et al. Early stage Parkinson’s disease patients and normal volunteers: comparative mechanisms of sequence learning. Hum Brain Mapp. 2003a;20:246–258. doi: 10.1002/hbm.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003b;60:612–619. doi: 10.1212/01.wnl.0000044154.92143.dc. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, Hacking A, et al. Functional networks in motor sequence learning: abnormal topographies in Parkinson’s disease. Hum Brain Mapp. 2001;12:42–60. doi: 10.1002/1097-0193(200101)12:1<42::AID-HBM40>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Raike RS, Jinnah HA, Hess EJ. Animal models of generalized dystonia. NeuroRx. 2005;2:504–512. doi: 10.1602/neurorx.2.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ramnani N, Passingham RE. Learning of sequences of finger movements and timing: frontal lobe and action-oriented representation. J Neurophysiol. 2002;88:2035–2046. doi: 10.1152/jn.2002.88.4.2035. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, et al. Impaired motor learning in mice expressing TorsinA with the DYT1 dystonia mutation. Neuroscience. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S, et al. Detection of thirty-second cognitive activations in single subjects with positron emission tomography: a new low-dose H2(15)O regional cerebral blood flow three-dimensional imaging technique. J Cereb Blood Flow Metab. 1993;13:617–629. doi: 10.1038/jcbfm.1993.80. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Neuroscience. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- Trošt M, Carbon M, Edwards C, Ma Y, Raymond D, Mentis MJ, et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann Neurol. 2002;52:853–856. doi: 10.1002/ana.10418. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Vasudevan A, Breakefield XO, Bhide PG. Developmental patterns of torsinA and torsinB expression. Brain Res. 2006;1073–1074:139–145. doi: 10.1016/j.brainres.2005.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–S62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, et al. The two sides of associative plasticity in writer’s cramp. Brain. 2006;129:2709–2721. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Zhong Y-M, Rockland KS. Inferior parietal lobule projections to anterior inferotemporal cortex (area TE) in macaque monkey. Cerebr Cortex. 2003;13:527–540. doi: 10.1093/cercor/13.5.527. [DOI] [PubMed] [Google Scholar]