Abstract
The purpose of this study was to investigate the site-specific expression pattern and the role of chondrocyte clusters in human OA knee. Cartilage explants were obtained from 45 varus knees of medial and lateral femoral condyle undergoing total knee replacement surgery. Cartilage degeneration, number of chondrocytes, and the cell arrangement were evaluated by live/dead assay and immunohistochemical analyses with antibodies of STRO-1, FGF2, and Ki-67. Chondrocytes from medial and lateral femoral condyle were cultured to compare the potential of cell proliferation and production of cartilaginous nodules. Finally, cartilage tissue from medial femoral condyle, which included cartilage cleft with chondrocyte clusters, was observed the histological alternation. As the results, chondrocyte density adjacent to severe cartilage degeneration was highest, whereas chondrocytes in lateral femoral condyle displayed low density with single type of cells. Over 80% of these chondrocyte clusters were survived, expressing STRO-1, FGF2, and Ki-67. Furthermore, chondrocyte clusters proliferated faster and produced more cartilaginous nodules than single type of chondrocytes. Cartilage clefts involving numerous chondrocyte clusters were filled with extracellular matrix during organ culture. In conclusion, chondrocyte clusters adjacent to severe cartilage degeneration have shown completely specific characteristics with progenitor and proliferative potential. Regulating chondrocyte clusters may offer new approaches to cartilage repair and OA therapy in the future.
Keywords: osteoarthritis, chondrocyte cluster, STRO-1
INTRODUCTION
Osteoarthritis (OA) is the most prevalent joint disease, and cartilage loss is a central event in its pathogenesis.1,2 OA is not merely a cartilage disease but consists of multiple factors lead to joint failure.3–6 Tissue degeneration and the remodeling process in OA-affected articular cartilage are due to abnormal cell activation.1
Chondrocyte morphology is altered with OA pathogenesis7 and chondrocyte clusters are recognized as a hallmark of OA.8,9 Many articles have revealed the characteristics of chondrocyte clusters, which express not only catabolic factors,10,11 such as Interleukin-1 beta (IL-1β) and matrix metalloproteinase-13 (MMP-13), but also anabolic factors such as Sox9 and type II collagen,12 suggesting several cell-signaling pathways and growth factors are associated with chondrocyte clusters.13,14 In addition, these clusters have been reported to express markers of progenitor cells,15 indicating that the role of chondrocyte clusters still need to be investigated.13
Most instance OA knee is exhibited in varus or valgus malalignment with an imbalance of mechanical stress.16 Abnormal mechanical stress might affect the expression of chondrocyte clusters, and particularly, fibroblast growth factor 2 (FGF2) is already known to induce chondrocyte clusters in response to mechanical stress.17–19 Although cartilage degeneration was observed to have site-specific differences, which might be associated with the function of chondrocyte clusters and mechanical stress, little is known about the expression pattern of chondrocyte clusters in OA. In this study, we focused on varus knee malalignment and investigated the site-specific expression of chondrocyte clusters to clarify the role of clusters in human knee OA.
MATERIALS AND METHODS
Cartilage Samples and Location
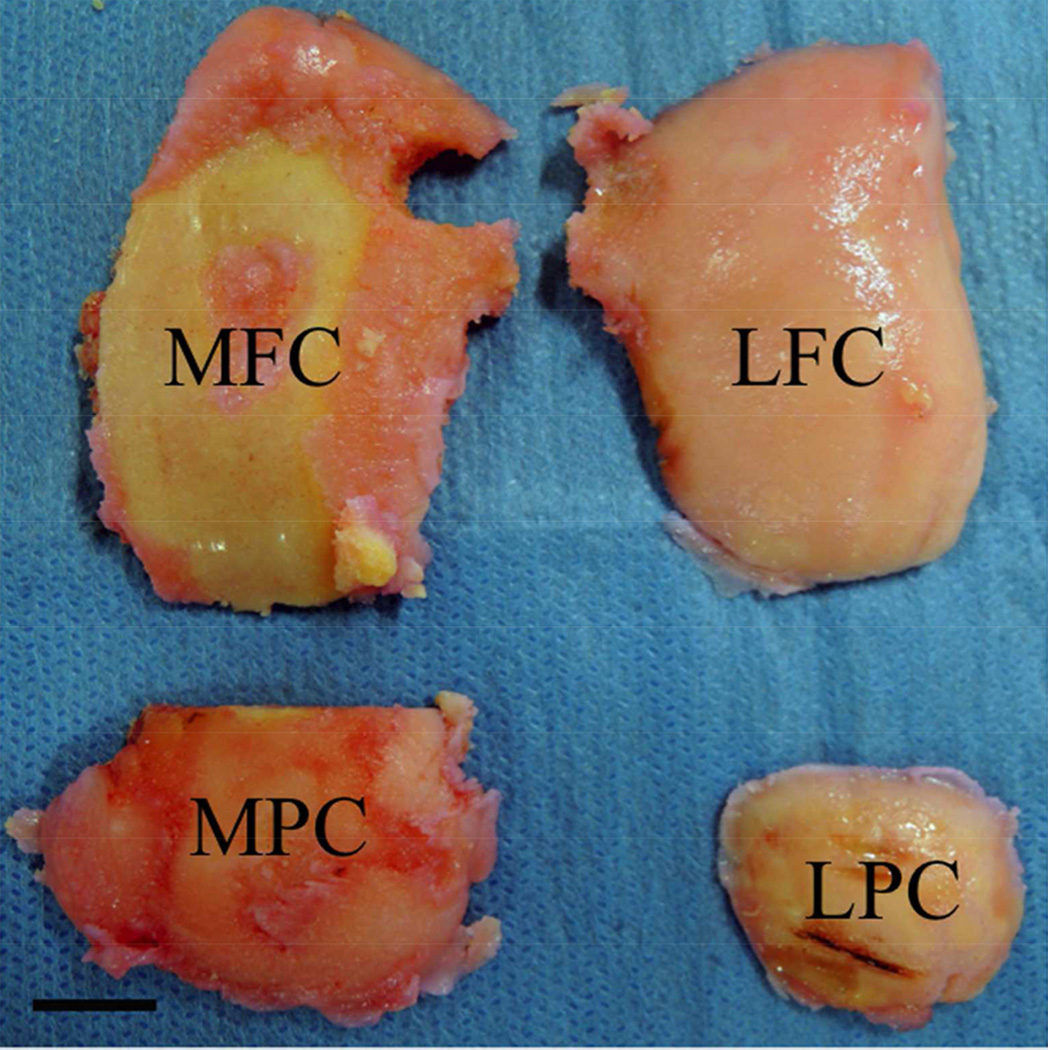
After receiving approval for this study from the Ethics Committee of Osaka Medical College (No. 0986), participants gave their written informed consent. Cartilage samples were obtained from 45 knees of 45 patients (34 women and 11 men), who had received total knee replacement surgery (TKR). The mean age was 74.6 ± 6.6 (57 – 86) years, and their body mass index (BMI) was 25.5 ± 3.9 (16.3 – 40.8) kg/m2. Knee malalignment was assessed by using the femorotibial angle (FTA), which was the lateral angle between the centerline of the femur and the tibia on the coronal radiograph at standing position.20 In this study, the average FTA was 183.3 ± 5.2 (176.0 – 197.0) degrees, which is nearly an 8-degree varus knee deformity (Table 1). Cartilage explants were harvested from 4 locations, which were the medial femoral condyle (MFC), the medial posterior femoral condyle (MPC), the lateral femoral condyle (LFC), and the lateral posterior femoral condyle (LPC) based on the International Cartilage Repair Society (ICRS) mapping system (Fig. 1).21
Table 1.
Donor information
| Characteristics | Value |
|---|---|
| Age, mean ± SD years | 74.6 ± 6.6 (57 – 86) |
| Female/male | 34 / 11 |
| Body mass index, mean ± SD kg/m2 | 25.5 ± 3.9 (16.3 – 40.8) |
| FTA, mean ± SD degrees | 183.3 ± 5.2 (176.0 – 197.0) |
FTA, femorotibial angle. SD, standard deviation
Figure 1.
Location of Cartilage Explants. Cartilage explants were harvested from 4 locations, which were the medial femoral condyle (MFC), the medial posterior femoral condyle (MPC), the lateral femoral condyle (LFC), and the lateral posterior femoral condyle (LPC) based on the International Cartilage Repair Society (ICRS) mapping system.
Cartilage Degeneration, Chondrocyte Density and Arrangement
Cartilage degeneration as well as chondrocyte density and arrangement were analyzed with safranin-O staining at each location. Cartilage degeneration was graded according to the Mankin score.9 Number of chondrocytes in the cartilage explants was counted three times by three observers in a 400 × 800 µm grid (using 100× field), and the density was analyzed. The arrangement of cells, which were clusters or single type of cells were also observed.
Histology and Immunohistochemistry
Cartilage explants were fixed with 4% formaldehyde neutral buffer (Nacali Tesque Inc., Kyoto, Japan) for 48 h and decalcified in Shandon TBD-2 decalcifier (Fisher Scientific, Pittsburgh, PA, USA) by resecting the subchondral bone for 24 h. Paraffin-embedded samples were sectioned at 5 µm, deparaffinized in a xylene substitute, passed through an ethanol series, and finally placed in xylene again for clear penetration. Immunohistochemical analysis was performed with antibodies of STRO-1 (R&D Systems, Minneapolis, MN, USA),15 proliferation marker FGF2 (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), and Ki-67 (Novus, CO, CA, USA). Sections were washed with phosphate-buffered saline (PBS) and blocked with skimmed milk at room temperature for 10 min. Primary antibodies STRO-1, diluted to 1/50; FGF2 and Ki67, diluted to 1/100; and negative controls were applied and incubated overnight at 4°C. After washing with PBS, sections were incubated with biotinylated goat anti-mouse IgM (Vector Laboratories, Burlingame, CA, USA) for STRO-1 and biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) for FGF2 and Ki-67 as secondary antibodies for 60 min at room temperature. After washing with PBS, all sections were incubated with streptavidin-horseradish peroxidase conjugate (HRP; Vector Laboratories, Burlingame, CA, USA) for 10 min at room temperature and stained with a 3,3'-diaminobenzidine tetrahydrochloride substrate kit (DAB; Invitrogen Corporation, Camarillo, CA, USA) for 3 min.22
Chondrocyte Death in Cartilage Explant (Live/ Dead Assay)
Cartilage explants, which were harvested from the MPC and the LPC, were cultured with Dulbecco's modified Eagle's medium (DMEM, Wako Pure Chemical Industries, Osaka, Japan) supplemented with calcein-AM (1.0 µM) and propidium iodide (0.5 µM) for 30 min using a fluorescent double-staining kit (Dojindo Laboratories, Kumamoto, Japan). Live (stained with calcein-AM) and dead cells (stained with propidium iodide) were simultaneously observed and counted with a confocal microscope (SP8; Leica, Wetzlar, Germany).
Chondrocyte Isolation and Culture Condition
Cartilage explants were digested with collagenase D (Roche Applied Science, Penzberg, Germany) for 4 h and cultured in DMEM supplemented with 10% calf serum for chondrocyte isolation. 0.5 × 106 cells were suspended in a culture dish for 2 weeks to determine cell proliferation and cartilaginous nodule production by toluidine blue staining (Muto Pure Chemicals, Tokyo, Japan).23
Response of Chondrocyte Clusters with Organ Culture
To evaluate the recovery of fibrillated cartilage histologically, explants from the medial condyle were cut and divided into two pieces. One piece was fixed immediately (Day 0), and another piece was cultured at 37°C and 5% CO2 in DMEM supplemented with 10% calf serum and antibiotics for 2 weeks (Day 14). The contiguous sides of day 0 and day14 sections were analyzed histologically by Hematoxylin and eosin stain and STRO-1.
Statistical Analysis
The four groups were compared using the Dwass-Steel procedure, followed by the Kruskal-Wallis procedure. Comparison between two groups was analyzed using the Wilcoxon signed-rank test. The results are reported as the mean ± standard deviation (SD). P-values of < 0.05 were considered to be significantly different. All statistical analyses were performed using SAS statistical software version 9.0.2 (SAS Institute Inc. Cary, NC).
RESULTS
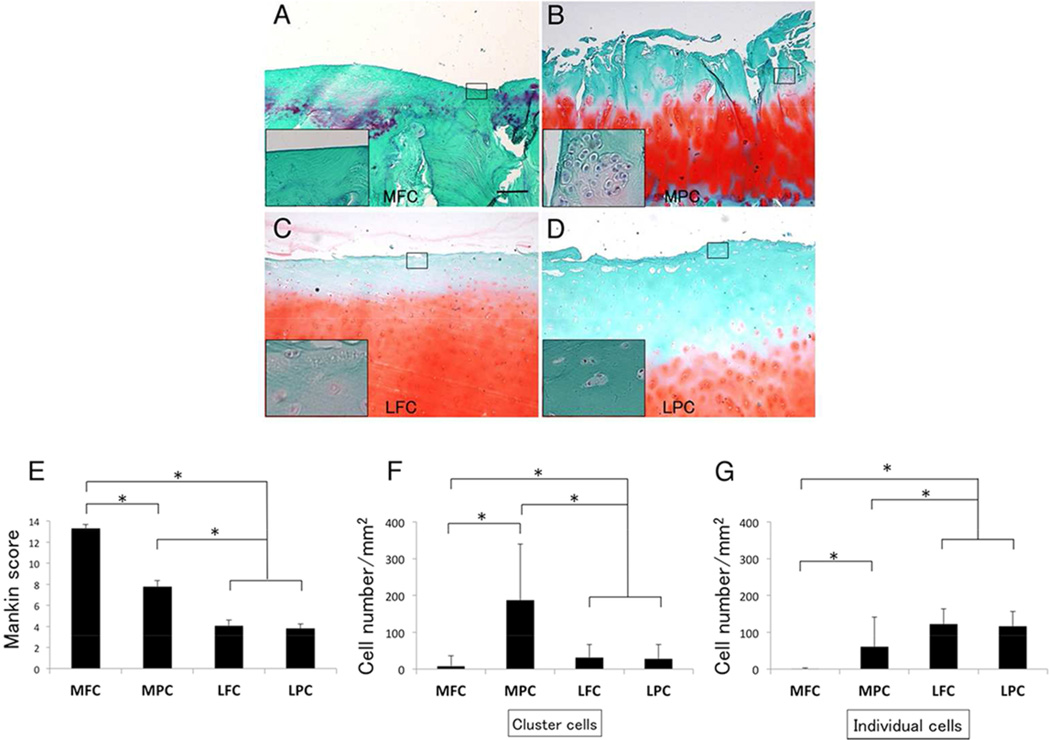
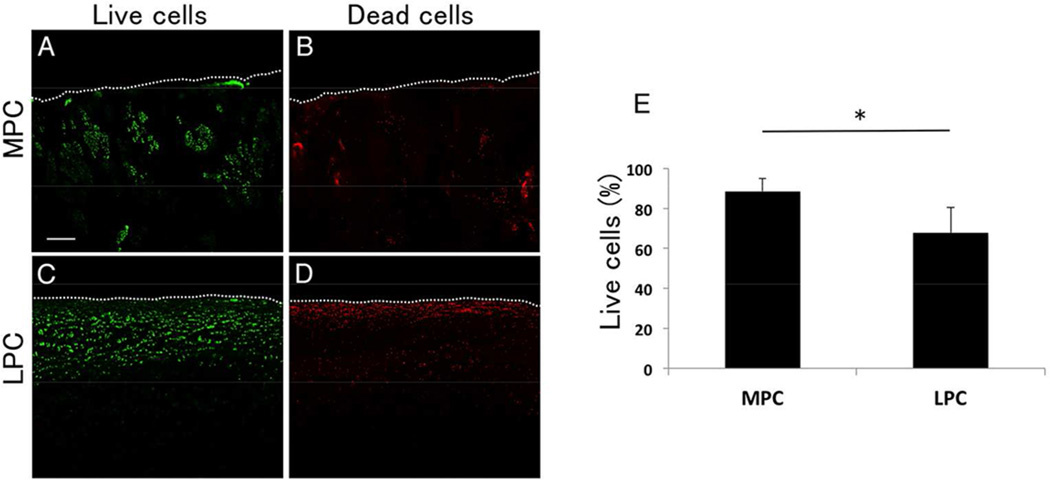
Most of cartilage in the MFC was severely degenerated and there were few chondrocytes (Fig. 2A and F). Cartilage in the MPC was fibrillated and clefts were extended from superficial to deep zone, however chondrocytes in fibrillated cartilage contained cluster formation with hypercellularity (Fig. 2B and F). Cartilage in the LFC and the LPC displayed a normal surface, but a moderate reduction was observed with safranin-O staining (Fig. 2C and D). Regarding the Mankin score, the MFC was much higher (13.3 ± 1.7) than any other locations such as the MPC (7.8 ± 2.9), the LFC (4.1 ± 2.7) and the LPC (3.8 ± 2.2; p < 0.001), suggesting medial weight bearing area had the most severe cartilage degeneration in varus knee joint (Fig. 2E). In terms of the chondrocyte density, the MPC (247.3 ± 130.9 /mm2) was significantly higher than the LFC (154.5 ± 38.0 /mm2), the LPC (145 ± 35.6 /mm2) and the MFC (9.0 ± 30.0 /mm2, p < 0.001). Chondrocyte arrangement in the MPC involved many clusters (cluster: 186.8 ± 152.9 /mm2, single: 60.5 ± 80.8 /mm2), whereas the LFC (cluster: 31.8 ± 34.9 /mm2, single: 122.5 ± 41.2 /mm2) or the LPC (cluster: 28.0 ± 38.9 /mm2, single: 116.7 ± 39.8 /mm2) mainly exhibited single type of cells (Fig. 2F). There seemed to be no significant difference in cartilage degeneration and chondrocyte cellularity in lateral knee cartilage. To determine the chondrocyte survival, live/dead assay showed that chondrocytes in the MPC had a higher survival rate (87.0 %) rather than the LPC (75.0%, Fig. 3).
Figure 2.
Cartilage degeneration and cell density at each location. (A) Most of cartilage in the MFC was severe degenerated with few chondrocytes. (B) Cartilage in the MPC was fibrillated and involved chondrocyte clusters. (C, D) Cartilage surfaces of the LFC and the LPC were smooth, and the chondrocytes were mainly arranged single type of cells. Original magnification was 40×, and 200× at small panels. (E) Mankin score of the MFC was higher than any other location (P < 0.001). (F) Chondrocyte density in the MPC was highest with cluster formation. Chondrocyte characteristics in the LFC or the LPC were mainly single type of cells. *p < 0.001; difference between cluster, #p < 0.001; difference between single cell.
Figure 3.
Live dead assay. Chondrocyte clusters in the MPC (87.0%) contained more live cells than those in the LPC (75.0%). Original magnification was 40×.
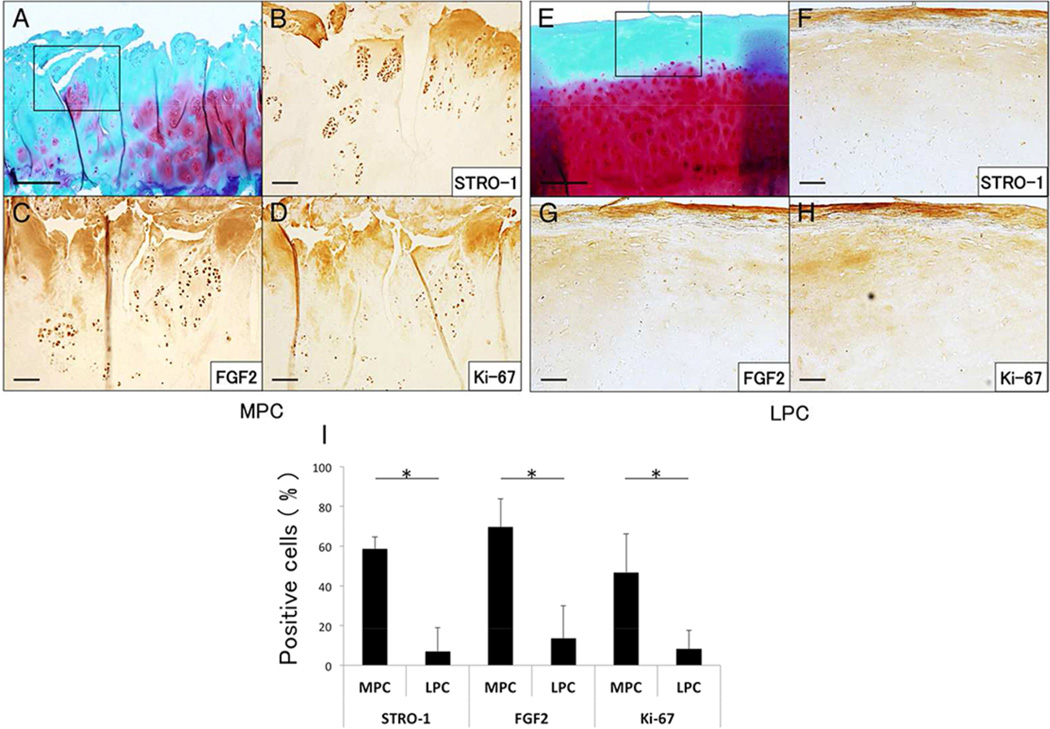
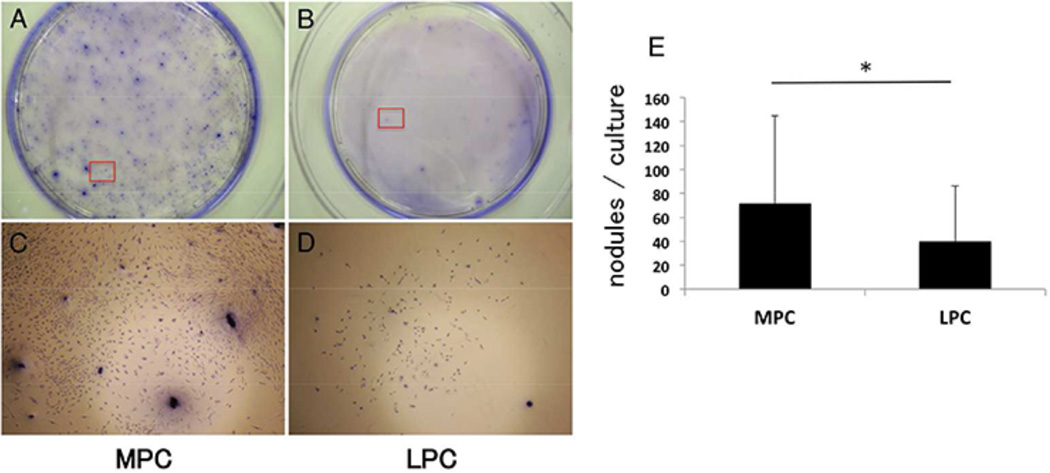
To identify the characteristics of chondrocytes, cartilage explants were stained with STRO-1, FGF2, and Ki-67. Expressions of these markers were significantly higher in the MPC (STRO-1; 58.7 ± 5.9%, FGF2; 69.7 ± 14.1% of, Ki-67; 46.9 ± 19.4%) compared with the LPC (STRO-1; 7.0 ± 11.9%, FGF2; 13.6 ± 16.5%, Ki-67; 8.2 ± 9.4%, Fig. 4). Furthermore, chondrocytes from the MPC showed higher proliferation and produced more cartilaginous nodules (Fig. 5A and C), compared with the chondrocytes from the LPC (Fig. 5B and D).
Figure 4.
Progenitor and proliferative characteristics of chondrocytes. (A-D) Most chondrocyte clusters in the MPC were expressed STRO-1, FGF2 and Ki-67. (E-H) On the other hand, single type of cells in the LPC were barely expressed with these markers. Bar = 100µm. (I) Expression of STRO-1, FGF2, and Ki-67 were significantly higher in the MPC compared with the LPC (*p < 0.001).
Figure 5.
Product of cartilage like nodules in vitro. (A, B) Toluidine blue staining showed a higher rate of chondrocyte proliferation and cartilaginous nodules production in the MPC compared with the LPC for 14 days culture. (C, D) The magnified view (40×) of the area was indicated by a red box in A and D, respectively.
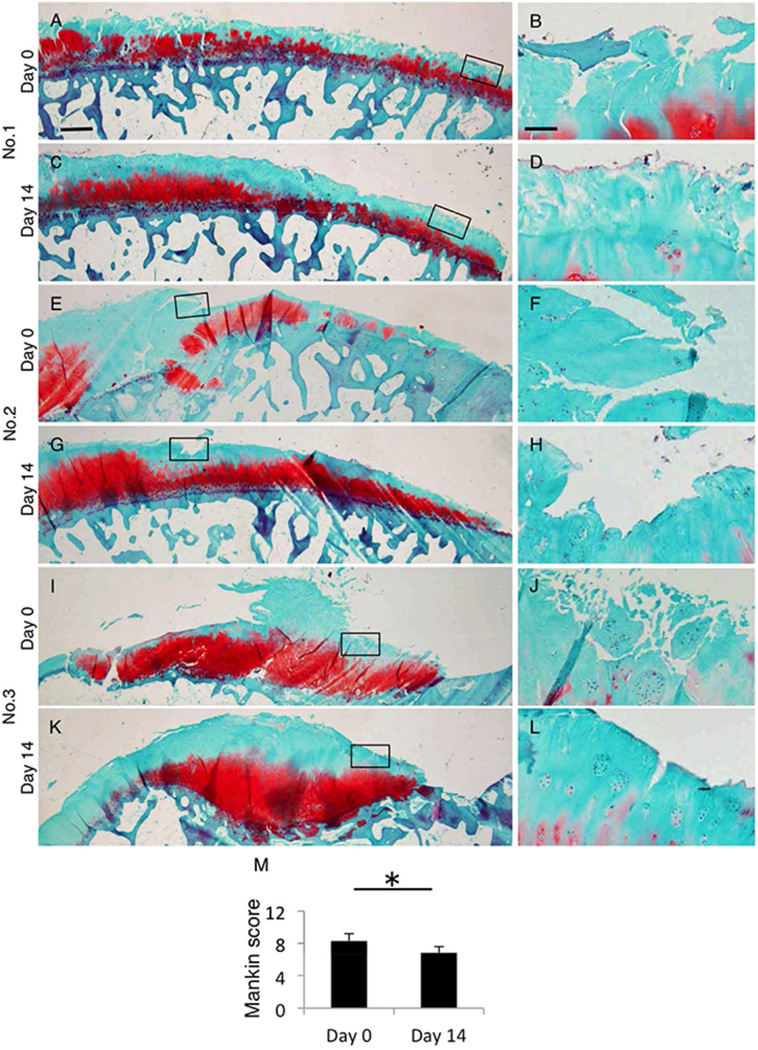
To further elucidate the response of chondrocyte clusters in degenerated cartilage, cartilage explants from the medial femoral condyle, which included much clusters, were cultured with high percentage of calf serum. Although cartilage clefts had been filled with extracellular matrix (ECM), chondrocyte clusters were decreased and chondrocyte density was reduced from 307.5 ± 65.5 /mm2 (day 0) to 193.4 ± 47.4 /mm2 (day14, Fig. 6A, B, D and E). STRO-1 positive cells were simultaneously reduced during this organ culture.
Figure 6.
Response of chondrocyte clusters with organ culture. (A, D) Clefts in cartilage explants were covered with new ECM for 14 days’ organ culture. (B, E) Higher magnification of black box in panel A and D were shown. Cluster formation around clefts was reduced, and STRO-1 positive cells were significantly reduced (C, F). Bar = 500µm for A, Bar = 50µm for B and C.
DISCUSSION
The most important finding in this study was that chondrocyte cellularity differed in a site-specific pattern in OA knee, and OA chondrocytes especially from cluster formation still have the potential of cell proliferation and produce cartilaginous nodules even in the end stage of OA. Although appropriate mechanical stress had been known to be critical for maintaining chondrocyte cellularity and function,24, 25 all samples in this study were harvested from end stage of OA knees due to varus knee malalignment, which had already been recognized to change the compression force in the knee joint.26 The center of the medial femoral condyle (MFC), which suffered the most excessive stress in varus, showed severe cartilage degeneration with few chondrocytes. The adjacent site of cartilage degeneration, which was the part of medial posterior condyle (MPC), included many chondrocyte clusters with higher percentage of live cells. Conversely, cartilage of the lateral condyle (LFC and LPC) included much single type of cells with lower percentage of live cells. All these suggest the difference of chondrocyte distribution and cellularity might be associated with the imbalance of mechanical stress and adjacent severe cartilage degeneration might be receiving most appropriate mechanical stress.
Function of several growth factors is associated with mechanical stress.27 Particularly, FGF2 is known to be released from ECM in response to mechanical stress,17,18 and critical for cartilage homeostasis.28 Incubation of wounded articular cartilage with exogenous FGF2 had reported to enhance cartilage repair through increased chondrocyte proliferation, which results in cluster formation.19 Ki-67 is a nuclear protein, in which positive cells indicate a high turnover with cell proliferation.29 Chondrocyte clusters, which are mainly located at MPC, showed a high percentage of FGF2 and Ki-67, rather than single type of chondrocytes in lateral condyle, suggesting that abnormal mechanical stress affects not only a form of chondrocyte cellularity, but also chondrocyte characteristics.
STRO-1 has been recognized to express in progenitor cells, which have multi-potential for differentiation towards adipocytes, osteoblasts, and chondrocytes,30–32 and we previously reported that cartilage, which contained many STRO-1 positive chondrocytes have a self-repair potential after cartilage injury with a porcine model.22 In this study, chondrocyte clusters, which expressed STRO-1, produced cartilaginous nodule rather than single type of chondrocytes in monolayer culture, indicating the ability of repair by producing cartilaginous nodules even in the human aged severe OA cartilage. To further determine the response of chondrocyte clusters in fibrillated cartilage, 2weeks’ organ culture was observed and the result showed that clefts in fibrillated cartilage were filled with new ECM. One reason of this reaction comes from culture medium, which contained several growth factors such as transforming growth factor (TGF), bone morphogenetic proteins (BMPs), and insulin-like growth factor (IGF).33 On the contrary, the number of chondrocyte clusters, as well as STRO-1 positives, were simultaneously reduced. Growth factors in culture medium may induce not only cartilage repair, but also accelerate cell migration33 because much chondrocytes were found outside cartilage explants in culture medium. Although several issues have still to be included, appropriate environment may contribute to cartilage regeneration,34,35 and activating and regulating chondrocyte clusters may offer new approaches to cartilage repair and OA therapy in the future.
Acknowledgements
We thank Yoshie Seki and Rintaro Oide for expert technical assistance.
This work was supported by the Japan Society for the Promotion of Science KAKENHI 24791571 (Grant-in-Aid for Young Scientists B), and The Nakatomi Foundation.
References
- 1.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Wada M, Kawahara H, et al. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englund M, Guermazi A, Roemer FW, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis. 2010;69:1796–1802. doi: 10.1136/ard.2009.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2012;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto S, Ochs RL, Komiya S, et al. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Mankin HJ, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52:424–434. [PubMed] [Google Scholar]
- 9.Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 10.Lee GM, Paul TA, Slabaugh M, et al. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarthritis Cartilage. 2000;8:44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- 11.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Yen CN, Lin YR, Chang MD, et al. Use of porous alginate sponges for substantial chondrocyte expansion and matrix production: effects of seeding density. Biotechnol Prog. 2008;24:452–457. doi: 10.1021/bp0702828. [DOI] [PubMed] [Google Scholar]
- 13.Lotz MK, Otsuki S, Grogan SP, et al. Cartilage cell clusters. Arthritis Rheum. 2010;62:2206–2218. doi: 10.1002/art.27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuki S, Taniguchi N, Grogan SP, et al. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res therapy. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma L, Song J, Felton DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 17.Vincent TL, Hermansson MA, Bolton M, et al. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent TL, Hermansson MA, Hansen UN, et al. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50:526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 19.Khan IM, Palmer EA, Archer CW. Fibroblast growth factor-2 induced chondrocyte cluster formation in experimentally wounded articular cartilage is blocked by soluble Jagged-1. Osteoarthritis Cartilage. 2010;8:208–219. doi: 10.1016/j.joca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Koshino T. The treatment of Spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am. 1982;64:42–58. [PubMed] [Google Scholar]
- 21.Otsuki S, Nakajima M, Lotz M, et al. Hyaluronic acid and chondroitin sulfate content of osteoarthritic human knee cartilage: Site-specific correlation with weight-bearing force based on femorotibial angle measurement. J Orthop Res. 2008;26:1194–1198. doi: 10.1002/jor.20571. [DOI] [PubMed] [Google Scholar]
- 22.Otsuki S, Grogan SP, Miyaki S, et al. Tissue Neogenesis and STRO-1 Expression in Immature and Mature Articular Cartilage. J Orthop Res. 2010;28:96–102. doi: 10.1002/jor.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschman A. Staining of fresh epiphyseal cartilage with toluidine blue Demonstration of previously unreported intracellular beta- and gamma- metachromatic granules. Histochemie. 1967;10:369–375. doi: 10.1007/BF00304320. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Videman T, Shimazaki K, et al. Experimental study on the repair of full thickness articular cartilage defects: effects of varying periods of continuous passive motion, cage activity, and immobilization. J Orthop Res. 1987;5:187–197. doi: 10.1002/jor.1100050205. [DOI] [PubMed] [Google Scholar]
- 25.Nugent-Derfus GE, Takara T, O'neill JK, et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566–574. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kettelkamp DB, Chao EY. A method for quantitative analysis of medial and lateral compression forces at the knee during standing. Clin Orthop Relat Res. 1972;83:202–213. doi: 10.1097/00003086-197203000-00037. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F, Fermor B, Keefe FJ, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 28.Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644–652. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- 29.Thomas S, Johannes G. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 31.Neumann K, Dehne T, Endres M, et al. Chondrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral cortico-spongious bone. J Orthop Res. 2008;26:1449–1456. doi: 10.1002/jor.20635. [DOI] [PubMed] [Google Scholar]
- 32.Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–612. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 33.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 34.D’Lima DD, Hashimoto S, Chen PC, et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 35.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]