Abstract
Calcific aortic valve disease (CAVD) is the most common cause of aortic stenosis. Matrix metalloproteinases (MMP) are upregulated in CAVD and contribute to valvular remodeling and calcification. We investigated the feasibility and correlates of MMP-targeted molecular imaging for detection of valvular biology in CAVD.
Methods
ApoE−/− mice were fed a Western diet (WD) for 3, 6 and 9 months (n= 108) to induce CAVD. Wild-type mice served as the control group (n=24). The development of CAVD was tracked with CT, echocardiography, MMP-targeted microSPECT imaging using RP805, a 99mTc-labeled tracer, and histological analysis.
Results
Key features of CAVD, leaflet thickening and valvular calcification were noted after 6 months of WD, and were more pronounced after 9 months. This was associated with a significant reduction in aortic valve leaflet separation and increase in trans-aortic valve flow velocity. On in vivo microSPECT/CT images, aortic valve area MMP signal was significantly increased in mice on WD for 6 months compared to control animals and decreased thereafter. The specificity of the signal was demonstrated by blocking using excess non-labeled precursor. Similar to MMP signal, MMP activity by in situ zymography and valvular inflammation by CD68 staining were maximal at 6 months. There was a significant correlation between RP805 uptake in vivo, and MMP activity (R2=0.94, p<0.05) or CD68 expression (R2=0.98, p<0.01) in CAVD.
Conclusions
MMP-targeted imaging detects valvular inflammation and remodeling in CAVD. If confirmed in humans, this may provide a tool for tracking the effect of emerging medical therapeutic interventions and predicting outcome in CAVD.
Keywords: Molecular imaging, Aortic valve, Inflammation, Calcification, Matrix metalloproteinases
INTRODUCTION
Aortic stenosis is the most common cause of valvular heart disease, and calcific aortic valve disease (CAVD) is the most common cause of aortic stenosis (1). Currently, treatment for CAVD is limited to valve replacement, valvuloplasty or transcatheter aortic valve replacement for symptomatic stenosis. Several medical treatments evaluated for slowing down or reversing the pathogenic process in CAVD have been found to be ineffective in clinical trials (2-4). It is speculated that this lack of efficacy may be due to initiation of therapeutic interventions at an irreversible stage of CAVD, or that because only a fraction of patients with aortic sclerosis develop symptomatic aortic valve stenosis it may be hard to demonstrate treatment efficacy in the presence of a large number of low risk subjects. The high prevalence of aortic sclerosis in general population (~25% in subjects >65 years old (5) would suggest that early diagnosis, by itself, may not be sufficient and it would be important to identify the subset of patients who would most benefit from preventive and therapeutic interventions. Molecular imaging targeted at relevant aspects of aortic valve biology can lead to detection of processes that determine disease progression. Ultimately, this can facilitate drug development and early assessment of the effect of therapeutic interventions.
Several aspects of aortic valve biology have been targeted in murine and rabbit models of CAVD by ex vivo near-infrared fluorescent (NIRF) imaging and magnetic resonance imaging and spectroscopy (6-8). While useful for mechanistic studies in animal models, these approaches have limited, if any possibility of “translation” to clinical imaging in humans using current imaging technology. Recent reports on 18F-fluorodeoxyglucose (FDG) and 18F- sodium fluoride (NaF) PET imaging of aortic valve demonstrate the feasibility non-invasive imaging of valvular biology by nuclear imaging in humans (9, 10). However, 18F-FDG imaging is notoriously non-specific and the clinical value of 18F-NaF imaging, which detects the calcification process, remains to be fully established. Given the key role of matrix metalloproteinases (MMPs) in valvular remodeling and inflammation and their high levels of expression in human CAVD (11), we investigated the feasibility of MMP-targeted microSPECT/CT (single photon computed tomography/computed tomography) imaging for in vivo detection of valvular biology and structure in a murine model of CAVD.
MATERIALS AND METHODS
Please refer to supplemental data for details of MATERIALS AND METHODS.
Animal Model
Four to five-week-old apoE−/− mice (originally from Jackson Laboratory) were fed a high fat Western diet (WD, 0.15% cholesterol and 40% calories from fat) for 2-4 months (3 months group, n=25), 5-7 months (6 months group, n=45) and 8-10 months (9 months group, n=38). Wild-type mice were fed with normal chow for 3-6 months and served as the control group (n=24). The experimental scheme is presented in Supplemental Figure 1. All experiments were performed according to regulations of Yale University and VA Connecticut Institutional Animal Care and Use Committees.
Imaging
Echocardiographic images were obtained using a VisualSonics Vevo 2100 system with a 30MHZ probe. RP805, a 99mTc-labeled tracer with specificity for activated MMPs, was provided by Lantheus Medical Imaging and labeled as described (12). MicroSPECT/CT imaging was performed and analyzed as described previously with minor modifications using a high resolution small animal imaging system (Gamma Medica X-SPECT) (13-15). After completion of in vivo imaging, the heart (with aortic valve) and aorta were harvested for ex vivo planar imaging and gamma-well counting.
Statistical Analysis
Values from continuous parameters were presented as mean±SE. Data from two groups were compared by Student’s t-test. Multiple groups were compared by one-way ANOVA followed by Bonferroni multiple comparison test. The linear relationship between two variables was addressed using Pearson’s correlation coefficient. The level of significance was set at p<0.05.
RESULTS
CAVD in Apolipoprotein E Deficient Mice
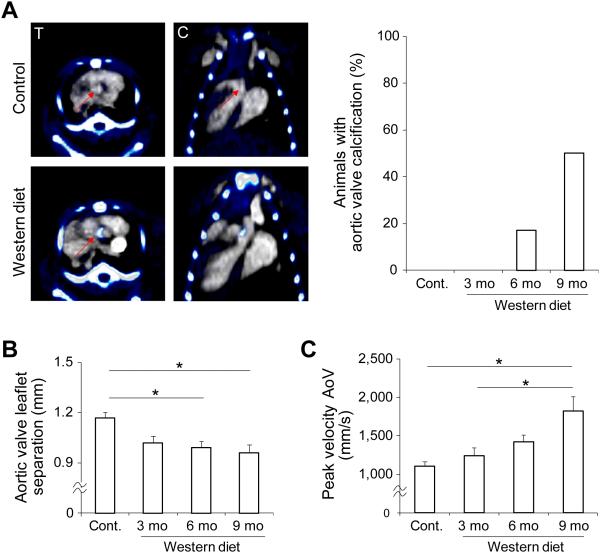
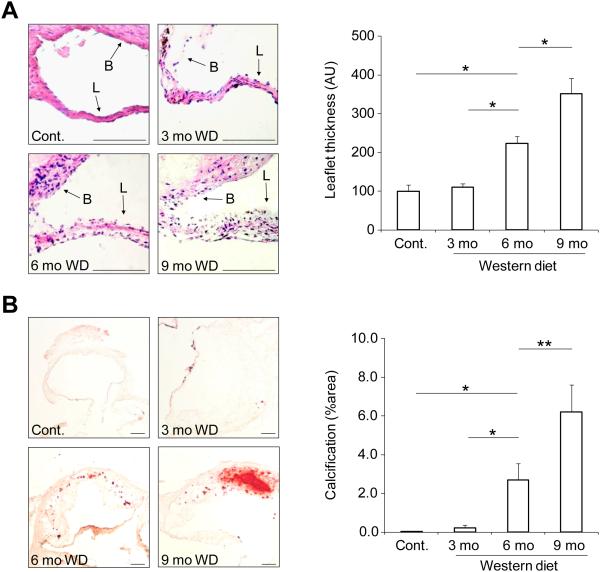
To induce calcific aortic valve disease (CAVD), we fed apoE−/− mice Western diet (WD) for up to 9 months. The development of valvular calcification in these animals was monitored by combining a non-contrast CT with a contrast-enhanced CT performed to localize aortic valve while the animal was kept in the same position, (Fig. 1A). While there was no detectable valvular calcification on in vivo CT images in control mice or those fed on WD for three months, valvular calcification could be seen in a subset (17%) of mice after 6 months of WD and the incidence of detectable calcification increased to 50% in animals fed on WD for 9 months. To investigate whether these animals develop aortic stenosis, we assessed aortic valve anatomy and physiology by echocardiography. Compared with control mice, leaflet separation was significantly reduced in apoE−/− mice fed on WD for 6 or 9 months (1.17±0.03, 1.02±0.04, 0.99±0.04, and 0.96±0.05 mm, respectively for control mice and 3, 6, and 9 months WD. n = 14-23 in each group, p < 0.05 for both, Supplemental Fig. 2A and Fig. 1B). Defining significant aortic stenosis as maximum leaflet separation less than mean-2.5 standard deviation of control animals, the incidence of significant aortic stenosis in animals on WD increased from 15.8% and 17.4% respectively at 3 and 6 months to 30% at 9 months. The reduction in aortic valve leaflet separation at 9 months in WD-fed mice relative to control animals was associated with a significant increase in peak flow velocity across aortic valve (but not in left ventricle outflow tract) in these animals (Fig. 1C and Supplemental Fig. 2B). Histological analysis of aortic valve tissue showed a gradual thickening of leaflets over time in animals on WD. As such, relative to leaflets of control mice or animals fed on WD for 3 months, aortic valve leaflet thickness was significantly larger after 6 months of WD and further increased after 9 months (n = 3 in each group, p < 0.001) (Fig. 2A). Consistent with CT data, alizarin-red staining showed a gradual increase in valvular calcification over time in WD-fed mice with considerable amount of calcification detectable after 6 months. Indeed, the percentage of alizarin-red positive area was significantly higher in aortic valves of mice at 6 and 9 months of WD relative to valves of control mice or animals fed on WD for three months (n = 3 in each group, p < 0.01 and < 0.001 respectively for 6 months vs control or 3 months WD, and 9 months vs 6 months WD) (Fig. 2B).
Figure 1.
CAVD development in apoE−/− mice. A) Representative examples of fused non-contrast (pseudo-colored) and contrast-enhanced CT images of a control (left panel, top row) and an apoE−/− mouse fed on Western diet for 6 months (left panel, bottom row) demonstrating aortic valve calcification in the high fat fed animal; and percent of animals with CT-detectable aortic valve calcification (right panel). Arrow: aortic valve area, T: transverse, C: coronal, n=7, 6, 23, and 16 for control, 3 months, 6 months, and 9 months WD, respectively. B) Quantification of M mode echocardiography-derived systolic aortic valve leaflet separation. *p<0.05, n=14-23 in each group. C) Echo Doppler-derived peak systolic flow velocity across aortic valve (AoV) *p<0.05, n=14-23 in each group. mo: months.
Figure 2.
Histological analysis of CAVD in apoE−/− mice. A) Examples of aortic valve leaflet H&E staining (left panel) and quantification of aortic valve leaflet thickness (right panel). L: leaflet, B: base. Scale bar: 100 um. *p<0.001, n=3 in each group. B) Examples (left panel) and quantification (right panel) of alizarin red staining of aortic valve calcification. Scale bar: 100 um. *p<0.01, **p<0.001, n=3 in each group. mo: months.
MMP Imaging of CAVD
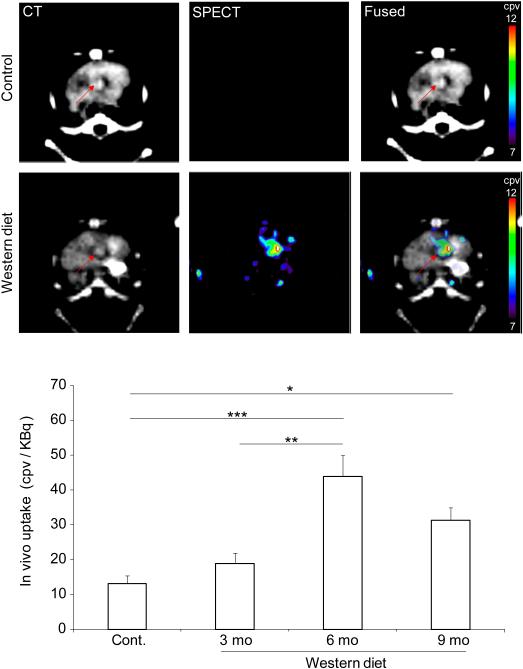
To investigate the feasibility of MMP-targeted imaging in murine CAVD, control animals and apoE−/− mice fed on WD for up to 9 months underwent microSPECT imaging with RP805, a 99mTc-labeled tracer with specificity for activated MMPs. SPECT was combined with contrast-enhanced CT to identify anatomic structures. RP805 signal in aortic valve area was best detectable on in vivo microSPECT/CT images acquired in animals fed on WD for 6 months (Fig. 3 and Supplemental Fig. 3). Quantification of aortic valve RP805 signal demonstrated significantly higher tracer uptake in animals fed on WD for 6 months compared to control mice or animals on WD for 3 months [13±2, 19±3, 44±6 and 31±6 counts per voxel (cpv)/KBq injected dose, respectively for control mice and 3, 6, and 9 months WD. n = 7-13 in each group, p < 0.0001 and < 0.01 for control and 3 months WD, respectively]. Despite their higher level of valvular calcification, in animals on WD for 9 months there was a non-statistically significant trend toward less aortic valve RP805 uptake compared to mice on WD for 6 months.
Figure 3.
In vivo imaging of MMP activation in CAVD. Examples of in vivo CT angiography, RP805 microSPECT, and fused transverse images in a control (top panel, top row) and an apoE−/− mouse fed on Western diet for 6 months (top panel, bottom row); and background-corrected quantification of RP805 uptake in CAVD (bottom panel). Arrows point to aortic valve area. cpv: counts per voxel. mo: months. n = 7-13 in each group, *p < 0.05, **p < 0.01, ***p < 0.0001.
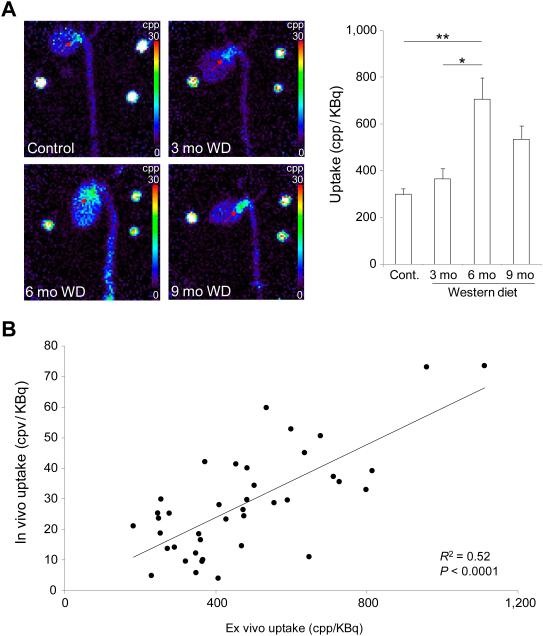
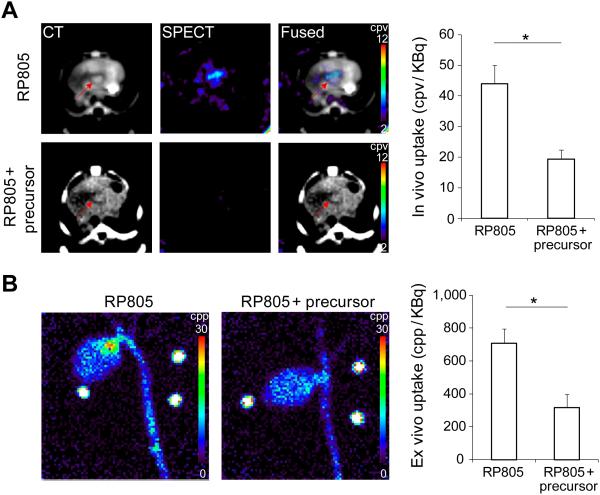
RP805 uptake in aortic valve was confirmed by ex vivo planar imaging (Fig. 4A). Consistent with in vivo imaging results, RP805 uptake in aortic valve area was significantly higher in animals fed on WD for 6 months than control mice or animals fed on WD for 3 months [299±25, 366±43, 707±90, and 535±56 counts per pixel (cpp)/KBq injected dose, respectively for control mice and 3, 6, and 9 months WD. n = 9-15 in each group, p < 0.001 and < 0.05 for control and 3 months WD, respectively). Importantly, in vivo quantification of RP805 uptake correlated well with its ex vivo quantification (R2 = 0.52, P < 0.0001, Fig. 4B). The small size of the valve and its leaflets did not allow more refined localization of RP805 uptake in the valve leaflets or its base by planar imaging. Therefore, we quantified tracer uptake in the leaflets and the base in a subset of animals by gamma-well counting. A significant correlation between RP805 signal in aortic valve leaflets and the base (n = 9, R2 = 0.47, p < 0.05, data not shown) indicated the involvement of the whole valve apparatus in tracer uptake. In order to investigate the specificity of tracer uptake, a group of apoE−/− mice (n = 4) on WD for 6 months were administered 400-fold excess unlabeled precursors immediately prior to RP805 administration. In vivo microSPECT/CT and ex vivo planar imaging showed a significant reduction in RP805 uptake in aortic valve following pre-administration of excess precursor, indicating the specificity of RP805 uptake in this model (p < 0.05 for both, Fig. 5A and B).
Figure 4.
Ex vivo imaging of MMP activation CAVD. A) Representative examples of ex vivo RP805 planar images of the heart and aorta in a control mouse and animals fed on Western diet (WD) for 3, 6, or 9 months (left panel); and background-corrected quantification of RP805 uptake in CAVD (right panel). Arrows point to aortic valve area. Three point sources are seen in the field of view. mo: months. n = 9-15 in each group, *p < 0.01, **p < 0.001. B) Correlation between in vivo and ex vivo quantification of RP805 uptake in CAVD. R2 = 0.52, p < 0.0001.
Figure 5.
Specificity of RP805 uptake in CAVD. A) Representative examples of in vivo CT angiography, RP805 microSPECT, and fused transverse images in apoE−/− mice fed on Western diet for 6 months without (top row) and with (bottom row) pre-administration of excess unlabeled precursor (left panel), and background-corrected quantification of RP805 uptake in CAVD without (n = 10) and with (n = 4) pre-administration of excess non-labeled precursor (right panel). Arrows point to aortic valve area. cpv: counts per voxel. *p < 0.05. B) Representative examples (left panel) and quantification (right panel) of RP805 uptake on ex vivo planar images in apoE−/− mice fed on WD for 6 months without (n = 13) or with (n = 4) pre-administration of excess non-labeled precursors. *p < 0.05. cpp: counts per pixel.
Correlates of MMP Signal in CAVD
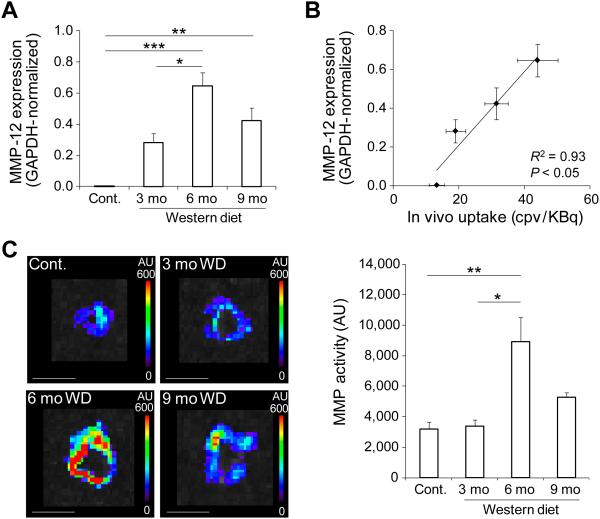
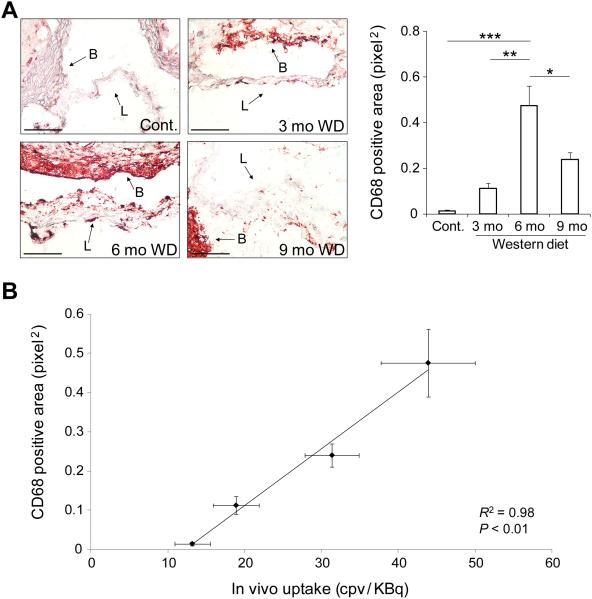
In line with the role of MMP activation in valvular remodeling and stenosis, the temporal pattern of MMP signal in CAVD (which was maximal at 6 months) was found to be different from the pattern of aortic valve leaflet separation, flow velocity and calcification. MMP-12 is the most upregulated MMP gene in CAVD (11). In the absence of reliable anti-murine MMP-12 (and other MMPs) antibodies for immunostaining, we relied on quantitative RT-PCR to assess MMP-12 expression in our model. Similar to RP805 uptake pattern, MMP-12 expression was maximal in apoE−/− mice after 6 months of WD (n = 3-5 in each group, p < 0.0001 and < 0.05 for 6 months WD vs control and vs 3m WD, respectively, Fig. 6A). Furthermore, there was a significant correlation between in vivo RP805 uptake and MMP-12 expression (R2 = 0.93, p < 0.05, Fig. 6B). Next, we assessed MMP activity in aortic valve by in situ zymography using a fluorogenic panMMP substrate. Aortic valve MMP activity was maximal in apoE−/− mice fed on WD for 6 months (n = 3 in each group, p < 0.01 and < 0.05 for 6m WD vs control and vs 3m WD, respectively, Fig. 6C). MMP activity in aortic valve leaflets detected by high resolution confocal microscope paralleled MMP activity of the whole valve, with the highest activity seen in the leaflets of animals fed on WD for 6 months (Supp. Fig. 4A). Consistent with RP805 targeting of MMP activation, there was a significant correlation between in vivo RP805 uptake in CAVD and MMP activity quantified by in situ zymography (R2 = 0.94, p < 0.05, Supp Fig. 4B). As inflammation is a major contributor to MMP production and activation, we examined the macrophage content of aortic valve in the course of CAVD development. In aortic valves of control animals, few, if any, macrophages could be detected by CD68 immunostaining (Fig. 7A). In apoE−/− mice fed on WD for three months, CD68 staining was detectable at low levels. Following a similar temporal pattern as RP805 uptake, aortic valve CD68 expression was maximal at 6 months of WD and decreased by 9 months with progression of valvular calcification (n = 3 in each group, p < 0.001 and < 0.01 for 6 months WD vs control and 3m WD, respectively). There was a significant correlation between in vivo RP805 uptake and CD68 expression (R2 = 0.98, p < 0.01, Fig. 7B).
Figure 6.
MMP expression and activation in CAVD. A) GAPDH-normalized MMP-12 expression assessed by quantitative RT-PCR in CAVD. n = 3-5 in each group, *p<0.05, **p<0.01, ***p<0.0001. B) Correlation between MMP-12 expression and in vivo RP805 uptake in CAVD. R2 = 0.93, p < 0.05. cpv: counts per voxel. C) Representative examples (left panel) and quantification (right panel) of MMP activation detected by in situ zymography in CAVD. Scale bar: 4 mm. WD: Western diet. AU: arbitrary units. n= 3 in each group, *p < 0.05, **p < 0.01. mo: months.
Figure 7.
Inflammation in CAVD. A) Representative examples (left panel) and quantification (right panel) of CD68 expression detected by immunohistochemistry in CAVD. L: leaflet, B: base, mo: months. Scale bar: 100 um. n = 3 in each group, *p < 0.05, **p < 0.01, ***p < 0.001. B) Correlation between CD68 expression and in vivo RP805 uptake in CAVD. R2 = 0.98, p < 0.01.
DISCUSSION
Our data demonstrate the development of CAVD in apoE−/− mice fed a Western diet for 9 months, establish the feasibility of MMP-targeted microSPECT/CT imaging of CAVD, and define the correlates of MMP activation in aortic valve, demonstrating a potential application of MMP-targeted imaging for tracking valvular remodeling in CAVD. Traditionally, the focus of clinicians who evaluate subjects with CAVD has been on detection and quantification of valvular stenosis. Somewhat similar to the relation between atherosclerosis and plaque rupture, high grade stenosis is the outcome only in a small subset of subjects with CAVD. In addition many patients with severe CAVD do not have critical aortic valve stenosis while others with less advanced disease become symptomatic. As such, CAVD and symptomatic valvular stenosis are distinct, yet related concepts. In vivo detection and quantification of pathogenic processes of CAVD is a pre-requisite for the development and evaluation of novel therapies aimed at preventing the progression of CAVD.
Murine models of CAVD are based on genetic and/or dietary interventions and lead to varying degrees of aortic stenosis (16). Here, we show that in Western diet-fed apoE−/− mice, aortic valve leaflets thicken over time and nearly half of the animals develop CT-detectable valvular calcification by 9 months. This valvular remodeling is associated with significant reduction in leaflet separation and increased aortic valve peak flow velocity. While accurate evaluation of aortic stenosis in humans can be difficult, the evaluation of aortic stenosis in the mouse is a rather challenging task with many pitfalls. The main echocardiography-based methods to evaluate aortic valve anatomy and function in the mouse are M-mode echocardiography which provides the necessary spatial and temporal resolution to assess aortic valve leaflet separation, and has been validated against invasive measurement of trans-valvular gradient (16), and Doppler to estimate transvalvular pressure gradient and valve area. Doppler measurements alone can be influenced by confounders such as aortic regurgitation, LV function and cardiac output. However, the combination of changes in M mode measurement of aortic valve leaflet separation and transvalvular Doppler velocity in the absence of a significant change in LV outflow track velocity indicates the development of aortic stenosis in WD fed apoE−/− mice.
Inflammation, extracellular matrix remodeling (including fibrosis), and calcification are hallmarks of CAVD (17, 18). Normal aortic valve leaflets are covered by an endothelial layer and contain several extracellular matrix proteins (e.g., collagen, elastin, fibronectin and laminin), proteoglycans and valvular interstitial cells. There are few resident leukocytes (mainly macrophages) in the normal aortic valve (17). Mechanical stress and deposition of lipid particles, among other factors, induce focal inflammation, which is believed to play a central role in the pathogenesis of CAVD (19). As such, inflammation triggers osteoblastic transformation of valvular interstitial cells, and ultimately valvular calcification. Pro-inflammatory cytokines produced by inflammatory cells trigger valvular MMP expression and matrix remodeling (18).
MMPs are a multi-gene family of endopeptidases that selectively digest individual components of the extracellular matrix (20). Most secreted MMPs are released into the extracellular space in a latent or proenzyme state. Activation of these latent MMPs exposes the catalytic domain targeted by 99mTc-RP805, the tracer used in our studies. Recent data show that activated MMPs bind to, and can be taken up, by neighboring cells (21). Several MMPs are expressed in CAVD and may contribute to valvular fibrosis and calcification (22-24). Indeed, expression profiling of healthy and (rather advanced) calcific human aortic valves has identified several MMPs amongst the top 10 upregulated genes and MMP-12 as the most upregulated gene in CAVD (11). This led us to investigate the temporal pattern of MMP-12 expression in our model. MMP-12 expression peaked at 6 months of WD, before the development of more advanced calcific lesions, raising the possibility of a similar finding in humans. Potential sources of MMP production and activation in CAVD are inflammatory cells, valvular interstitial cells and endothelial cells (17). Several mediators of valvular inflammation and remodeling, including cytokines, periostin, oxidative stress, and hemodynamics regulate MMP expression and activation (25, 26). Similarly, a number of key regulators of aortic valve calcification, including transforming growth factor (TGF)-β1 and RANKL, as well as mechanical stretch can induce MMP activation in cell or organ culture (27-29). As such, MMP expression and activation appear to be closely linked to valvular remodeling and calcification in CAVD, highlighting the potential of MMP-targeted imaging for detection of the remodeling process in CAVD.
Some aspects of CAVD biology have been targeted by ex vivo optical imaging in the mouse and these studies have provided important insight on aortic valve pathobiology (6, 7). However, noninvasive optical imaging of CAVD lacks a clear path to clinical translation. Unlike optical imaging, nuclear imaging techniques are highly quantitative in nature and can be readily applied to non-invasive imaging in humans. As a first step toward potential clinical application, in this first in vivo imaging study we demonstrated the feasibility of microSPECT/CT-based MMP-targeted imaging of CAVD in the mouse. CAVD is often associated with atherosclerosis, and there is ongoing debate regarding their similarities and differences. The differentiation of leaflet-specific processes from those at the base is impossible and without any practical implication. We found a good correlation between tracer uptake in the leaflets and the base of the valve, and MMP activity in the leaflets paralleled MMP tracer uptake. Together, these data indicate that various components of the valve apparatus contribute to valvular RP805 signal in vivo. We ascertained the validity of our in vivo quantification methodology by correlating the data obtained in vivo with the results of ex vivo imaging. The time course of MMP activation in CAVD suggests that MMP-targeted imaging can be used for tracking the remodeling process in vivo. Indeed, peak MMP activation in aortic valve was noted at 6 months, preceding the development of advanced valvular calcification and stenosis. Not surprisingly, MMP activation in the valve paralleled CD68 expression, suggesting that it may also be useful as a surrogate marker for early assessment of the effect of emerging therapeutic interventions aimed at preventing the progression of the disease.
The failure of lipid lowering, anti-inflammatory treatments in preventing the progression of CAVD in subjects with mild to moderate stenosis (2-4) may be due to limited understanding of pathophysiology and/or poor selection of subjects who might benefit from such interventions. Molecular imaging provides an investigational tool for mechanistic studies of aortic valve pathology. Unique to noninvasive approaches studied here, nuclear-based molecular imaging allows for serial assessment of aortic valve biology in the same subject. Our data suggest that MMP-targeted molecular imaging may be used to detect valvular inflammation, potentially identifying those subjects prone to more rapid disease progression. The ability to do so would have practical implications for the choice of therapeutic interventions, for example when a patient with moderate aortic stenosis is to undergo bypass surgery. In addition, this can help focus clinical trials on subjects who would most benefit from emerging therapies, and help track the effectiveness of interventions. Given the absence of optimal large animal models of CAVD, it is likely that clinical implementation of this approach requires direct validation in patients with early stage aortic stenosis. The cost and added value are among factors to consider in bringing a new imaging technique to the clinic. Approaches such as MMP imaging which target fundamental biological processes (e.g., remodeling and inflammation) have a broad range of potential clinical applications and therefore, are economically more viable for further development for clinical use.
CONCLUSION
The multimodality imaging approach to CAVD anatomy, physiology and biology employed here provides a comprehensive picture of CAVD. If validated in humans, this approach could have a major impact on the management of subjects with CAVD.
Supplementary Material
Acknowledgements
We thank Nicole Mikush for performing and assistance in reading the echocardiography studies.
Funding Sources
This work was supported by National Institutes of Health R01 HL112992, R01 HL114703, and Department of Veterans Affairs Merit Award I0-BX001750 to MMS and P01 HL062984 to DDH.
Footnotes
Disclosures
Simon Robinson is employee of Lantheus Medical Imaging. Mehran M. Sadeghi received experimental tracers from Lantheus Medical Imaging.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 3.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 4.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 5.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 6.Aikawa E, Nahrendorf M, Sosnovik D, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters EA, Chen J, Allen JS, Zhang H, Lanza GM, Wickline SA. Detection and quantification of angiogenesis in experimental valve disease with integrin-targeted nanoparticles and 19-fluorine MRI/MRS. J Cardiovasc Magn Reson. 2008;10:43. doi: 10.1186/1532-429X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marincheva-Savcheva G, Subramanian S, Qadir S, et al. Imaging of the aortic valve using fluorodeoxyglucose positron emission tomography increased valvular fluorodeoxyglucose uptake in aortic stenosis. J Am Coll Cardiol. 2011;57:2507–2515. doi: 10.1016/j.jacc.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 11.Bosse Y, Miqdad A, Fournier D, Pepin A, Pibarot P, Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 12.Su H, Spinale FG, Dobrucki LW, et al. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Nie L, Razavian M, et al. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation. 2008;118:1953–1960. doi: 10.1161/CIRCULATIONAHA.108.789743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razavian M, Zhang J, Nie L, et al. Molecular imaging of matrix metalloproteinase activation to predict murine aneurysm expansion in vivo. J Nucl Med. 2010;51:1107–1115. doi: 10.2967/jnumed.110.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razavian M, Nie L, Challa A, et al. Lipid lowering and imaging protease activation in atherosclerosis. J Nucl Cardiol. 2014;21:319–328. doi: 10.1007/s12350-013-9843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akat K, Borggrefe M, Kaden JJ. Aortic valve calcification: basic science to clinical practice. Heart. 2009;95:616–623. doi: 10.1136/hrt.2007.134783. [DOI] [PubMed] [Google Scholar]
- 18.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 19.Helske S, Kupari M, Lindstedt KA, Kovanen PT. Aortic valve stenosis: an active atheroinflammatory process. Curr Opin Lipidol. 2007;18:483–491. doi: 10.1097/MOL.0b013e3282a66099. [DOI] [PubMed] [Google Scholar]
- 20.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. Febs J. 2011;278:28–45. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- 21.Koppisetti RK, Fulcher YG, Jurkevich A, et al. Ambidextrous binding of cell and membrane bilayers by soluble matrix metalloproteinase-12. Nat Commun. 2014;5:5552. doi: 10.1038/ncomms6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol. 2000;9:281–286. doi: 10.1016/s1054-8807(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 23.Satta J, Oiva J, Salo T, et al. Evidence for an altered balance between matrix metalloproteinase-9 and its inhibitors in calcific aortic stenosis. Ann Thorac Surg. 2003;76:681–688. doi: 10.1016/s0003-4975(03)00529-0. [DOI] [PubMed] [Google Scholar]
- 24.Fondard O, Detaint D, Iung B, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 25.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 26.Hakuno D, Kimura N, Yoshioka M, et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark-Greuel JN, Connolly JM, Sorichillo E, et al. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83:946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Kaden JJ, Dempfle CE, Kilic R, et al. Influence of receptor activator of nuclear factor kappa B on human aortic valve myofibroblasts. Exp Mol Pathol. 2005;78:36–40. doi: 10.1016/j.yexmp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296:H756–764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.