Abstract
Here we expand the set of tools for genetically manipulating Saccharomyces cerevisiae. We show that puromycin-resistance can be achieved in yeast through expression of a bacterial puromycin-resistance gene optimized to the yeast codon bias, which in turn serves as an easy to use dominant genetic marker suitable for gene disruption. We have constructed a similar DNA cassette expressing yeast codon-optimized mutant human dihydrofolate reductase (DHFR) that confers resistance to methotrexate and can also be used as a dominant selectable marker. Both of these drug-resistant marker cassettes are flanked by loxP sites allowing for their excision from the genome following expression of cre-recombinase. Finally, we have created a series of plasmids for low-level constitutive expression of cre-recombinase in yeast that allows for efficient excision of loxP-flanked markers.
Keywords: Saccharomyces cerevisiae, dominant markers, puromycin, methotrexate, cre-recombinase
Introduction
The budding yeast Saccharomyces cerevisiae has many intrinsic advantages as a model system to study a wide-range of biological processes. Beyond research, yeast is increasingly being used for synthetic production of proteins and compounds in industry. The S. cerevisiae genome is relatively small with few introns, and the completion of its sequencing (Goffeau, et al., 1996) has led to a range of tools for global operations and analysis. Examples include microarray analysis and use of genomic libraries, such as GFP-tag, TAP-tag, gene deletion and overexpression collections (DeRisi, et al., 1997; Ghaemmaghami, et al., 2003; Huh, et al., 2003; Winzeler, et al., 1999), these tools collectively provide a tremendous resource. As research targets larger protein complexes and networks, and industrial cell factory projects become more ambitious, demand increases for multiple gene modifications within a single experimental strain.
Conventional prototrophic markers are useful in yeast, but they rely on specific auxotrophic strains and can limit plasmid transformation options. These difficulties have been over-come in 2 main ways. One is using dominant selectable markers that confer drug-resistance, one example being the heterologous expression of the E. coli aminoglucoside 3′ phosphotransferase (kanr) that confers resistance to the aminoglycoside antibiotic gentamicin / G418 (Wach, et al., 1994). Other dominant drug-resistance yeast markers that confer resistance to certain drugs have been described, for example nourseothricin and hygromycin B (Goldstein and McCusker, 1999), phelomycin (Gatignol, et al., 1987), cycloheximide (del Pozo, et al., 1991) and others. A second way to provide for multiple gene manipulations is using markers that can be subsequently excised and reused. For example, a marker flanked by tandem hisG repeats can accomplish this upon intragenic recombination (Alani, et al., 1987) or markers flanked by loxP sites can be excised from the genome using Cre-recombinase, which was first shown for a loxP containing KanMX cassette (Güldener, et al., 1996). Later, other markers, such as URA3 from Kluyveromyces lactis and his5+ from Schizosaccharomyces pombe, which complements S. cerevisiae his3Δ mutants (Gueldener, et al., 2002), were also developed. Despite various selectable markers, projects can still be constrained by consumption of such markers. In addition, some of the most effective drugs are not efficacious enough to eliminate a sequent laborious screening process to eliminate false positives. Finally, projects that require subsequent use of the same marker often use the Cre-lox system to regenerate markers. Commonly, Cre-recombinase production is controlled by the GAL1 promoter. However, many laboratory strains do not grow robustly on galactose, presenting a major problem with using GAL1-driven Cre.
In an effort to expand the repertoire of genetic tools for yeast, we have generated two new dominant marker cassettes that can be used efficiently to delete a gene or register another genetic modification. These heterologous markers confer resistance to the drugs puromycin and methotrexate to wild-type parental strains of both haploid and diploid S. cerevisiae. Both cassettes are flanked by loxP sites, allowing easy marker excision using Cre-recombinase. Additionally, cassettes are housed in the backbone of a series of commonly used PCR template vectors (Gueldener, et al., 2002), so that previously designed oligonucleotides are compatible with the new cassettes. Finally, we have optimized low-level constitutive expression of Cre-recombinase to allow fast marker retrieval, and created a series of selectable and counter-selectable cre-expression plasmids.
Materials and Methods
Reagents
A list of plasmids and yeast strains used and generated in this study are listed in Tables 1 and 2, respectively. Codon optimized versions of the bacterial pac gene and mutant human DHFR were designed for expression in S. cerevisiae and chemically synthesized by GenScript, Piscataway, NJ. The sequence data for the PCR template puror and mtxr cassette vectors and Cre expression plasmids containing different nutritional markers have been deposited at NCBI GenBank and are available upon request from Addgene (The GenBank accession numbers and Addgene plasmid numbers are listed in Table 3).
Table 1. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| BY4741 | MATa his3Δ leu2Δ met15Δ ura3Δ | (Brachmann et al., 1998) |
| BY4742 | MATα his3Δ leu2Δ lys2Δ ura3Δ | (Brachmann et al., 1998) |
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | (Robinson et al., 1988) |
| EE10 | MATa SEY6210 | From Scott Moye-Rowley |
| X2180-1A | MATa SUC2 mal mel gal2 | (Mortimer and Johnston, 1986) |
| X2180-1B | MATα SUC2 mal mel gal2 | (Mortimer and Johnston, 1986) |
| PLY4008 | BY4742; pdr5Δ:: his5+ ubi4Δ::kanr | This study |
| PLY4541 | BY4742; pdr5Δ:: his5+ ubi4Δ::puror | This study |
| PLY4225 | SEY6210; bro1Δ::his5+ | (Pashkova et al., 2013) |
| PLY4650 | SEY6210; bro1Δ::mtxr | This study |
| PLY4689 | BY4742; ade2Δ::puror | This study |
| PLY4752 | Diploid; BY4741 × BY4742 | This study |
| PLY4792 | Diploid; BY4741 × BY4742 ade2Δ::puror | This study |
| PLY4774 | BY4742; pdr5Δ::kanr | (Winzeler et al., 1999) |
| PLY4772 | BY4742; pdr1Δ::kanr | (Winzeler et al., 1999) |
| PLY4773 | BY4742; pdr3Δ::kanr | (Winzeler et al., 1999) |
| PLY4775 | BY4742; erg6Δ::kanr | (Winzeler et al., 1999) |
| PLY4757 | BY4742; ade2Δ::puror ; met15Δ::mtxr | This study |
| PLY4760 | Diploid; BY4741 × BY4742 ade2Δ::mtxr | This study |
| PLY4778 | BY4742; ade2Δ::puror met15Δ::mtxr sna3Δ::his5+ | This study |
| PLY4797 | BY4742; ade2Δ::loxP met15Δ::loxP sna3Δ::loxP | This study |
| PLY4721 | BY4742; ade2Δ::loxP | This study |
| PLY4682 | BY4742; ade2Δ::URA3 | This study |
| PLY4641 | BY4742; trp1Δ::his5+ | This study |
| PLY4721 | BY4742; trp1Δ::loxP | This study |
| PLY4678 | BY4742; met15Δ::mtxr | This study |
| PLY4719 | BY4742; met15Δ::loxP | This study |
Table 2.
Plasmids used in this study.
| Plasmid | Description | Source |
|---|---|---|
| pRS416 | Low copy yeast shuttle plasmid containing URA3 marker | (Sikorski and Hieter, 1989) |
| pRS414 | Low copy yeast shuttle plasmid containing TRP1 marker | (Sikorski and Hieter, 1989) |
| pPL5103 | pRS416 expressing pac from TEF1* promoter (promoter expresses at 0.16× of wild-type TEF) | This study |
| pSH47 | pRS416 expressing cre from GAL1 promoter | (Gueldener et al., 2002) |
| pPL5071 | pRS416 expressing cre from TEF1* promoter | This study |
| pPL5608 | pRS414 expressing cre from TEF1* promoter | This study |
| pPL5606 | pRS41-ADE2 expressing cre from TEF1* promoter | This study |
| pPL5628 | pRS41-MET15 expressing cre from TEF1* promoter | This study |
| pUG27 | pRS400 series backbone containing loxP flanked SpΨhis5+ gene driven from Ag§TEF1 promoter | (Gueldener et al., 2002) |
| pUG72 | pRS400 series backbone containing loxP flanked URA3 gene driven from Ag§TEF1 promoter | (Gueldener et al., 2002) |
| pPL5617 | pRS400 series backbone containing loxP flanked pac gene driven from Ag§TEF1 promoter | This study |
| pPL5618 | pRS400 series backbone containing loxP flanked DHFR* gene driven from Ag§TEF1 promoter | This study |
Spψ = Schizosaccharomyces pombe
Ag§ = Ashbya gossypii
Table 3. Plasmid request information.
| Plasmid | Short Description | GenBank Accession # | Addgene Plasmid # |
|---|---|---|---|
| pPL5617 | loxP-puror-loxP PCR template | KP159511 | 60928 |
| pPL5618 | loxP-puror-loxP PCR template | KP159512 | 60929 |
| pPL5071 | URA3 marked TEF1*-cre | KP159513 | 60930 |
| pPL5608 | TRP1 marked TEF1*-cre | KP159514 | 60931 |
| pPL5606 | ADE2 marked TEF1*-cre | KP159515 | 60932 |
| pPL5628 | MET15 marked TEF1*-cre | KP159516 | 60933 |
Cell culture
Yeast Extract Peptone Dextrose (YPD) rich media (2% glucose, 2% peptone, 1% yeast extract) and synthetic complete (SC) minimal media (2% glucose, 1× yeast nitrogen base; Research Products International, Mount Prospect, IL), with appropriate amino acid and base drop out compositions for selections were used (Formedium, Norfolk, UK). Rich media containing 1 mg/ml lead nitrate was prepared using a modified recipe (4% glucose, 0.3% peptone, 0.5% yeast extract, 0.02% ammonium sulfate). Geneticin (G418; Research products International, Mount Prospect, IL) was used at a concentration of 250 μg/ml in rich media. Puromycin (Gold Biotechnology, St. Louis, MO) was used at 4 mM for selection of strains carrying the pdr5Δ mutation and 20 mM for wild-type background strains. Methotrexate (Sigma-Aldrich, St. Louis, MO) was used at a final concentration of 25 nM in synthetic complete plates. To minimize the expense of puromycin containing plates we routinely use 35 × 10 mm plates containing 2 mls of solidified agar media. Sulphanilamide (Fisher Scientific, Pittsburgh, PA) was added to methotrexate containing plates at a final concentration of 5 mg/ml. 5-fluoroorotic acid (5-FOA; GoldBiotechnology, St. Louis, MO) was added to SC plates at a final concentration of 1 mg/ml. 5-fluoroanthranillic acid (5-FAA; Matrix Scientific, Columbia, SC) was used in SC media at a concentration of 0.5 mg/ml.
Homologous recombination for gene deletion and plasmid construction
Yeast gene deletions were carried out by PCR based homologous recombination to integrate knockout cassette into desired locus. Oligonucleotides were designed containing ~20 nts of 3′ sequence to prime from template DNA and ~50 nts of 5′ sequence with homology to the region of integration. Most plasmids were also generated by homologous recombination, with amplified PCR products containing ~50 bp homology to the host vector, which was first linearized by restriction digest followed by gel extraction and purification (Qiagen, Valencia, CA). The generation of the MET15 marked TEF1*-cre plasmid (pPL5628) was carried out in the methionine auxotroph strain, BY4741. Plasmids generated by homologous recombination were rescued via SURE Escherichia coli cells (Stratagene, La Jolla, CA) and general DNA propagation was carried out in Top10 Escherichia coli cells (Invitrogen Life Technologies, Grand Island, NY). The final PCR template plasmids containing puror and mtxr cassettes were created by standard sub-cloning methods, as detailed in Supplemental Figure S1.
Drug sensitive growth assays
Yeast cultures were grown overnight in SC media, or SC-URA media for cultures transformed with the TEF1-pac expression plasmid (pPL5103), diluted and grown for 6 hours to mid log phase. Cultures were then added to a sterile 96 well plate containing drugs as indicated. A 9-step, 10-fold serial dilution for each condition was created with a final volume of 250 μl in every well. Plates were incubated at 30°C overnight in a humidified incubator, mixed and then the average (n = 3) optical density at 600 nm (OD600) was used to measure yeast growth. Graphs depict OD600 compared with a control well lacking drug. These kill curves were used to estimate the lethal dose of either puromycin or methotrexate required for selection of cells expressing resistance markers. The sensitivity of yeast to certain drug concentrations was also confirmed on solid agar media.
Competitive fitness growth assays
Haploid yeast strains containing dominant drug marker integrations were grown to log phase for 6 hours in YPD media alongside control cell counterparts. Equal volumes of cultures containing either the puror or mtxr cassettes were then mixed with control cells and then diluted in a large volume of YPD media to allow continually growth at exponential phase. Cells were harvested at 1 hour, 24 hour and 48 hour time points, diluted 6000-fold and 9000-fold (pertaining to OD600 = 0.5) before 200 μl of each sample was spread on a non-selective YPD plate and a drug selection plate. Plates were incubated at 30°C to allow growth prior to manual counting of colonies formed.
Immunoblot analysis
Yeast cells transformed with either pSH47 (GAL1-cre) or pPL5071 (TEF1*-cre) were grown to mid-log phase in SC-URA media. Cultures expressing cre from the GAL1 promoter were grown in media containing 2% glucose, 2% raffinose or 2% galactose. Equivalent amounts of cells were harvested from each culture and incubated in 0.2 N NaOH for 3 minutes. Cells were then pelleted again and lysates generated by incubation in Laemmli sample buffer (50 mM Tris.HCl pH 6.8, 5% SDS, 10% glycerol) containing 8 M urea. Lysates were resolved by SDS-PAGE before transfer to nitrocellulose membrane for immunoblotting. Membranes were incubated in a blocking solution (5% milk) for 30 minutes prior to incubation with polyclonal anti-Cre-recombinase antibodies (catalogue #: PRB-106P-200; Covance Antibody Products, Dedham, MA). Immunoblot analysis of Carboxypeptidase Y was carried out as described (Macdonald, et al., 2012a) to serve as a loading control. Membranes were then probed with appropriate secondary antibodies conjugated to HRP. Protein levels were visualized by chemiluminescence and images were collected directly using a digital camera.
PCR analysis of genomic loci
Yeast cultures were grown to saturation in rich media before harvesting and 16 hours incubation in TE (50 mM Tris.HCl pH = 8.0, 20 mM EDTA, 1% 2-mercaptoethanol) buffer containing 200 μg zymolyase T-100. Genomic DNA was isolated by 2× phenol/chloroform/isoamyl (25:24:1) extractions followed by ethanol precipitation. DNA was resuspended in water and used as a template for PCR reactions.
Routine protocol for using new reagents
Puromycin (puror) and methotrexate (mtxr) resistance cassettes were amplified by PCR using 50 ng of template plasmid. DNA was ethanol precipitated, resuspended in water and transformed into competent yeast using standard Lithium acetate methods wherein yeast were pelleted from LiOAc-Sorbital-PEG suspension, resuspended in water, and plated directly onto the drug-containing plate (Gietz and Woods, 2002). Puromycin-resistant yeast from wild-type parents were selected for on small (2 ml) YPD plates containing 20 mM puromycin. Reduced drug concentrations are sufficient for selection of hypersensitive strains, discussed in Figure 3. Methotrexate-resistant yeast were selected on plates containing SC minimal media replete with all amino acids and containing 5 mg/ml sulphanilamide and 50 nM methotrexate. In practice, we added drugs after autoclaving media, however, we found no reduced efficacy of sulphanilamide if the drug is added prior to autoclave sterilization. Drug-resistant single colonies were then patched on fresh selection plates before yeast is grown for isolation of genomic DNA and genotyping. For excision of loxP-flanked cassettes, yeast were transformed with the TEF1*-cre plasmid. Yeast were selected on minimal media plates lacking the appropriate nutrient (e.g. SC-Ura for pPL5071). Single colonies were patched onto fresh plates before preparation of genomic DNA for genotyping.
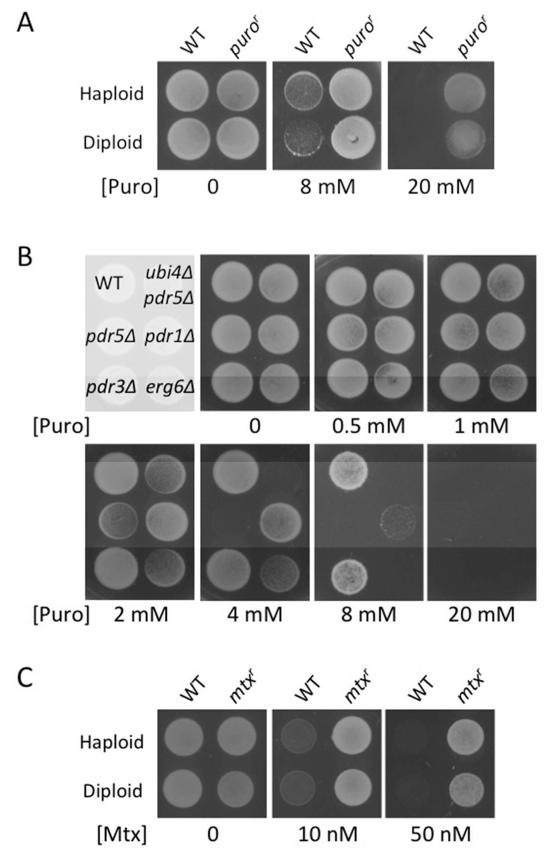
Figure 3. Puromycin and methotrexate selections on solid agar media.
A) Haploid and diploid cells, both wild-type parents and strains carrying a puror cassette, were grown to mid-log phase before dilution and plating. Cultures were plated on rich media containing either 8 mM or 20 mM puromycin, and a control YPD plate that lacked drug.
B) Wild-type cells or strains carrying gene deletions indicated were grown to mid-log phase before plating on YPD plates containing different concentrations of puromycin.
C) Haploid and diploid cells, either wild-type or containjing a mtxr cassette were grown in liquid culture, diluted and then plated on SC media. Cultures were also plated on SC media containing 5 mg/ml sulphanilamide and either 10 nM or 80 nM methotrexate.
Results & Discussion
Puromycin-resistance as a dominant marker in yeast
Puromycin is an antibiotic derived from Streptomyces alboniger that induces chain termination during protein translation by releasing the ribosome (Traut and Monro, 1964). Puromycin is inactivated by puromycin N-Acetyltransferase encoded by the S. alboniger pac gene (Lacalle, et al., 1989; Perez-Gonzalez, et al., 1985; Vara, et al., 1985). Exogenous expression of pac has been used as a dominant marker in mammalian cells (de la Luna, et al., 1988; Vara, et al., 1986), and more recently in C. elegans (Semple, et al., 2010). We titrated concentrations of puromycin in growth media cultures of S. cerevisiae to test whether puromycin could effectively kill wild-type yeast. We also tested cells lacking the multi-drug transporter Pdr5, which are hypersensitive to a wide range of drugs (Ernst, et al., 2005). We found that growth of wild-type yeast is completely inhibited in the presence of 20 mM puromycin, and that growth of pdr5Δ cells was completely inhibited at 2.5 mM (Figure 1A). We took advantage of the hypersensitive pdr5Δ strain, which also harbours a Kanamycin-resistance (kanr) cassette at the UBI4 locus, to test if puromycin-resistance was a viable selection in yeast. We chemically synthesized a version of the pac gene with an optimized codon sequence for expression in yeast and targeted its integration by homologous recombination between the TEF1 promoter and terminator present in the kanr cassette (Figure 1B). Puromycin-resistant (puror) colonies were apparent after 48 hours following transformations containing the pac gene PCR product, with no colonies in the control containing only carrier DNA (Figure 1C). Correct insertion of the pac gene at the UBI4 locus corresponded with replacement of the kanr cassette, demonstrated by failure of puror clones to grow in the presence of G418, unlike the parental strain (Figure 1D). Amplifying the UBI4 locus by PCR was used to confirm the genotype of the puror strains (Figure 1E). We next determined how well the puror cassette functioned in wild-type cells by first introducing it on a low copy CEN-based plasmid (pPL5103). We found that pac gene expression was sufficient to confer resistance to 20, 30 and 40 mM puromycin, concentrations that prevent growth of wild-type cells (Figure 1F).
Figure 1. Puromycin-resistance as a selective marker in yeast.
A) Wild-type and pdr5Δ cells were grown to mid-log phase in SC media before addition to a 2-fold serial dilution of puromycin in a 96-well plate. Each well contained a 250 μl culture of yeast cells at an OD600 = ~0.005. The puromycin gradient ranged from 40 mM to 0.04 mM, with a final well containing no drug. Cells were incubated at 30°C for 72 hours, mixed, and then the OD600 was measured. Growth of wild-type (blue) and mutant pdr5Δ (green) cells is indicated.
B) Schematic diagram of homologous recombination strategy to replace the Kanamycin-resistance (kanr) cassette at the UBI4 locus with that of the pac gene conferring Puromycin-resistance (puror), retaining the original TEF1 promoter and 3′ untranslated (UTR) terminator sequences for expression of the new marker.
C) ubi4Δ::kanr cells (also containing pdr5Δ mutation) were transformed with either a PCR product encoding the pac gene or a carrier DNA control. Cells were plated on YPD plates containing 4 mM puromycin and colonies were imaged after 48 hours.
D) Puromycin-resistant clones from (C) were patched onto plates containing puromycin or G418. As a control the parental kanr is included.
E) PCR analysis of the UBI4 locus from genomic DNA isolated from wild-type yeast or yeast containing either the kanr or puror cassettes at the UBI4 locus.
F) Growth assay as described in (A) of wild-type cells expressing TEF1-pac from a low-copy CEN plasmid (pPL5103) (red). The sensitivity of wild-type cells to puromycin from (A) is indicated (dotted blue).
Methotrexate-resistance as a dominant marker in yeast
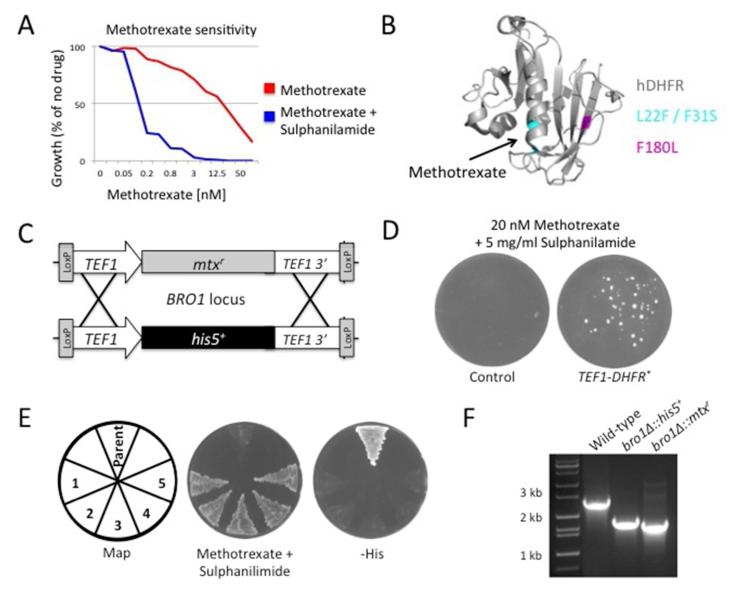
The Dihydrofolate reductase (DHFR) enzyme catalyses the NADPH-dependent conversion of dihydrofolate to tetrahydrofolate, an essential co-factor in metabolic processes. Methotrexate is a commonly used drug that inhibits DHFR, with subsequent loss of tetrahydrofolate that induces cell death; conversely, cells expressing high levels of DHFR are resistant to methotrexate (Biedler, et al., 1972; Chang and Littlefield, 1976; Littlefield, 1969). Methotrexate-resistance through increased expression of DHFR was developed as a dominant marker in mammalian cells (Christman, et al., 1982; Murray, et al., 1983; Wigler, et al., 1980). This concept was then adapted for use in yeast (Miyajima, et al., 1984). As the authors documented, methotrexate is inefficient at killing yeast cells unless sulfanilamide is added, to block the de novo synthesis of dihydrofolate (Brendel, et al., 1975; Wickner, 1975). Similarly we found growth is completely inhibited in yeast grown in the presence of 20 nM methotrexate and 5 mg/ml sulphanilamide, but a limited level of growth is detected at all concentrations of methotrexate tested in cultures lacking sulphanilamide (Figure 2A). It is unclear why sulfanilamide, which blocks synthesis of the dihydrofolate precursor, dihydropterate, from the de novo pathway is required because DHFR and methotrexate function after these pathways converge, presumably some functionality of thymidine synthesis bypasses this canonical route. Regardless, the combined lethal effect of methotrexate and sulphanilamide provides a useful system to test exogenous expression of DHFR to overcome methotrexate-sensitivity. It was originally hypothesized that naturally occurring mutants of DHFR with decreased affinity for methotrexate were more resistant to the drug (Nakamura and Littlefield, 1972). Later biochemical studies were carried out using murine DHFR showing various mutants do decrease the binding affinity of DHFR to methotrexate (Thillet, et al., 1988). Combining two of these mutants, L22F and F31S, located in the methotrexate binding region of DHFR, (Figure 2B) results in a version of DHFR that is approximately 10,000 times less sensitive to methotrexate than endogenous yeast DHFR (Remy, et al., 2007). Because this was such an effective selection tool for carrying out protein interaction complementation assays (Tarassov, et al., 2008), we created a codon optimized double mutant DHFR gene for use as a methotrexate-resistant (mtxr) dominant marker. A homologous recombination strategy was used to insert the gene between the transcriptional (TEF1) machinery of a his5+ knockout cassette (Figure 2C). Recombination was carried out in a bro1Δ::his5+ deletion strain (Pashkova, et al., 2013), and selected for on methotrexate plates containing sulphanilamide (Figure 2D). Methotrexate-resistant transformants were then unable to grow on media lacking histidine (-His), demonstrating that the mutant DHFR gene had successfully recombined at the BRO1 locus and conferred resistance to methotrexate, this was also confirmed by PCR analysis of the BRO1 locus (Figure 2E & F).
Figure 2. Mutant DHFR confers methotrexate-resistance in yeast.
A) Wild-type cells were grown to mid-log phase before addition to the wells of a 96-well plate containing a 2-fold serial dilution of methotrexate. After 72 hours incubation at 30°C growth measured by absorbance at 600 nm was calculated as a percentage of yeast growth in media lacking drug. Growth across the methotrexate gradient is indicated (red), as is growth in the same gradient containing 5 mg/ml sulphanilamide (blue).
B) The crystal structure of human DHFR in complex with the methotrexate ligand, (PDB accession number 1U72) is depicted. hDHFR is shown in grey cartoon format, the two mutations that reduce affinity to methotrexate (L22F and F31S) are shown in cyan and an arrow indicates the methotrexate-binding pocket. The location of an additional mutation (F180L) acquired in the selection for methotrexate-resistant yeast clones is shown in magenta.
C) Schematic diagram of homologous recombination strategy to replace the his5+ cassette between the TEF1 machinery at the BRO1 locus with that of the mutant form of human DHFR carrying the L22R and F31S mutations (DHFR* / mtxr).
D) bro1Δ::his5+ cells were transformed with either a PCR product encoding DHFR* or carrier DNA control. Cells were plated on SC plates containing 20 nM methotrexate and 5 mg/ml sulphanilamide.
E) Methotrexate-resistant clones from (D) and the parental bro1Δ::his5+ strain were patched onto plates containing methotrexate and sulphanilamide and plates lacking histidine (-His).
F) Genomic DNA isolated from wild-type, bro1Δ::his5+, and bro1Δ::mtxr yeast were analysed by PCR using oligonucleotides in the 5′ and 3′ untranslated regions of the BRO1 locus.
New loxP flanked marker cassettes
Having shown that expression of the pac gene and mutant DFHR in yeast confers resistance to puromycin and methotrexate, respectively, we next cloned the dominant marker cassettes into vectors for use as PCR templates. Upon sequencing, the mtxr cassette rescued from methotrexate-resistant yeast was found to contain an additional mutation (F180L), besides the two intentional L22F and F31S amino acid changes that reduce DHFR affinity for methotrexate. The conservative F180L substitution is housed in a ß-sheet domain distinct from the methotrexate-binding domain: a hydrophobic pocket facing the opposite direction (Figure 2B). Based on the location of the F180L mutation it is unlikely to impact resistance to methotrexate, although we cannot exclude the possibility it enhanced survival chances of the original methotrexate-resistant yeast. Because this version of mutant DHFR was very effective at its intended purpose (see below), no further modification of it was undertaken.
We chose a vector backbone commonly used for generation of dominant marker cassettes, plasmid pUG27, to host the puromycin and methotrexate-resistant markers (Supplemental Figure S1). pUG27 encodes a his5+ marker flanked by loxP sites (Gueldener, et al., 2002), so we simply inserted the puror and mtxr genes in place of his5+, retaining the same promoter and terminator sequences and the loxP sites for recombination based excision (see below). Another advantage to this strategy is that the puror and mtxr cassettes can be amplified using oligonucleotides designed for use with existing pUG vectors. To generate cassettes, oligonucleotides that anneal to the common vector sequences should be designed contiguous with 40-50 nts of 5′ DNA sequence homologous to the region of integration that is being targeted.
In order to test these cassettes, we next generated a puror PCR product to integrate in place of the ADE2 gene of S. cerevisiae. Resistant clones were used to compare the drug-sensitivity of yeast on solid agar. We used the kill curves derived from liquid cultures (Figure 1) as an estimate of drug concentrations required and found the selectivity to be similar. We found that 8 mM puromycin was a sub-lethal dose but had a marked effect on the growth of both haploid and diploid wild-type cells; increasing the concentration of puromycin to 20 mM was sufficient to completely inhibit growth (Figure 3A). By contrast, both diploid and haploid yeast containing the puror cassette could grow normally at 20 mM puromycin.
It has recently been proposed that wild-type yeast cells are insensitive to puromycin, and that a combination of gene deletions (pdr1Δ, pdr3Δ and erg6Δ) are required to sensitize cells to the drug (Cary, et al., 2014). In agreement with this study we find perturbations in the ERG (ergosterol biosynthesis) and PDR (pleiotrophic drug response) pathways do sensitize cells to puromycin. The most effective single gene deletion to sensitize cells to puromycin was PDR5. As mentioned in Figure 1, the ubi4Δ pdr5Δ strain (originally used to integrate the pac gene) cannot grow in puromycin concentrations of ~2.5 mM. This is supported by observations on solid agar where 4 mM puromycin is sufficient to kill ubi4Δ pdr5Δ cells, and 2 mM puromycin inhibits growth significantly (Figure 3B). We attribute this drug sensitivity to the absence of Pdr5, because single pdr5Δ mutant cells exhibit the same sensitivity. Deletion of PDR1 or ERG6 did sensitize cells to puromycin but to a lesser extent. Taking into account the inherent slow growth defect observed in erg6Δ null cells (Gaber, et al., 1989; Welihinda, et al., 1994), erg6Δ and pdr3Δ cells have very similar puromycin-sensitivity, where most growth was inhibited at 8 mM. The growth of pdr3Δ cells in the presence of puromycin was indistinguishable from wild-type, as shown previously for other drugs (Prunuske, et al., 2012); it may be the puromycin-sensitivity imparted through this mutation is too subtle for detection at the concentrations tested. Our finding that PDR5 deletion increases drug sensitivity more than cells lacking either of the Pdr5 upstream transcriptional regulators, Pdr1 or Pdr3, is consistent with previous findings (Dexter, et al., 1994; Meyers, et al., 1992). Although deletion of such genes allows use of lower concentrations of puromycin - for puror selection or study of translation inhibition - the myriad defective phenotypes associated with each deletion might undermine the physiological relevance to such experiments. We would recommend simply using the higher doses of puromycin documented herein, an approach that is not obliged to any particular genotype.
We also tested methotrexate-sensitivity of haploid and diploid cells by integrating a PCR product encoding the mtxr cassette at the MET15 locus. We find the sub-lethal concentration of 10 nM methotrexate dramatically slows growth of haploid and diploid wild-type yeast and concentrations above 20 nM is sufficient to effectively kill both strains (Figure 3C). We routinely use 50 nM methotrexate for selection, a concentration at which both haploid and diploid yeast carrying an integrated mtxr cassette grow efficiently.
We find the puromycin and methotrexate concentrations required to affect lethality in yeast were remarkably similar in liquid and solid media. The concentrations we document should provide useful estimates for drug titrations to determine the sensitivity of other experimental strains. We also find the ploidy state does not alter the required drug concentrations to select for yeast expressing the respective resistance cassettes. Finally, we carried out competitive fitness growth assays of yeast integrated with either the puror or mtxr cassettes and find them to be phenotypically neutral (Supplemental Figure S2), as has been shown for other dominant drug resistance markers (Goldstein and McCusker, 1999; Vorachek-Warren and McCusker, 2004). Together, these features expand the potential use of these dominant markers.
Constitutive Cre-recombinase expression plasmids for marker retrieval
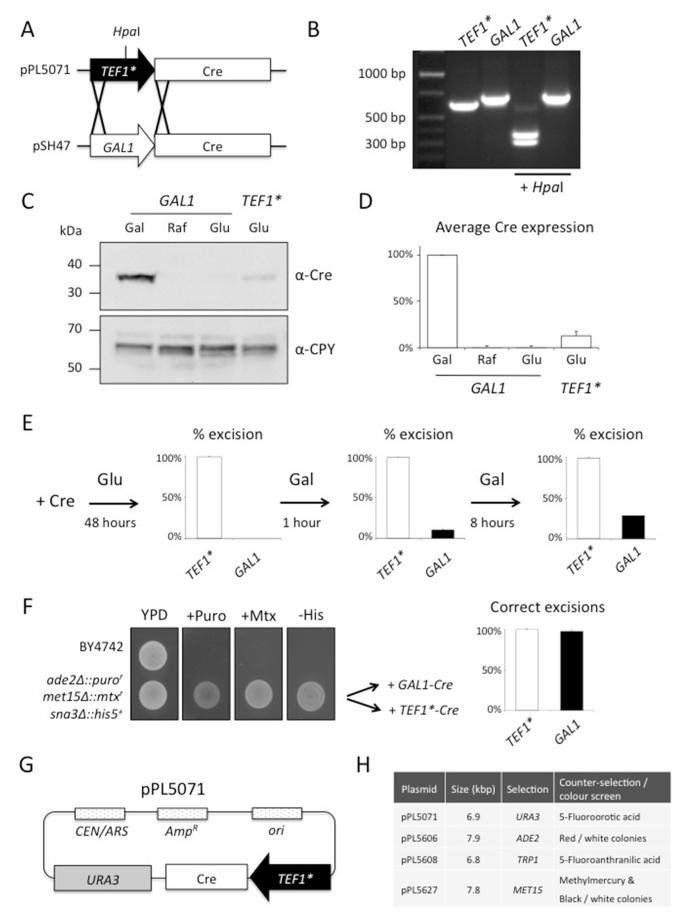
The ability to remove integrated markers allows further use of that selection tool in subsequent rounds of genetic manipulation. A widely used system for this is based on the site-specific recombination of short DNA sequences, called loxP sites, by Cre-recombinase in phage P1 (Abremski, et al., 1983; Hoess and Abremski, 1985; Sternberg and Hamilton, 1981). Expression of cre in yeast to excise a loxP flanked LEU2 marker to return cells to a Leu− phenotype was the first documentation of this system in eukaryotes (Sauer, 1987). This study induced expression of cre from the GAL1 promoter, which was useful in demonstrating specificity of Cre activity, and has since been used to remove other integrated loxP containing dominant marker cassettes (Gueldener, et al., 2002). This approach involves shifting the carbon energy source to galactose in order to induce cre expression, a significant physiological change highlighted by a slow growth phenotype (Johnston, 1987; Lohr, et al., 1995). Indeed, certain commonly used strains grow very poorly on galactose (Supplemental Figure S2). To bypass extended incubation times we designed a plasmid that constitutively expresses cre-recombinase from a modified TEF1 promoter of moderate strength (TEF1*). The TEF1* promoter shares 95% sequence identity with the wild-type version but sustains only a 16% level of expression (Nevoigt, et al., 2006). We exchanged the GAL1 promoter of plasmid pSH47 (Güldener, et al., 1996) with that of the TEF1* by homologous recombination (Figure 4A), as confirmed by PCR analysis (Figure 4B) and sequencing.
Figure 4. Expression of cre-recombinase from a constitutive promoter.
A) Schematic diagram depicting the homologous recombination strategy used to create TEF1*-cre plasmid (pPL5071) using the GAL1-cre parent plasmid, pSH47 (Güldener, et al., 1996).
B) Confirmation of the replacement of GAL1 promoter with that of TEF1* by PCR using oligonucleotides that anneal in common regions either side of the promoter sequences. The slightly smaller PCR product generated from the TEF1* template migrates faster. HpaI digestion, a restriction site unique to the TEF1* promoter, was used to confirm the GAL1 promoter has been exchanged.
C) Yeast transformed with the GAL1-cre plasmid were grown to mid-log phase in SC-Ura media supplemented with either galactose (Gal), raffinose (Raf) or glucose (Glu). Yeast retaining the TEF1*-cre plasmid were also grown in glucose containing SC-Ura media. Cells were harvested and treated with 0.2 N NaOH for 3 minutes before lysates were generated by addition of Laemmli sample buffer containing 8 M urea. Lysates were resolved by SDS-PAGE followed by transfer to nitrocellulose and probing with polyclonal antibodies raised against Cre and CPY.
D) Quantitation of Cre levels driven from either the GAL1 promoter in different carbon sources or the TEF1* promoter. Densitometry of Cre immunoblots was carried out using Fiji image analysis software and the mean and SD shown from 3 experiments are shown.
E) bro1Δ::loxP-his5+-loxP cells were transformed with TEF1*-cre (white) or GAL1-cre (black) plasmids were selected for on glucose containing SC-Ura plates. Single colony transformants (n = 48 each) were patched on SC-His plates and the percentage of cells that had successfully excised the loxP-his5+-loxP marker are shown. Transformants were also washed 3x in SC-Ura galactose media and then grown for 1 hour or 8 hours in galactose media before being washed 3 times in SC-Ura glucose media to repress GAL1 induction of Cre. Cultures were struck out on SC-Ura glucose plates and the percentage of single colony transformants that had excised the his5+ marker were identified by patching on SC-His plates.
F) The triple null ade2Δ met15Δ sna3Δ strain containing loxP flanked puror, mtxr and his5+ markers, respectively, was grown on YPD plates, YPD 20 mM puromycin, SC + 50 nM methotrexate, or SC-His plates. As a control the parental wild-type strain By4742 was included. The excision of loxP markers from ade2Δ met15Δ sna3Δ cells transformed with either TEF1*-cre (white) or GAL1-cre (black) is shown. Cre efficiency was calculated by PCR analysis of the ADE2, MET15 and SNA3 loci from genomic DNA isolated from single colony transformants of each plasmid (24 each), shown in supplemental Figure S3.
G) Plasmid map of pPL5071 encoding TEF1*-cre from a low-copy URA3 marked CEN plasmid.
H) Table of TEF1*-cre plasmids including the size of each vector, the positive selection marker, and the means to remove plasmid.
We next measured the levels of Cre by immunoblot analysis of yeast lysates generated from cells transformed with either the GAL1 or TEF1* driven cre plasmids. As expected, the GAL1 promoter produced high levels of Cre in cells when grown in media containing galactose, and there were no detectable levels when cells were grown in media containing glucose (repressed state) or raffinose (Figure 4C). The constitutive expression of cre from the TEF1* promoter was markedly less than GAL1 induced levels. Immunoblots from multiple experiments were quantified by densitometry and show Cre levels expressed from the TEF1* promoter are ~12% of that from the GAL1 promoter induced with galactose (Figure 4D). We next compared the how efficient each plasmid was at mediating Cre-induced recombination between loxP sites in cells containing a loxP-his5+-loxP marker cassette at the BRO1 locus. We found cells expressing theTEF1*-cre plasmid formed colonies after 48 hours and 100% of transformants tested had correctly excised the loxP-flanked marker cassette, as indicated by histidine auxotrophy. Cells containing GAL1-cre also formed colonies on glucose plates in 48 hours, and none of these had excised the loxP-his5+-loxP marker, consistent with glucose repression of Cre production. When these cells were patched onto solid agar containing galactose they efficiently excised the his5+ marker, but patches took an additional 48 hours to grow. An alterative strategy to avoid extended incubations when using the GAL1-cre plasmid is to expose them to galactose for “pulse” periods, however, we found that even an 8 hour galactose pulse only resulted in ~28% of cells successfully excising the loxP flanked his5+ marker (Figure 4E).
The TEF1*-cre plasmid achieves maximal excision efficiency in much less time than the GAL1-cre plasmid, and does not require additional manipulations to shift cells to another carbon source. It has been shown in yeast containing multiple loxP sites, promiscuous Cre activity can force undesired recombination events, sometimes inducing severe chromosomal rearrangements (Delneri, et al., 2000). To ensure that low-level constitutive expression of cre in cells harbouring the TEF1*-cre plasmid did not significantly increase the likelihood of such promiscuous activity, we generated a yeast strain containing three loxP flanked markers. First, the nutritional marker genes ADE2 and MET15 were deleted using the puror and mtxr marker cassettes, respectively. Then a his5+ cassette was used to delete SNA3, which encodes a small membrane protein involved with endocytic trafficking (MacDonald, et al., 2012b) (Figure 4F). We then excised the markers from the triple null strain using either the TEF1*-cre or GAL1-cre expression plasmids. Genomic DNA was isolated from 24 single colony transformants from each test group and PCR analysis was used to confirm marker excisions at each locus. We found that all 72 loxP-marked loci were correctly excised when Cre was generated using the TEF1* plasmids (Figure 4F and Supplemental Figure S3). We found similar success rates with the GAL1 plasmid, although it took much longer to obtain transformants lacking the three markers. From these studies we conclude that TEF1*-cre is no worse at inducing promiscuous Cre activity than the GAL1-cre version, and it is possible that the approximately 10-fold reduced Cre levels in cells carrying the TEF1*-cre plasmid actually minimize these unwanted effects. Promiscuous Cre activity observed in our experimental conditions were less than documented by Delneri et al, who found ~75% success from a strain housing 4 loxP marked cassettes. Presumably the total number of loxP sites in a strain, the particular marker cassettes integrated, and the genomic localization all contribute to the likelihood of undesired recombination events. It is also possible that some chromosomal rearrangements prove lethal and are selected against during cre expression. We caution that proper genotyping of any modified loci should be carried out following genetic manipulation to ensure the desired modification has been achieved.
The TEF1*-cre vector discussed above (pPL5071) is a CEN based low copy expression plasmid containing a URA3 selection marker (Figure 4F). We created additional versions of this cre expression plasmid carrying different selection markers: ADE2 (pPL5606), TRP1 (pPL5608) and MET15 (pPL5627). URA3, TRP1, and MET15 versions can be removed by counter-selection, additionally, the ADE2 and MET15 versions can be removed by growth in non-selective media, and identified by a simple change in colony colour upon plasmid loss (Figure 4G; discussed in more detail below).
Use of new tools for gene deletion and marker retrieval
To document the utility of the reagents discussed in this manuscript we used them to create additional auxotroph versions of the commonly used lab strain BY4742 (Brachmann, et al., 1998). Firstly, a puromycin-resistance cassette was integrated at the ADE2 locus and puror colonies were tested for growth on puromycin and SC-Ade plates (Figure 5A). The resultant ade2Δ::puror strain was transformed with our URA3 marked TEF*-cre plasmid, in order to remove the puror cassette and create an ade2Δ::loxP strain. The URA3-containing cre plasmid was then removed by selecting cells on SC plates containing 5-fluoroorotic acid (5-FOA: Figure 5A, right), a counter selection tool that only allows growth of cells that have lost the URA3-containing plasmid (Boeke, et al., 1984). URA3-containing Cre plasmids cannot be used in Ura+ strains, which presents a problem for strains in which a loxP-URA3-loxP cassette had been deployed (Gueldener, et al., 2002). Similar to before, we deleted the endogenous copy of ADE2 using a loxP-URA3-loxP marker and also made an ADE2 version of TEF1*-cre, which efficiently excised the URA3 marker (Figure 5B). Although there is no known counter selection for ADE2 to select for cells lacking the ADE2- TEF1*-cre plasmid, there is an easy counterscreen. Yeast that had successfully lost the ADE2-cre plasmid were easily identifiable by their red colony colour phenotype (Figure 5B), due to the accumulation of phosphoribosylaminoimidazole, the Ade2 substrate that generates a red polymer following oxidization in ade2Δ auxotrophs (Smirnov, et al., 1967). This tool could be used to confirm loss of TEF1*-cre in any ade2 mutant yeast, such as the commonly used W303 (Rothstein, 1983) and YNN/YPH (Sikorski and Hieter, 1989) strains.
Figure 5. Use of new dominant markers and TEF1*-cre plasmids.
A) The ADE2 gene of wild-type yeast (BY4742) was deleted using the loxP-puror-loxP cassette. The puror cassette was removed using pPL5071, a URA3 marked plasmid expressing cre-recombinase from a mutant TEF1* promoter. pPL5071 was removed from ade2Δ::loxP cells by growth on 5-FOA, single colonies were selected and plated on 5-FOA and -Ura media to confirm plasmid loss.
B) As with (A) the ADE2 gene of BY4742 was deleted, this time using a URA3 deletion cassette generated from PCR template pUG72 (Gueldener, et al., 2002). The URA3 marker was excised by cre expression from the ADE2 marked version (pPL5606), which was subsequently removed by growth on rich media and screening for red single colonies. Cells were plated on SC minimal media and incubated for an extra 24 hours to develop red colour for clarity.
C) The MET15 gene of BY4742 was deleted using the loxP-mtxr-loxP dominant marker cassette. The mtxr cassette was removed by expression of cre-recombinase from pPL5627, which carries a MET15 marker. This plasmid was removed by growth on rich media, and cells lacking the plasmid could be easily identified on modified YPD media containing Pb2+ ions. Cells were incubated for an extra 48 hours at 4°C to develop colour difference for documentation in this image.
D) The TRP1 locus of BY4742 was deleted using a his5+ cassette amplified from pUG27 (Gueldener, et al., 2002). The marker was removed using the TRP1 marked cre expression plasmid (pPL5608), which was later removed by selective growth on SC media containing 5-FAA.
We next used the mtxr cassette to delete the MET15 gene, which causes a methionine auxotroph phenotype (Masselot and De Robichon-Szulmajster, 1975). We transformed a version of TEF1*-cre plasmid containing the MET15 nutritional marker into met15Δ::mtxr cells to excise the marker (Figure 5C). There are two options available to removal of the MET15-TEF1*-cre plasmid. First, there was counter selection for cells resistant to methylmercury, which selects against MET15 expressing yeast as documented previously (Singh and Sherman, 1974). Second, was a blackening of colony colour when met15Δ auxotrophs are grown on modified rich media containing Pb2+ ions (Cost and Boeke, 1996). This allows visual identification of colonies that have lost the cre plasmid and was found to be easily implemented (Figure 5C) thus avoiding use of hazardous methymercury. Finally, we created a version of the TEF1*-cre plasmid carrying the TRP1 marker. Figure 5D shows that trp1Δ::his5+ cells could be cured of their TRP1-TEF1*-cre plasmid following growth on plates with 5-fluoroanthranillic acid (5-FAA), which selects against cells expressing TRP1 (Toyn, et al., 2000).
Summary
In this paper we document that there are sufficient levels of puromycin that can effectively inhibit yeast growth and be used to select for yeast that express a puromycin-resistance gene. This is a useful addition to the list of options for dominant marker selection in yeast. It was previously documented that three deletions (pdr1Δ, pdr3Δ and erg6Δ) were necessary to elevate intracellular drug levels to allow puromycin-based inhibition of translation (Cary, et al., 2014). It is useful to document that higher doses of puromycin can also achieve this goal, if the desired experiments were to be carried out in a strain other than pdr1Δ, pdr3Δ erg6Δ, a strain that is not ideal for many experimental questions. We also show that methotrexate-resistance in yeast can be used as convenient dominant selection tool. The cloning of the puror and mtxr markers into the vector backbone of the pUG PCR template vector series allows easy incorporation of these tools with existing methods. Finally, we document the use of plasmids that constitutively express cre-recombinase that allows for quick excision of chromosomal regions flanked by loxP sites. This series of plasmids containing different prototrophic markers, all of which can be removed easily, allows use in a variety of yeast strain backgrounds.
Supplementary Material
Supplemental Figure S1
Strategy used to sub-clone puror and mtxr cassettes into parental vector pUG27. The restriction enzymes used replace the his5+ marker gene with either the pac (puror) or mutant DHFR (mtxr) genes. The original pUG27 promoter and terminator sequences (from the TEF1 untranslated regions) are retained, as are the flanking loxP sequences. The sequences distal to the loxP sites that can be used for design of oligonucleotides, alongside sequence that will target integrations, to PCR amplify the cassettes are also labelled. The full sequence of plasmids pPL5617 and pPL5618 has also been deposited in the GenBank database.
Supplemental Figure S2
Phenotypic Neutrality of puror and mtxr cassettes A) Yeast containing the mtxr cassette at the MET15 locus (PLY4678; met15Δ::mtxr) and a control strain lacking the dominant marker (PLY4719; met15Δ::loxP) were grown to mid-log phase in rich media. Cultures were diluted to optical density OD600 = 0.5 and then 1 ml of each was added to a fresh flask containing 18 mls of YPD. The culture containing both strains was incubated at 30°C for 1 hour before a 1 ml sample was collected and diluted approximately 6000-fold and 9000-fold (dilutions based on an OD600 = 0.5). 200 μl of each dilution was then plated on non-selective YPD or plates containing 50 mM methotrexate, as shown on the right. The number of colonies on each plate was then counted. This process was repeated at 24 hour and 48 hour time points. During the 48-hour incubation cultures were diluted several times in YPD to maintain growth at exponentially dividing phase.
B) The percentage of colonies that grew on methotrexate selection plates compared with colony number on non-selective YPD plates was plotted over culture time. The mean and standard deviation calculated from different dilutions are shown. Right, we used a similar competitive fitness assay to test if the puror cassette was phenotypically neutral. The method was essentially the same except ade2Δ::puror cells (PLY4689) were mixed with met15Δ::mtxr cells prior to growth and plating on YPD and methotrexate plates. The number of puror colonies was therefore calculated from the discrepancy between the colony number on YPD and methotrexate plates, and was plotted over culture time.
Supplemental Figure S3
A series of commonly used lab strains (both a and alpha mating types) were grown in SC media to log phase before being plated on SC media supplemented with glucose, raffinose or galactose. A ten-fold serial dilution was created to show relative growth of strains. Growth was visualised after incubation at 30°C for 24 and 48 hours.
Supplemental Figure S4
Upper, a strain containing three loxP flanked markers, (PLY4778: ade2Δ::puror met15Δ::mtxr sna3::his5+) was transformed with TEF1*-cre and the genomic DNA was isolated from 24 single colonies. gDNA was used as a PCR template for PCR reactions using oligonucleotides specific to the ADE2 (top), MET15 (middle), and SNA3 (bottom) loci. As a control gDNA was isolated from wild-type and ade2Δ met15Δ sna3Δ yeast and included in the PCR analysis.
Lower, the same analyses as above were carried out for ade2Δ met15Δ sna3Δ cells transformed with GAL1-cre. Transformants were first struck out on galactose containing SC-Ura plates.
Acknowledgements
This work was supported by AHA post-doctoral fellowship award 13POST 14710042 to CM and NIHRO1 GM058202 to RP. We thank Scott Moye-Rowley for helpful discussion. (Brachmann, et al., 1998) (Robinson, et al., 1988) (Mortimer and Johnston, 1986) (Pashkova, et al., 2013)(Winzeler, et al., 1999)(Sikorski and Hieter, 1989)(Gueldener, et al., 2002)
References
- Abremski K, Hoess R, Sternberg N. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell. 1983;32:1301–11. doi: 10.1016/0092-8674(83)90311-2. [DOI] [PubMed] [Google Scholar]
- Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–5. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler JL, Albrecht AM, Hutchison DJ, Spengler BA. Drug response, dihydrofolate reductase, and cytogenetics of amethopterin-resistant Chinese hamster cells in vitro. Cancer research. 1972;32:153–161. [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Molecular & general genetics : MGG. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–32. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brendel M, Fath WW, Laskowski W. Isolation and characterization of mutants of Saccharomyces cerevisiae able to grow after inhibition of dTMP synthesis. Methods Cell Biol. 1975;11:287–94. doi: 10.1016/s0091-679x(08)60329-5. [DOI] [PubMed] [Google Scholar]
- Cary GA, Yoon SH, Torres CG, Wang K, Hays M, Ludlow C, Goodlett DR, Dudley AM. Identification and characterization of a drug-sensitive strain enables puromycin-based translational assays in Saccharomyces cerevisiae. Yeast. 2014;31:167–78. doi: 10.1002/yea.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Littlefield JW. Elevated dihydrofolate reductase messenger RNA levels in methotrexate-resistant BHK cells. Cell. 1976;7:391–396. doi: 10.1016/0092-8674(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Christman JK, Gerber M, Price PM, Flordellis C, Edelman J, Acs G. Amplification of expression of hepatitis B surface antigen in 3T3 cells cotransfected with a dominant-acting gene and cloned viral DNA. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:1815–1819. doi: 10.1073/pnas.79.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost GJ, Boeke JD. A useful colony colour phenotype associated with the yeast selectable/counter-selectable marker MET15. Yeast (Chichester, England) 1996;12:939–941. doi: 10.1002/(SICI)1097-0061(199608)12:10%3C939::AID-YEA988%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- de la Luna S, Soria I, Pulido D, Ortín J, Jiménez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene. 1988;62:121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]
- del Pozo L, Abarca D, Claros MG, Jimenez A. Cycloheximide resistance as a yeast cloning marker. Curr Genet. 1991;19:353–8. doi: 10.1007/BF00309595. [DOI] [PubMed] [Google Scholar]
- Delneri D, Tomlin GC, Wixon JL, Hutter A, Sefton M, Louis EJ, Oliver SG. Exploring redundancy in the yeast genome: an improved strategy for use of the cre-loxP system. Gene. 2000;252:127–35. doi: 10.1016/s0378-1119(00)00217-1. [DOI] [PubMed] [Google Scholar]
- DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–6. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- Dexter D, Moye-Rowley WS, Wu AL, Golin J. Mutations in the yeast PDR3, PDR4, PDR7 and PDR9 pleiotropic (multiple) drug resistance loci affect the transcript level of an ATP binding cassette transporter encoding gene, PDR5. Genetics. 1994;136:505–15. doi: 10.1093/genetics/136.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst R, Klemm R, Schmitt L, Kuchler K. Yeast ATP-Binding Cassette Transporters: Cellular Cleaning Pumps. Elsevier; 2005. pp. 460–484. [DOI] [PubMed] [Google Scholar]
- Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989;9:3447–56. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatignol A, Baron M, Tiraby G. Phleomycin resistance encoded by the ble gene from transposon Tn 5 as a dominant selectable marker in Saccharomyces cerevisiae. Mol Gen Genet. 1987;207:342–8. doi: 10.1007/BF00331599. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–41. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. Life with 6000 genes. Science. 1996;274(546):563–7. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–53. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic acids research. 2002;30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic acids research. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess RH, Abremski K. Mechanism of strand cleavage and exchange in the Cre-lox site-specific recombination system. J Mol Biol. 1985;181:351–62. doi: 10.1016/0022-2836(85)90224-4. [DOI] [PubMed] [Google Scholar]
- Huh W-K, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987;51:458–76. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalle RA, Pulido D, Vara J, Zalacaín M, Jiménez A. Molecular analysis of the pac gene encoding a puromycin N-acetyl transferase from Streptomyces alboniger. Gene. 1989;79:375–380. doi: 10.1016/0378-1119(89)90220-5. [DOI] [PubMed] [Google Scholar]
- Littlefield JW. Hybridization of hamster cells with high and low folate reductase activity. Proceedings of the National Academy of Sciences of the United States of America. 1969;62:88–95. doi: 10.1073/pnas.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D, Venkov P, Zlatanova J. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J. 1995;9:777–87. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- Macdonald C, Buchkovich NJ, Stringer DK, Emr SD, Piper RC. Cargo ubiquitination is essential for multivesicular body intralumenal vesicle formation. EMBO Rep. 2012a;13:331–338. doi: 10.1038/embor.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C, Stringer DK, Piper RC. Sna3 Is an Rsp5 Adaptor Protein that Relies on Ubiquitination for Its MVB Sorting. Traffic. 2012b doi: 10.1111/j.1600-0854.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masselot M, De Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol Gen Genet. 1975;139:121–32. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- Meyers S, Schauer W, Balzi E, Wagner M, Goffeau A, Golin J. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr Genet. 1992;21:431–6. doi: 10.1007/BF00351651. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Miyajima I, Arai K, Arai N. Expression of plasmid R388-encoded type II dihydrofolate reductase as a dominant selective marker in Saccharomyces cerevisiae. Molecular and cellular biology. 1984;4:407–414. doi: 10.1128/mcb.4.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. Genealogy of principal strains of the yeast genetic stock center. Genetics. 1986;113:35–43. doi: 10.1093/genetics/113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Kaufman RJ, Latt SA, Weinberg RA. Construction and use of a dominant, selectable marker: a Harvey sarcoma virus-dihydrofolate reductase chimera. Molecular and cellular biology. 1983;3:32–43. doi: 10.1128/mcb.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Littlefield JW. Purification, properties, and synthesis of dihydrofolate reductase from wild type and methotrexate-resistant hamster cells. The Journal of biological chemistry. 1972;247:179–187. [PubMed] [Google Scholar]
- Nevoigt E, Kohnke J, Fischer CR, Alper H, Stahl U, Stephanopoulos G. Engineering of promoter replacement cassettes for fine-tuning of gene expression in Saccharomyces cerevisiae. Applied and environmental microbiology. 2006;72:5266–5273. doi: 10.1128/AEM.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkova N, Gakhar L, Winistorfer SC, Sunshine AB, Rich M, Dunham MJ, Yu L, Piper RC. The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev Cell. 2013;25:520–33. doi: 10.1016/j.devcel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez JA, Vara J, Jiménez A. The mechanism of resistance to puromycin and to the puromycin-precursor O-demethyl-puromycin in Streptomyces alboniger. Journal of general microbiology. 1985;131:2877–2883. doi: 10.1099/00221287-131-11-2877. [DOI] [PubMed] [Google Scholar]
- Prunuske AJ, Waltner JK, Kuhn P, Gu B, Craig EA. Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1. Proc Natl Acad Sci U S A. 2012;109:472–7. doi: 10.1073/pnas.1119184109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I, Campbell-Valois FX, Michnick SW. Detection of protein-protein interactions using a simple survival protein-fragment complementation assay based on the enzyme dihydrofolate reductase. Nature protocols. 2007;2:2120–2125. doi: 10.1038/nprot.2007.266. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–48. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–11. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2087–96. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JI, Garcia-Verdugo R, Lehner B. Rapid selection of transgenic C. elegans using antibiotic resistance. Nature methods. 2010;7:725–727. doi: 10.1038/nmeth.1495. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Sherman F. Association of methionine requirement with methyl mercury resistant mutants of yeast. Nature. 1974;247:227–229. doi: 10.1038/247227a0. [DOI] [PubMed] [Google Scholar]
- Smirnov MN, Smirnov VN, Budowsky EI, Inge-Vechtomov SG, Serebrjakov NG. Red pigment of adenine-deficient yeast Saccharomyces cerevisiae. Biochemical and biophysical research communications. 1967;27:299–304. doi: 10.1016/s0006-291x(67)80096-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150:467–86. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. An in vivo map of the yeast protein interactome. Science. 2008;320:1465–70. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- Thillet J, Absil J, Stone SR, Pictet R. Site-directed mutagenesis of mouse dihydrofolate reductase. Mutants with increased resistance to methotrexate and trimethoprim. The Journal of biological chemistry. 1988;263:12500–12508. [PubMed] [Google Scholar]
- Toyn JH, Gunyuzlu PL, White WH, Thompson LA, Hollis GF. A counterselection for the tryptophan pathway in yeast: 5-fluoroanthranilic acid resistance. Yeast (Chichester, England) 2000;16:553–560. doi: 10.1002/(SICI)1097-0061(200004)16:6<553::AID-YEA554>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Traut RR, Monro RE. The puromycin reaction and its relation to proteins synthesis. Journal of molecular biology. 1964;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- Vara J, Perez-Gonzalez JA, Jiménez A. Biosynthesis of puromycin by Streptomyces alboniger: characterization of puromycin N-acetyltransferase. Biochemistry. 1985;24:8074–8081. doi: 10.1021/bi00348a036. [DOI] [PubMed] [Google Scholar]
- Vara JA, Portela A, Ortín J, Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic acids research. 1986;14:4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorachek-Warren MK, McCusker JH. DsdA (D-serine deaminase): a new heterologous MX cassette for gene disruption and selection in Saccharomyces cerevisiae. Yeast. 2004;21:163–71. doi: 10.1002/yea.1074. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Welihinda AA, Beavis AD, Trumbly RJ. Mutations in LIS1 (ERG6) gene confer increased sodium and lithium uptake in Saccharomyces cerevisiae. Biochim Biophys Acta. 1994;1193:107–17. doi: 10.1016/0005-2736(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Wickner RB. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine 5′-monophosphate into DNA in vivo. Methods Cell Biol. 1975;11:295–302. doi: 10.1016/s0091-679x(08)60330-1. [DOI] [PubMed] [Google Scholar]
- Wigler M, Perucho M, Kurtz D, Dana S, Pellicer A, Axel R, Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–6. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1
Strategy used to sub-clone puror and mtxr cassettes into parental vector pUG27. The restriction enzymes used replace the his5+ marker gene with either the pac (puror) or mutant DHFR (mtxr) genes. The original pUG27 promoter and terminator sequences (from the TEF1 untranslated regions) are retained, as are the flanking loxP sequences. The sequences distal to the loxP sites that can be used for design of oligonucleotides, alongside sequence that will target integrations, to PCR amplify the cassettes are also labelled. The full sequence of plasmids pPL5617 and pPL5618 has also been deposited in the GenBank database.
Supplemental Figure S2
Phenotypic Neutrality of puror and mtxr cassettes A) Yeast containing the mtxr cassette at the MET15 locus (PLY4678; met15Δ::mtxr) and a control strain lacking the dominant marker (PLY4719; met15Δ::loxP) were grown to mid-log phase in rich media. Cultures were diluted to optical density OD600 = 0.5 and then 1 ml of each was added to a fresh flask containing 18 mls of YPD. The culture containing both strains was incubated at 30°C for 1 hour before a 1 ml sample was collected and diluted approximately 6000-fold and 9000-fold (dilutions based on an OD600 = 0.5). 200 μl of each dilution was then plated on non-selective YPD or plates containing 50 mM methotrexate, as shown on the right. The number of colonies on each plate was then counted. This process was repeated at 24 hour and 48 hour time points. During the 48-hour incubation cultures were diluted several times in YPD to maintain growth at exponentially dividing phase.
B) The percentage of colonies that grew on methotrexate selection plates compared with colony number on non-selective YPD plates was plotted over culture time. The mean and standard deviation calculated from different dilutions are shown. Right, we used a similar competitive fitness assay to test if the puror cassette was phenotypically neutral. The method was essentially the same except ade2Δ::puror cells (PLY4689) were mixed with met15Δ::mtxr cells prior to growth and plating on YPD and methotrexate plates. The number of puror colonies was therefore calculated from the discrepancy between the colony number on YPD and methotrexate plates, and was plotted over culture time.
Supplemental Figure S3
A series of commonly used lab strains (both a and alpha mating types) were grown in SC media to log phase before being plated on SC media supplemented with glucose, raffinose or galactose. A ten-fold serial dilution was created to show relative growth of strains. Growth was visualised after incubation at 30°C for 24 and 48 hours.
Supplemental Figure S4
Upper, a strain containing three loxP flanked markers, (PLY4778: ade2Δ::puror met15Δ::mtxr sna3::his5+) was transformed with TEF1*-cre and the genomic DNA was isolated from 24 single colonies. gDNA was used as a PCR template for PCR reactions using oligonucleotides specific to the ADE2 (top), MET15 (middle), and SNA3 (bottom) loci. As a control gDNA was isolated from wild-type and ade2Δ met15Δ sna3Δ yeast and included in the PCR analysis.
Lower, the same analyses as above were carried out for ade2Δ met15Δ sna3Δ cells transformed with GAL1-cre. Transformants were first struck out on galactose containing SC-Ura plates.