Abstract
Background
Pharmacology frequently fails for the treatment of epilepsy. Although surgical techniques are effective, these procedures are highly invasive. We describe feasibility and efficacy of minimally invasive mapping and ablation for the treatment of epilepsy.
Methods
Mapping and radiofrequency ablations were performed via the venous system in eleven baboons and three dogs.
Results
Mapping in deep cerebral areas was obtained in all animals. High-frequency pacing was able to induce seizure activity of local cerebral tissue in 72% of our attempts. Cerebral activity could be seen during mapping. Ablative lesions were deployed at deep brain sites without steam pops or sudden impedance rise. Histologic analysis showed necrosis at the sites of ablation in all primates.
Conclusion
Navigation through the cerebral venous system to map seizure activity is feasible. Radiofrequency energy can be delivered transvenously or transcortically to successful ablate cortical tissue in this animal model using this innovative approach.
Keywords: epilepsy, catheter ablation, venous system, radiofrequency, cerebral cortex
Introduction
Epilepsy affects 0.5% to 1% of the world's population (Engel, Wiebe et al. 2003). Despite the best pharmacologic treatments available, 40% of patients remain refractory to therapy (Engel 1998; Stephen, Kelly et al. 2006). Epilepsy surgery is an alternative way to treat refractory patients and can achieve adequate results (Choi, Sell et al. 2008). However, it is highly invasive and technically difficult in deep areas of the brain (Wyllie, Chee et al. 1993). New and less invasive experimental techniques have been developed to achieve access to critical areas of the cerebral cortex for epilepsy treatment using the venous system (Henz, Friedman et al. 2008; Bower, Stead et al. 2013). The aim of this study was to assess the feasibility and efficacy of minimally invasive transvenous mapping and ablation for the treatment of epilepsy using novel catheters in animal models.
Methods
Animal preparation
Eleven baboons (25-30 kg) and three canines (beagles) were studied under general anesthesia using isoflurane (1% to 3%). Intravenous heparin was given with a target ACT of 250 seconds. The studies were performed with the approval of the Mayo Clinic Institutional Animal Care and Use Committee.
Retrograde cerebral venous access and arterial access was obtained via the internal jugular vein, femoral vein, internal carotid artery, and femoral artery for angiography and mapping. A 4-Fr multipurpose catheter was inserted near the ostium of the jugular vein for venous angiography and in the internal carotid artery for arterial phase angiography. Both vessels were used for subsequent placement of mapping and ablation catheters in both the venous and arterial systems. Following transvenous mapping and ablation, craniotomy was performed with exposure of the parietal cortex. Induction, mapping, and ablation of the presumed seizure focus were then repeated under direct visualization through the craniotomy.
Electrical mapping and ablation
Transvenous mapping and ablation
Transvenous mapping of cortical electrograms from the occipital, temporal, and parietal lobes was performed through the cortical veins using the following experimental catheters: (1) over-the-wire 4.5-Fr mapping and temperature controlled irrigated ablation catheter (Figure 1A) to negotiate the tortuous cortical veins and (2) a 4.5-Fr open-irrigation, balloon-virtual electrode venous ablation catheter (Figure 1B) for larger lesion creation in the high impedance cerebral cortex environment. Mapping was also performed using commercially available catheters, a 2.7-Fr octapolar microelectrode catheter (Revelation Cardima™, Fremont CA) and a 6-Fr 4-mm tip, deflectable catheter (Blazer Boston Scientific™, Natick, MA). Electrograms were recorded using a multichannel recording system (Prucka™, General Electric, Milwaukee, USA) with filter settings of 0.5-500 Hz.
Figure 1. Engineering design of novel catheters used for CNS cortical mapping and ablation.
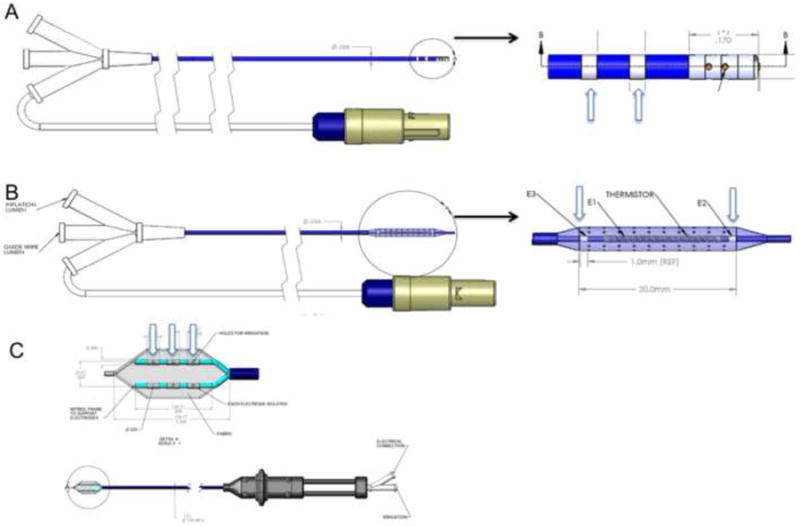
Panel A – Over-the-wire, open-irrigated catheter: There are 3 electrodes that are separated by 3-mm spacing. Panel B – Open-irrigation balloon with virtual electrode ablation catheter: There are 6 electrodes (2 rows with 3 electrodes on each row), with spacing between rows of electrodes 5 mm apart, and spacing between electrodes 2 mm apart. Panel C – Glove catheter (designed for subdural ablation): There are 3 electrodes; 2 mapping electrodes that are 1 mm in length and a coil ablation electrode that is 15mm in length). The spacing between the mapping electrodes is 18mm, and the spacing between the electrode and ablation electrode is 1.5 mm.
Seizures were induced by one of two techniques: pacing maneuvers (10 baboons) or cortical penicillin injection (4 baboons and 1 dog). The mapping catheter was navigated to cortical veins at different locations and pacing was performed at high (200 Hz) and low (50-200 Hz) frequencies. We obtained stimulation threshold values by varying energy from 2 mA to 20 mA at a pulse width of 2 ms and assessing for lowest value required for cerebral tissue activity. Pacing was then used to induce seizures. Alternatively, 2500 U of crystalline penicillin was injected over a skull aperture, in pre-determined areas located close to the site of catheter ablation. Penicillin injections induced large amplitude periodic epileptiform spikes. These injections were made to produce partial seizure activity in order to capture from the venous system brain signals close to ablation sites.
The induced seizure was mapped using the transvenously introduced catheter. Radiofrequency energy was delivered at pre-specified cortical locations using 500 kHz output of a generator grounded to two 100-cm2 patches. Ablation settings were different for each catheter evaluated (Table 1). The operator specified the cortical location where RF energy was delivered, and the lesions were verified at necropsy. The local electrograms before and after ablation were recorded from the four electrodes at the ablation catheter tip in the venous system producing two bipolar signals.
Table 1. Catheters and settings used in cortical ablation.
| Catheter | Power (W) | Irrigation flow (ml/min) | Temperature (°C) |
|---|---|---|---|
| 4 mm non-irrigated catheter | 20 | - | 50 |
| Balloon catheter | 50 | 30-60 | 50 |
| Over-the-wire irrigated catheter | 10-30 | 17-60 | 50 |
| Glove catheter | 5-40 | 17 | - |
Transcranial mapping and ablation
Following transvenous mapping and ablation, the penicillin-induced seizure was mapped on the cortical surface through a craniotomy. An experimental ‘glove’ catheter was then used to deploy larger lesions from the subdural space (Figure 1C).
Magnetically-guided navigation
The NIOBE II system (Stereotaxis, Saint-Louis, Missouri, USA) utilizes a magnetic field to remotely navigate a micro-catheter (1.1 Fr) with magnetic steel in the tip. This system provides greater maneuverability through tortuous intracranial veins and was used to guide catheter placement to a desired intracerebral location, navigating through the venous system in two canine animal models (Kara, Leinveber et al. 2012).
Necropsy and histology
At the end of the study, the animals were euthanized by ventricular fibrillation induction. A craniotomy was performed and brain removed. Gross examination was done and histological sections of ablative lesions were taken. Venous and arterial vasculature was examined for extravascular bleeding and presence of cerebral infarction.
Results
Successful mapping and navigation was achieved in 11 baboons and 3 canines without any signs of hemorrhagic or embolic complications.
Mapping
The central nervous system was able to be mapped in all primates. Navigation through the venous system accessed the occipital lobe, temporal lobe, and the base of the brain in all animals. Guided by fluoroscopy, the catheters were advanced through the jugular vein and the intra-cerebral electrograms were recorded in 10 ± 4 min (Figure 2). During high frequency pacing maneuvers in these areas, local capture was achieved 72% of the time, with a mean threshold of 14.4 ± 8.3 mA at a pulse length of 2 ms. In all captured sites, partial seizures were seen during stimulation. Immediately after cerebral pacing stimulation, intense cerebral activity was recorded from the catheter electrodes, as shown in Figure 2A and 2B. During the resting state, slow cerebral activity could be seen at the catheter tip as showed in Figure 2C and 2D. Cerebral activity after penicillin injection under partial seizure is shown in Figure 3.
Figure 2. Capture of seizure activity during cerebral high output pacing stimulation.
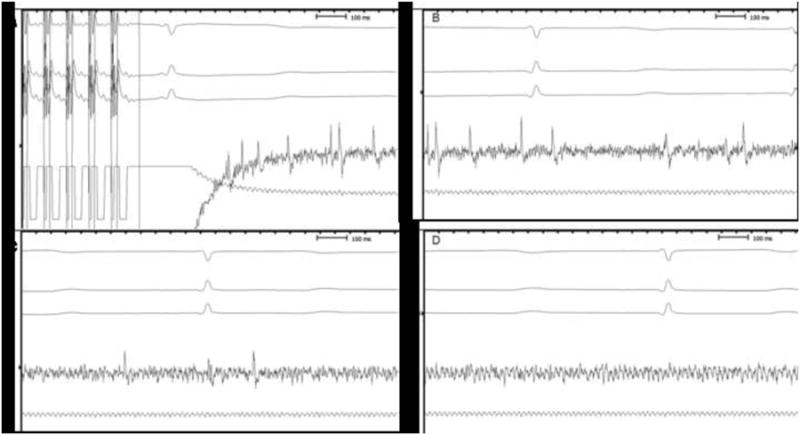
Capture of seizure activity during cerebral high output pacing stimulation. Pacing stimulation of the cerebral cortex (70 ms S1-S1) was performed. Tracings show cerebral activity after high output pacing with local capture and partial seizure during stimulation. Cerebral activity is decreasing from Figure 2A (end of stimulation) to complete rest (Figure 2D). Three electrocardiogram derivations are presented at the top of the figure (I-II-aVF).
Intracerebral distal: bipolar signal captured from the two distal electrodes at the tip of the catheter. Intracerebral proximal: bipolar signal captured from the two proximal electrodes from the tip of the catheter.
Figure 3. Cerebral activity after Penicillin injection.
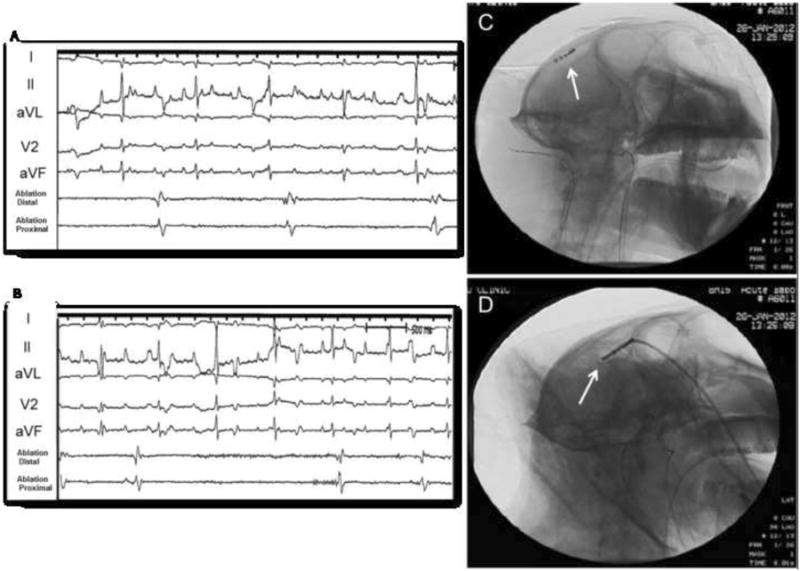
Recordings of cerebral activity during partial seizure (A) and after (B) 2500 U of penicillin injection. Panels A and B show cerebral activity presented at the tip of the ablation catheter. Panel C: Cine image from catheter location during seizure activity (AP view); Panel D: Lateral view.
Catheter Ablation
Radiofrequency energy delivery was performed in all animals following cortical mapping through the venous system. The radiofrequency characteristics of the three catheters used for ablation are presented in Table 2.
Table 2. Ablative data from cortical lesions using different catheters.
| Catheter | Power (W) | Temperature (°C) | Impedance (Ω) |
|---|---|---|---|
| 4 mm non-irrigated catheter | 11±2 | 91±1 | 137±31 |
| Over-the-wire irrigated catheter | 16±10 | 28±3 | 149±31 |
| Balloon catheter | 28±8 | 39±17 | 202±33 |
| Glove catheter | 25±12 | 107±17 |
During RF delivery, steam pops or sudden impedance rise were not observed. Char was present on the tip of the catheter in three ablative lesions.
Magnetic Navigation
The micro-catheter was successfully navigated to the brain by gentle changes in the magnetic field, through the sinus temporalis, sigmoideus, and cavernosus (Figure 2B), without evidence of venous dissection, acute thromboembolism, or bleeding during mapping and catheter movement.
Pathology
At gross pathology, the ablative lesions were identified in all animals, without any evidence of coagulum, venous dissection, or cerebral hemorrhage. Histological analysis of cortical tissue from areas of RF lesion showed evidence of cortical necrosis in 11/11 primates (Figure 4).
Figure 4. Gross pathology and histology post cerebral ablation.
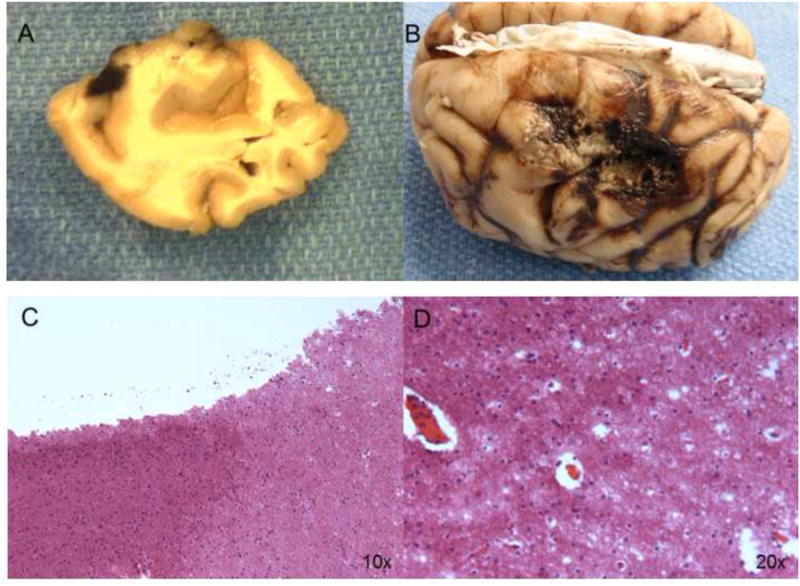
Panel A: Baboon brain with a small ablative lesion made by a 4 mm catheter. Panel B: Large ablative lesion at the temporal lobe made using the glove catheter. Panel C: Histologic findings at cerebral tissue after ablative lesion. Under HE stain we observed the transition between normal and damaged tissue at 10×. Panel D: Magnification at 20×.
Discussion
Epilepsy surgery is an established treatment for drug refractory focal epilepsy, however, seizure outcomes following surgery are suboptimal with certain techniques (Najm, Jehi et al. 2013). Catheter-guided mapping using multipolar catheters are commonly used to acquire electrical signals from the heart (Haissaguerre, Shah et al. 2000; Haissaguerre, Shah et al. 2000), and to create activation maps to define early electrical activity and eliminate cardiac arrhythmias through ablation. We previously described the feasibility of cortical mapping and ablative lesion creation inside cortical tissue using the venous system in a pig model (Henz, Friedman et al. 2008).
We report the feasibility of cortical mapping and ablation in eleven primates using novel, over-the-wire catheters guided to deep areas of cortical tissue and deployed for effective radiofrequency lesions, as well as a subdural ablation catheter (Glove catheter) to produce larger and more superficial lesions as necessary.
Compared to the previous study done using the swine model (Henz, Friedman et al. 2008), the primate model has some advantages due to accessibility to the cerebral venous system using the internal jugular vein, and thereby improving the navigability and ease of catheter manipulation. This led to decreased procedure times and facilitated the deployment of ablative lesions in the deep brain.
During mapping and high output stimulation, we captured brain tissue frequently, created partial and generalized seizures, and after filter improvements and adjustments, could clearly see local brain activity captured by the intravenous catheters. Penicillin was used as an adjunctive model of seizure induction (Van Gompel, Bower et al. 2011), with the advantage of creating partial seizures in areas located near the tip of the ablation catheter for fine-tuning the generated electrical data.
These soft catheters using over-the-wire techniques improved the safe accessibility to deep cerebral areas. In addition, the ability to insert a guide wire into the venous circulation and manipulate catheters protected by the wire improved procedure security and decreased possible complications such as venous laceration. The irrigated-tip catheters and virtual electrode balloon could produce deep and large lesions without steam pops, vein perforation, or vein thrombosis. The differences in catheters facilitate type of ablation produced such as a deep lesion (irrigated and balloon catheter), or larger and more superficial lesion in other cases (glove catheter).
Future studies are needed in an animal model to evaluate how the lesions mature in a chronic phase and to evaluate the possible complications produced by this therapeutic intervention.
Limitations
Since these procedures are performed under general anesthesia, this could influence the capture threshold and the captured sites to produce seizures. It is also possible that the electrogram quality is affected when compared to a non-sedated state. Furthermore, since these were acute studies, it is unknown what the progression of lesion development would be if followed longer. The impact of transvenous radiofrequency ablation on seizure inducibility needs further study.
Conclusions
The current study establishes the feasibility of navigating through the cerebral venous system using novel catheters to map seizure activity. We also demonstrate that radiofrequency energy can be delivered transvenously and transcortically to successfully ablate cortical tissue in a primate model. Magnetic navigation of soft micro-catheters establishes a new standard of safety for interventions in the CNS, even in vascular structures with complex anatomy. This may provide a theoretical basis for using this technology for other innovative therapies in the CNS, such as in stroke treatment or deep brain stimulation.
Highlights.
Localization of epileptiform activity via venous mapping catheter
Catheter ablation of epileptiform activity via venous ablation catheter
RF ablation of cortical tissue through a transvenous or transcortical approach
Efficacy of catheter ablation for either superficial or deep tissue ablation
CNS ablation catheters with magnetic steerability to navigate tortuous vessels
Acknowledgments
SJA and TK were supported by European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Bower MR, Stead M, et al. Intravenous recording of intracranial, broadband EEG. J Neurosci Methods. 2013;214(1):21–26. doi: 10.1016/j.jneumeth.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Sell RL, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300(21):2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51(5):1243–1244. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Wiebe S, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Shah DC, et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;102(20):2463–2465. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Shah DC, et al. Mapping-guided ablation of pulmonary veins to cure atrial fibrillation. Am J Cardiol. 2000;86(9A):9K–19K. doi: 10.1016/s0002-9149(00)01186-3. [DOI] [PubMed] [Google Scholar]
- Henz BD, Friedman PA, et al. Successful radiofrequency ablation of the cerebral cortex in pigs using the venous system: possible implications for treating CNS disorders. Epilepsy Res. 2008;80(2-3):213–218. doi: 10.1016/j.eplepsyres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Kara T, Leinveber P, et al. Endovascular brain intervention and mapping in a dog experimental model using magnetically-guided micro-catheter technology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012 doi: 10.5507/bp.2012.076. [DOI] [PubMed] [Google Scholar]
- Najm I, Jehi L, et al. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54(5):772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Kelly K, et al. Pharmacological outcomes in older people with newly diagnosed epilepsy. Epilepsy Behav. 2006;8(2):434–437. doi: 10.1016/j.yebeh.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Bower MR, et al. Swine model for translational research of invasive intracranial monitoring. Epilepsia. 2011;52(6):e49–53. doi: 10.1111/j.1528-1167.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie E, Chee M, et al. Temporal lobe epilepsy in early childhood. Epilepsia. 1993;34(5):859–868. doi: 10.1111/j.1528-1157.1993.tb02103.x. [DOI] [PubMed] [Google Scholar]


