Abstract
Congenital abnormalities of the urogenital tracts form a major part of clinical practice for paediatric urologists, but their knowledge of normal and abnormal development is often limited. Advances in understanding frequently come from studying experimental findings from animal models, however, most clinicians underestimate both the power and perils of extrapolating scientific knowledge from animals. In this review, the key issues that urologists need to understand in order to link animal studies to clinical practice are discussed. Urologists must avoid the traps of anthropomorphism (assuming humans are always the same as animal models) or anthropocentrism (assuming humans are too different from animal models). This review used two common disorders: hypospadias and undescended testes.
Keywords: Animal models, Hypospadias, Undescended testes
Introduction
Many young paediatric urologists aspire to investigate the underlying causes of the common congenital anomalies that they clinically treat, such as hypospadias and cryptorchidism, by using animal models. However, like all doctors, they have spent most of their education and career focused on patients. Therefore, if paediatric urology is going to advance (and their academic career is going to flourish) they need to understand how to use animal models of normal and abnormal urogenital development. A significant barrier to using results from animal models for medicine is the very limited knowledge that many surgeons have of broad biological principles.
Two of the authors are paediatric urologists (John Hutson and Larry Baskin) and two are scientists and anatomists (Gail Risbridger and Gerald Cunha), and they have all learnt how to extrapolate from animal models to clinical practice the hard way. It is hoped that others might be inspired to follow a similar path, and that these thoughts about extrapolation will be useful. These words may be useful for the young paediatric urologist contemplating a career as a surgeon-scientist.
Lack of exposure to biology
During premedical studies many doctors are not exposed to a deep analysis of the mechanisms of evolution, with studies in biology, embryology and anatomy totally preoccupied with the human. This is likely to lead to them (as well as other surgeons) having had little exposure to comparative anatomy and embryology, where differences between species might either be ignored or not appreciated for the insight they might bring to the comparable human process or structure.
Ignoring species differences: anthropomorphism
Underestimating the differences between species might lead surgeons to take for granted that the results of an animal experiment can be automatically translated into a human context. For some surgeons, it is as if the mouse or rat is the same as the child. This type of thinking is more common when the science and experiment looks at lower orders of bodily structure, such as the gene, the cell and the tissue. It is less prevalent at higher orders of structure, such as gross anatomy, where the differences between species are more obvious. However, the moment one stops to think, differences are to be expected, albeit minor, at every level of structure. The recent discovery that Hox genes, which have been recognised to control embryonic segmentation in the fruit fly and are also regulating segmentation of the human embryo, shows the power of extrapolation from one species to another [1]. However, Hox genes in all mammals, including humans, are more complicated than in the fruit fly, because of replication of the primitive gene set over the eons into a large cluster of related genes with overlapping functions. This ‘descent with minor variation’ is a powerful example of Charles Darwin’s view that all species share a common inheritance, in this case, in the specific genes involved in segmental development of the embryo (Charles Darwin, 1859). Therefore, the important message for doctors is that the biology of animals is not identical, but only slightly different from humans. These differences tend to be in quirky or idiosyncratic areas of embryology or anatomy that lead to different body shapes or functions, while the fundamental processes of embryology are likely to be comparable.
Anthropocentrism: humans are different
Some doctors bring a view to medicine that ‘humans are special’ and not like animals at all. This was the worldview of all doctors prior to Darwin’s publication of On the origin of Species in 1859. At first, the notion that ‘men are related to monkeys’ was ridiculed, but for the last 50 years the principles of evolution have not only been accepted, but also actively taught in biology classes. However, the rise of conservative religious groups in many countries, where the Bible (or the Koran) may be interpreted literally, means that a significant number of doctors (and also paediatric surgeons) in practice today may have been shielded from the full implications of seeing the world as an evolving system. This may lead to some beliefs that ‘it’s just a rat experiment’, and, thus, not relevant for children.
In order to bring the benefit of animal models to a child with a urogenital anomaly, the differences in development between animal models, such as the mouse or rat, and the human need to be understood. These differences need to be taken into account during translation to clinical medicine, so that it is not assumed that everything is the same. Bear in mind the problems of Galen and medieval doctors, who inherited anatomy books based on dissection of animals alone, as human dissection was forbidden. Once human cadaver dissection became acceptable, these important differences were eventually corrected. However, there is a classic error of extrapolation between animals and humans recorded for posterity in a famous drawing of a human fetus within the uterus by Leonardo da Vinci, where the fetus itself is extremely life-like, but the placenta is drawn with multiple cotyledons, like a cow, rather than like a human placenta.
Sometimes, differences between animals and humans can reveal important insights into human biology. An example of this is the asymmetry of the genitalia seen in disorders of sex development (DSD) with mixed chromosomes, where mosaicism in the sex chromosomes leads to gonadal and genital duct asymmetry, rather than an even mixture of different cells. It is more common in mixed gonadal dysgenesis (45,X/46,XY DSD) and in ovo-testicular DSD, for example, for the right side to be more masculine than the left side, with a descended testis in the right hemiscrotum and an undescended intra-abdominal left ovary, ovotestis or streak gonad [2]. From where did this asymmetry suddenly spring? A review of the evolution of sexual development immediately reveals that the asymmetry was embedded in the genome of animals all along, and is normal in all female birds, where the left gonad in the urogenital ridge forms an ovary and the Müllerian duct differentiates into a uterus, while on the right side the gonad forms an ovotestis and the right Müllerian duct regresses [3]. In humans, the asymmetry is much more subtle and usually invisible, but is revealed in DSD when the right side is more likely to be ‘masculine’ and the left side more ‘feminine’.
To highlight the strength, as well as the limitations, of animal models of urogenital development, development of the penis and testicular descent will be described, as these two examples underlie the two most common problems in paediatric urology: hypospadias and cryptorchidism. In both of these examples, the power as well as the peril of animal models is evident.
Hypospadias
This is a common anomaly that all paediatric urologists understand well; there are three characteristic features: (i) proximal urethral opening on the undersurface of the penis (hence, ‘hypospadias’ or hole underneath); (ii) dorsal hooded foreskin with ventral deficiency; and (iii) chordee, with inadequate growth of ventral periurethral tissues compared with the corpora cavernosa dorsally, leading to ventral curvature that is worse with erection.
Sixty years ago the famous French biologist, Alfred Jost, showed that sexual development is regulated mostly by androgens from the fetal testis [4]. He removed the gonads of fetal rabbits and showed that by replacement with a crystal of testosterone, external male development was normal. He also noted that exogenous testosterone did not make the Müllerian ducts regress and hence proposed the existence of a non-steroidal hormone, now known as Müllerian Inhibiting Substance or anti-Müllerian hormone (MIS/AMH), which is required to control regression of the embryonic female genital tract in males [4].
Deficiency of androgens leads to 46,XY DSD, with severe phallic hypoplasia with penoscrotal or perineal hypospadias. Although the primary role of androgens in masculinisation of the embryonic genital tubercle into a penis is well accepted, the aetiology of hypospadias is more complex and likely to be multifactorial. Mutations in genes for the oestrogen receptor beta (ERβ), activating transcription factor 3 (ATF3) and 5-alpha reductase-2 (SRD5A2) have been implicated [5–9].
Determining the relevance of these different putative causes of hypospadias, however, has been hampered by inaccurate extrapolation of the anatomy from rodent models. Even the definition of ‘hypospadias’ in mice has been fraught with difficulty, as the normal anatomy of the penis is different in certain respects from the human [10,11]. The mouse penis is completely covered by hair-bearing skin on the lower abdominal wall, known as the ‘prepuce’. The mouse penis is described as being formed by two elements: the shaft or body, which is attached to the pubic bones, and a long ‘glans’, which is joined to the shaft at a right angle. A distal projection of the glans, which is known as the male urogenital mating protuberance (MUMP), is fused with a circumferential ridge (MUMP ridge) to form the urethral meatus. Just proximal to the MUMP ridge is another fold of skin, which surrounds the glans to form the ‘internal prepuce’. Very recently, the latter has been discovered to be closely homologous with the human prepuce [11]. By contrast, the hair-bearing skin of what we would now call the ‘outer prepuce’ of the mouse penis has no human homologue, and appears to be an adaptation to protect the penis from trauma in four-legged animals. When these anatomical differences are taken into account, the ‘hypospadias’ in a mouse or rat can be defined more accurately by the anatomical relationship between the urethral opening, the MUMP and MUMP ridge, and the ‘internal prepuce’ (Fig. 1).
Figure 1.
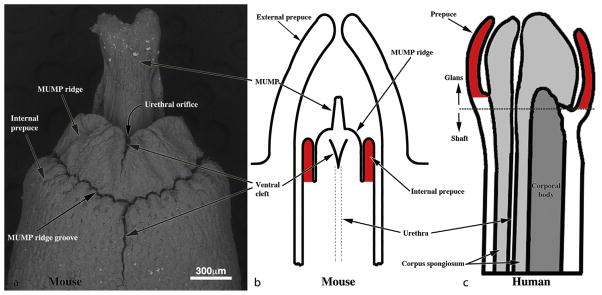
Re-evaluation of penile anatomy to establish mouse–human homology. (a) SEM of the adult mouse penis. The penile glans lies within an extensive preputial space beginning at the opening of the preputial space distally in the hair-bearing prepuce (a prominent elevation in the perineum labelled ‘External prepuce’ in (b)) and ending proximally near the glans–body junction. Drawings of mouse (b) and human (c) morphology demonstrating the homology of the human prepuce and the mouse ‘internal prepuce’ (both red) in so far as both are integral to the distal penis and encircle the glans. (Reproduced with permission from Blaschko et al., The Anatomical Record, 2013). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Another small, but crucial, difference between murine and human penile development is related to timing. In humans, the external genitalia masculinise between 8 and 15 weeks’ gestation, with the urethra reaching the future coronal groove at about 10 weeks and the glanular urethra forming between 10 and nearly 20 weeks [12], while growth of the shaft and increase in penile size occurs right up to term [13]. In the mouse penis, a significant amount of postnatal development occurs, as sexual differentiation in response to male hormones occurs between prenatal embryonic days 14–17, but also in the first 10 postnatal days [10] (Fig. 2). Therefore, to be able to extrapolate a hormonal anomaly of the phallus causing ‘hypospadias’ in the mouse to the human, the mouse needs to be at least 10 days old, if not adult. Comparison of mice at birth with the human, therefore, is an error, as the distal urethra and inner prepuce have not yet formed.
Figure 2.
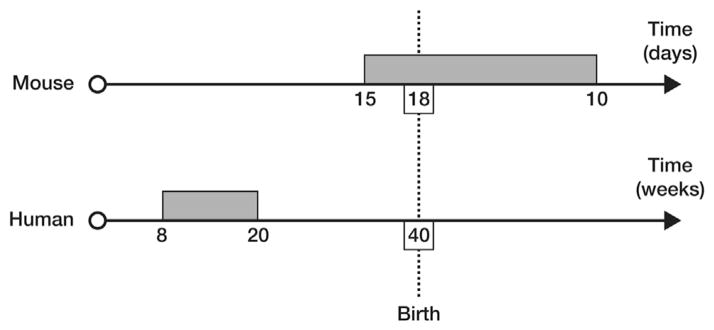
Schema of timing of penile development in the mouse (day 15 of embryological development to at least postnatal day 10) compared with the human (8–20 weeks of gestation).
Once these two anatomical differences and timing have been incorporated, the basic development of the normal rodent penis and its response to abnormal hormone levels is essentially the same as in baby boys. With this in mind, recent studies of the hormonal control of penile development in rodents suggest an important role for not only androgens, but also oestrogens. This is because the oestrogen alpha and beta receptors (ERα, ERβ) are present in the glans, prepuce and distal urethra of both mice and children [10,11]. Although it has been known for some time that the enzyme aromatase is present in peripheral tissues, such as the external genitalia and phallus, a specific role for oestrogens was not known [14]. It can be speculated that aromatase has allowed an expansion of the ways in which androgens can regulate anatomical development of the penis; it can act directly via androgen receptors (AR), but also indirectly via ERα or ERβ after conversion to oestradiol. This allows more potential permutations for anatomical regulation, but also predisposes the fetus to the potential harmful effects of exogenous oestrogens from the environment, such as has been suggested recently for ‘endocrine disruptors’ [15]. It is quite likely that hypospadias is caused by derangement in the way oestrogen receptors modulate the closure of the terminal urethra. Distal hypospadias may be related to abnormal oestrogenic function, while proximal hypospadias may reflect significant androgen deficiency in the critical window of embryonic development.
Cryptorchidism
The second example of the power of extrapolation is cryptorchidism. Undescended testis or cryptorchidism is a very common developmental anomaly in boys, perhaps reflecting the relatively recent evolution of testicular descent in mammals only 200 million years ago. Marsupials, such as the kangaroo, normally have descended testes just like the human, but with important differences in anatomy. In the male marsupial fetus, the scrotum initially develops independently of androgens in the same location as the mammary buds in females (in the groin, just cranial to the pubis). Testicular descent occurs in two stages, which is similar to rodents and humans, but as the scrotum in marsupials is located over the external inguinal ring (and cranial to the penis) the inguinoscrotal phase is much simpler than in humans; not surprisingly, cryptorchidism is rare [16,17] (Fig. 3). The other critical feature of marsupial testicular descent is the close relationship between the gubernaculum and the mammary bud before androgens induce male sexual development. This has revealed that there is a similar link between the gubernaculum and the mammary line in rodents, and it is suspected that this is also important in humans. This is an example of where marsupial anatomy has led researchers to find something in rodents that was never suspected. Moreover, it may finally explain why the mammary line may persist right down to the inguinal region in humans, despite the fact that there are no breasts in the groins.
Figure 3.
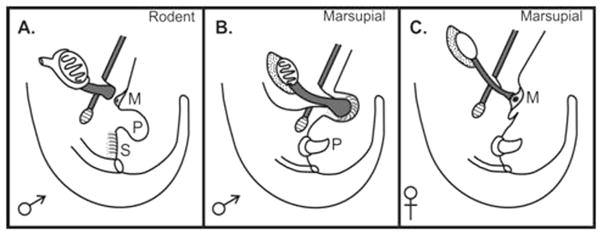
Diagram showing anatomical arrangement of the gubernaculum in the rodent before inguinoscrotal descent vs. the equivalent arrangement in male and female marsupials.
Rodents have a perineal scrotum similar to humans, but there are other minor differences in anatomy. The major difference with humans is how the processus vaginalis forms to allow the intra-abdominal testis to reach the scrotum while still inside the peritoneal cavity [18]. During the transabdominal phase of descent in rodents and humans, the gubernaculum enlarges with a swelling reaction in response to insulin-like hormone 3 (INSL3). To initiate the inguinoscrotal phase in rodents, the gubernaculum remodels from a solid intra-abdominal pyramid, with its base embedded in the abdominal wall, to form a hollow, protruding cone lined by peritoneum and with a central cord attached to the caudal epididymis and testis. A significant amount of the extracellular matrix formed in the swelling reaction is resorbed prior to eversion to allow formation of the gubernacular cone and its elongation to the scrotum.
In humans, the processus vaginalis grows into the proximal part of the swollen gubernaculum to make an intraperitoneal space for the testis, but most of the bulk of the extracellular matrix in the distal gubernaculum persists until after elongation and migration to the scrotum is complete. It then resorbs rapidly, but in premature babies is often still palpable in the scrotum as a soft swelling immediately caudal to the testis. Unlike the rodent, the human gubernaculum does not evert, but instead elongates steadily out of the abdominal wall in a manner similar to an embryonic limb bud (Fig. 4).
Figure 4.
Testicular descent in the human fetus vs. that in a rodent. In both species the process occurs in two separate phases: the transabdominal and inguinoscrotal stages. Migration of the gubernaculum is similar, except that in rodents the extracellular matrix in the gubernaculum regresses before migration begins at birth, while in humans this occurs after the gubernaculum reaches the scrotum, and the entire process is prenatal. Also in humans, the last step after descent is closure of the processus vaginalis (to prevent inguinal hernia), while in rodents the processus remains open and a fat pad on the epididymis, which plugs the inguinal canal, prevents herniation.
As is well known to paediatric urologists, the processus vaginalis normally obliterates after testicular descent is complete just before or after birth, and failure of which leads to inguinal hernia or hydrocele [19]. By contrast, in four-legged animals, such as rodents, the processus vaginalis remains patent after testicular descent, and inguinal hernia is prevented by a large fat pad attached to the head of the epididymis, which acts as a plug in the inguinal canal (Fig. 4). Therefore, the rodent testis remains throughout life within the peritoneal cavity and is on a mesentery (the mesorchium), similar to the bowel. When the testes of a rodent retract, they ascend into the peritoneal cavity through the open inguinal canal. By contrast, in humans, this is only possible when there is a complete inguinoscrotal inguinal hernia. The human testis is inside a satellite peritoneal cavity, the tunica vaginalis, on a mesentery, the mesorchium, which is why when it is abnormally long it can predispose to intra-vaginal testicular torsion.
Another minor, but important, difference in anatomy between rodents and humans is the length of the gubernacular cord. In humans this is very short, so that the gubernaculum and its contained testis (inside the processus vaginalis) migrate to the scrotum together (Fig. 4). By contrast, the cord is quite long in rodents, so that even though inguinoscrotal gubernacular migration occurs at 1–10 days after birth the testis usually remains within the abdominal cavity until puberty begins at three to four weeks of age.
In humans, these minor differences in timing of development mean that at birth, normal testicular descent should be complete (by about 35 weeks’ gestation), while in rodents only the first phase of descent is complete. The inguinoscrotal phase in rodents occurs postnatally, with migration of the gubernaculum to the scrotum complete by 7–10 days in rats and 5–7 days in mice. The testis remains intra-abdominal (or inguinal), because of the longer gubernacular cord, until puberty.
Delayed descent of the testis to the low-temperature environment of the scrotum leads to another important difference between rodents and children: how the abnormally high temperature of the cryptorchid testes affects postnatal germ cell development. This leads to differences in the effects of cryptorchidism on malignancy risk. Congenital undescended testes lead to a 5–10 fold increased risk of cancer in men, but not in rodents [20]. The reason for this difference is unknown, but the differences in timing of the testis reaching the scrotum could well account for it.
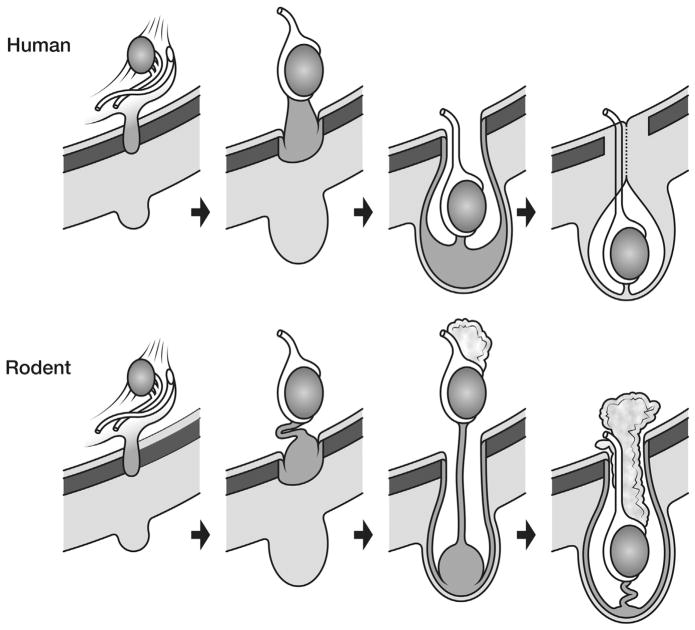
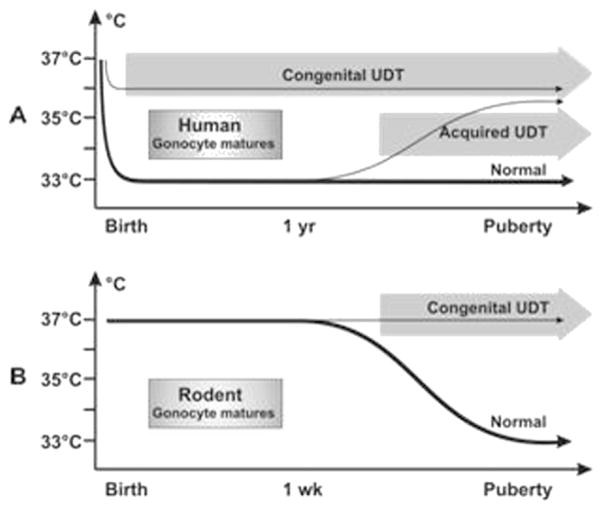
In children with cryptorchidism, the undescended testis is at the wrong temperature from the moment of birth, as when the baby boy is born the descended testes rapidly adapt to the new environmental temperature of the scrotum (33 °C) within a few weeks. Cryptorchidism in humans leads to very early interruption of the testicular physiology, including germ cell maturation, so that the first anomaly seen is interference with the transformation of neonatal gonocytes into type A spermatogonia (thought to be the stem cells for sperm) at 3–6 months of age. Failure of gonocyte transformation leads to some gonocytes (which are totipotential stem cells) remaining in the testis later in life, as the high temperature probably inhibits not only the transformation, but also apoptosis of any remaining gonocytes. This putative failure of apoptosis in human cryptorchid testis may be the reason why men with previous cryptorchidism are at risk of cancer, as there is indirect evidence that the gonocyte may be the origin of the malignancy.
In rodents with cryptorchidism the effect of abnormal temperature is not apparent until later, as the gubernaculum normally does not reach the scrotum until 5–10 days of age and the testis does not normally descend fully into the scrotum (and hence the lower temperature) until 2–3 weeks of age. This is after gonocyte transformation/ apoptosis is normally finished even if later the testis is cryptorchid. It is speculated that early germ cell development, in the first year in humans and the first week in rodents, is important not only to form the unipotential stem cells for spermatogenesis, but also to eliminate any remaining neonatal gonocytes (which are totipotential stem cells) by apoptosis [20]. Cryptorchidism, with its abnormal temperature of the inguinal testis, will only interfere with these early steps in humans but not in rodents, as the abnormal testicular temperature of an inguinal undescended testis is not present until after the gonocyte has transformed or undergone apoptosis normally. By the time the undescended rodent testis is exposed to an abnormally high temperature there are likely to be no abnormal totipotential germ cells remaining within the testis that have a cancer risk (Fig. 5). This would imply that rodent models with congenital cryptorchidism more closely mimic acquired cryptorchidism in humans, where the effect of abnormally high temperature in the testis does not appear until later in childhood, after the key steps in early germ cell development are complete.
Figure 5.
Testicular temperature versus age germ cell development in humans and rodents. (A) Human gonocytes mature in the first year when the testicular temperature is 33 °C. Congenital UDT have abnormal temperature early, which interferes with gonocyte development. By contrast, acquired UDT only interferes with subsequent survival of stem cells. (B) In rodents, gonocytes mature before a change in temperature occurs, so that gonocyte development is unaffected by congenital UDT similar to acquired UDT in humans. (Reproduced with permission from Hutson et al., Frontiers in Endocrinology, 2012).
Once all these minor differences in anatomy and timing between rodents and humans are appreciated, rodent models of cryptorchidism provide powerful insights into normal and abnormal testicular descent in humans, and also allow these models to predict the effects of at least acquired cryptorchidism in men.
In conclusion, animal models of normal and abnormal urogenital development provide powerful insights into the human condition, but the interpretation of the results of animal studies requires some finesse so that extrapolation to humans is valid. Where the animal biology is slightly different, these differences may actually illuminate what is happening in humans when the biological differences are put in their proper context. Therefore, if animal models are ‘handled with care’ they can make an invaluable contribution to understanding the causes and consequences of urogenital anomalies in children.
Footnotes
Competing interests/funding
None declared.
References
- 1.Goff DJ, Tabin CJ. Analysis of Hoxd-13 and Hoxd-11 misexpression in chick limb buds reveals that Hox genes affect both bone condensation and growth. Development. 1997;124:627–36. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- 2.Hutson JM, Warne GL, Grover SR. Disorders of sex development: an integrated approach to management. Berlin: Springer; 2012. [Google Scholar]
- 3.Hutson JM, MacLaughlin DT, Ikawa H, Budzik GP, Donahoe PK. Regression of the Mullerian ducts during sexual differentiation in the chick embryo. A reappraisal. Int Rev Physiol. 1983;27:177–224. [PubMed] [Google Scholar]
- 4.Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953;8:379–418. [Google Scholar]
- 5.Beleza-Meireles A, Lundberg F, Lagerstedt K, et al. FGFR2, FGF8, FGF10 and BMP7 as candidate genes for hypospadias. Eur J Hum Genet. 2007;15:405–10. doi: 10.1038/sj.ejhg.5201777. [DOI] [PubMed] [Google Scholar]
- 6.Beleza-Meireles A, Kockum I, Lundberg F, Soderhall C, Nordenskjold A. Risk factors for hypospadias in the estrogen receptor 2 gene. J Clin Endocrinol Metab. 2007;92:3712–8. doi: 10.1210/jc.2007-0543. [DOI] [PubMed] [Google Scholar]
- 7.Beleza-Meireles A, Barbaro M, Wedell A, Tohonen V, Nordenskjold A. Studies of a co-chaperone of the androgen receptor, FKBP52, as candidate for hypospadias. Reprod Biol Endocrinol. 2007;5:8. doi: 10.1186/1477-7827-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beleza-Meireles A, Tohonen V, Soderhall C, et al. Activating transcription factor 3: a hormone responsive gene in the etiology of hypospadias. Eur J Endocrinol. 2008;158:729–39. doi: 10.1530/EJE-07-0793. [DOI] [PubMed] [Google Scholar]
- 9.Samtani R, Bajpai M, Vashisht K, Ghosh PK, Saraswathy KN. Hypospadias risk and polymorphism in SRD5A2 and CYP17 genes: case-control study among Indian children. J Urol. 2011;185:2334–9. doi: 10.1016/j.juro.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2011;84:261–8. doi: 10.1016/j.diff.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaschko SD, Mahawong P, Ferretti M, et al. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anat Rec (Hoboken) 2012;296:1127–41. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999;64:115–22. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed SF, Rodie M. Investigation and initial management of ambiguous genitalia. Best Pract Res Clin Endocrinol Metab. 2010;24:197–218. doi: 10.1016/j.beem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim KS, Liu W, Cunha GR, et al. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145–53. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- 15.Yiee JH, Baskin LS. Environmental factors in genitourinary development. J Urol. 2010;184:34–41. doi: 10.1016/j.juro.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 16.Coveney D, Shaw G, Hutson JM, Renfree MB. Effect of an antiandrogen on testicular descent and inguinal closure in a marsupial, the tammar wallaby (Macropus eugenii) Reproduction. 2002;124:865–74. doi: 10.1530/rep.0.1240865. [DOI] [PubMed] [Google Scholar]
- 17.Renfree MB, Short RV, Shaw G. Sexual differentiation of the urogenital system of the fetal and neonatal tammar wallaby, Macropus eugenii. Anat Embryol (Berl) 1996;194:111–34. doi: 10.1007/BF00195006. [DOI] [PubMed] [Google Scholar]
- 18.Hutson JM, Southwell BR, Li R, et al. The regulation of testicular descent and the effects of cryptorchidism. Endocr Rev. 2013 doi: 10.1210/er.2012-1089. [DOI] [PubMed] [Google Scholar]
- 19.Clarnette TD, Rowe D, Hasthorpe S, Hutson JM. Incomplete disappearance of the processus vaginalis as a cause of ascending testis. J Urol. 1997;157:1889–91. [PubMed] [Google Scholar]
- 20.Hutson JM, Li R, Southwell BR, Petersen BL, Thorup J, Cortes D. Germ cell development in the postnatal testis: the key to prevent malignancy in cryptorchidism? Front Endocrinol (Lausanne) 2012;3:176. doi: 10.3389/fendo.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]