Abstract
The ability to dynamically use various aspects of cognition is essential to daily function, and reliant on dopaminergic transmission in corticostriatal circuitry. Our aim was to investigate both striatal and cortical dopaminergic changes in patients with Parkinson’s disease with mild cognitive impairment, who represent a vulnerable group for the development of dementia. We hypothesized severe striatal dopamine denervation in the associative (i.e. cognitive) region and cortical D2 receptor abnormalities in the salience and executive networks in Parkinson’s disease with mild cognitive impairment compared with cognitively normal patients with Parkinson’s disease and healthy control subjects. We used positron emission tomography imaging with dopaminergic ligands 11C-dihydrotetrabenazine, to investigate striatal dopamine neuron integrity in the associative subdivision and 11C-FLB 457, to investigate cortical D2 receptor availability in patients with Parkinson’s disease (55–80 years of age) with mild cognitive impairment (n = 11), cognitively normal patients with Parkinson’s disease (n = 11) and age-matched healthy control subjects (n = 14). Subjects were administered a neuropsychological test battery to assess cognitive status and determine the relationship between dopaminergic changes and cognitive performance. We found that patients with mild cognitive impairment had severe striatal dopamine depletion in the associative (i.e. cognitive) subdivision as well as reduced D2 receptor availability in the bilateral insula, a key cognitive hub, compared to cognitively normal patients and healthy subjects after controlling for age, disease severity and daily dopaminergic medication intake. Associative striatal dopamine depletion was predictive of D2 receptor loss in the insula of patients with Parkinson’s disease with mild cognitive impairment, demonstrating interrelated striatal and cortical changes. Insular D2 levels also predicted executive abilities in these patients as measured using a composite executive z-score obtained from neuropsychological testing. Furthermore we assessed cortical thickness to ensure that D2 receptor changes were not confounded by brain atrophy. There was no difference between groups in cortical thickness in the insula, or any other cortical region of interest. These findings suggest that striatal dopamine denervation combined with insular D2 receptor loss underlie mild cognitive impairment in Parkinson’s disease and in particular decline in executive function. Furthermore, these findings suggest a crucial and direct role for dopaminergic modulation in the insula in facilitating cognitive function.
Keywords: Parkinson’s disease, mild cognitive impairment, positron emission tomography, dopamine
Introduction
Patients with Parkinson’s disease experiencing mild cognitive impairment (MCI) represent a group at risk for the development of dementia; however, it is not yet possible to predict who will progress to functional impairment and how fast (Caviness et al., 2007). Nigrostriatal dopamine depletion in Parkinson’s disease contributes directly to motor symptoms, however, accumulating evidence suggests that dopamine depletion spreading from the motor subdivisions into the associative (i.e. cognitive) striatum (Haber et al., 2000) disrupts corticostriatal communication and thus recruitment of cortical regions involved in cognitive tasks (Kish et al., 1988; Cools, 2006; Grahn et al., 2008). Dopamine receptors located throughout the cortex dynamically regulate cortical activity (Seamans and Yang, 2004), and in particular dopamine D2 receptors are involved in subserving behavioural flexibility (Floresco and Magyar, 2006). Although the prefrontal cortex has generally been a critical region in studies involving dopamine and cognition, the insula has more recently been considered as an integral hub for many diverse brain regions used to dynamically switch between different networks (e.g. the central executive and default mode networks), facilitating access to attentional, working memory and other higher order cognitive processes (Menon and Uddin, 2010; Chang et al., 2013). Recent evidence suggests that insular activity may be a precursor for cognitive processes, acting in conjunction with the anterior cingulate cortex as part of a salience network to selectively initiate the recruitment of brain regions relevant to the task at hand (Seeley et al., 2007; Sridharan et al., 2008). Additionally, the insula is highly connected with the central executive network (Menon and Uddin, 2010), including the dorsolateral prefrontal cortex and posterior parietal cortex, which notoriously display abnormal activation in Parkinson’s disease. These cortical regions in turn communicate with the striatum through corticostriatal circuits to govern cognitive performance (Alexander and Crutcher, 1990). Based on these premises, we sought to determine for the first time whether associative striatal dopamine depletion was more severe in Parkinson’s disease with MCI compared to cognitively normal Parkinson’s disease patients and healthy control subjects, and whether this depletion could predict D2 receptor changes in the insula, anterior cingulate cortex, dorsolateral prefrontal cortex and posterior parietal cortex. We also sought to determine whether neuropsychological performance, and in particular attentional and/or executive performance, were related to either striatal dopamine depletion or D2 receptor changes in those cortical areas. To achieve this, we took advantage of PET imaging to assess striatal dopamine nerve terminal degeneration in the associative striatum (including the caudate and anterior putamen) with 11C-dihydrotetrabenazine (DTBZ), a biomarker of dopamine neuron integrity, and cortical D2 receptor availability with 11C-FLB 457, a cortical D2 receptor ligand (Meyer, 2008). Non-displaceable binding potential (BPND) of these ligands at rest was compared between three groups of subjects: Parkinson’s disease with MCI as diagnosed by recent consensus criteria (Litvan et al., 2012), cognitively normal patients with Parkinson’s disease and age-matched healthy subjects (Table 1). Groups were assessed for performance in executive function, attention, visuo-spatial function, memory and language with a neuropsychological test battery.
Table 1.
Demographic and clinical variables
| Healthy control subjects (n = 14) | Cognitively normal patients with Parkinson’s disease (n = 11) | Patients with Parkinson’s disease with MCI (n = 11) | |
|---|---|---|---|
| Age in years (SD) | 67.5 (7.43) | 68.8 (3.71) | 70.8 (7.01) |
| Education in years (SD) | 17.1 (2.74) | 15.7 (2.87) | 16.2 (1.47) |
| Male/female | 3M/11F | 3M/8F | 8M/3F |
| Unified Parkinson’s Disease Rating Scale-III | – | 23.3 (12.03) | 31.45 (18.5) |
| Disease duration in years (SD) | – | 6.3 (4.20) | 7.1 (3.42) |
| LEDD total (mg/day) | – | 725 (570.85) | 663.7 (397.53) |
| Dopamine agonist LEDD (mg/day) | – | 166.67 (28.87) | 216.67 (160.73) |
| Montreal Cognitive Assessment | 27.6 (2.21) | 26.5 (1.81) | 23.2 (2.79)a |
| Beck Depression Inventory | 4 (3.85) | 5.9 (1.87) | 5.54 (5.87) |
SD = standard deviation; LEDD = levodopa equivalent daily dose.
Values are listed as mean (SD).
Independent sample t-tests were used to compare demographic variables between groups. The patients with Parkinson’s disease with MCI group had significantly lower Montreal Cognitive Assessment scores than healthy control subjects (P < 0.001) and cognitively normal patients (P < 0.01). There were no significant differences in age, education, Unified Parkinson’s Disease Rating Scale III, disease duration, levodopa equivalent daily dose (calculated according to Evans. et al., 2004) or Beck Depression Inventory.
Materials and methods
Participants and experimental design
A total of 36 subjects; 22 patients with Parkinson’s disease meeting the UK Parkinson’s Disease Society Brain Bank Criteria, and 14 aged-matched healthy controls participated in the study. We excluded patients with any other neurological or psychiatric conditions, or patients meeting the criteria for dementia (Emre et al., 2007). Patients were also matched based on disease severity with the Unified Parkinson’s Disease Rating Scale III and levodopa equivalent daily dose, which was based on theoretical equivalence to L-DOPA as follows: L-DOPA dose + L-DOPA dose × 1/3 if on entacapone + bromocriptine (mg) × 10 + cabergoline or pramipexole (mg) × 67 + ropinirole (mg) × 20 + pergolide (mg) × 100 + apomorphine (mg) × 8 (Evans et al., 2004). Fourteen healthy control subjects were recruited from advertisement and had no history of neurological or psychiatric disorder, and no evidence of cognitive impairment as assessed by a neuropsychological test battery. All participants were matched on the basis of age (within 5 years) and categories of estimated premorbid IQ according to the Weschler Test of Adult reading. All participants were also screened for depression using the Beck Depression Inventory (Beck et al., 1961). Demographic and clinical variables are presented in Table 1. Neuropsychological assessment was administered to all subjects to assess cognitive status (Table 2).
Table 2.
Neuropsychological data
| Healthy control subjects (n = 14) | Cognitively normal patients with Parkinson’s disease (n = 11) | Patients with Parkinson’s disease with MCI (n = 11) | |
|---|---|---|---|
| Digit Span forward | 0.53 (1.04) | 0.56 (0.50) | −0.05 (0.73) |
| California Verbal Learning Test delayed free recall | 0.25 (0.92) | 0.54 (0.72) | −0.55 (1.09) |
| Letter-number sequencing | 0.15 (0.68) | 0.30 (0.64) | 0.002 (0.95) |
| Visual Verbal total shifts | −0.4 (0.49) | −1.38 (2.03) | −2.57 (1.52)a |
| Judgement of Line Orientation total correct | 0.14 (0.84) | −0.07 (1.23) | −1.92 (1.37)a,b |
| Stroop: Colour Naming, completion time | 0.4 (0.91) | 0.12 (0.54) | −0.54 (1.18)a |
| Stroop: Inhibition, completion time | 0.33 (1.02) | 0.30 (0.71) | −0.14 (0.85) |
| Category fluency | 0.85 (0.89) | 0.95 (0.96) | −0.74 (1.06)a,b |
Values are listed as group based mean z-scores (SD).
Independent sample t-test: healthy control subjects > patients with Parkinson’s disease with MCI, Visual verbal (P = 0.002), Judgement of Line Orientation (P = 0.001), Colour naming of the Stroop task (P = 0.01) and Category (semantic) fluency (P < 0.0001).
Independent sample t-test: cognitively normal patients with Parkinson’s disease versus patients with Parkinson’s disease with MCI, Judgement of Line Orientation (P = 0.003), and Category (semantic) fluency (P < 0.0001).
Patients underwent 12-h overnight withdrawal from anti-parkinsonian medication before PET scans, which is standard practice in Parkinson’s disease research (Defer et al., 1999) and ensures that the effect of medication is standardized and minimized while patients are still functional. Each subject was scanned with 11C-DTBZ, a vesicular monoamine transporter (VMAT2) PET radioligand, as a marker of dopamine neuronal integrity. The selection of VMAT2 (now known as SLC18A2) as the present ‘gold standard’ dopamine marker is based on extensive animal data suggesting that levels of VMAT2 are more resistant to drug-compensatory regulation affecting other dopamine markers such as the dopamine transporter (Kilbourn et al., 1996; Wilson et al., 1996; Kemmerer et al., 2003), and is well established in the literature as a tool for measuring striatal dopamine neuron degeneration in Parkinson’s disease (Frey et al., 1996; Bohnen et al., 2006; Martin et al., 2008; Stoessl, 2011). Subjects were also scanned with 11C-FLB 457, which binds to D2 receptors. 11C-FLB 457 can be used to assess postsynaptic D2 binding in cortical regions (but not in the striatum). The recent development of high-affinity dopaminergic ligands such as 11C-FLB 457, permit an accurate assessment of dopaminergic function in the human cortex. Subjects also underwent a high resolution structural MRI for registration with PET images. Both PET scans and MRI scans were performed on separate days to avoid excessive fatigue. Neuropsychological testing and both PET scans were performed within a 6-month span of enrolment in the study to ensure no significant change in cognitive status. This study was approved by the Research Ethics Committees, for the Centre for Addiction and Mental Health and the University Health Network of the University of Toronto. All subjects provided written informed consent to participate according to the Declaration of Helsinki.
Neuropsychological assessment
Patients were diagnosed as Parkinson’s disease with MCI according to recent criteria proposed by the Movement Disorders Society commissioned task force (Litvan et al., 2012). Patients were classified as Parkinson’s disease with MCI according to the Level 1 criteria requiring at least 1.5 standard deviations below the normative mean on at least two core variables of tests in a neuropsychological test battery. All subjects were administered a neuropsychological test battery assessing cognitive function in the domains of executive function, attention/working memory, language, visuospatial function and memory. Core variables from each test were selected for use in the diagnosis of Parkinson’s disease with MCI. The neuropsychological tests and core variables included: Wechsler Memory Scale-III Digit Span (attention) and Letter-Number Sequencing total score (attention/working memory) (Wechsler, 1997); California Verbal Learning Test-II long delay free recall score (memory) (Delis et al., 2002); Visual Verbal Test total number of shifts (executive) (Wicklund et al., 2004); Judgement of Line Orientation total score (visuospatial) (Benton et al., 1994); Delis-Kaplan Executive Function System: Colour-Word Interference (Stroop task), time to complete condition 1: colour naming (attention) and time to complete condition 3: inhibition (executive); and Delis-Kaplan Executive Function System Verbal Fluency, total score for category fluency (language/executive) (Delis et al., 2001). Neuropsychological data are displayed in Table 2. The Disability Assessment for Dementia was used to ensure that no patients had any functional impairment due to cognition (Gelinas et al., 1999). For assessing relationships between PET imaging measures and cognition, composite z-scores were calculated for each domain of cognition. When the cognitive domain had only one test, the individual test score was used. For executive function the average of the z-scores from the visual verbal test, inhibition segment of the Stroop task and verbal fluency were used, while for attention/working memory the average of the z-scores from the digit span, letter-number sequencing and colour-naming segment of the Stroop were used. The Judgement of Line Orientation was used as a measure of visuospatial function, the California Verbal Learning Test long delay free recall was used as a measure of memory function and although also a measure of executive function, the category fluency score (alone) was used as a measure of language.
Positron emission tomography imaging
The PET scanning was performed using a 3D high-resolution research tomograph (HRRT) brain tomograph (Siemens), which measures radioactivity in 207 brain sections with a thickness of 1.22 mm each. The detectors of the HRRT are a LSO/LYSO phoswich detector, with each crystal element measuring 2 × 2 × 10 mm3. A 10-min transmission scan, measured using a single photon point source, 137Cs (t1/2 = 30.2 years, Eγ = 662 keV) was acquired immediately before the acquisition of the emission scan to correct for attenuation. Custom-made thermoplastic facemasks together with a head-fixation system (Tru-Scan Imaging) were used to minimize subject head movement in the scanner. Upon completion of the acquisition, the emission list mode data were rebinned into a series of 3D sinograms. For each 3D sinogram, the data were normalized with attenuation and scatter correction before applying Fourier rebinning to convert the 3D sinograms into 2D sinograms (Defrise et al., 1997). The 2D sinograms were then reconstructed into image space using a 2D filtered back projection algorithm, with a ramp filter at Nyquist cut-off frequency. Each subject underwent a 11C-DTBZ PET scan and a 11C-FLB 457 PET scan on separate days. For each scan, the radioactive tracer was injected as a bolus into an intravenous line placed in an antecubital vein. Emission data were acquired over 90 min for 11C-FLB 457 and 60 min for 11C-DTBZ while subjects were at rest. Subjects underwent an MRI scan on a separate day to obtain high-resolution T1-weighted and proton density weighted structural MRIs (GE 3 T, 1 mm slice thickness).
11C-DTBZ analysis
As in previous studies (Boileau et al., 2008; Tong et al., 2008), a region of interest analysis was performed for 11C-DTBZ to measure BPND in striatal regions of interest. Regions of interest were delineated as described in Rusjan et al. (2006) using ROMI software [based on Talairach and Tournoux (1988) and Kabani et al. (1998) atlases]. They included the associative (our primary region of interest, including the anterior putamen and caudate nucleus), sensorimotor and limbic striatal subdivisions, which were delineated according to previously specified criteria (Mawlawi et al., 2001; Rusjan et al., 2006). A standard brain template (International Consortium for Brain Mapping/Montreal Neurological Institute 152 MRI) containing predefined subcortical regions of interest was non-linearly transformed using Statistical Parametric Mapping software (SPM8), (Wellcome Department of Imaging Neuroscience, London, UK) to fit the individual high-resolution MRI (GE 3 T, proton density weighted images, 1 mm slice thickness). The resulting individual regions of interest were aligned and resliced to match the PET images using a normalized mutual information algorithm. The occipital cortex was selected as the region of reference as it is devoid of significant levels of VMAT2 (Scherman et al., 1988). 11C-DTBZ binding to VMAT2 was estimated in each region of interest using the simplified reference tissue model (Lammertsma and Hume, 1996; Gunn et al., 1997) and the occipital cortex time activity curve as an input function (Koeppe et al., 1996; Chan et al., 1999). This was performed using Receptor Parametric Mapping software (RPM). The outcome measure derived from this analysis is BPND, which refers to the specific to non-specific partition coefficient equal to Bmax/KD where Bmax is unoccupied VMAT2 density, and 1/KD is the in vivo affinity of 11C-DTBZ. The simplified reference tissue model has been shown to be an appropriate model for quantifying DTBZ data in humans without arterial input function (Koeppe et al., 1996; Chan et al., 1999). To address the possibility of volume loss in the patient groups potentially affecting the analysis, partial volume effect correction on time activity curve data was implemented using the Rousset algorithm (Rousset et al., 1998).
Statistical analysis
Comparisons between 11C-DTBZ across groups of subjects were performed using an ANCOVA, to test for significant differences between the three groups in the associative striatum (region of interest), while controlling for age, motor disease severity with the Unified Parkinson’s Disease Rating Scale III and levodopa daily equivalent dose. A multivariate ANCOVA was also used to assess binding within the three striatal subdivisions, the associative striatum, limbic striatum and sensorimotor striatum while controlling for age, Unified Parkinson’s Disease Rating Scale III and levodopa daily equivalent dose. Post hoc independent sample t-tests were used to examine between group differences using SPSS (Version 13.0) software. Results were considered significant at the threshold of P < 0.05 after Bonferroni correction for multiple comparisons.
11C-FLB 457 analysis
As in our previous imaging studies (Strafella et al., 2005; Monchi et al., 2006; Cho and Strafella, 2009; Ko et al., 2009), the high-resolution MRI (GE 3 T, T1-weighted images, 1 mm slice thickness) of each subject’s brain was transformed into standardized stereotaxic space (Talairach and Tournoux, 1988) using non-linear automated feature-matching to the Montreal Neurological Institute (MNI) template (Collins et al., 1994). PET frames were smoothed, realigned, summed, registered to the corresponding MRI (Woods et al., 1993) and transformed into standardized stereotaxic space (Talairach and Tournoux, 1988) using the transformation parameters of the individual structural MRIs. All PET images were smoothed with an isotropic Gaussian of 6 mm full-width at half-maximum to accommodate for inter-subject anatomical variability. For 11C-FLB 457 (Olsson et al., 1999; Ito et al., 2001), voxel-wise 11C-FLB 457 binding potentials (BPND) were calculated using the simplified reference tissue model with the cerebellum as a reference region (Lammertsma and Hume, 1996; Gunn et al., 1997) to generate statistical parametric images of change in BPND (Aston et al., 2000).
Statistical analysis
The statistical map was constructed representing voxel-by-voxel change in BPND and statistical analysis was performed in MATLAB version 7.4 (Mathworks Inc.) using SPM8. Comparisons between 11C-FLB 457 BPND across the groups of subjects were conducted using an ANCOVA in SPM8 to test for main effect of group while controlling for covariates including age, disease severity (Unified Parkinson’s Disease Rating Scale III) and levodopa daily equivalent dose followed by voxel-wise post hoc independent sample t-tests to examine differences between groups (SPM8). T-map images were thresholded at P < 0.005 uncorrected with an extent threshold of k = 10 voxels. All coordinates of significant clusters are reported in MNI space. Regions of changes in BPND at the extrastriatal level were considered significant at the voxel-level threshold of P < 0.05 after family-wise error (FWE) correction for multiple comparisons. Correction within our a priori regions of interest was using small volume correction for the specified brain region (Aston et al., 2000). BPND values, from each group of patients, were extracted from volumes of interest defined by a sphere of 4 mm radius drawn on the individual MRI at the level of the statistical peak defined by the parametric map. Individual BPND was entered into a multiple regression analysis with neuropsychological test scores from the test battery to determine whether there was a relationship between cognitive functions in each cognitive domain, (i.e. executive, memory, language, attention/working memory and visuospatial function) and the amount of binding. Age, Unified Parkinson’s Disease Rating Scale III and levodopa daily equivalent dose were entered as covariates in the analysis to control for the effects on BPND measurements.
Cortical thickness analysis
We performed a secondary cortical thickness analysis to investigate whether there was a significant cortical thinning in regions where 11C-FLB 457 binding was significantly different between groups. The cortical thickness analysis was performed using FreeSurfer software (FreeSurfer 4.0.5, freely available at: http://surfer.nmr.mgh.harvard.edu) (Dale et al., 1999; Fischl et al., 1999), a widely used and automated program for the analysis of brain structure (Fischl and Dale, 2000). In brief, FreeSurfer processing streams included the removal of non-brain tissue, the segmentation of grey and white matter, and the alignment of each image volume to a standardized space (i.e. MNI space). For the estimation of cortical thickness specifically, after intensity normalization, grey and white tissue segmentation was used as the starting point for a deformable surface algorithm that was applied to extract the pial and grey/white surfaces (Dale et al., 1999). All surface models were visually inspected for accuracy. Cortical thickness was calculated as the closest distance from the grey/white boundary to the grey/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000).
Statistical analysis
Regional differences between groups in cortical measures were assessed using a vertex-by-vertex general linear model. Maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a full width at half maximum of 15 mm. Z Monte Carlo simulations with 10 000 iterations were applied to cortical thickness maps to provide clusterwise correction for multiple comparisons, and the results were thresholded at a corrected P-value of 0.05 (Z = 1.3). The statistical analysis was performed using the Qdec module and the results were visualized on maps of cortical curvatures.
Results
Neuropsychological testing
Based on the results of our neuropsychological test battery (Table 2), 11 of our patients with Parkinson’s disease met the criteria for Parkinson’s disease with MCI. The remaining 11 patients with Parkinson’s disease did not meet the criteria, and were therefore considered cognitively normal, as were all of the healthy control subjects. Of the Parkinson’s disease with MCI group (n = 11), eight had executive impairments, six had visuospatial impairments, four had language impairments, four patients had memory impairments and two had attention/working memory impairments. There were significant differences between the three groups in executive function, visuospatial function and attention/working memory. The Visual Verbal Abstraction performance (executive) was significantly different between groups [F(2,33) = 6.95, P = 0.003] and post hoc independent sample t-tests demonstrated lower z-scores in Parkinson’s disease with MCI compared with healthy control subjects (P = 0.002). The verbal fluency task performance, also measuring executive function, was also significantly different between groups [F(2,33) = 11.959, P < 0.0001] with lower z-scores in Parkinson’s disease with MCI compared with healthy controls (P < 0.0001) and cognitively normal patients with Parkinson’s disease (P < 0.0001). There were group differences on the Judgement of Line Orientation, a visuospatial test [F(2,33) = 8.364, P = 0.001] with lower z-scores in the Parkinson’s disease with MCI group compared to healthy controls (P = 0.001) and cognitively normal patients with Parkinson’s disease (P = 0.003). Performance on the colour-naming segment of the stroop task (attention/working memory) was also significantly different between groups [F(2,33) = 3.24, P < 0.05] with patients with Parkinson’s disease with MCI having lower scores than healthy controls (P = 0.01).
Severity of striatal dopamine denervation
In our PET imaging analysis, we sought to investigate the degree of associative striatal dopamine nerve terminal integrity with 11C-DTBZ in patients with Parkinson’s disease with MCI. We found significant group differences in 11C-DTBZ binding in the sensorimotor striatum [F(2,33) = 60.628, P < 0.0001] with significant dopamine denervation in both patient groups compared with healthy control subjects (P < 0.0001), as is expected in Parkinson’s disease. We found significant differences in 11C-DTBZ binding between groups in the associative striatum, our region of interest, which remained significant after controlling for age, motor disease severity and daily dopaminergic medication intake [F(2,30) = 4.981, P < 0.05, Fig. 1]. Post hoc independent sample t-tests demonstrated that the Parkinson’s disease with MCI group had significantly reduced binding compared with healthy control subjects (P < 0.001) and cognitively normal patients with Parkinson’s disease (P = 0.03), indicative of more severe dopamine neuron degeneration in this group (Fig. 1). There were significant group differences in the limbic striatum [F(2,33) = 7.393, P = 0.002] with cognitively impaired patients (P = 0.001) and cognitively normal patients (P < 0.05) having reduced binding compared with healthy control subjects; however, no significant difference was seen between patient groups in this region (P = 0.17).
Figure 1.
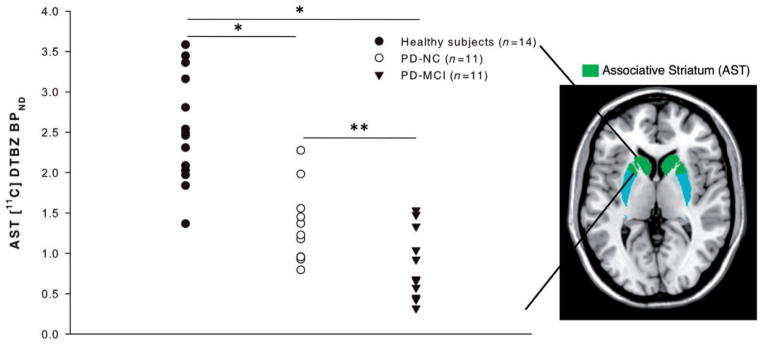
Severity of associative striatal dopamine depletion in Parkinson’s disease with MCI. Patients with Parkinson’s disease with MCI (PD-MCI) show reduced 11C-DTBZ BPND compared with healthy control subjects and cognitively normal patients with Parkinson’s disease (PD-NC): *P < 0.001 compared with healthy control subjects (Bonferroni corrected), **P < 0.05 compared to cognitively normal patients with Parkinson’s disease (Bonferroni corrected). AST = associative striatum.
Cortical D2 receptor availability
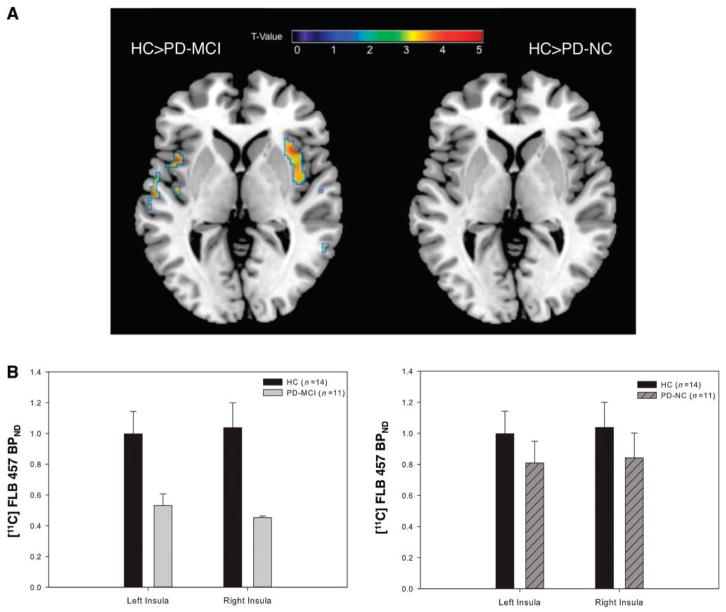
We then asked whether dopamine D2 receptor availability was affected in the insula, anterior cingulate cortex, dorsolateral pre-frontal cortex or posterior parietal cortex, the cortical regions defined in our a priori hypothesis. We found a significant difference in D2 receptor availability between our three groups in the insula [F(2,30) = 7.74, P = 0.03]. Post hoc voxel-wise t-tests demonstrated a reduction in D2 receptor availability in Parkinson’s disease with MCI in the bilateral insula compared with healthy control subjects (P < 0.05; Fig. 2A and Supplementary Table 1) and cognitively normal patients (P < 0.05; Fig. 3 and Supplementary Table 2). Mean BPND was extracted from the regions on the parametric map of significant change and plotted to display reduced D2 receptor binding in Parkinson’s disease with MCI (Figs 2B and 3B). When healthy control subjects and cognitively normal patients with Parkinson’s disease were compared, however, there were no significant clusters of reduced D2 binding (Fig. 2A). The lack of any difference between patients with normal cognition and healthy controls emphasized that D2 receptor changes seen in the insula were specific to patients with declining cognition. In addition to our a priori regions, we detected a reduction in D2 availability in the right parahippocampal gyrus of the Parkinson’s disease with MCI group, when we extended our search to the whole brain (P = 0.03; Supplementary Table 1).
Figure 2.
(A) D2 receptor reductions in Parkinson’s disease with MCI (PD-MCI) compared with healthy control subjects (HC). Statistical parametric map (left brain) demonstrating reduced D2 availability in the bilateral insula [right: x = 46, y = −4, z = 14, voxels (k) = 350 T = 3.76, left: x = −44, y = −12, z = 2, k = 22, T = 3.33 P < 0.05 FWE small volume corrected] in patients with MCI compared with healthy control subjects. Both left and right insula were significant after controlling for age, disease severity and daily medication intake. Conversely, right brain demonstrates no significant clusters of reduced binding in the healthy control subjects versus cognitively normal patients with Parkinson’s disease (PD-NC) contrast. (B) Plot on the left of extracted BPND values from volumes of interest in the left and right insula confirming significantly reduced D2 binding in Parkinson’s disease with MCI compared with healthy control subjects. Plot on the right of extracted BPND values from identical regions demonstrates no significant difference between healthy controls and cognitively normal Parkinson’s disease. Error bars represent SEM.
Figure 3.
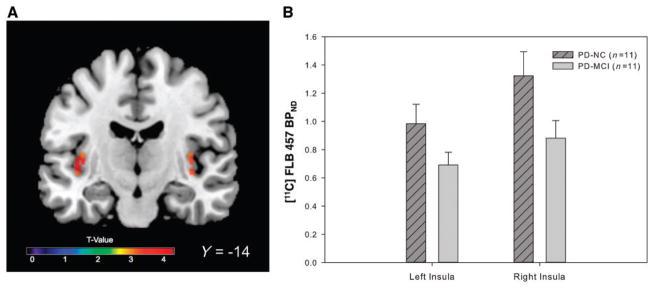
(A) D2 reductions in Parkinson’s disease with MCI (PD-MCI) compared with cognitively normal patients with Parkinson’s disease (PD-NC). Statistical parametric map demonstrating reduced D2 availability in the bilateral insula (left: x = −40, y = −14, z = 2, k = 46 voxels, T = 3.53, right: x = 38, y = −18, z = 2, k = 26 T = 3.06 P < 0.05 FWE small volume corrected) in patients with MCI compared with cognitively normal patients with Parkinson’s disease. (B) Plots demonstrate extracted mean BPND values from volumes of interest in the left and right insula. Error bars represent SEM.
Relationship between striatal and cortical dopaminergic dysfunction
Next, we sought to determine if there was a relationship between the extent of dopamine depletion in the associative striatum and D2 receptor changes in the cortex. Using a whole-brain voxel-wise regression analysis we found that higher associative striatal dopamine levels in patients with Parkinson’s disease with MCI were predictive of higher D2 receptor availability in the right anterior insula, which remained significant after accounting for age, disease severity and daily medication intake (P = 0.01; Fig. 4A). Mean 11C-FLB BPND was extracted from the significant cluster in the right insula and plotted against associative striatal 11C-DTBZ BPND confirming the linear positive relationship (R2 = 0.679; Fig. 4B). This suggested that the striatal and cortical dopaminergic changes seen in the cognitively impaired group were in fact, interrelated.
Figure 4.
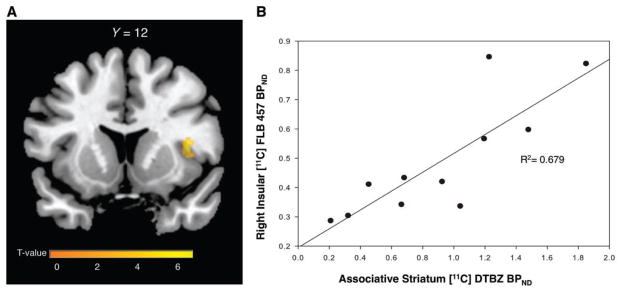
Relationship between striatal dopamine and insular D2 levels in Parkinson’s disease with MCI. (A) Parametric map displaying region of significant positive relationship between associative striatal dopamine preservation and D2 binding in the right anterior insula (x = 40, y = 12, z = 10, k = 59, T = 6.34 P < 0.05 FWE small volume corrected, R2 = 0.679). (B) 11C-FLB 457 BPND extracted from cluster in the right insula of the parametric map and plotted against 11C-DTBZ BPND from the associative striatum in patients with Parkinson’s disease with MCI demonstrating a positive correlation.
Relationship between dopaminergic changes and cognitive performance
Last, we tested which cognitive domain was best related to dopaminergic changes found in the associative striatum and insula. The whole brain voxel-based regression analysis revealed a significant positive relationship between D2 receptor binding and composite executive z-scores in patients with Parkinson’s disease with MCI in the right anterior insula (P = 0.03; Fig. 5A). Thus, the higher the D2 receptor availability, the better the executive performance in patients with Parkinson’s disease with MCI. Mean 11C-FLB BPND was extracted from the significant cluster in the right insula and plotted against composite executive z-scores demonstrating a positive association (R2 = 0.7518; Fig. 5B). There were no significant relationships between any cognitive domain (i.e. executive, attention, visuospatial function, language or memory) and D2 availability in any other subject group. No relationship was found between 11C-DTBZ binding in the associative striatum and any cognitive measure.
Figure 5.
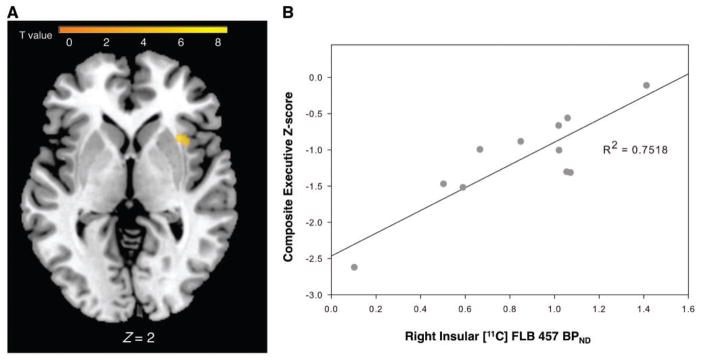
Relationship between insular D2 levels and executive performance in Parkinson’s disease with MCI. (A) Parametric map displaying region of significant positive relationship between D2 binding and composite executive scores in the right anterior insula (x = 36, y = 16, z = 0, k = 29, T = 4.69 P < 0.05, FWE small volume corrected, R2 = 0.7518). (B) Plot showing mean 11C-FLB BPND values extracted from the statistical peak of the parametric map confirming a significant positive relationship between BPND and composite executive scores in the right anterior insula.
To ensure that the observed dopaminergic receptor changes in the insula were not the result of atrophic changes, we performed an additional cortical thickness analysis. The analysis showed no significant differences in cortical thickness in the left or right insula or any other cortical region among groups, suggesting that D2 receptor changes were not attributed to brain atrophy.
Discussion
This study established that dopamine innervation in the associative striatum declines stepwise from cognitively normal patients with Parkinson’s disease to patients with Parkinson’s disease with MCI and that D2 receptor loss in the insula is unique to patients with MCI. Thus, what distinguished the Parkinson’s disease with MCI group from the other patients was the presence of associative striatal dopamine depletion combined with D2 receptor loss in the insula. In addition, we established that the level of dopamine depletion in the associative striatum was predictive of D2 receptor levels in the insula of cognitively impaired patients. Together, these observations suggest that insular dopaminergic changes are closely related to associative striatal dopamine depletion and interfering with cognitive processing. Furthermore, we found a linear positive relationship between D2 receptor levels and executive performance in patients with MCI in the right anterior insula suggesting that D2 receptors in this region are directly related to executive function, the domain that was most significantly impaired in our MCI group. Interestingly, but not surprisingly, no relationship was observed between insular D2 receptor availability and other cognitive domains. Our finding that D2 receptors in the anterior insula were strongly linked to diverse executive abilities confirms that the insula (in particular the anterior region) is indeed an essential hub for governing cognitive processes (Menon and Uddin, 2010). This is in agreement with studies demonstrating that behavioural flexibility is dependent upon the modulation of cortical activity through D2 signalling in animals and humans (Floresco and Magyar, 2006; van Holstein et al., 2011). Thus, the loss of D2 in the insula could directly affect executive performance by disrupting the modulation of activity in this region, impairing its role in the recruitment of or switching between brain networks (Menon and Uddin, 2010). The lack of a direct relationship between associative striatal dopamine depletion and executive performance, but strong association with insular D2 levels suggests that although striatal dopamine depletion is worse in patients with MCI, performance may only be affected once dopaminergic changes are manifested cortically (i.e. insula). In favour of this possibility was the observation that cognitively normal patients with Parkinson’s disease showed less severe associative striatal dopamine depletion than those patients with Parkinson’s disease with MCI, and no significant dopaminergic changes in the insula. The proportional changes at both the striatal level and cortical level are also consistent with the strong functional connectivity of the insula to the subcortical substantia nigra and ventral tegmental area, which are key components of the salience network (Seeley et al., 2007).
A possible reconciliation for the lack of D2 changes, but frequently reported abnormal blood oxygen level-dependent signal changes (i.e. activation) measured with functional MRI in the dorsolateral prefrontal and posterior parietal cortices of patients with Parkinson’s disease (Monchi et al., 2004, 2007) is that D2 receptor loss may impact the ability of the insula to effectively recruit these regions during cognitive tasks. This is consistent with the known role of D2 receptor stimulation in modulating cortical activation (Seamans and Yang, 2004) as well as the finding that the insula plays a critical and causal role in the activation of the central executive network including the dorsolateral prefrontal and posterior parietal cortices (Sridharan et al., 2008). The fact that our patients were imaged at rest could explain the lack of D2 changes in those cortical areas that might have only been detected during the performance of an executive task. Previous studies using 18F-DOPA PET to examine changes in presynaptic dopamine turnover in Parkinson’s disease, have found reduced 18F-DOPA in frontal cortical regions in patients with severe cognitive impairment (Hilker et al., 2005; Klein et al., 2010). Taken together, these observations suggest that, in these patients, pre- and postsynaptic dopaminergic function in cortical regions may decline with cognition over time. The D2 reductions seen in the right parahippocampal gyrus in cognitively impaired patients could also be related to cognitive decline, as the right parahippocampal gyrus has a known role in memory consolidation and retrieval, especially with relation to object location (Owen et al., 1996). Although the patients from our sample did not show substantial memory deficits at the group level, spatial memory was not assessed, and thus could have been affected in the cognitively impaired group.
In conclusion, the functional coupling of the insula to subcortical regions and the parallel loss of dopaminergic function in these regions seem to suggest that the pathological changes are not distinct, but interrelated, and importantly not a result of brain atrophy. This relationship is not universal to all patients with Parkinson’s disease, but specific to patients with cognitive impairment possibly at risk for dementia. Thus, dopaminergic function and the relationship between striatal and insular dopamine should be considered as important contributors to cognitive decline.
Supplementary Material
Acknowledgments
Funding
This study was supported by the National Parkinson Foundation and Canadian Institutes of Health Research (MOP 110962). A.P.S. is supported by the Canada Research Chair program. Leigh Christopher is supported by a scholarship from Parkinson’s Society Canada. Connie Marras is supported by a New Investigator Award from the Canadian Institutes of Health Research.
Abbreviations
- BPND
non-displaceable binding potential
- DTBZ
dihydrotetrabenazine
- MCI
mild cognitive impairment
- VMAT2
vesicular monoamine transporter 2
References
- Aarsland D, Brønnick K, Alves G, Tysnes OB, Pedersen KF, Ehrt U, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2009;80:928–30. doi: 10.1136/jnnp.2008.166959. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage. 2000;12:245–56. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. Inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KD, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. Orlando: Psychological Assessment Resources; 1994. [Google Scholar]
- Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–6. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–7. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Chan GL, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T, et al. Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med. 1999;40:283–9. [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Neelin P, Peters T, Evans A. Automatic 3D intersubject registration of MR volumetric data in standardized talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marié RM, Rémy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999;14:572–84. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16:145–58. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Examiner’s manual for the Delis–Kaplan executive function system. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California verbal learning test. 2. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;9:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, et al. Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann Neurol. 1996;40:873–4. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- Gelinas I. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–81. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Gunn R, Lammertsma A, Hume S, Cunningham V. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–22. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- Ito H, Sudo Y, Suhara T, Okubo Y, Halldin C, Farde L. Error analysis for quantification of [(11)C]FLB 457 binding to extrastriatal D(2) dopamine receptors in the human brain. Neuroimage. 2001;13:531–9. doi: 10.1006/nimg.2000.0717. [DOI] [PubMed] [Google Scholar]
- Kabani NJ, Collins DL, Evans AC. A 3d neuroanatomical atlas. Presented at the 4th International Conference on Functional Mapping of the Human Brain; 7–12 June, 1998; Montreal, Quebec, Canada. 1998. [Google Scholar]
- Kemmerer ES, Desmond TJ, Albin RL, Kilbourn MR, Frey KA. Treatment effects on nigrostriatal projection integrity in partial 6-OHDA lesions: comparison of L-DOPA and pramipexole. Exp Neurol. 2003;183:81–6. doi: 10.1016/s0014-4886(03)00096-7. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Frey KA, Vander Borght T, Sherman PS. Effects of dopaminergic drug treatments on in vivo radioligand binding to brain vesicular monoamine transporters. Nucl Med Biol. 1996;23:467–71. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–92. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Ko JH, Ptito A, Monchi O, Cho SS, Van Eimeren T, Pellecchia G, et al. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: a [11C]FLB 457 PET study. Neuroimage. 2009;46:516–21. doi: 10.1016/j.neuroimage.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC, et al. Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab. 1996;16:1288–99. doi: 10.1097/00004647-199611000-00025. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord. 2012;27:349–56. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WRW, Wieler M, Stoessl AJ, Schulzer M. Dihydrotetrabenazine positron emission tomography imaging in early, untreated Parkinson’s disease. Ann Neurol. 2008;63:388–94. doi: 10.1002/ana.21320. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH. Applying neuroimaging ligands to study major depressive disorder. Semin Nucl Med. 2008;38:287–304. doi: 10.1053/j.semnuclmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. Neuroimage. 2006;33:907–12. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–10. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130:233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–73. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC. A specific role for the right Parahippocampal Gyrus in the retrieval of object-location: a positron emission tomography study. J Cogn Neurosci. 1996;8:588–602. doi: 10.1162/jocn.1996.8.6.588. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904–11. [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Scherman D, Raisman R, Ploska A, Agid Y. [3H]dihydrotetrabenazine, a new in vitro monoaminergic probe for human brain. J Neurochem. 1988;50:1131–6. doi: 10.1111/j.1471-4159.1988.tb10583.x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl AJ. Neuroimaging in Parkinson’s disease. Neurotherapeutics. 2011;8:72–81. doi: 10.1007/s13311-010-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci. 2005;22:2946–52. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tong J, Wilson AA, Boileau I, Houle S, Kish SJ. Dopamine modulating drugs influence striatal (+)-[11C]DTBZ binding in rats: VMAT2 binding is sensitive to changes in vesicular dopamine concentration. Synapse. 2008;62:873–6. doi: 10.1002/syn.20573. [DOI] [PubMed] [Google Scholar]
- Van Holstein M, Aarts E, van der Schaaf ME, Geurts DEM, Verkes RJ, Franke B, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology. 2011;218:567–78. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale. 3. NY, USA: The Psychological Corporation; 1997. [Google Scholar]
- Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004;26:347–55. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.