Abstract
The first aim of the present study was to compare performance of people with tic disorders (TD) and controls on executive function and a range of skilled motor tests requiring complex performance, guided movements, hand co-ordination, and fine control of steadiness. The second aim was to investigate the effect of cognitive behaviour therapy (CBT) on motor performance. A total of 55 patients with TD were recruited at baseline from participants in a behavioural management programme. A comparison group of 55 patients suffering from a variety of habit disorders (HD) involving complex manual movements, were matched on age and level of education to 34 non-psychiatric controls. Participants were evaluated pre- and post-treatment and post-waitlist with a neuropsychological evaluation focusing on executive function (Wisconsin Card Sorting Test, WCST) and skilled motor performance (Purdue Pegboard, Hole Steadiness Test, and the Groove Test). Results revealed WCST scores in the normal range, while motor performance differed significantly on the Purdue Pegboard Tests in both TD and HD as compared to the control group. Cognitive-behavioural treatment selectively improved motor performance in both clinical groups compared to waitlist control, and this improvement related to clinical outcome measures.
INTRODUCTION
Tics are defined as repetitive non-voluntary muscle contractions and can be simple (e.g., eye blinking, coughing) or complex (e.g., nail biting, repeating sentences). The DSM-IV identifies three subtypes of tic disorder (TD): motor or phonic tic disorder, intermittent tic disorder, and Gilles de la Tourette syndrome (TS). There has been controversy about current criteria for TS (Tourette Syndrome Study Group, 1993) but the diagnosis is currently dichotomous, not dimensional, and depends crucially on the existence of a phonic tic. However, clinician consensus suggests a continuum of severity, in particular between chronic motor tic disorder and TS. Neurobiological hypotheses have centred on basal ganglia dysfunction similar to other movement disorders, in particular the orbital-frontal-basal ganglia loop (Casey, Tottenham, & Fossella, 2002; Peterson et al., 1999). Another hypothesis is that TDs show abnormally high levels of sensori-motor activation. This results in problems with visuo-motor co-ordination, chronic muscle tension, and over-generalised responding. This hypothesis could also partially account for the success of relaxation and habit reversal techniques in tic management (Peterson & Azrin, 1993).
In clinical practice, TD patients frequently show motor restlessness and hyperactivity in their style of planning action, often attempting too much at once and creating frustration and tension (O’Connor, Brisebois, Brault, Robillard, & Loiselle, 2003). Adequate motor functioning is essential in the performance of almost all tasks of daily living, and performance in motor dexterity is predictive of optimal cognitive and occupational functioning (Asikainen, Nybo, Muller, Sarna, & Kaste, 1999), but there have been few studies explicitly describing motor performance in TD. Neuropsychological studies have reported abnormalities in severe TD patients with motor skills tasks like the Purdue Pegboard and Groove Test in children (Bornstein, Baker, Bazylewich, & Douglass, 1991; Hagin, Beecher, Pagano, & Kreeger, 1982), pre-adolescents (Bornstein, 1990), and adults (Bornstein, 1991). Other investigations have shown that TD patients were particularly disadvantaged in responding to various conflicting stimulus-response configurations (Georgiou, Bradshaw, Phillips, Bradshaw, & Chiu, 1995). Hence, any impairment in movement control found in TD patients could be due to disorders of cognition and/or of motor activation. In a further set of studies, Cope, Georgiou, Bradshaw, Iansek, and Phillips (1996) found that patients with hyperkinetic basal-ganglia disorders (i.e., Huntington’s disease and TD) had difficulty with motor tasks where the response location was either compatible or incompatible with the stimulus pointer, while patients with Parkinson’s disease and with hypokinetic basal ganglia performed in the normal range (for their age). But more direct evidence of cortical involvement in TD comes from a functional magnetic resonance imaging (fMRI) study of the motor cortex in a TD sample, during a finger-tapping task. This study revealed an overactivation of the sensori-motor and supplementary motor area and recruitment of larger portions of these areas in the execution of a finger tapping task (Biswal et al., 1998), so suggesting a distinct pattern of motor cortex activation in patients with TD. In order to further refine these results, Fattapposta et al. (2005) evaluated the cortical motor circuit in a patient with TS during a self-paced voluntary movement in either a repetitive, bilateral index finger (habitual) or a little finger (non-habitual) tapping task. The results showed similar activation during both tasks and suggested that the patient with TD was unable to switch from a habitual to a non-habitual mode but rather responded to each task as non-habitual. People with TD may have more difficulty regulating and adapting their motor responses optimally. This hypothesis is consistent with what has been observed in TD with less severe symptomatology while performing a stop-go countermanding task. O’Connor, Robert, Dubord, and Stip (2000) reported no group differences in “go” time, but the TD group was significantly slower than a control group to “stop” automated responses, and they also had greater difficulty regulating the controlled motor response. Lower correlations were also found between motor output (response preparation) and electrocortical activity in TD and a habit disorder group compared to controls (O’Connor, Lavoie, Robert, Stip, & Borgeat, 2005). So, there is some evidence that motor regulation is affected in TD groups as reflected at cortical as well as subcortical level, which varies with task demand.
Pharmacotherapy is currently the treatment of choice for TD. Pharmacological agents that increase dopamine functioning such as L-dopa, stimulant medication (Golden, 1974; Price, Leckman, Pauls, Cohen, & Kidd, 1986) or neuroleptic withdrawal exacerbate TD symptoms (Riddle, Hardin, Towbin, Leckman, & Cohen, 1987). Conversely, drugs that lower or block the action of dopamine, including typical (Shapiro et al., 1989) and atypical (Lombroso et al., 1995) neuroleptics tend to improve tic symptoms. However, unwanted side effects occur in about 80% of individuals, and Peterson, Campise, and Azrin (1994) estimated that only about 20–30% of clients continue their medication over an extended period of time.
A variety of behavioural treatments have shown some success with tic management (Azrin & Peterson, 1988; Bergin, Waranch, Brown, Carson, & Singer, 1998). The most compelling method for managing the tics themselves seems to be “habit reversal” (HR) (Azrin & Peterson, 1988). This cognitive-behavioural package involves multiple stages, including relaxation, awareness, contingency training and positive reinforcement of not performing the tic and the crucial element of practice of a competing antagonistic response. This latter technique involves tensing the muscle antithetical and incompatible with the tic-implicated muscle. Awareness training and competing response training seem the most crucial elements of the programme (Miltenberger, Fuqua, & Woods, 1998), which can be applied to both tics and habit disorders (Rapp, Miltenberger, & Long, 1998). Azrin and Peterson (1988) reported an improvement of 64–100% in a review of studies using this method in populations with both simple tics and/or Tourette syndrome. In a recent waitlist controlled treatment trial, a cognitive-behavioural package based on HR showed significant post-treatment clinical improvement for 52% of both TD and habit disordered patients at 2-year follow-up (O’Connor et al., 2001). This treatment package integrated conventional CBT components with rehabilitation to improve motor planning and coordination. Improvement in planning was related to relapse prevention at 2-year follow-up, so suggesting that improved motor function related to improved tic management in both TD and HD.
Habit disorder (HD) is a term covering a variety of destructive impulse habits including trichotillomania, bruxism, onychophagia, and scabiomania. TD and HD have both been viewed as part of the obsessive-compulsive disorder (OCD) spectrum (Hollander, 1998). Although TD and HD have been compared independently with OCD, there has been little systematic inquiry into the common or distinguishing features between TD and HD. HDs are clearly distinct from tics and tied to emotional state, but involve repetitive manual actions that can resemble complex tics. The tension-reducing or emotion regulating function of both tics and habits would suggest the presence of a heightened state of behavioural arousal in both cases (Christenson, Ristvedt, & Mackenzie, 1993; Dean, Nelson, & Moss, 1992). There already exists evidence that some subtypes of HD show abnormalities on visuo-motor processing and spatial memory. For example, people with trichotillomania have shown poor performance on pursuit rotor tasks (Rettew et al., 1991) and Keuthen et al. (1996) reported deficits in executive function. Stanley, Hannay, and Breckenridge (1997) reported lower scores in tasks requiring divided attention (Paced Auditory Serial Addition test, Trail Making B and Stroop Test), but found lower performance correlated with negative mood states. Furthermore our own clinical work with this HD group has shown a similar style of planning profile to TD and a comparable response to our CBT package (see O’Connor et al., 2001). Hence, HD forms a highly appropriate clinical comparison group for examining motor function in TD.
The first aim of the present study was to provide more information on both central and peripheral-motor function in TD, and to compare executive function, visuo-motor performance involving aiming movements, hand co-ordination and fine control of steadiness, in a group of TD, an HD comparison group, and a non-pathological control group. The second aim was to evaluate the effect of CBT on motor performance when the client groups were tested pre- and post-successful completion of a CBT management programme.
The hypotheses were that: (1) at baseline the TD group would score lower on all motor performance measures than HD or controls, with the controls scoring highest; (2) the TD and HD clients who completed a CBT programme to improve control over tics or habits would show improved motor performance compared to baseline and waitlist at three months retest; and (3) baseline and post-CBT clinical parameters of tic symptomatology would relate to motor performance.
Participants
Study participants were 110 tic and habit disorder participants (55 TD, of whom 13 were diagnosed with TS, 55 HD) recruited at baseline from clients participating in a behavioural management programme. Thirty-four controls were matched on age and level of education to the client groups. The diagnosis was made by a certified psychiatrist (E.S. and F.B.). In addition, the Tourette Syndrome Global Scale (TSGS; Harcherik, Leckman, Detlor, & Cohen, 1984) was administered in semi-structured interview by an independent psychologist to assess tic severity. The final diagnosis was based on the consensus between the evaluation of the psychiatrist and the semi-structured interview (TSGS) conducted by the psychologist. The inclusion criteria for the TD group were based on the DSM-III-R (American Psychiatric Association, 1987) for Tourette disorder (307.23) or chronic tic disorder (307.22). Criteria for inclusion for the TD group were the presence of at least one simple motor and/or phonic tic occurring daily. The inclusion criteria for the HD group were the presence of at least one complex motor habit occurring daily. Participants in the HD group had problems of: trichotillomania (n = 16); onychophagia (n = 13); bruxism (n = 9); skin picking/scratching (n = 6); and other habits (n = 11). The TD group contained as principal tics: eye tics (n = 17); head/neck tics (n = 22); face tics (n = 6); legs/trunk tics (n = 8); and phonic tics (n = 2). The TSGS score was in the mild–moderate range for the TD (range = 12–35).
Criteria for exclusion for all groups were the presence of diagnosis on Axis I, such as schizophrenia, mood disorders, somatoform disorders, dissociative disorders, substance-related disorders and any other disorders diagnosed during infancy, childhood, or adolescence (except attention deficit hyperactivity disorder); Axis II, the presence of personality disorders; Axis III, medical conditions such as neurological problems (e.g., Parkinson’s, hemifacial spasms, Meige syndrome, cerebral sclerosis, Huntington’s disease, Wilson’s disease); Axis IV, any psychosocial stressors such as current behavioural, social or family problems, any severe stressor (e.g., marital rupture), or any other psychological problems requiring attention and abuse of alcohol or drugs. Subjects currently receiving treatment from a psychologist, acupuncturist, hypnotherapist, or massotherapist and those showing a lack of availability were also excluded. Controls were screened for pathology by interview and all groups completed questionnaires at baseline. Questionnaire measures to assess psychosocial function and psychopathology included: the Social Self-Esteem Inventory (SSEI: Lawson, Marshall, & McGrath, 1979), the Speilberger State–Trait Anxiety Inventory (STAI; Speilberger, Gorsuch, & Lushene, 1970), the Beck Depressive Inventory (BDI; Beck, 1970), the General Health Questionnaire (GHQ) – 12-item version (Goldberg, 1972), the Maudsley Obsessional-Compulsive Inventory (MOCI; Rachman & Hodgson, 1980), and the Style of Planning Questionnaire (O’Connor, 2005). Demographic and questionnaire data are given in Table 1.
TABLE 1.
Demographic and baseline clinical data and questionnaire for tic, habit disorder and control group
| Tic disorder (n = 55)
|
Habit disorder (n = 55)
|
Control (n = 34)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 38.93 | 10.30 | 36.89 | 10.54 | 37.57 | 11.46 |
| Female/male | 21/34 | – | 33/22 | – | 21/13 | – |
| Chronicity of problem | 26.17 | 11.24 | 23.02 | 11.10 | – | – |
| GHQ | 25.60 | 5.49 | 25.23 | 5.89 | 22.06 | 4.82 |
| STAI – State Anxiety | 40.79 | 9.91 | 40.92 | 13.52 | 34.21 | 8.25 |
| STAI – Trait anxiety | 43.68 | 9.94 | 41.80 | 11.78 | 35.35 | 9.70 |
| STOP – Total | −9.93 | 35.31 | −12.72 | 34.29 | 22.49 | 18.68 |
| MOCI | 7.96 | 4.29 | 8.13 | 3.94 | – | – |
| BDI | 9.82 | 6.58 | 11.69 | 8.14 | – | – |
| SSEI | 129.70 | 26.22 | 132.15 | 28.58 | – | – |
GHQ: General health Questionnaire; STAI: State–Trait Anxiety Inventory; MOCI: Maudsley Obsessive–Compulsive Inventory; BDI: Beck Depression Inventory; SSEI: Social Self–Esteem Inventory; STOP: Style of Planning Questionnaire.
Treatment and waitlist procedure
A total of 110 TD and HD participants were recruited. One third, 37 of this group (22 CTD, 15 HD) (one in every three consecutive referrals) were allocated at random to a waitlist control condition for 3 months. The 37 in the waitlist group were retested post-waitlist, and then received CBT. The rest immediately received a 3-month CBT treatment package. Eighty two participants were retested post-CBT at 3 months. The CBT programme was inspired by Azrin and Nunn’s (1973), and Azrin and Peterson’s (1988) HR techniques, although it addressed overall cognitive and behavioural restructuring of action as part of implementing a competing response.
The treatment was individualised, manual-based, and was carried out by therapists who were licensed psychologists with 10 years experience of CBT with tic disorder and OCD. The programme was progressive and passed through seven major steps, lasting a total of 4 months: information, awareness training, constructing a situational profile, relaxation and muscle discrimination exercises, modifying background style of action, development of alternative competing responses using cognitive and behavioural strategies, and preventing relapse. In addition, the key HR strategy of implementing a competing behavioural response to the tic/habit was developed alongside a more general cognitive and behavioural restructuring of the person’s approach to the high-risk tic situation, which addressed anticipations and appraisals concerning the appearance of the tics. The strategies were cumulative in the sense that each week the person built on the exercises of the previous week.
As part of the behavioural strategy of retraining sensori-motor activation, overactive style of action and perfectionist concerns with personal organisation were specifically addressed, including: the efficacy of concentrating on one task at a time and screening out distractions; countering thoughts likely to lead to overactive performance; developing realistic feedback on performance ability; avoiding strategies that create tension and frustration (e.g., trying always to be further advanced and “ahead of oneself” in performance); establishing a right to relax; and structuring a timetable efficiently. This style of action was monitored by a specially developed style of planning questionnaire (STOP), which measures excessive overactivation and overpreparation in daily settings. Training in incompatible responses took three forms: prevention by relaxation (localised to counteract onset of the tic in the high-risk situations); normalisation (a more normal response substituted for the tic or habit to replace the overactive response, e.g., correcting excessive blinking through training in the use of correct muscles and rhythm); and the behaviourally antagonist response incompatible with the tic. Behavioural strategies incompatible with the tic were developed in line with alternative evaluations of the situation. The cognitive aspect of restructuring action and planning action aimed to introduce flexibility into judgements and anticipations about intended action, both in high-risk and other situations. The entire treatment package was administered for a standard period of 12 weekly sessions with a further 1-month home practice and then full post-treatment evaluation (see O’Connor et al., 2001).
Executive functioning and motor tasks
The motor performance tests included the Purdue Pegboard, the Groove Steadiness Test, and the Hole Type Steadiness Test (Lafayette Instrument Company). The Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993) assessed abstraction ability and the ability to shift cognitive strategy. The WCST measures functions related to dorsolateral frontal lobe functioning (Demakis, 2003; Milner, 1963; Taylor, 1979). Use of the WCST follows other studies of TS that have reported its sensitivity to changes in executive functioning in TS groups (Schultz, Carter, Scahill, & Leckman, 1999).
The Purdue Pegboard Test has been validated as a measure of sensori-motor performance efficiency in both normal and clinical populations, and is scored as the total number of small pegs placed in a series of aligned holes by dominant and non-dominant hands separately. The Groove Test was scored as the distance in centimetres (max: 25 cm) travelled along the groove until the probe touched the side. The score was averaged over 10 trials. The Hole Steadiness Test performance was measured as the number of holes for which the person was able to maintain the probe steady for 10 seconds without more than one contact, each hole having an increasingly small diameter. The total number of possible holes was nine for each of three successive replications. The score was the mean of the total number of holes completed without contact over three replications for the dominant and non-dominant hands. The WCST was scored according to the manual direction instructions. Scores included: total number of categories sorted, number of trials administered, correct responses, errors, perseverative and non-perseverative errors, and percentage conceptual level responses and learning to learn (conceptual efficiency across categories). Together these WCST sub-scores yield a total score.
Clinical measures
In order to examine links between clinical status of clients and motor task performance and improvement post-treatment, selected correlation coefficients were computed between three specific clinical measures relevant to tic status and motor performance. The three clinical measures reported were: degree of control reported over the tics/habits, frequency of tics/habits, and the total scores on the subscales measuring overactivity and overpreparation in tic disorders from the STOP questionnaire.
Degree of control was the extent to which the person rated being able to resist or control tic onset. Frequency was the subjective estimate of number of tics occurring within a set uniform time period (see O’Connor et al., 2001, for further details). This measure of frequency was validated through comparison with an external rater and video counter estimates. All three measures showed acceptable concordance (see O’Connor et al., 2001). Both control and frequency were monitored in daily diaries throughout the treatment period.
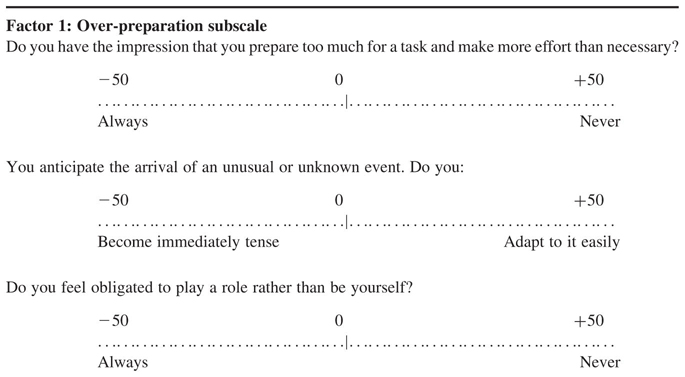
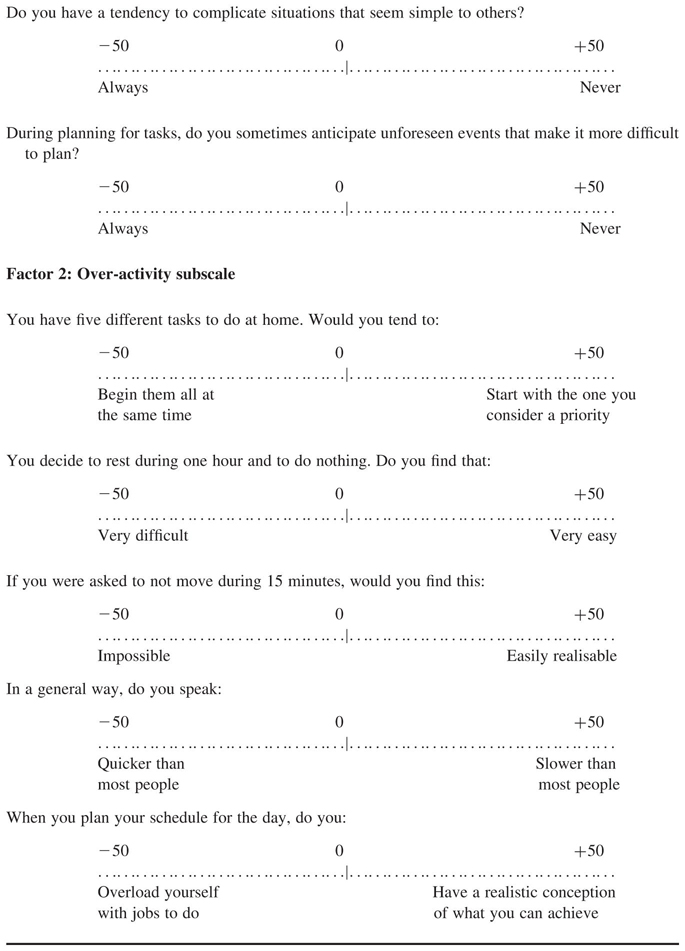
The Style of Planning Questionnaire (STOP) is a 30-item questionnaire that measures alternative ways in which people plan activities on a scale from −50 to +50. A more negative raw score indicates greater pathology. Factor analysis has revealed two robust dimensions, namely over-activity and over-preparation. The STOP has satisfactory test-retest reliability for clinical groups (O’Connor, 2005; O’Connor et al., 2001). High scoring items on the STOP can also form the targets of treatment for addressing sensori-motor activation within the CBT management programme. The five high loading items on each of these factors together form two scales that reliably discriminate between TD, OCD, anxiety disorders, and controls (O’Connor, 2005; see Appendix). Furthermore, scores change following successful therapy and the degree of change on selected items has proved a predictor of relapse (O’Connor et al., 2001).
RESULTS
The scores on all tests are given in Tables 2 and 3. All measures met constraints of sphericity and equality of variance. Effect sizes were computed via partial etas squared (η2): weak > 0.02; medium > 0.13; large > 0.26. A posteriori power is also given for significant effects following Bonneferoni corrections for number of comparisons.
TABLE 2.
Wisconsin Card Sorting Test for each client group (baseline only)
| Tic disorder (n = 54)
|
Habit disorder (n = 51)
|
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Wisconsin | ||||
| Categories completed | 2.85 | 0.49 | 2.89 | 0.38 |
| Trials to complete | 13.61 | 6.40 | 15.80 | 10.42 |
| Correct | 38.57 | 10.44 | 41.64 | 12.71 |
| Errors | 15.31 | 16.44 | 16.76 | 14.86 |
| Perseverative responses | 9.44 | 12.13 | 9.93 | 10.79 |
| Perseverative errors | 8.41 | 10.14 | 8.82 | 9.09 |
| Non-perseverative errors | 6.91 | 7.16 | 7.93 | 6.78 |
| % perseverative errors | 12.59 | 8.15 | 13.18 | 6.45 |
| % conceptual level responses | 71.04 | 18.03 | 69.77 | 15.54 |
| Failure to maintain set | 0.31 | 0.61 | 0.60 | 1.07 |
| Learning to learn | −3.62 | 10.24 | −2.09 | 9.27 |
TABLE 3.
Motor performance at the Purdue Pegboard (no of pegs placed), the Groove Test (distance travelled in centimetres), and the Hole Steadiness Test (mean number of holes without contact) for each group at baseline
| Baseline
|
||||
|---|---|---|---|---|
| Tic (n = 55) | Habit disorder (n = 55) | Control (n = 34) | p< | |
| Groove Test | ||||
| Dominant hand | 21.55 (1.43) | 21.35 (1.71) | 21.61 (1.30) | <.676 |
| Non–dominant hand | 20.11 (2.17) | 20.56 (1.62) | 20.41 (1.74) | <.461 |
| Purdue Pegboard | ||||
| Dominant hand | 45.41 (3.66) | 46.65 (5.06) | 49.15 (5.66) | <.005 |
| Non–dominant hand | 43.87 (3.70) | 45.23 (4.88) | 46.69 (6.16) | <.031 |
| Hole Steadiness Test | ||||
| Dominant hand | 5.27 (1.03) | 5.10 (1.05) | 4.76 (1.23) | <.115 |
| Non–dominant hand | 4.79 (1.09) | 4.73 (1.03) | 4.40 (1.21) | <.274 |
Baseline comparisons
In accordance with the first hypothesis, analysis of variance was calculated to compare motor performance between groups with significance level set at p < .01.
Wisconsin Card Sorting Test (Table 2)
There were no differences between TD and HD groups in any of the WCST scores, and all scores fell within the 16% or greater percentile range of the age-matched norms of WCST published in Heaton et al. (1993). So there was no evidence of abnormal WCST performance in TD or HD groups in our sample.
Purdue Pegboard (Table 3)
There was no difference between TD and HD groups in total pegs placed by the dominant hand, the non-dominant hand, or by both hands simultaneously. All scores were in the top 10 percentile of the norms given by Lafayette and co-authors in the instruction manual. There was, however, a significant difference in performance between clinical groups and the control subjects in the number of pegs placed by dominant, and the total of both hands: dominant F(2, 139) = 5.52, p < .005, η2 = 0.074, observed power = 0.85; non-dominant F(2, 139) = 3.56, p < .03, η2 = 0.049, observed power = 0.65; total (dominant plus non-dominant) F(2, 139)=9.89, p < .001, η2 = 0.13, observed power = 0.98. The controls performed better than the clinical groups and placed a greater number of pegs.
Groove Test (Table 3)
There were no significant differences between TD or HD groups or between the two clinical groups and the control group in mean distance travelled.
Hole Type Steadiness Test (Table 3)
There was no significant difference between TD and HD groups in performance or between clinical and control groups in number of holes completed.
Pre- versus post-treatment
The second hypothesis concerning improvement post-CBT was tested by repeated measures MANOVA. Eighty-two participants completed post-treatment tests (42 TD, 40 HD) (Table 4). The scores on each of the motor performance tests were examined pre- and post-treatment with significance level set at p < .01. Only the Purdue Pegboard showed a significant main treatment effect of improvement post-treatment for the dominant hand performance, F(1, 81) = 7.12, p < .009, η2 = 0.81, observed power = 0.75, and a highly significant main treatment effect for the non-dominant hand performance, F(1, 81) = 14.31, p < .0001, η2 = 0.16, observed power = 0.96. There were no differences in improvement between TD and HD groups, and the tendency in both groups was for better performance post-treatment.
TABLE 4.
Clinical measures of affect, style of planning, and self-reported tic frequency, and degree of tic control pre- and post-treatment. Motor performance at the Purdue Pegboard (number of pegs placed), the Groove Test (distance travelled in centimetres), and the Hole Steadiness Test (mean number of holes without contact) for each group pre- and post-treatment
| Pre-treatment
|
Post-treatment
|
p | |||
|---|---|---|---|---|---|
| Tic (n = 42) | Habit disorder (n = 40) | Tic (n = 42) | Habit disorder (n = 40) | ||
| STOP | |||||
| Over-activity scale | −23.94 (69.12) | 6.42 (68.42) | 9.34 (54.55) | 16.05 (62.77) | <.000 |
| Over-preparation scale | 6.37 (95.67) | 14.34 (109.49) | 17.46 (77.63) | 38.05 (82.12) | <.127 |
| Tic frequency | 19.62 (35.09) | 9.80 (12.04) | 6.70 (21.53) | 2.57 (3.78) | <.005 |
| Tic control | 36.19 (26.20) | 47.41 (26.12) | 74.10 (22.16) | 86.13 (14.09) | <.000 |
| STAI | |||||
| Anxiety State | 39.80 (9.03) | 39.64 (13.14) | 36.41 (9.28) | 35.52 (11.76) | <.003 |
| Anxiety Trait | 43.20 (10.34) | 39.95 (13.14) | 39.10 (9.39) | 37.26 (10.97) | <.000 |
| SSEI | 128.56 (27.76) | 136.09 (26.31) | 131.90 (26.09) | 144.02 (24.47) | <.000 |
| BDI | 10.00 (7.22) | 11.63 (8.07) | 6.11 (5.33) | 6.33 (7.70) | <.000 |
| GHQ | 25.36 (5.89) | 25.13 (5.96) | 21.65 (5.79) | 21.10 (5.64) | <.000 |
| Groove Test | |||||
| Dominant hand | 21.59 (1.50) | 20.38 (1.70) | 21.69 (1.19) | 21.46 (1.30) | <.626 |
| Non-dominant hand | 20.36 (1.94) | 20.58 (1.42) | 21.06 (1.51) | 20.72 (1.58) | <.043 |
| Purdue Pegboard | |||||
| Dominant hand | 45.61 (3.57) | 46.90 (5.00) | 46.76 (4.62) | 48.05 (5.21) | <.009 |
| Non-dominant hand | 44.10 (3.50) | 45.00 (4.59) | 45.40 (3.35) | 46.70 (3.38) | <.000 |
| Hole Steadiness Test | |||||
| Dominant hand | 5.39 (0.89) | 5.22 (1.03) | 5.37 (0.97) | 5.40 (1.00) | <.456 |
| Non-dominant hand | 4.85 (0.95) | 4.79 (1.06) | 4.93 (0.95) | 4.90 (0.97) | <.350 |
Comparison between scores of the TD and HD groups post-treatment and controls at baseline on the Purdue Pegboard Test revealed no significant differences for either dominant or non-dominant hand. In other words, differences present at baseline had disappeared post-treatment.
Pre- versus post-waitlist control
The waitlist group was retested after an equivalent period of time to the CBT group but without treatment with significance level set at p < .01. There was no significant improvement over time in the waitlist group on clinical measures or for dominant or non-dominant hand performance on any of the motor tasks. However a medium effect size indicated some practice effect in the dominant hand performance of the Purdue Pegboard and Hole Test in the TD group and the Groove Test for the HD group (see Table 5).
TABLE 5.
Pre-treatment–post-Waitlist change in the waitlist group on motor performance measures
| Pre-treatment
|
Post-Waitlist
|
ANOVA
|
||||
|---|---|---|---|---|---|---|
| Tic (n = 22) | Habit disorder (n = 15) | Tic (n = 22) | Habit Disorder (n = 15) | p (Tic) | p (Habit disorder) | |
| Groove Test | ||||||
| Dominant hand | 21.39 (1.21) | 20.13 (2.23) | 21.47 (1.29) | 21.36 (1.47) | <.742 | <.013 |
| Non-dominant hand | 19.52 (2.53) | 20.31 (1.77) | 20.48 (1.75) | 20.83 (1.75) | <.125 | <.163 |
| Purdue Pegboard | ||||||
| Dominant hand | 46.32 (3.43) | 47.20 (6.55) | 47.82 (4.44) | 48.53 (8.10) | <.039 | <.232 |
| Non-dominant hand | 43.64 (3.54) | 44.93 (6.12) | 44.27 (4.80) | 44.93 (5.97) | <.506 | <1.000 |
| Hole Steadiness Test | ||||||
| Dominant hand | 5.14 (1.19) | 4.85 (1.07) | 5.59 (0.96) | 4.80 (1.32) | <.023 | <.868 |
| Non-dominant hand | 4.86 (1.30) | 4.40 (1.02) | 4.94 (1.02) | 4.53 (1.15) | <.772 | <.531 |
| Tic frequency | 27.81 (30.98) | 10.44 (12.57) | 21.40 (31.23) | 7.72 (8.68) | <.365 | <.442 |
| Tic control | 32.60 (21.72) | 50.78 (35.15) | 36.82 (29.48) | 59.40 (29.13) | <.326 | <.113 |
Relationship between clinical and motor performance measures
The third hypothesis concerned the link between motor performance and clinical measures in TD and HD groups. A Pearson product moment correlation was computed to see if there was a significant link at baseline between severity of clinical measures and poorer motor performance. After Bonferroni correction, the significance level was set at p < .005 (one tailed). At baseline there was no consistent relationship between tic frequency or control over tics, STOP subscales and any motor task performance. Baseline Hole Task performance and Groove Test performance were significantly correlated for both dominant and non-dominant hands: dominant r(139) = .21, p < .005; non-dominant r(138) = .27, p < .001. Purdue Pegboard performance was unrelated to the Hole Test but did relate to dominant hand performance of the Groove Test, r(140) = .23, p < .003.
Both the clinical measures and the Purdue Pegboard performance significantly improved post-treatment. The relationship between clinical improvement and Purdue Pegboard improvement was examined by correlating respective change measures calculated as (Pre−Post)*100/Pre. Significance level was set at p < .01 (one tailed).
Among completers, improvement in control over tics was correlated with both improvement in dominant Pegboard performance, r(77) = .26, p < .01, and non-dominant performance r(77) = .25, p < .01. Among those completers showing a more clinically significant improvement (≥35% improvement), there was also a significant relation between change in STOP preparation subscale and the dominant Purdue Pegboard performance, r(46) = .36, p < .01, but the relation with non-dominant hand performance fell short of significant (p < .04).
Affect and performance
Differences in motor function have been related to affect and distress and may confound genuine changes in motor function (Stanley et al., 1997). Hence, product moment correlations were calculated between measures of anxiety, depression, and self-esteem, and motor performance at baseline. There were no significant correlations between any affect measure and motor performance for any tasks at baseline. Furthermore, improvement in the Purdue Pegboard was unrelated to change in any mood measure.
DISCUSSION
The first aim of the current study was to compare skilled motor performance involved in aiming movements, hand co-ordination, and fine control of steadiness in groups of adult TD, HD, and a control group. Our current results revealed that there were consistent differences between both TD and HD groups and the control group at baseline, indicating that both clinical groups showed poorer performances in skilled hand co-ordination, but not in aiming or steadying movements. Furthermore, there were no consistent differences between TD and HD groups, either pre- or post-treatment, so suggesting similar motor functioning in both disorders. The relationship between these two diagnostic categories is sometimes clinically unclear because the complex movements in HD can often resemble the complex tics of TD. These findings support previous investigations into electrocortical activity where both TD and HD groups showed low correlations between cerebral activity related to motor preparation and its execution (O’Connor et al., 2005). Hence, there may be similarities in motor organisation between these TD and HD groups.
However, although the motor performance of both clinical groups was inferior to the control group, it was not in the abnormal range. A number of executive function dimensions, such as the ability to form abstract concept, to shift and maintain set, and utilise feedback, were intact as measured by the WCST. The initial baseline comparisons with controls indicated that there was no absolute deficit in executive functioning as measured by the WCST; a finding also reported in another cohort of our patients (Lavoie, Thibault, Stip, & O’Connor, 2007) and in other tic populations (e.g., Channon, Pratt, & Robertson, 2003). This result suggests that impairment is more related to motor action than to a problem of mental flexibility or executive function, so supporting a sensori-motor activation hypothesis rather than a dorsolateral frontal dysfunction. However, the intact WCST in clinical groups does not necessarily generalise to other aspects of executive functioning. There is currently a lack of consensus on how to measure executive function (Miyake & Shah, 1999) with no test measuring purely executive functions (Whitney, Jameson, & Hinson, 2004). It is possible that the WCST was not sufficiently sensitive for our clinical population. Our TD group had in general less tic severity than a typical clinic TS population. Further investigations are thus needed to complement the description with more symptomatic patients.
The second aim was to look at the effect of CBT on motor performance. Our results showed that selected motor performance can be improved following successful tic management. In fact, after Bonferroni correction, only performance on the Purdue Pegboard showed a significant difference between controls and TD and HD samples, and again after correction, only the Purdue Pegboard showed improvement in TD and HD samples post-CBT. Whether the post-treatment improvement is due to the reduction in tics or the acquisition of improved strategies of motor control remains to be established. However, in both HD and TD groups, there was a significant correlation between improvement in the clinical parameters of tic control and the STOP preparation subscale and improvement in Purdue Pegboard performance.
The effect of therapy was to produce changes on the test involving complex goal-directed guided movements, namely the Purdue Pegboard. This task requires co-ordination in the context of forward feedback planning, whereas both the Hole Steadiness Task and the Groove Test are largely static, requiring hand posture feedback and tremor control through somesthetic positioning. The effect of therapy may be to improve the co-ordination and control over effort and movement execution, particularly in effortful complex guided tasks. Since the aim of therapy was to replace automated reflex actions with a more controlled awareness of action and a mastery over involuntary action, the results on the motor tasks would be concordant with the goal of the therapy. Post-therapy, there was no longer any significant difference between control and clinical groups in Purdue Pegboard for either dominant or non-dominant hand.
Behaviour therapy for TD explicitly addresses motor responses in terms of restructuring antagonist or competing responses to the tic situation. In our particular programme, in addition, overall style of action was addressed to deal with the tendency to complete everyday tasks in an over-effortful, over-active, and tension-producing mode. Successful outcome in therapy was associated with improvement on style of action as measured by the STOP questionnaire. In fact, such change has previously proved a robust predictor of relapse prevention at two-year follow-up (see O’Connor et al., 2001). So, the normalising effect of therapy on motor performance found in the current study echoes previous clinical results.
However, the current effects of therapy on discrete tests of motor performance inform us further on the nature of motor change. Differences in the Purdue Pegboard Test imply differences in complex goal-oriented motor performance. The client group however showed equal performance to the control group in the Hole Steadiness Test, which requires regulation of position on the basis of proprioceptive and kinesiological feedback. The Groove Test entails a lower level combination of the two other tasks involving both steadying and guiding actions, and although there was a trend towards lower performance in TD and HD groups, perhaps here neither parameter was sufficiently taxing to elicit group differences. The results of the Groove Test and Hole Steadiness Test were highly correlated at baseline, so suggesting a similarity in task demand, compared to the Purdue Pegboard. However, the Purdue Pegboard was positively correlated with the Groove Test at baseline, so supporting an intermediate status of the Groove Test between the skills of the other two tasks.
In our case, mood factors were unrelated either to baseline motor performance or change post-treatment. There is always the possibility that motor performance could be affected by peripheral factors such as the presence of fewer tics during performance post-CBT (Channon et al., 2003). However, the selective nature of the relationships between clinical and motor results supports a direct effect of CBT on motor processes. These correlations between clinical measures, style of planning, and motor performance support the possibility that change in motor function can be modified through behavioural change. The overactive style of planning dimension contains items relating to keeping calm and reducing level of movement, whereas the over-preparation style of planning dimension reflects over-investment and over-complication of task performance. It would seem reasonable that the over-preparation dimension would relate more precisely to tasks including complex effortful performance. But it could mean that the differential improvement in either of the two styles of planning dimensions may be selectively detected by distinct motor tasks.
The limitations of the current study lie in the restricted number of neuropsychological tests which may have precluded a firmer conclusion about the role of executive functioning. The participants in our TD group were not in the severe symptomatic range. More extensive measures of executive function might permit more conclusive evidence for whether CBT does indeed affect motor processes relevant to TS, or whether the relationship is mediated by a third psychological or physiological factor. Also, generalisation of the improvement found post-CBT to more ecologically valid behavioural situations requiring manual dexterity was not evaluated.
The current study addressed cognitive components in behaviour change, particularly beliefs in the need for overactive and over-prepared planning of action. If habit reversal may be better conceived as a rehabilitation for some aspects of executive function, then cognitive and even meta-cognitive factors will require more integration in CBT. The current CBT focus is on behavioural training via habit reversal whereas in motor skills theory cognitive mechanisms of feedforward and feedback form key factors in motor control.
Future work might examine the relationship between motor dexterity and more complex goal-directed tasks and how CBT impacts on more complex performance. It would also be informative to see how selectively CBT affects motor compared to other types of processing in TD (e.g., sensory, cognitive, affective) post-treatment. Sensory and motor-related electrocortical potentials as well as autonomic and electromyographic measures during performance might complement behavioural measures and allow us to more clearly distinguish motor from other processes.
In conclusion, the present results align themselves with other studies showing that cognitive-behavioural therapies can induce changes in both functional brain processes and neuropsychological performance and therefore highlight the importance of considering the role of cognitive-behavioural therapy as a rehabilitation strategy in tic disorders.
Acknowledgments
This work was supported by a clinical research operating grant number 930673/04-8470 from the Fonds de la Recherche en Santé du Québec, and by the laboratory infrastructure support from the Fernand-Seguin Research Centre. We wish to express our gratitude to Sophie Robillard, Josée Dubord and Josée Loiselle for their clinical and technical support.
APPENDIX. Subscales of the Style of Planning Questionnaire (STOP)
|
|
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- Asikainen I, Nybo T, Muller K, Sarna S, Kaste M. Speed performance and long-term functional and vocational outcome in a group of young patients with moderate or severe traumatic brain injury. European Journal of Neurology. 1999;6(2):179–185. doi: 10.1111/j.1468-1331.1999.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Nunn RG. Habit-reversal: A method of eliminating nervous habits and tics. Behaviour Research and Therapy. 1973;11(4):619–628. doi: 10.1016/0005-7967(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Peterson AL. Habit reversal for the treatment of Tourette syndrome. Behaviour Research and Therapy. 1988;26(4):347–351. doi: 10.1016/0005-7967(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression. Philadelphia: University of Pennsylvania Press; 1970. [Google Scholar]
- Bergin A, Waranch HR, Brown J, Carson K, Singer HS. Relaxation therapy in Tourette syndrome: A pilot study. Pediatric Neurology. 1998;18(2):136–142. doi: 10.1016/s0887-8994(97)00200-2. [DOI] [PubMed] [Google Scholar]
- Biswal B, Ulmer JL, Krippendorf RL, Harsch HH, Daniels DL, Hyde JS, Haughton VM. Abnormal cerebral activation associated with a motor task in Tourette syndrome. American Journal of Neuroradiology. 1998;19(8):1509–1512. [PMC free article] [PubMed] [Google Scholar]
- Bornstein RA. Neuropsychological performance in children with Tourette’s syndrome. Psychiatry Research. 1990;33(1):73–81. doi: 10.1016/0165-1781(90)90150-4. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. Neuropsychological performance in adults with Tourette’s syndrome. Psychiatry Research. 1991;37(3):229–236. doi: 10.1016/0165-1781(91)90059-x. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Baker GB, Bazylewich T, Douglass AB. Tourette syndrome and neuropsychological performance. Acta Psychiatrica Scandinavica. 1991;84(3):212–216. doi: 10.1111/j.1600-0447.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette’s syndrome. Neuropsychology. 2003;17(2):247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- Christenson GA, Ristvedt SL, Mackenzie TB. Identification of trichotillomania cue profiles. Behaviour Research and Therapy. 1993;31(3):315–320. doi: 10.1016/0005-7967(93)90030-x. [DOI] [PubMed] [Google Scholar]
- Cope MT, Georgiou N, Bradshaw JL, Iansek R, Phillips JG. Simon effect and attention in Parkinson’s disease: A comparison with Huntington’s disease and Tourette’s syndrome. Journal of Clinical and Experimental Neuropsychology. 1996;18(2):276–290. doi: 10.1080/01688639608408282. [DOI] [PubMed] [Google Scholar]
- Dean JT, Nelson E, Moss L. Pathologic hair-pulling: A review of the literature and case reports. Comprehensive Psychiatry. 1992;33(2):84–91. doi: 10.1016/0010-440x(92)90003-9. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17(2):255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Fattapposta F, Restuccia R, Colonnese C, Labruna L, Garreffa G, Bianco F. Gilles de la Tourette syndrome and voluntary movement: A functional MRI study. Psychiatry Research. 2005;138(3):269–272. doi: 10.1016/j.pscychresns.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA, Chiu E. The Simon effect and attention deficits in Gilles de la Tourette’s syndrome and Huntington’s disease. Brain. 1995;118(Pt 5):1305–1318. doi: 10.1093/brain/118.5.1305. [DOI] [PubMed] [Google Scholar]
- Goldberg DP. The detection of psychiatric illness by questionnaire: A technique for the identification and assessment of non-psychotic psychiatric illness. London: Oxford University Press; 1972. [Google Scholar]
- Golden GS. Gilles de la Tourette’s syndrome following methylphenidate administration. Developmental Medicine and Child Neurology. 1974;16(1):76–78. doi: 10.1111/j.1469-8749.1974.tb02715.x. [DOI] [PubMed] [Google Scholar]
- Hagin RA, Beecher R, Pagano G, Kreeger H. Effects of Tourette syndrome on learning. Advances in Neurology. 1982;35:323–328. [PubMed] [Google Scholar]
- Harcherik DF, Leckman JF, Detlor J, Cohen DJ. A new instrument for clinical studies of Tourette’s syndrome. Journal of the Academy of Child and Adolescent Psychiatry. 1984;23(2):153–160. doi: 10.1097/00004583-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual. Revised and Expanded. Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- Hollander E. Treatment of obsessive-compulsive spectrum disorders with SSRIs. British Journal of Psychiatry. 1998;35(Suppl):7–12. [PubMed] [Google Scholar]
- Keuthen NJ, Savage CR, O’Sullivan RL, Brown HD, Shera DM, Cyr P, Jenike MA, Baer L. Neuropsychological functioning in trichotillomania. Biological Psychiatry. 1996;39:747–749. doi: 10.1016/0006-3223(95)00613-3. [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Thibault G, Stip E, O’Connor K. Memory and executive functions in adults with Gilles de la Tourette syndrome and chronic tic disorders. Cognitive Neuropsychiatry. 2007;12:165–181. doi: 10.1080/13546800600826371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JS, Marshall WL, McGrath P. The social self-esteem inventory. Educational and Psychological Measurement. 1979;39:803–811. [Google Scholar]
- Lombroso PJ, Scahill L, King RA, Lynch KA, Chappell PB, Peterson BS, McDougle CJ, Leckman JF. Risperidone treatment of children and adolescents with chronic tic disorders: A preliminary report. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(9):1147–1152. doi: 10.1097/00004583-199509000-00011. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:90–100. [Google Scholar]
- Miltenberger RG, Fuqua RW, Woods DW. Applying behavior analysis to clinical problems: Review and analysis of habit reversal. Journal of Applied Behavior Analysis. 1998;31:447–469. doi: 10.1901/jaba.1998.31-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of maintenance and executive control. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- O’Connor KP. Cognitive behavioral management of tic disorders. New York: John Wiley & Sons; 2005. [Google Scholar]
- O’Connor KP, Brault M, Loiselle J, Robillard S, Borgeat F, Stip E. Evaluation of a cognitive-behavioral program for the management of chronic tic and habit disorders. Behaviour Research & Therapy. 2001;39:667–681. doi: 10.1016/s0005-7967(00)00048-6. [DOI] [PubMed] [Google Scholar]
- O’Connor KP, Brisebois H, Brault M, Robillard S, Loiselle J. Behavioral activity associated with onset in chronic tic and habit disorders. Behavior Research and Therapy. 2003;41(2):241–249. doi: 10.1016/s0005-7967(02)00051-7. [DOI] [PubMed] [Google Scholar]
- O’Connor KP, Lavoie ME, Robert M, Stip E, Borgeat F. Brain-behavior relations during motor processing in chronic tic and habit disorder. Cognitive and Behavioral Neurology. 2005;18(2):79–88. doi: 10.1097/01.wnq.0000151131.06699.af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KP, Robert M, Dubord J, Stip E. Automatic and controlled processing in chronic tic disorders. Brain and Cognition. 2000;43(1–3):349–352. [PubMed] [Google Scholar]
- Peterson AL, Azrin NH. Behavioral and pharmacological treatment for Tourette’s syndrome: A review. Applied and Preventive Psychology. 1993;2:231–242. [Google Scholar]
- Peterson AL, Campise RL, Azrin NH. Behavioral and pharmacological treatments for tic and habit disorders: A review. Journal of Developmental and Behavioral Pediatrics. 1994;15(6):430–441. [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Arnsten A, Anderson GM, Staib LH, Gore JC, Bronen RA, Malison R, Scahill L, Cohen DJ. Neuroanatomical circuitry. In: Leckman JF, Cohen DJ, editors. Tourette’ syndrome. Tics, obsessions, compulsions. Developmental psychopathology and clinical care. New York: John Wiley & Sons; 1999. pp. 230–260. [Google Scholar]
- Price RA, Leckman JF, Pauls DL, Cohen DJ, Kidd KK. Gilles de la Tourette’s syndrome: Tics and central nervous system stimulants in twins and nontwins. Neurology. 1986;36(2):232–237. doi: 10.1212/wnl.36.2.232. [DOI] [PubMed] [Google Scholar]
- Rachman S, Hodgson R. Obsessions and compulsions. New York: Prentice Hall; 1980. [Google Scholar]
- Rapp JT, Miltenberger RG, Long ES. Augmenting simplified habit reversal with an awareness enhancement device: Preliminary findings. Journal of Applied Behavior Analysis. 1998;31(4):665–668. doi: 10.1901/jaba.1998.31-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew DC, Cheslow DL, Rapoport JL, Leonard HL, Lenane MC, Black B, Swedo SE. Neuropsychological test performance in trichotillomania: A further link with obsessive-compulsive disorder. Journal of anxiety Disorders. 1991;5:225–235. [Google Scholar]
- Riddle MA, Hardin MT, Towbin KE, Leckman JF, Cohen DJ. Tardive dyskinesia following haloperidol treatment in Tourette’s syndrome. Archives of General Psychiatry. 1987;44(1):98–99. doi: 10.1001/archpsyc.1987.01800130110023. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Carter AS, Scahill L, Leckman JF. Neuropsychological findings. In: Leckman JF, Cohen DJ, editors. Tourette’s syndrome: Tics, obsessions, compulsions. Developmental psychopathology and clinical care. New York: John Wiley & Sons; 1999. pp. 80–103. [Google Scholar]
- Shapiro E, Shapiro AK, Fulop G, Hubbard M, Mandeli J, Nordlie J, Phillips RA. Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette’s syndrome. Archives of General Psychiatry. 1989;46(8):722–730. doi: 10.1001/archpsyc.1989.01810080052006. [DOI] [PubMed] [Google Scholar]
- Speilberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory (test manual) Palo Alto: Consulting Psychologist Press; 1970. [Google Scholar]
- Stanley MA, Hannay HJ, Breckenridge JK. The neuropsychology of trichotillomania. Journal of Anxiety Disorders. 1997;11(5):473–488. doi: 10.1016/s0887-6185(97)00024-8. [DOI] [PubMed] [Google Scholar]
- Taylor IB. Psychological assessment of neurological patients. In: Rasmussen T, Marino R, editors. Functional neurosurgery. New York: Raven Press; 1979. [Google Scholar]
- Tourette Syndrome Study Group. Definitions and classification of tic disorders. The Tourette Syndrome Classification. Archives of Neurology. 1993;50(10):1013–1016. doi: 10.1001/archneur.1993.00540100012008. [DOI] [PubMed] [Google Scholar]
- Whitney P, Jameson T, Hinson JM. Impulsiveness and executive control of working memory. Personality and Individual Differences. 2004;37(2):417–428. [Google Scholar]


