Abstract
Objective
This study examined electrophysiological indices of preparation (readiness potential, RP) and execution (movement-associated potential, MAP) during automated and controlled reaction time (RT) in 13 chronic tic disorder, 17 habit disorder, and 14 control participants.
Background
Both tic and habit disorders are hypothesized to involve states of heightened activation, which could impede initiation and the control of complex motor actions.
Method
The electrophysiological signal was recorded from 4 electrodes (Fz, C3, C4, Pz) during a fixed 4-second foreperiod reaction time task.
Results
During automated responses, controls showed a shorter RP peak onset, and during controlled responses a longer MAP peak onset, compared with both clinical groups. The controls were the only group who showed a consistent linear relationship between RP and RT.
Conclusions
Patients with chronic tic as well as habit disorder may not modulate cortical activation optimally in planning and executing both automated and controlled responses.
Keywords: chronic tic disorder, habit disorder, motor preparation, event-related potentials
Tics are defined as repetitive nonvoluntary contractions of functionally related groups of skeletal muscles in one or more parts of the body. Simple tics include blinking, cheek twitches, head or knee jerks, and shoulder shrugs.1–3 Complex tics involve sequences of movements and may also take the form of repetitive actions or mannerisms such as gestures and dystonic postures involving limbs, face, head, torso, or extremities. The Diagnostic and Statistical Manual of Psychiatric Disorders (DSM-IV) distinguishes between chronic Tic Disorder (TD) with one principal motor or phonic tic and Gilles de la Tourette Syndrome (TS) with multiple tics and at least one phonic tic. Habit Disorders (HD) is a term covering a variety of destructive impulsive habits including trichotillomania, bruxism, onychophagia, and scabiomania. There seems to be a consensus among researchers that TD and TS share enough common aspects to be considered on a continuum of severity.4 There has been controversy about current criteria for TS,5,6 but the diagnosis of TS is currently dichotomous, not dimensional, and depends crucially on the existence of a vocal tic.
TD and HD have been viewed as part of the Obsessive-Compulsive Disorder (OCD) spectrum.7 Although TD and HD have been compared independently with OCD, there has been little systematic inquiry into the common or distinguishing features between TD and HD. The tension-reducing or emotion-regulating function of both tics and habits would suggest the presence of a heightened state of behavioral activation or arousal,8,9 but we do not know if there is also heightened cortical arousal. Although several electrophysiological studies have specifically investigated the TS population, none has specifically compared patients with TD/TS and HD.
Neuropsychological research with TS patients consistently reports problems in visuomotor integration and motor skill tasks such as the Purdue and Groove Pegboard in children,10,11 preadolescents,12 and adults.13 More direct evidence, based on fMRI studies of the motor cortex in five patients with TS, has reported a heightened arousal of the sensorimotor and supplementary motor area.14 Consistent with this finding, Ziemann, Paulus, and Rothenberger15 reported decreased motor inhibition following transcranial magnetic stimulation at the level of the motor cortex. Previous studies have measured gross motor output but failed to consider different stages of processing such as motor preparation and execution. Moreover, there is little information on how motor output (reaction time) relates to electrocortical activity in these populations. Recruitment of larger portions of sensorimotor cortex in the execution of a finger-tapping task suggests a distinct pattern of motor cortex arousal in patients with TS as compared with controls, but impaired motor regulation could be caused by problems in planning and/or executing motor activity.
Motor action itself can be decomposed into different stages, each associated with a distinct cortical event-related potential (ERP) component. For instance, ERP components such as the readiness potential (RP) and movement-associated potential (MAP) are thought to reflect, respectively, processes of response preparation16–18 and response execution.19 The RP typically shows a slow negative-going deflection within 1 second before movement and maximal at the precentral motor cortex. The RP precedes voluntary movement and is largest when preparation for movement involves planned effort.20 RP seems higher during activation of selective complex responses, particularly during performance of sequential complex finger movements.21,22 It is nearly or entirely absent during reflex actions,23 although tics themselves may sometimes appear semivoluntary with accompanying RP.24,25 Movement itself produces a potential maximal over the vertex called the movement-associated potential (MAP). The MAP represents a composite index of precentral cortex motor activation while generating the pyramidal tract volley following response and may also be a function of reafferent and proprioceptive feedback to cortical areas from subcortical structures.26–28
In accordance with the stage model, motor processing begins with a planning stage, involving a generalized motor program. A preparation stage “tunes” the response, and an execution stage with decreasing degrees of freedom accompanied by error correction, if necessary, terminates in an action endpoint.29 The preparation stage, then, does not necessarily overlap with the execution stage. The separate localization of preparation-related potentials and execution-related potentials during hand movements have been reported by Leuthold and Jentzsch.30 RP shows higher amplitude in the sensorimotor areas, whereas MAP are localized more posteriorly. Hence RP and MAP measures appear as independent processes, but their electrocortical topography is sensitive to type of planned motor execution.31 In particular, the parameters of movement may vary depending on whether the intended action is controlled or automated. All movements involve some degree of controlled and automated components, but these components may be differentially emphasized depending on task demand and complexity of the act required. The key influence on whether controlled or automated planning has priority seems related to the complexity of movement.32 There is evidence that separate cortical systems are linked to automated timing (motor and premotor circuits) and cognitively controlled tasks (prefrontal and parietal circuits).33 Thus, anticipation of the nature of the act, its complexity, and consequences becomes of paramount importance in determining preparation and enactment of controlled acts.
Repetitive (automated) motor action can, as a part of its feedback system, affect limbic reward centers by stimulating the hippocampus through limbic cholinergic pathways.34,35 An automated action is more likely to augment or at least persist under stress or high arousal because an indirect consequence of feedback from stimulation of limbic cholinergic pathways may be a stress reduction effect.36 In other words, chronically high levels of arousal may predispose the tic affected person to perform automated ballistic actions as a way of trying to regulate proprioceptive (sensory) feedback on state. Under conditions of stress, the person with a tic may find it more difficult to initiate a controlled action and to stop an automated action. This hypothesis was partially supported in previous studies examining automated and controlled performance during countermanding stop and go paradigms.37,38 These studies reported that whereas the control group showed faster reaction time (RT) for automated than controlled response conditions, the TD group showed no condition effects.37 Furthermore, O’Connor et al38 reported no differences among TD, HD, and control groups in go reaction time (GO RT) but a difference in the ability to inhibit an automated response (STOP RT) as reflected in a slower STOP RT to automated than controlled responses in the TD group. The control group (CON) showed a practice effect over time in both STOP and GO RT, whereas the patient groups showed no practice effect in either STOP or GO RT over two trial blocks. The results were interpreted as showing that in TD there is a difficulty in modulating motor arousal level in accordance with task demand and that such patients have a tendency to be chronically overaroused. In the present study, we extended our investigation to include electrocortical measures to investigate the time course of brain activation related to automated and controlled processing.
The primary aim of the current report was to examine the electrocortical chronometry of motor processing in clinical groups affected with either TD or HD compared with a non-psychiatric CON group, as recorded before (RP) and during (MAP) the generation of controlled and automated RT. In accordance with our previous RT results and with brain-imaging studies, we hypothesized that the TD and HD group would show a heightened cortical arousal compared with the CON group, and consequently, (1) the TD and HD group would show no systematic difference in RP or MAP peak latency or amplitude between automated and controlled performance, whereas the CON group would show a significant difference in both RP and MAP peak latency and amplitude between automated and controlled response conditions, and (2) the TD and HD group would show no practice effect for either the RP or MAP amplitude or RT across two consecutive trial blocks because of a ceiling level effect of arousal, whereas the CON group would show an increase in RP and MAP amplitude and a shorter RT on the second compared with the first trial block. The second aim was to explore the relationship between electrocortical indices of the planning (RP) and the behavioral execution stage (MAP and RT) in TD and HD. We predicted that the correlation between RP latency and MAP latency, and between RP latency and RT, would be lower in TD compared with the CON group, where suboptimal modulation of response planning in TD would presumably produce a more variable relationship between preparation and the processes involved in response execution. Finally, the clinical similarities between HD and TD led us to hypothesize that the HD group would also show a lower correlation between RP and MAP and between RP and RT than the CON group, thereby resembling the TD rather than the CON group.
MATERIALS AND METHODS
Participants
Participants entering a behavioral management program for tics were recruited pretreatment. Eighteen TD, 24 HD, and 24 controls (CON) originally participated in our study. After elimination because of movement artifact or technical problems, 44 participants remained (14 CON, 17 HD, and 13 TD). In the TD group were 8F, 5M (all right handed, as assessed by the Edinburgh Handedness Questionnaire),39 age 39.54 ± 11.4 years. Six had eye tics, four head tics, two vocal tics, and one shoulder tic. In the HD group, 12F, 5M (15 right handed, age 38.3 ± 10.3), seven had trichotillomania, eight onychophagia, and two had bruxism. Mean chronicity of the problem in the TD group was 26.4 ± 11.2 years, and for the HD group 22.3 ± 9.2. In the nonpsychiatric control group were 9F, 5M (13 right handed, age 36.4 ± 9.5). The patients recruited into the study predominantly suffered from one major simple or complex tic or habit disorder for which they were seeking treatment, and without significant comorbidity. Those diagnosed with chronic tic disorder scored between 2.5 and 12.0 on the global calculation of the Tourette Symptom Global Scale (TSGS),40 which is in the mild–moderate range. Typically, apart from the presence of tics causing distress and at least some disruption of activities, social and other functioning was not significantly impaired with the exception of some degree of motor restlessness.
Participants were initially screened by telephone for suitability in terms of geographic accessibility/motivation to attend; absence of grave psychiatric or medical history; no current psychotropic medication or other psychotherapy; no current behavioral or social or familial problems; abuse of alcohol or drugs. Criteria for inclusion were age 18–60 and presenting a chronic simple/complex tic or motor habit disorder for at least 1 year and occurring daily. Those included had a diagnosis of either TD or HD as the principal presenting problem. Exclusion criteria were any major medical problem or other psychiatric problem on Axis I or II, TS severe or extreme; neurologic problems (eg, Parkinson disorder, hemi-facial spasms); Meige syndrome or sclerosis; Huntington disease; currently receiving treatment from psychiatrist, psychologist, acupuncturist, hypnotherapist, or massotherapist; currently receiving anxiolytic, antidepressant, or antipsychotic medication; abuse of alcohol or drugs. After passing the telephone screening, an appointment was made with 1 of the clinical psychiatrists (E.S., F.B.) collaborating on the project for psychiatric screening. The standard psychiatric interview assessed participants according to DSM-III-R criteria and on personal history of tic disorder and severity of symptom rating using the TSGS.40 The recognized habit disorders (bruxism, onychophagia, trichotillomania) were assessed according to recommendations in the literature (bruxism41; onychophagia42; trichotillomania43,44). The nonpsychiatric controls were screened for psychopathology by interview and a battery of standardized questionnaires. All participants read and, if they were willing, signed a consent form approved by the local ethics committee.
The Traffic Light Task
The traffic light paradigm45 was a fixed 4-second foreperiod reaction time task. The 4-second warning period permitted adequate preparation for controlled and automated responses. The interstimulus interval (ISI) was constant at 4 seconds, and the intertrial interval (ITI) varied randomly between 5 and 15 seconds. The longer foreperiod permitted adequate distinction between early sensory components and ensured that RP was unconfounded by orienting processes.21,31 The paradigm allowed us to compare cortical events during the time to initiate an automated and controlled response. Two sets of three lights appeared side by side on a computer screen in the form of traffic lights. One set signaled an automated and the other a controlled response sequence. Each trial began with one of the two yellow READY lights signaling that in four seconds, the green GO light would appear and the participant would have to make either a controlled or an automated response. Subsequent to response initiation, on half the trials, a red STOP sign would appear, indicating that the response should be terminated. All movement parameters (fingers involved, extent of movement, type of movement) were standardized across conditions except for movement complexity. The most popular way of experimentally operationalizing response complexity is through number and regularity of key presses.46,47 Such response complexity effects obtained using key presses seem generalizable to other motor programs.48 The automated response was 3 taps on a lever with the two fingers of the dominant hand (– – –). The controlled response was more complex and was three taps of Morse code “dash-dot-dash” (– · –) on another lever but using the same two fingers of the dominant hand. The Morse code sequence was identical for all participants. The controlled response differed solely in motor complexity and was equivalent to the simple response in terms of all other biomechanical and movement parameters. After a period of acclimatization of 20 trials, all subjects received two replications of 52 trials, with a short rest period between replications. The controlled and automated conditions were presented in random order across trials. Participants were requested to abstain from unnecessary movement during trial period but were not explicitly asked to suppress tics.
EEG Recordings and ERP Extraction
Scalp voltages were recorded from 4 placements (Ag/AgCl electrodes) at Fz, C3, C4, and Pz (10–20 system) to give a topographical and lateral view of the signal distribution and obtain EEG signal from the motor cortex. The vertical and horizontal eye movements were monitored through two electrodes placed at the external canthi. All electrodes were referenced to linked earlobes and were grounded at Fpz. Impedance was kept below 5000 Ω for all recordings. The signal was fed through Grass preamplifiers (model 12c) with pass-band filter between 0.01 and 100 Hz (10 seconds time constant) at a sampling rate of 250 Hz. EOG was corrected offline in the frequency domain (Woestenburg method InstEP-TALO). The raw EEG signal was automatically averaged offline, time-locked to the response with digitally marked criteria (which is accepted procedure for RP analysis) in two replication blocks of the two conditions (controlled/automated).
The two response levers for automated and controlled response were side by side and were part of the same box assembly. They input into the same display and permitted a continuous analogue input to monitor both the response and screen for any anticipatory responses. The levers modulated a voltage level that was input into a Grass amplifier. The two lever channels and an additional digital signal marker channel were recorded in synchrony with the ongoing EEG signal. The criterion for motor response onset and hence RT calculation was a nontransient change in voltage from baseline in the analogue input. The GO RT was calculated automatically, and the RT criterion was verified offline with a digital marker set manually to exclude incomplete and premature responses. The traffic light test was conceived as a countermanding paradigm intended to measure ability to countermand a response already undertaken (STOP RT). Previous results have supported the validity of the test to elicit differences in STOP RT between TD and controls.37,38 However, recording ERP before STOP RT is impossible because the response is already under way. Hence, the ERP analysis in the present study was limited to the period preceding GO RT onset.
Trials with behavioral and recording artifacts not caused by EOG (amplitude not exceeding 150 μV), and anticipatory or partial responses were eliminated through offline editing by an experienced EEG technician (M.R.), who was blind to group membership and the research hypotheses. There were no significant differences between groups in number of trials included for averaging. The RP amplitude was scored as the highest negative point within a window of 400 milliseconds before the initial GO-response onset. The maximal peak RP can occur at any point within the 1000 milliseconds preceding the GO-response, but preliminary visual analysis suggested that a window of 400 milliseconds was sufficient to detect all peak RP in our sample. If a peak was not detectable, the trial was eliminated. The MAP amplitude was scored as the highest negative point in the window within a 200-millisecond postinitial GO-response.
Statistical Analyses
The RT, RP, and MAP data were subjected separately to a repeated-measure ANOVA with conditions (automated/controlled) and replication blocks (first/second repetition) as within-subjects factors and groups (HD/TD/CON) as a between-subject factor. Specifically for the electrophysiological data, the 4 leads (Fz, C3, C4, Pz) constituted an additional within-subject factor. In all analyses, the significance level was set at 5% (two-tailed) with Greenhouse-Geisser corrections for degrees of freedom where necessary and Scheffé tests for post-hoc comparison.
Separate correlation (Pearson) analyses were applied to assess the correlation between behavioral (RTs) and electrocortical responses (RP and MAP latency). The relationship between these variables was further explored through a linear regression analysis with the RTs as the predictor (independent factor) and the RP latency as dependent variable. Separate linear regressions were also conducted with the MAP latency and RT.
RESULTS
Behavioral Data
The GO reaction time (RT) results presented in Table 1 mirrored previous findings.38 There were no overall group or condition effects. Only the CON group showed a practice effect with reduced RT over blocks (F[1,13] = 4.45, P < 0.04).
TABLE 1.
Descriptive Statistics of Reaction Time for All Groups
| Type of Responses | Replications | Control
|
Habit Disorder
|
Chronic Tic Disorder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Min | Max | Mean | SE | Min | Max | Mean | SE | Min | Max | ||
| Automated | 1 | 283.39 | 25.35 | 232.11 | 334.67 | 286.29 | 23.01 | 239.76 | 332.83 | 272.77 | 28.60 | 214.92 | 330.62 |
| 2 | 259.00 | 23.23 | 212.02 | 305.98 | 288.65 | 21.08 | 246.02 | 331.29 | 260.64 | 26.21 | 207.63 | 313.64 | |
| Controlled | 1 | 290.04 | 22.77 | 243.97 | 336.10 | 286.65 | 20.67 | 244.85 | 328.46 | 282.00 | 25.69 | 230.03 | 333.97 |
| 2 | 253.25 | 21.20 | 210.36 | 296.14 | 273.22 | 19.24 | 234.30 | 312.14 | 253.77 | 23.92 | 205.39 | 302.16 | |
SE, standard error; Min, minimum; Max, maximum.
Electrophysiological Data
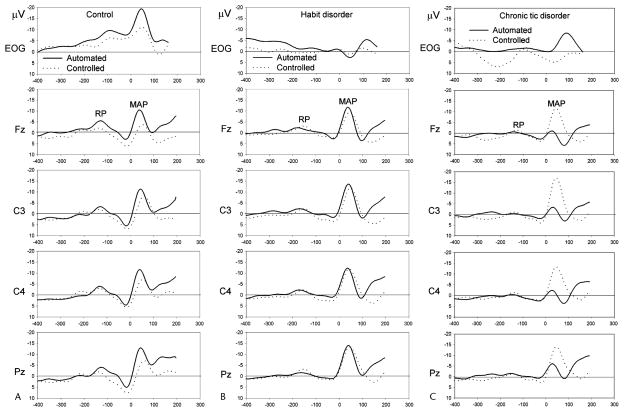
ERP waveforms showing RP and MAP averaged over each group separately for both the controlled and automated response conditions (pooled over blocks) are shown in Figure 1.
FIGURE 1.
Response-locked ERP waveforms pooled across blocks in the Control (A), Habit Disorders (B), and Chronic Tic Disorders (C) groups for the automated (bold line) and controlled (dashed line) type of response. The RP designates the readiness potential (mean latency 172 milliseconds before the beginning of response) and movement-associated potentials (MAP, mean latency 75 milliseconds after the beginning of response).
Peak amplitude of MAP showed a main electrode effect indicating a significant difference in amplitude at electrode placements (F[2.89,104.75] = 8.46, P < 0.0001) with frontal amplitude lower than both central (FZ versus C3: t[44] = 3.15, P < 0.003; FZ versus C4: t[44] = 2.18, P < 0.03) and parietal placements (FZ versus PZ: t[44] 4.36, P < 0.000). This electrode effect was similar across all groups. The MAP amplitude was larger in the automated as compared with the controlled condition at Pz (t[44] = −2.40, P = 0.021), accounting for the condition-by-significant electrode interaction (F [2.89,104.75] = 4.61, P <0.007). The CON group only showed a smaller MAP in block 1 as compared with block 2 trials. Both clinical groups showed a nonsignificant tendency, across blocks, in the opposite direction, which accounted for the group-by-block interaction effect (F[2,41] = 4.12, P < 0.02).
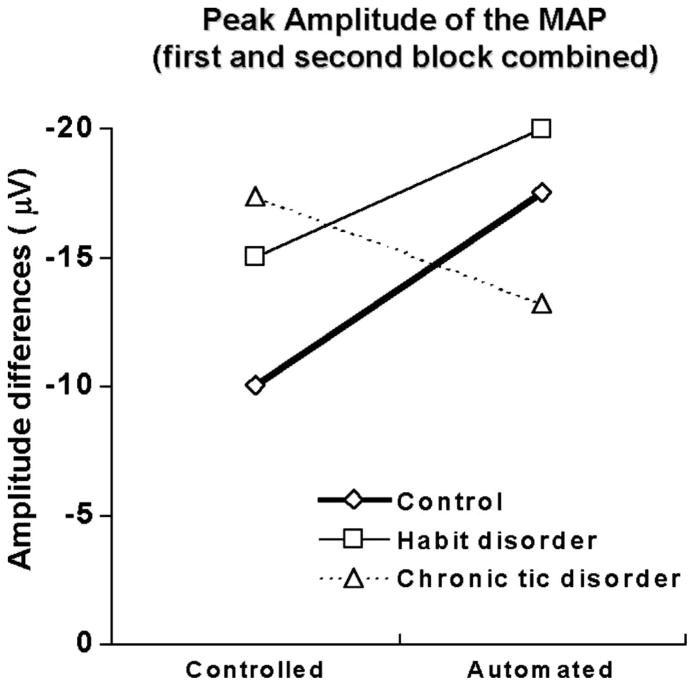
Most importantly, there was a significant group-by-condition interaction (F[2,41] = 4.01, P < 0.03). A subsequent ANOVA, comparing TD and CON groups, revealed that the interaction was mainly caused by the difference between TD and CON, which produced a significant group-by-condition interaction (F[1,25] = 7.31, P < 0.02). However, the ANOVA comparing HD and CON groups showed no significant interaction (F[1,29] = 0.358, P = 0.55). Figure 2 illustrates the CON group’s higher MAP amplitude under automated compared with controlled response conditions across both blocks, an effect significant in all but one electrode site (Fz, P < 0.02; C3, P < 0.13; C4, P < 0.03; Pz, P < 0.01). The HD group showed the same pattern of results than the CON, but there were no significant condition differences for all electrode sites (Fz, P < 0.10; C3, P < 0.19; C4, P < 0.08; Pz, P < 0.13). The TD group showed a nonsignificant inverse tendency with larger MAP amplitude in the controlled than in the automated condition (Fz, P <0.23; C3, P <0.08; C4, P <0.15; Pz, P <0.77).
FIGURE 2.
Group comparison based on peak amplitude (μV) of the MAP, pooled across electrode locations, as a function of controlled and automated condition. The CON group showed higher amplitude under the automated condition, which was not the case for the TD.
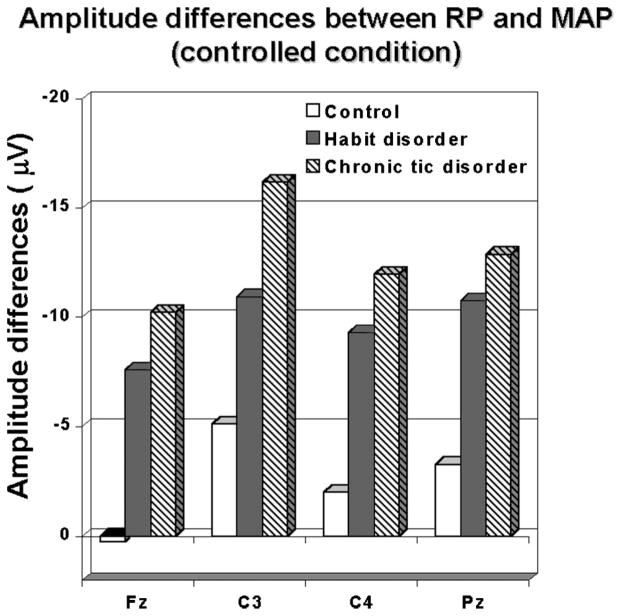
These group differences in MAP amplitude were reflected more significantly in the discrepancy between RP and MAP amplitude across groups (Fig. 3). This difference measure (RP minus MP peak amplitude) showed a significant group-by-condition effect (F[2,41] = 5.11, P < 0.01) over all electrode sites during the controlled condition, especially in the first block of trials. The Scheffé post hoc test revealed that the frontal (Fz, F[2,41] = 5.70, P <0.01) and right central (C4, F[2,41] = 7.66, P < 0.001) amplitude difference measure significantly discriminated among all 3 groups (Fig. 3), with the TD group showing the largest difference and the CON group the least difference. The other two electrode sites (C3, Pz) only discriminated between both clinical groups and CON group (C3, F[2,41] = 5.32, P < 0.01; PZ, F[2,41] = 6.36, P < 0.001).
FIGURE 3.
Amplitude differences between peak RP and peak MAP for all groups over all electrode placements under the controlled conditions (A, CON; B, HD; C, TD).
There was a significant positive correlation between RP and MAP peak amplitude for both automated and controlled conditions in both HD and CON groups for all electrode sites (P < 0.01). Nevertheless, there was no systematic correlation in the TD group.
Peak latency of MAP showed an electrode effect (F[2.50,104.97] = 2.66, P < 0.05) that revealed a longer latency at Pz in comparison with the Fz location (FZ versus PZ: t[44] = 2.02, P = 0.04). A significant group-by-condition-by-block effect (F[2,41] = 4.45, P < 0.02) showed that, for the CON group, MAP latency was longer in controlled than in automated conditions, but only in the first block of trials (t[13] = 4.62, P < 0.001). Under the automated response condition, there was no difference in latency between groups. In the controlled condition, however, the Scheffé post-hoc test revealed that the CON group showed longer MAP peak latency than the TD (P < 0.02) and HD (P < 0.03) groups, but, again, only during the first block of trials (see Table 2).
TABLE 2.
Descriptive Statistics for the Motor-Associated Potential Amplitude and Latency for All Groups
| Type of Responses | ERP Measures | Replications | Control
|
Habit Disorder
|
Chronic Tic Disorder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Min | Max | Mean | SE | Min | Max | Mean | SE | Min | Max | |||
| Automated | Amplitude | 1 | −13.17 | 4.21 | −21.68 | −4.66 | −23.21 | 3.82 | −30.93 | −15.49 | −12.10 | 4.37 | −20.93 | −3.26 |
| 2 | −21.92 | 4.09 | −30.18 | −13.67 | −16.83 | 3.71 | −24.32 | −9.33 | −12.04 | 4.24 | −20.61 | −3.47 | ||
| Latency | 1 | 63.66 | 10.14 | 43.19 | 84.13 | 68.24 | 9.20 | 49.66 | 86.81 | 71.48 | 10.50 | 50.24 | 92.73 | |
| 2 | 78.75 | 12.67 | 53.15 | 104.35 | 68.09 | 11.50 | 44.86 | 91.32 | 84.03 | 13.19 | 57.48 | 110.60 | ||
| Controlled | Amplitude | 1 | −8.15 | 3.47 | −15.16 | −1.13 | −14.99 | 3.15 | −21.36 | −8.62 | −18.84 | 3.60 | −26.12 | −11.56 |
| 2 | −11.93 | 3.67 | −19.34 | −4.53 | −15.09 | 3.33 | −21.81 | −8.37 | −15.01 | 3.80 | −22.69 | −7.33 | ||
| Latency | 1 | 97.86 | 8.59 | 80.50 | 115.21 | 69.78 | 7.80 | 54.03 | 85.53 | 70.19 | 8.91 | 52.18 | 88.20 | |
| 2 | 74.55 | 8.11 | 58.17 | 90.94 | 76.03 | 7.36 | 61.16 | 90.90 | 74.80 | 8.42 | 57.80 | 91.81 | ||
SE, standard error of the mean; Min, minimum; Max, maximum.
Peak Amplitude of the RP
In the control group the RP amplitude showed an expected laterality effect with maximal negativity on the side contralateral to the response, C3 versus C4 (t[13] = 1.72, P < 0.05, one-tailed). During the second block of trials under the automated condition, the CON group had a larger RP amplitude than the other two clinical groups, leading to a group-by-condition-by-block interaction (F[2,41] = 3.29, P < 0.05).
Peak latency of the RP showed a significant group-by-condition-by-electrode interaction (F[5.03,105.58] = 3.28, P < 0.008). In the controlled condition there were no significant group differences, but in the automated condition the CON showed shorter peak latency than the two clinical groups (F[2,41] = 3.22; P < 0.05) at the central (C4) and parietal (Pz) region (Table 3).
TABLE 3.
Descriptive Statistics for the Readiness Potential Peak Amplitude and Latency for All Groups
| Type of Responses | ERP Measures | Replications | Control
|
Habit Disorder
|
Chronic Tic Disorder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Min | Max | Mean | SE | Min | Max | Mean | SE | Min | Max | |||
| Automated | Amplitude | 1 | −7.37 | 2.51 | −12.45 | −2.30 | −9.53 | 2.28 | −14.14 | −4.93 | −2.90 | 2.61 | −8.16 | 2.37 |
| 2 | −10.18 | 2.38 | −14.99 | −5.37 | −5.35 | 2.16 | −9.72 | −0.98 | −5.71 | 2.47 | −10.71 | −0.72 | ||
| Latency | 1 | −185.27 | 18.26 | −222.15 | −148.39 | −159.49 | 16.57 | −192.95 | −126.02 | −170.48 | 18.95 | −208.75 | −132.21 | |
| 2 | −177.50 | 17.78 | −213.41 | −141.59 | −173.97 | 16.13 | −206.55 | −141.39 | −163.46 | 18.45 | −200.72 | −126.20 | ||
| Controlled | Amplitude | 1 | −8.32 | 2.10 | −12.55 | −4.08 | −4.66 | 1.90 | −8.51 | −0.82 | −3.87 | 2.18 | −8.27 | 0.53 |
| 2 | −6.69 | 2.40 | −11.53 | −1.85 | −6.10 | 2.18 | −10.50 | −1.71 | −4.31 | 2.49 | −9.33 | 0.72 | ||
| Latency | 1 | −203.30 | 16.62 | −236.87 | −169.73 | −152.87 | 15.08 | −183.33 | −122.40 | −166.63 | 17.25 | −201.47 | −131.80 | |
| 2 | −177.77 | 11.92 | −201.84 | −153.70 | −177.56 | 10.82 | −199.41 | −155.72 | −159.04 | 12.37 | −184.02 | −134.06 | ||
SE, standard error of the mean; min, minimum; Max, maximum.
Relationships Between Behavioral (RT) and Electrophysiological (RP, MAP) Measures
Regression Between RP Peak Latency and RT
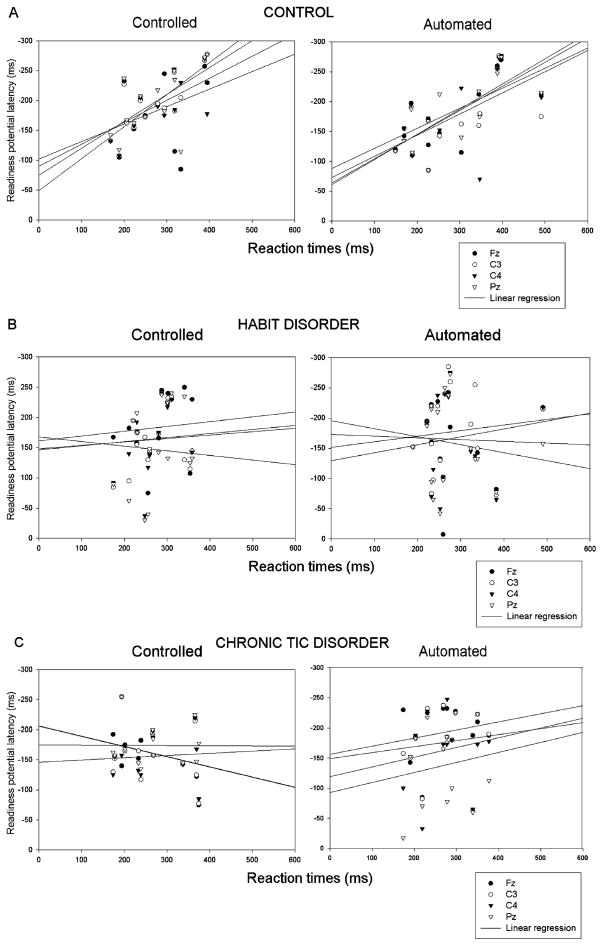
The CON group showed a linear relationship between RP latency and RTunder automated and controlled conditions in all electrode sites. The HD and TD groups showed no linear relationship between RP latency and RTunder any performance conditions (Table 4). This latter finding was not confounded by heterogeneity of variance across groups and represented a genuine difference in association between response time and preparation processes in the clinical groups. The ranges of linear regression R2 coefficients for the controlled and automated conditions, across channels, and for each group are given in Table 4 and illustrated in Figure 4 (a, CON; b, HD; c, TD).
TABLE 4.
Summary of the Linear Regression Analysis Showing the Relationship Between Reaction Times and Electrophysiological Data
| ERP Measures | Type of Responses | Electrodes | Control
|
Habit Disorder
|
Chronic Tic Disorder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Slope | Intercept | P | R2 | Slope | Intercept | P | R2 | Slope | Intercept | P | |||
| Preparation potential | Automated | Fz | 0.55 | −30.38 | −0.56 | 0.00 | 0.02 | −129.33 | −0.13 | 0.60 | 0.04 | −156.02 | −0.14 | 0.51 |
| C3 | 0.52 | −28.69 | −0.54 | 0.00 | 0.01 | −151.27 | −0.09 | 0.70 | 0.01 | −148.66 | −0.10 | 0.70 | ||
| C4 | 0.42 | −45.84 | −0.51 | 0.01 | 0.00 | −172.94 | 0.03 | 0.91 | 0.03 | −119.26 | −0.16 | 0.60 | ||
| Pz | 0.54 | −28.50 | −0.57 | 0.00 | 0.02 | −195.85 | 0.13 | 0.59 | 0.02 | −92.76 | −0.17 | 0.62 | ||
| Controlled | Fz | 0.14 | −101.79 | −0.29 | 0.19 | 0.02 | −161.51 | −0.08 | 0.56 | 0.11 | −206.32 | 0.17 | 0.26 | |
| C3 | 0.64 | −49.06 | −0.54 | 0.00 | 0.01 | −148.22 | −0.06 | 0.67 | 0.07 | −205.81 | 0.17 | 0.37 | ||
| C4 | 0.35 | −89.73 | −0.37 | 0.03 | 0.01 | −146.61 | −0.07 | 0.66 | 0.01 | −145.95 | −0.04 | 0.80 | ||
| Pz | 0.37 | −74.70 | −0.45 | 0.02 | 0.01 | −167.95 | 0.08 | 0.69 | 0.00 | −174.74 | 0.00 | 0.98 | ||
| Motor potential | Automated | Fz | 0.21 | −152.01 | 0.33 | 0.10 | 0.06 | −12.33 | −0.22 | 0.33 | 0.00 | −29.21 | −0.04 | 0.86 |
| C3 | 0.26 | −163.14 | 0.34 | 0.06 | 0.10 | 4.70 | −0.31 | 0.21 | 0.08 | 20.90 | −0.26 | 0.34 | ||
| C4 | 0.25 | −155.34 | 0.32 | 0.07 | 0.02 | −41.43 | −0.13 | 0.57 | 0.00 | −38.81 | −0.02 | 0.91 | ||
| Pz | 0.20 | −177.84 | 0.36 | 0.11 | 0.05 | −27.30 | −0.22 | 0.37 | 0.01 | −45.88 | −0.07 | 0.72 | ||
| Controlled | Fz | 0.03 | −16.12 | −0.05 | 0.58 | 0.05 | −18.47 | −0.12 | 0.39 | 0.01 | −81.40 | 0.08 | 0.79 | |
| C3 | 0.12 | −89.44 | 0.15 | 0.23 | 0.13 | −5.13 | −0.20 | 0.16 | 0.00 | −77.44 | −0.01 | 0.98 | ||
| C4 | 0.01 | −49.68 | 0.03 | 0.74 | 0.05 | −17.75 | −0.13 | 0.37 | 0.01 | −84.68 | 0.08 | 0.74 | ||
| Pz | 0.02 | −27.32 | −0.05 | 0.65 | 0.05 | −30.72 | −0.12 | 0.40 | 0.00 | −75.08 | 0.03 | 0.91 | ||
Data in bold characters are significant at 0.05.
FIGURE 4.
Group comparison illustrating the relationship (linear regression) between the readiness potential and the reaction time latency for each electrode pooled across blocks in controls (A), Habit Disorder (B), and Chronic Tic Disorder (C) groups. The control group showed a significant relationship between the readiness potential and the reaction times that was not found in either clinical group.
In sum, the TD group showed no correlation between electrocortical and behavioral processes engaged in motor preparation and those reflecting execution of the motor responses. In the CON group, these two stages were highly associated. The HD group fell midway between TD and CON groups.
Regression Between MAP Peak Latency and RT
There were no significant correlations between MAP peak latency and RT for any group. All groups likewise showed no linear relationship between MAP latency and RT under either condition for any electrode site.
DISCUSSION
We initially hypothesized that the TD group would show less consistent differences between automated and controlled conditions and no habituation of either the RP or MAP across trial blocks. Results revealed that the CON group was better able than the clinical groups to modulate RP and MAP peak amplitude in accordance with practice and task demand. For instance, the effect of practice in the CON group was to shorten RT and increase RP and MAP peak amplitude. The CON group also showed larger MAP amplitude in the automated condition, so indicating that execution of the response was more readily achieved under automated processing. This would concur with previous findings indicating that under normal conditions, MAP amplitude varies inversely with extent of motor programming.49,50 Peak RP latency of the CON group was earlier for the automated condition, and the peak MAP latency onset was later for the controlled condition. The HD group showed a similar but nonsignificant pattern to the CON group. In the TD group, however, the pattern across conditions was the reverse of that in the CON group (see Fig. 2) but also nonsignificant. Effectively, we can conclude that in contrast to the CON group, neither clinical group showed a systematic distinction in either preparatory or motor associated responses between controlled and automated responses. All groups showed expected amplitude variations over frontal versus parietal regions; however, electrode placements were not sufficient to permit a full mapping procedure.
The lack of a systematic condition or practice effects in TD and HD groups could suggest a ceiling level of cortical arousal, perhaps induced by impaired dopamine modulation.51–53 Alternatively, the difficulty in TD may be to inhibit nonrelevant activation, which would impede selective efficient preparation for the corresponding response execution, particularly under complex task demand; this problem may be exacerbated as the planned act becomes more complex, requiring more open-loop control and control of more parameters.54
Although the RT results reflected differences in practice effects between groups, there were no overall group differences in GO RT. There was a nonsignificant trend in all groups toward expected longer GO RT to controlled than to automated conditions during the first block of trials. The current traffic light task was designed as a countermanding paradigm rather than as a go–no go or choice versus simple RT task. This task, inspired in part by previous findings showing a lack of difference between TD and controls in GO-RT, was designed specifically to elicit differences in STOP RT that have been reported elsewhere.38 The fact that the initial response in both automated and controlled tasks was manual depression of lever (followed subsequently by complex or simple movements) rather than a button press may have lengthened RT and so masked GO RT condition effects.
We also predicted a lower correlation between electrophysiological and behavioral response measures in the TD group. The linear regression results between RT and RP peak latency revealed a strong linear association in the CON group, so indicating a temporal synchrony between central motor decisions and response times. This synchrony was absent in both the HD and the TD group, where there was no association between RP peak latency and RTunder any conditions. The TD group showed no systematic relationship between RT and RP latency but also a lack of correlation between electrocortical measures of planning (RP) and execution (MAP). There was a larger difference between RP and MAP amplitude in the TD group, indicating less connection between the measures of cortical activation associated with response preparation and execution (see Fig. 3). The HD group fell midway between CON and TD in their relationship between behavioral and electrocortical indices of activation during planning and execution stages. Although, as noted in the introduction, RP and MAP seem generated by separate pools of neurons,21,22 the closer the RP to response onset, the more one might expect overlap between movement preparation and response execution processes.27 The precise association between primary and supplementary motor areas and motor task demand remains controversial.21,22 Assuming a time-ordered progression of increasingly selective motor planning before response onset, one might predict that the more selective the response planning, the greater the overlap between premotor areas and motor cortex activation. Conversely, a more general or abstract level of motor planning would offer less possibility of a specific parallel activation between premotor and motor areas. The lack of association between the MAP and RT in all groups is not surprising because the MAP seems more sensitive to degree and extent of muscle activation and motor neuron excitability than to response time.17,55 However, our present results remain speculative because of limited lead placements.
Biswal et al14 have previously reported recruitment of larger portions of the motor cortex in TS during finger tapping. The lack of difference between TD and CON in MAP amplitude in the present study despite a trend suggesting higher MAP and hence more muscle activation during controlled responding might be a result of the inclusion of a TD rather than a TS population.
The participants in the current study were diagnosed with TD rather than TS. However the restricted range of scores on the TSGS in our TD group was largely caused by the absence of impairment in social and other functioning in everyday life. Motor tic frequency covered the complete range (1–5) of the scale. Because tics are the defining characteristic of both TS and TD, it seems reasonable to speculate that the current results would generalize to a more severe TS group.
In clinical practice, both TS and TD patients frequently show motor restlessness and hyperactivity in their style of planning action, often attempting too much at once and creating frustration and tension.56,57 The current differences in cortical indices of preparation support the hypothesis that difficulties in planning actions optimally are characteristic of chronic tic disorders. In the present study, we manipulated, controlled, and automated processing in terms of straightforward motor response and through manipulating limited parameters of movement complexity. In future studies, it might be important to manipulate task demand in terms of time constraints, task load, and the amount to be accomplished to allow characteristic clinical patterns of planning difficulties to emerge within a complex motor preparation paradigm. Another clinical implication lies with the potential use of electrophysiological measures to confirm nosological categories of TD and HD. There is a benefit in distinguishing between complex tics and impulsive habits because of differential responses to treatment. The relationship between these two diagnostic categories is sometimes clinically unclear because the complex movements in HD can often resemble complex tics. However, our findings give support to a dimensional model of classification with HD falling between TD and controls along a continuum of motor arousal potentially quantifiable by ERP chronometry.
Acknowledgments
This research was funded by the Fonds de la Recherche en Santé du Québec (FRSQ) and by the Louis-H Lafontaine Foundation.
References
- 1.Lohr JB, Wisniewski A. Movement Disorders: A Neuropsychiatric Approach. New York: Wiley; 1987. [Google Scholar]
- 2.Shapiro E, Shapiro A. Semiology, nosology and criteria for tic disorders. Rev Neurol. 1986;142:824–832. [PubMed] [Google Scholar]
- 3.Shapiro AK, Shapiro ES, Young JG, et al. Gilles de la Tourette Syndrome. 2. New York: Raven Press; 1988. [Google Scholar]
- 4.Spencer T, Biederman J, Harding M, et al. The relationship between tic disorders and Tourette’s syndrome revisited. J Am Acad Child Adolesc Psychiatry. 1995;34:1133–1139. doi: 10.1097/00004583-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 5.First MB, Frances A, Pincus HA. DSM-IV criteria for Tourette’s: reply. J Am Acad Child Adolesc Psychiatry. 1995;34:402. [Google Scholar]
- 6.The Tourette Syndrome Classification Study Group. Definitions and classification of tic disorders. Arch Neurol. 1993;50:1013–1016. doi: 10.1001/archneur.1993.00540100012008. [DOI] [PubMed] [Google Scholar]
- 7.Hollander E, Benzaquen SD. The obsessive-compulsive spectrum disorders. Int Rev Psychiatry. 1997;9:99–109. [Google Scholar]
- 8.Christenson GA, Ristvedt SL, Mackenzie TB. Identification of trichotillomania cue profiles. Behav Res Ther. 1993;31:315–320. doi: 10.1016/0005-7967(93)90030-x. [DOI] [PubMed] [Google Scholar]
- 9.Dean JT, Nelson E, Moss L. Pathologic hair-pulling: A review of the literature and case reports. Compr Psychiatry. 1992;33:284–291. doi: 10.1016/0010-440x(92)90003-9. [DOI] [PubMed] [Google Scholar]
- 10.Hagin RA, Beecher R, Pagano G, et al. Effects of Tourette syndrome on learning. Adv Neurol. 1982;35:323–328. [PubMed] [Google Scholar]
- 11.Bornstein RA, Baker GB, Bazylewich T, et al. Tourette syndrome and neuropsychological performance. Acta Psychiatr Scand. 1991;84:212–216. doi: 10.1111/j.1600-0447.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 12.Bornstein RA. Neuropsychological performance in children with Tourette’s syndrome. Psychiatr Res. 1990;33:73–81. doi: 10.1016/0165-1781(90)90150-4. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein R. Neuropsychological performance in adults with Tourette’s syndrome. Psychiatr Res. 1991;37:229–236. doi: 10.1016/0165-1781(91)90059-x. [DOI] [PubMed] [Google Scholar]
- 14.Biswal B, Ulmer JL, Krippendorf RL, et al. Abnormal cerebral activation associated with a motor task in Tourette syndrome. Am J Neuroradiol. 1998;19:1509–1512. [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: Evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 16.Kornhuber HH, Deecke L. Cerebral potentials and the initiation of voluntary movement. In: Desmedt JE, editor. Attention, Voluntary Contraction and Event-Related Cerebral Potentials. Basel: Karger; 1977. [Google Scholar]
- 17.Brunia CHM. What is wrong with legs in motor preparation? Motivation, motor and sensory processes of the brain. Prog Brain Res. 1980;54:232–236. doi: 10.1016/S0079-6123(08)61631-3. [DOI] [PubMed] [Google Scholar]
- 18.Brunia CH, Damen EJ. Distribution of slow brain potentials related to motor preparation and stimulus anticipation in a time estimation task. Electroencephalogr Clin Neurophysiol. 1988;69:234–243. doi: 10.1016/0013-4694(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 19.Rektor I, Bares M, Kubova D. Movement-related potentials in the basal ganglia: a SEEG readiness potential study. Electroencephalogr Clin Neurophysiol. 2001;112:2146–2153. doi: 10.1016/s1388-2457(01)00662-9. [DOI] [PubMed] [Google Scholar]
- 20.Kornhuber HH. Cortex, basal ganglia and cerebellum in motor control. In: Cobb WA, Van Duijn H, editors. Contemporary Clinical Neurophysiology. Amsterdam: Elsevier; 1978. [PubMed] [Google Scholar]
- 21.Cui RQ, Egkner A, Huter D, et al. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin Neurophysiol. 2000;111:1847–1859. doi: 10.1016/s1388-2457(00)00388-6. [DOI] [PubMed] [Google Scholar]
- 22.Cui RQ, Huter D, Egkher A, et al. High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Exp Brain Res. 2000;134:49–57. doi: 10.1007/s002210000449. [DOI] [PubMed] [Google Scholar]
- 23.Obeso J, Rothwell J, Marsden CJ. Simple tics in Gilles de la Tourette’s syndrome are not prefaced by a normal premovement EEG potential. J Neurol Neurosurg Psychiatry. 1981;44:735–738. doi: 10.1136/jnnp.44.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp BI, Porter S, Toro C, et al. Simple motor tics may be preceded by a premotor potential. J Neurol Neurosurg Psychiatry. 1996;61:103–106. doi: 10.1136/jnnp.61.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggal HS, Haque Nizamie S. Bereitschaftspotential in tic disorders: A preliminary observation. Neurol India. 2002;50:487–489. [PubMed] [Google Scholar]
- 26.Brunia CHM, Vuister FM. Spinal reflexes as indicator of motor preparation in man. Physiol Psychol. 1979;7:377–380. [Google Scholar]
- 27.Brunia CHM, Scheirs JGM, Haagh AVM. Changes of Achilles tendon reflex amplitudes during a fixed foreperiod of four seconds. Psychophysiology. 1982;19:63–70. doi: 10.1111/j.1469-8986.1982.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 28.Rektor I, Louvel J, Lamarche M. Intracerebral recording of potentials accompanying simple limb movements: a SEEG study in epileptic patients. Electroencephalogr Clin Neurophysiol. 1998;107:277–286. doi: 10.1016/s0013-4694(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R. Motor and action perspectives on motor behaviour. In: Meijer O, Roth K, editors. Motor Behavior. Amsterdam: Elsevier-North Holland; 1988. [Google Scholar]
- 30.Leuthold H, Jentzsch I. Distinguishing neural sources of movement preparation and execution. An electrophysiological analysis. Biol Psychol. 2002;60:173–198. doi: 10.1016/s0301-0511(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor KP. Applications of the CNV in psychophysiology. In: Venables P, Martin I, editors. Techniques in Psychophysiology. Chichester: Wiley; 1980. pp. 396–430. [Google Scholar]
- 32.Osman A, Hornblum S, Meyer D. Response times in a countermanding paradigm. J Exp Psychol. 1990;16:183–198. doi: 10.1037//0096-1523.16.1.183. [DOI] [PubMed] [Google Scholar]
- 33.Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:1–6. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 34.Gray JA. A theory of anxiety: the role of the limbic system. Encephale. 1983;9(4 Suppl 2):161B–166B. [PubMed] [Google Scholar]
- 35.McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor KP. Individual differences and motor systems. In: Ney Y, Gale A, editors. Smoking and Human Behaviour. Chichester: Wiley; 1989. pp. 141–170. [Google Scholar]
- 37.O’Connor KP, Serawaty M, Stip E. Simple and complex motor processing in chronic tic disorders. Brain Cogn. 1999;40:211–215. [Google Scholar]
- 38.O’Connor KP, Robert M, Dubord J, et al. Automatic and controlled processing in chronic tic disorders. Brain Cogn. 2000;43:349–352. [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analyses of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Harcherik DF, Leckman JF, Detlor J, et al. A new instrument for clinical studies of Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1984;23:153–160. doi: 10.1097/00004583-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–743. [PubMed] [Google Scholar]
- 42.Hadley NH. Fingernail Biting. Theory, Research and Treatment. New York: Spectrum Publications; 1984. [Google Scholar]
- 43.Rothbaum BO, Ninan PT. The assessment of trichotillomania. Behav Res Ther. 1994;32:651–662. doi: 10.1016/0005-7967(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 44.Minichiello WE, O’Sullivan RL, Osgood-Hynes D, et al. Trichotillomania: Clinical aspects and treatment strategies. Harv Rev Psychiatry. 1994;1:336–344. doi: 10.3109/10673229409017100. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor KP, Lapierre GD. The traffic light test. Montréal: Stelate Systems. 1994 [Google Scholar]
- 46.Rosenbaum DA, Hindorff V, Munro EM. Scheduling and programming of rapid finger sequences: Tests and elaborations of the hierarchical editor model. J Exp Psychol. 1987;113:372–393. doi: 10.1037//0096-1523.13.2.193. [DOI] [PubMed] [Google Scholar]
- 47.Canic MJ, Franks IM. Response preparation and latency in patterns of tapping movements. Hum Move Sci. 1989;8:123–139. [Google Scholar]
- 48.Sternberg S, Monsell S, Knoll RL, et al. The latency and duration of rapid movement sequences: Comparisons of speech and typewriting. In: Stelmach GE, editor. Information Processing in Motor Control and Learning. New York: Academic Press; 1978. pp. 117–152. [Google Scholar]
- 49.Kitamura J, Shibasaki H, Takagi A, et al. Enhanced negative slope of cortical potentials before sequential as compared with simultaneous extensions of two fingers. Electroencephalogr Clin Neurophysiol. 1993;86:176–182. doi: 10.1016/0013-4694(93)90005-g. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura J, Shibasaki H, Kondo T. A cortical slow potential is larger before an isolated movement of a single finger than simultaneous movement of two fingers. Electroencephalogr Clin Neurophysiol. 1993;86:252–258. doi: 10.1016/0013-4694(93)90106-6. [DOI] [PubMed] [Google Scholar]
- 51.Singer HS, Butler IJ, Tune LE, et al. Dopaminergic dysfunction in Tourette syndrome. Ann Neurol. 1982;12:361–366. doi: 10.1002/ana.410120408. [DOI] [PubMed] [Google Scholar]
- 52.Malison RI, McDougle CJ, van Dyck CH, et al. [1231]beta-CIT SPECT imaging of striatal dopamine transporter binding in Tourette’s disorder. Am J Psychiatry. 1995;152:1359–1361. doi: 10.1176/ajp.152.9.1359. [DOI] [PubMed] [Google Scholar]
- 53.Ridley RM. The psychology of perseverative and stereotyped behavior. Prog Neurobiol. 1994;44:221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich R, Leuthold H, Sommer W. Motor programming of response force and movement direction. Psychophysiology. 1998;35:721–728. [PubMed] [Google Scholar]
- 55.Decety J, Ingvar DH. Brain structures participating in mental simulation of motor behavior: a neuropsychological interpretation. Acta Psychol. 1990;73:13–34. doi: 10.1016/0001-6918(90)90056-l. [DOI] [PubMed] [Google Scholar]
- 56.O’Connor KP, Aardema F, Brisebois H. Validation of a style of planning action (STOP) as a discriminator between tic disorder, obsessive-compulsive disorder and generalized anxiety disorder. Poster presented at WCBCT; Vancouver, BC. July 2001. [Google Scholar]
- 57.O’Connor KP, Brisebois H, Brault M, et al. Behavioral activity associated with onset in chronic tic and habit disorder. Behav Res Ther. 2003;41:241–24. doi: 10.1016/s0005-7967(02)00051-7. [DOI] [PubMed] [Google Scholar]