Abstract
Bacterial polyester polyhydroxyalkanoates (PHAs) have been produced in engineered Escherichia coli, which turned into an efficient and versatile platform by applying metabolic and enzyme engineering approaches. The present study aimed at drawing out the latent potential of this organism using genome-wide mutagenesis. To meet this goal, a transposon-based mutagenesis was carried out on E. coli, which was transformed to produce poly(lactate-co-3-hydroxybutyrate) from glucose. A high-throughput screening of polymer-accumulating cells on Nile red-containing plates isolated one mutant that produced 1.8-fold higher quantity of polymer without severe disadvantages in the cell growth and monomer composition of the polymer. The transposon was inserted into the locus within the gene encoding MtgA that takes part, as a non-lethal component, in the formation of the peptidoglycan backbone. Accordingly, the mtgA-deleted strain E. coli JW3175, which was a derivate of superior PHA-producing strain BW25113, was examined for polymer production, and exhibited an enhanced accumulation of the polymer (7.0 g/l) compared to the control (5.2 g/l). Interestingly, an enlargement in cell width associated with polymer accumulation was observed in this strain, resulting in a 1.6-fold greater polymer accumulation per cell compared to the control. This result suggests that the increase in volumetric capacity for accumulating intracellular material contributed to the enhanced polymer production. The mtgA deletion should be combined with conventional engineering approaches, and thus, is a promising strategy for improved production of intracellularly accumulated biopolymers.
Introduction
Polyhydroxyalkanoates (PHAs) are bacterial polyesters that can be developed as commodity plastic materials and also applicable for environmental and biochemical applications [1–4]. The material properties of PHAs are governed by their monomer composition, molecular weight and copolymer microstructure [5]. In addition, the efficient conversion of inexpensive and renewable feedstock into PHA results in value-added products that are competitive with their petroleum counterparts [6]. The primary aims of the metabolic engineering of PHA, therefore, include controlling the different factors that determine these polymer properties and optimizing yield. In this regard, a recombinant Escherichia coli system, which incorporates natural and engineered PHA biosynthetic pathways, is useful for achieving high-yield production of various tailor-made PHAs [7–9]. In addition, unlike many natural PHA produces, in which PHA accumulation is induced under the nitrogen and/or phosphate limited conditions, recombinant E. coli is able to produce PHAs under the nutrient rich condition probably because E. coli possesses no regulators controlling PHA biosynthesis.
To date, many rationally designed approaches have been examined to increase a flux toward PHA, such as reinforcement in the activities of PHA biosynthetic enzymes by means of gene dosage [10–12], and enzyme engineering [13–16]. In addition, disruption of competing pathways was also effective to improve the polymer production [17]. On the other hand, optimization of fermentation parameters such as pH, aeration rate etc, was useful to achieve high cell density and production per unit time [18,19]. These strategies for the metabolic and fermentation engineering of PHA producers were implemented either individually or in combination.
For further improvement in polymer production, we have explored positive factor(s) indirectly contributing to improved polymer production that is unable to be predicted based on rational approaches. Previously we evaluated the effect of deletion of four non-essential sigma factors, which is a global regulator governing the transcription of over 100 genes, of E. coli on the production of lactate-based polyester poly(lactate-co-3-hydroxybutyrate) [P(LA-co-3HB)], an engineered PHA with semitransparent and flexible properties (for detail, see [20]). This experiment aimed at exploring a global gene suppression that eventually increased polymer production. As a result, the rpoN deletion was found to improve P(LA-co-3HB) production in recombinant E. coli [21]. This result suggested the potential of this organism to be modified for enhanced polymer production. Therefore, in this study, we designed a transposon-based genome-wide mutagenesis of P(LA-co-3HB)-producing E. coli and high-throughput screening of highly-accumulating mutants. This approach expectedly isolates beneficial single gene knockouts that increased polymer production among all non-lethal gene disruptants. Indeed, one strain bearing a disruption of MtgA, which is involved in formation of the peptidoglycan strand [22–24], was isolated as a positive mutant. Interestingly, the selected mutant exhibited phenotype of enlarged cell size, which was associated with polymer accumulation. To the best of our knowledge, this is the first case of the single gene deletion that induced both cell size enlargement (a so-called fat cell), and enhanced polymer production.
Materials and Methods
Plasmids, strains, and growth conditions
E. coli strains used in this study are listed in Table 1. The expression vector pTV118NpctphaC1 Ps(ST/QK)AB, which harbors genes encoding propionyl-CoA transferase from Megasphaera elsdenii (pct) [25], engineered PHA synthase with LA-polymerizing activity [phaC1 Ps(ST/QK)] from Pseudomonas sp. 61–3 [26] and 3HB-CoA supplying enzymes β-ketothiolase and acetoacetyl-CoA reductase (phaA, and phaB) from Ralstonia eutropha [27], was used for P(LA-co-3HB) production [16,28]. The synthetic pathways of P(LA-co-3HB) was shown in Fig 1. pTS52 was used as a helper plasmid for conjugation [29]. For polymer production, recombinant E. coli harboring pTV118NpctphaC1 Ps(ST/QK)AB were grown on 1.7 ml LB medium containing 20 g/l glucose and 10 mM calcium pantothenate at 30°C for 48 h with reciprocal shaking at 180 rpm. Ampicillin (Amp; 100 μg/ml), kanamycin (Km; 25 μg/ml), and chloramphenicol (Cm; 25 μg/ml) were added when needed. pCA24N-mtgA was obtained from ASKA clone [30].
Table 1. E. coli strains used in this study.
| Strains | Genotype | Purpose | Reference |
|---|---|---|---|
| S17-1 | MG1655 RP4-2-tc::[ΔMu1::aac(3)IV-ΔaphA-Δnic35-ΔMu2::zeo] ΔdapA::(erm-pir) ΔrecA | Transposon mutagenesis of JM109 | [48,49] |
| JM109 | endA1 glnV44 thi-1 relA1 gyrA96 recA1 mcrB+ Δ(lac-proAB) e14- [F' traD36 proAB+ lacIq lacZΔM15] hsdR17(rK-mK+) | Host strain of transposon mutagenesis | [49] |
| JM109 C21 | JM109 ΔmtgA∷Tn5 | Selected mutant of JM109 as a enhanced polymer producer | this study |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) lacIp- 4000(lacIq) λ- rph-1 Δ(rhaD-rhaB)568 hsdR514 | Parent strain of Keio collection mutants | [38] |
| JW3175 | ΔmtgA::FRT-kan-FRT | A mutant in Keio collection | [38] |
Fig 1. Model of polymer accumulation in fat E. coli cell with mtgA deletion.
MtgA is a dispensable monofunctional glycosyltransferase catalyzing the polymerization of lipid II for the extension of glycan strands but not cross-linking. Penicillin-binding proteins (PBPs), which are bifunctional transpeptidases-transglycosylases and monofunctional transpeptidases, play a central role in the peptidoglycan formation. The mtgA deletion had no obvious effect on cell morphology without polymer accumulation, but generated a fat cell phenotype with polymer production. P(LA-co-3HB) production from glucose in E. coli was achieved by expressing four heterologous enzymes; β-ketothiolase (PhaA), acetoacetyl-CoA reductase (PhaB), propionyl-CoA transferase (PCT) and lactate-polymerizing engineered polyhydroxyalkanoate synthase [PhaC1(ST/QK)]. D-Lactate dehydrogenase (LDH) is an intrinsic enzyme. The polymer synthesis may elevate turgor pressure, which expands the cell to form the fat-cell and allowed to accumulate the additional amount of polymer.
Construction of mutant library of E. coli JM109 and screening of the positive mutants
E. coli S17-1 λ-pir carrying pUTmini-Tn5 Km (Ampr, Biomedical, Seville, Spain [31]) was used for the conjugative transfer of the mini-Tn5 transposon (Kmr) to recombinant E. coli JM109 harboring pTV118NpctphaC1 Ps(ST/QK)AB (Ampr) and pST52 (Cmr). The S17-1 and JM109 cells were conjugated on an LB agar plate at 30°C for 16 h. The cells were suspended in 10 mM MgSO4 and grown on LB plates containing Cm, Km and Amp. Cm was used to get rid of S17-1 cells. Km was used to select transposon-inserted JM109 cells. Amp was used to maintain pTV118NpctphaC1 Ps(ST/QK)AB. The plates were incubated at 30°C for 16 h. The transposon-inserted JM109 harboring pTV118NpctphaC1 Ps(ST/QK)AB was screened on LB plates containing 2% glucose, Amp, Km and 0.5 μg/ml Nile red (Sigma-Aldrich). Colonies emitting strong fluorescence were chosen as candidates under a transilluminator and subjected to HPLC analysis for determining polymer production as described previously [32]. In brief, cells were directly treated with concentrated sulfuric acid at 100°C to convert polyester into unsaturated carbonic acids, which were measured using UV detector at 210 nm [33,34]. The concentration of glucose in the supernatant was determined by HPLC equipped with a refractive index detector, as previously described [35].
Identification of transposon insertion site
The transposon insertion site was identified using inverse PCR method. Chromosomal DNA of E. coli was digested with PstI, self-ligated [36] and amplified by PCR using a pair of primers: 5′-AAGGTGATCCGGTGGATGAC-3′ and 5′-CAATCGGCTGCTCTGATGCCGC-3′, which annealed to the Km resistance gene in the transposon [37]. The amplified fragment was sequenced to identify the transposon insertion site in E. coli chromosome.
Measurement of cell density using flow cytometry
The volumetric cell density (cells/l) was measured by flow cytometry using a SH800 cell sorter (SONY). Cells grown under aforementioned conditions were harvested at 48 h (OD600 between 20 and 25) and 10-fold diluted sample with water was analyzed. The flow rate was set to 11 μl/min (pressure 2). All FSC (forward scatter) and SSC (side scatter) images were recorded using SH800 software (SONY).
Determination of cell size
Cells were grown on polymer-producing conditions and harvested at 48 h. The cell images were captured using a microscopy BZ-X700 (Keyence). On the digital images, the length of polar axis (x) and diameter (y) of 150–200 cells for each condition were measured using ImageJ software (http://rsb.info.nih.gov/ij/index.html). Based on the " lemon-shaped " morphology of polymer accumulating cells, the cell volume (V) was approximated by that of oval sphere, and thus, calculated using the following formula (1).
| (1) |
Results and Discussion
Transposon mutagenesis and screening of the highly polymer-producing mutant
A mutant library of the E. coli JM109 strain producing P(LA-co-3HB) was prepared by using the transposon mini-Tn5. A high-throughput screening of polymer-accumulating cells was performed by means of plate assay using Nile red-containing agar plates. The dye-staining allowed us to readily screen the candidates with higher polymer production than that of original recombinant (parent control). The strain JM109 was used as a host because the cells were efficiently stained with Nile red and our group has developed several in vitro evolved enzymes involved in PHA biosynthesis using the screening method [15]. Among approximately 10,000 colonies, 100 colonies were chosen as the first stage candidates. The polymer production in the candidates was determined using HPLC, and eventually, one mutant C21, which exhibited an enhanced polymer accumulation, was isolated. The mutant C21 produced 5.1 g/l polymer compared to the parent recombinant (2.9 g/l, S1 Table).
The deletion of mtgA gene contributed to the enhanced polymer production
Nucleotide sequence analysis of chromosomal DNA of C21 revealed that the transposon was inserted into the mtgA gene. Accordingly, this knowledge was applied to a superior P(LA-co-3HB)-producing strain E. coli BW25113, which has been extensively used for the polymer production [17,32]. An mtgA-deleted derivative of BW25113, E. coli JW3175, was obtained from Keio collection [38,39]. Recombinant JW3175 and BW25113 harboring pTV118NpctphaC1 Ps(ST/QK)AB (referred as rJW and parent recombinant, respectively) were grown on glucose to induce polymer accumulation (Table 2). rJW produced increased amount of P(LA-co-3HB) (7.0 g/l) compared to the parent recombinant (5.2 g/l) as observed in C21. To confirm a contribution of mtgA deletion to the enhanced polymer production, a complementary experiment of rJW was carried out by heterologous expression of mtgA gene [30]. The complementation recovered the phenotype the parent recombinant (Table 2). Thus, it was concluded that the deletion of the mtgA gene led to an increase in P(LA-co-3HB) production in E. coli. The mtgA-deletion had little effect on the LA/3HB ratio in the copolymer (Table 2).
Table 2. P(LA-co-3HB) production in mtgA-deleted and complemented strains.
| Genotype | Plasmid | Cell dry weight (g/l) | True cell weight (g/l) | Polymer production (g/l) | ||
|---|---|---|---|---|---|---|
| Total | LA | 3HB | ||||
| Wild type | pTV118Npct phaC1 Ps(ST/QK)AB | 9.2 ± 0.2 | 4.1 ± 0.3 | 5.2 ± 0.1 | 0.8 ± 0.1 | 4.4 ± 0.1 |
| ΔmtgA | pTV118Npct phaC1 Ps(ST/QK)AB | 11.6 ± 1.0 | 4.6 ± 0.9 | 7.0 ± 0.4 | 1.0 ± 0.1 | 6.0 ± 0.4 |
| ΔmtgA a | pTV118Npct phaC1 Ps(ST/QK)AB + pCA24N-mtgA | 8.0 ± 0.7 | 3.2 ± 0.3 | 4.9 ± 0.3 | 0.9 ± 0.1 | 3.9 ± 0.3 |
E. coli BW25113 (wild type) and JW3175 (ΔmtgA) harboring pTV118NpctphaC1 Ps(ST/QK)AB were grown on LB medium containing 20 g/l of glucose at 30°C for 48 h with reciprocal shaking at 180 rpm. The data represent the average ± standard deviation of three independent trials. pCA24N-mtgA bears the mtgA gene, which is expressed by lac promoter.
a 100 μM IPTG was added.
The time profile of polymer accumulation and glucose consumption are shown in Fig 2. At initial stage, rJW produced slightly less amount of polymer than parent recombinant, but at 24 h, achieved higher production. Notably rJW consumed glucose more rapidly than parent recombinant. The yield of the polymer from glucose in rJW (3.6 g/g) was slightly higher than the control (3.1 g/g). Therefore, mtgA-deletion increased both of the glucose consumption and conversion efficiency into polymer.
Fig 2. Time course of P(LA-co-3HB) production in the mtgA-deleted E. coli.
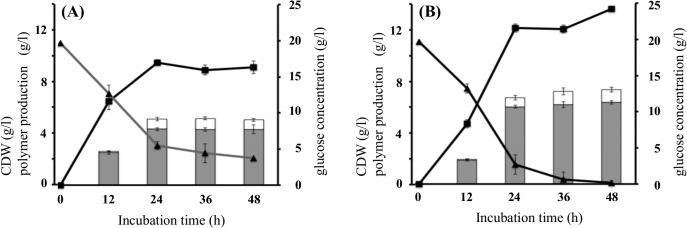
E. coli BW25113 (wild type) (A) and JW3175 (ΔmtgA) (B) harboring pTV118NpctphaC1 Ps(ST/QK)AB were grown on LB medium containing 20 g/l glucose. Triangle, glucose concentration in the medium. Square, cell dry weight. Gray bar, amount of 3HB unit in the polymer. White bar, amount of LA unit in the polymer. The data represent the average ± standard deviation of three independent trials. The cells were inoculated at time zero.
Cell size enlargement caused by polymer accumulation in mtgA-deleted strain
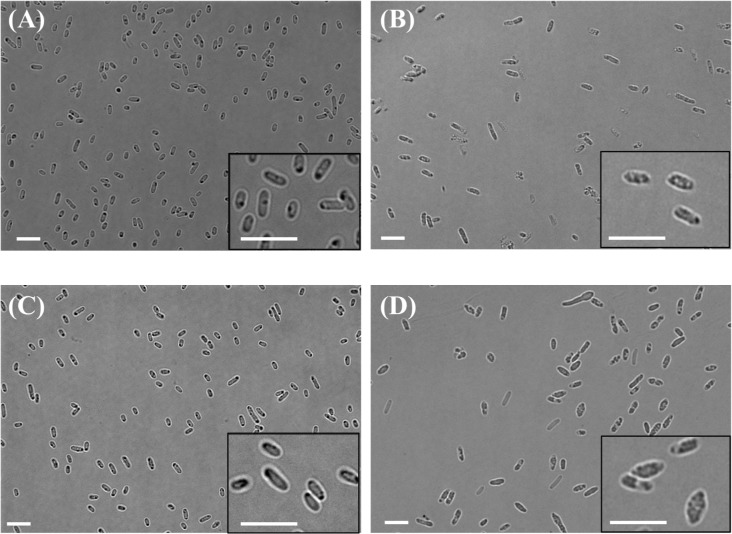
The mtgA gene has been reported to encode a monofunctional peptidoglycan transglycosylase involved in the polymerization of lipid II molecules into glycan strands of peptidoglycans [40]. This fact prompted us to observe the cell morphology of the mtgA-deleted strain with polymer accumulation. Under the non polymer-producing conditions, as expected, JW3175 exhibited similar cell size to the parent strain BW25113 (Table 3). Thus, the mtgA deletion alone did not affect the cell morphology. On the contrary, under the polymer-producing conditions, rJW cells remarkably increased in size (1.4-fold) compared to the parent recombinant (Table 3). Interestingly, the mtgA deletion led to an increase in only the cell diameter but not length of polar axis, thus cells became fat rather than tall. Furthermore, the complemented strain of rJW exhibited cell size similar to the parent recombinant (data not shown), supporting that the mtgA deletion contributed to the cell enlargement. The transposon-inserted strain JM109 C21 exhibited similar cell morphology (data not shown).
Table 3. Correlation between polymer production and cell volume in recombinant E. coli with mtgA deletion.
| Genotype | Plasmid | Cell density (l-1) | Single cell dry weight (g) | Cellular polymer content (wt%) | Polymer production in single cell (g) | Size of cell | ||
|---|---|---|---|---|---|---|---|---|
| Polar axis (μm) | Diameter (μm) | Volume (μm3) | ||||||
| Wild-type | pTV118N | 0.73×1012 | 2×10–12 | N.D. | N.D. | 2.26 ± 0.56 | 1.10 ± 0.12 | 1.43 |
| Wild-type | pTV118NpctC1(STQK)AB | 1.2×1012 | 8×10–12 | 56.8 ± 1.8 | 4.5×10–12 | 3.36 ± 0.82 | 1.15 ± 0.16 | 2.56 |
| ΔmtgA | pTV118N | 0.75×1012 | 2×10–12 | N.D. | N.D. | 2.49 ± 0.62 | 1.14 ± 0.23 | 1.69 |
| ΔmtgA | pTV118NpctC1(STQK)AB | 0.96×1012 | 12×10–12 | 60.9 ± 3.5 | 7.3×10–12 | 3.48 ± 0.84 | 1.42 ± 0.25 | 3.67 |
E. coli BW25113 (wild type) and JW3175 (ΔmtgA) harboring pTV118NpctphaC1 Ps(ST/QK)AB were grown on LB medium at 30°C for 48 h. Cell density was measured using a flow cytometory. Cellular polymer content was defined as a ratio of polymer weight over total cell dry weight. The data represent the average ± standard deviation of three independent trials. The size of cells was determined using microscopic images of 150–200 cells. N.D., not detectable.
The cell morphology of rod-shaped bacteria such as E. coli is determined by a balance between elongation and septation of the cells, in which the peptidoglycan synthesis plays a central role. E. coli peptidoglycan is synthesized by multiple penicillin-binding proteins (PBPs), which are categorized into three classes; bifunctional transpeptidases-transglycosylases (class A), monofunctional transpeptidases (class B) and endopeptidases (class C) [40]. In the case of E. coli, deletion of class B PBP3 produced filamentous cells because the cells are unable to septate, whereas deletion of class B PBP2 resulted in an increase in the diameter of the cell [41]. These results suggest that the removal of cross-linking enzymes influenced the cell morphology. On the other hand, MtgA, a dispensable monofunctional transglycosylase, has been thought to play an auxiliary role in peptidoglycan synthesis. In fact, mtgA deletion alone exhibited no obvious effect on cell morphology (Fig 3) [42]. However, the result of present study showed that the cells lacking MtgA increased diameter presumably by outward force from the intracellularly synthesized polymer (Table 3), suggesting that MtgA does contribute to the peptidoglycan synthesis in E. coli. In addition, mtgA deletion did not induce filamentation of the cells, suggesting no critical effect on septation. This phenotype was contrast to the cell elongation induced by inhibition of FtsZ [43]. Currently, the three-dimensional structure of peptidoglycan remains elusive and there is ongoing argument on this issue [44–47], and therefore, an effect of mtgA deletion on the peptidoglycan structure also remains uncharacterized. The mtgA deletion may increase the flexibility of cell wall that allowed cells to expand in width and to accumulate extra amount of polymer.
Fig 3. Effect on the cell volume exerted by polymer production in the wild-type and mtgA-deleted E. coli.
The cells were grown on LB medium containing 2% glucose. E. coli BW25113 (wild-type) harboring pTV118N (empty vector) (A) and pTV118NpctphaC1 Ps(ST/QK)AB (B). E. coli JW3725 (ΔmtgA) harboring pTV118N (C) and pTV118NpctphaC1 Ps(ST/QK)AB (D). The cells harboring pTV118N did not produce detectable amount of polymer. Scale bars = 5 μm.
Quantitative analysis of the polymer production in fat cell
Volumetric polymer production (P) is determined using the following formula:
In order to gain an advantage of the cell enlargement, the impact on cell growth is a primarily important factor. The growth of rJW was slightly slower than the control at the initial stage (Fig 3), but the decrease in the cell density (number of cells per volume. Cell density was measured using a flow cytometory.) of polymer-accumulating rJW at 48 h from parent recombinant was as small as 20% (Table 3), suggesting that there was no severe influence of mtgA-deletion on cell growth. In addition, the mtgA-deleted strain appeared robust and no cell lysis was observed (S1 Fig).
Another important value is the polymer production per cell. Based on the cell dry weight and cellular polymer content, the polymer production in a single cell of rJW was estimated to be 7.3 × 10–12 g/cell, which was 1.6-fold greater than the parent recombinant (4.5 × 10–12 g/cell). This result clearly indicated that the rJW fat cell possessed higher capacity of accumulating polymer. The increase in cell size should be necessarily accompanied with an increase in the amount (area) of cell membranes. In fact, the true cell weight (subtraction of polymer weight from total cell dry weight) of rJW was greater than parent recombinant when cells accumulated polymer (Table 3). However, the benefit of the increased accumulation capacity for PHA outweighed the additional consumption of carbon source for cell formation, and overall, the polymer production (g/l) and the polymer yield over glucose consumption (g/g) in rJW were increased. This fact conversely indicates that the size of intracellular space has been a limiting factor in the polymer accumulation in E. coli, and the mtgA deletion loosened the limitation.
In summary, P(LA-co-3HB) production in recombinant E. coli was elevated by disruption of the mtgA gene that led to a formation of fat cell. The same phenomenon was observed for P(3HB) production in mtgA-deleted strain (S2 Fig and S2 Table), indicating that the beneficial effect of mtgA deletion is not limited to P(LA-co-3HB) but should be applicable to wide range of intracellularly accumulated compounds. In addition, a synergy of combining mtgA deletion and conventional engineering approaches would be useful for further increasing polymer production.
Supporting Information
Cells were grown on LB medium at 30°C for 48 h. Proteins in culture medium was concentrated with acetone and applied to SDS-PAGE. M, size marker. 1, BW25113. 2. JW3175. There was no significant difference in protein level in the medium, indicating that no cell lysis was promoted by mtgA deletion.
(EPS)
E. coli BW25113 (wild-type) harboring pGEMphaC1 Ps(ST/QK)AB grown on LB medium containing glucose (A). E. coli JW3725 (ΔmtgA) harboring pGEMphaC1 Ps(ST/QK)AB grown on LB medium containing glucose (B). The cells produced P(3HB) under the presence of glucose. Scale bar = 5 μm.
(EPS)
All strains were grown in 1.7 ml of LB medium containing 20 g/l of glucose at 30°C for 48 h with reciprocal shaking at 180 rpm. The data represent the average ± standard deviation of three independent trials.
(DOCX)
E. coli BW25113 (wild type) and JW3175 (ΔmtgA) harboring pGEMphaC1 Ps(ST/QK)AB [50] were grown on LB medium containing 20 g/l of glucose at 30°C for 48 h with reciprocal shaking at 180 rpm. The data represent the average ± standard deviation of three independent trials.
(DOCX)
Acknowledgments
We thank the National BioResource Project, Japan for providing the Keio collection strains and the mtgA-overexpression plasmid in the ASKA clone. The plasmid of pTUmini-Tn5Km was provided by Dr. Yoshitoshi Ogura (University of Miyazaki). This study was partly supported by CREST, JST.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a Core Research for Evolutional Science and Technology (CREST) program “Creation of essential technologies to utilize carbon dioxide as a resource through the enhancement of plant productivity and the exploitation of plant produces” (http://www.jst.go.jp/kisoken/crest/index.html) from the Japan Science and Technology Agency (JST) (http://www.jst.go.jp) (RK and ST). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chung AL, Zeng GD, Jin HL, Wu Q, Chen JC, Chen GQ. Production of medium-chain-length 3-hydroxyalkanoic acids by beta-oxidation and phaC operon deleted Pseudomonas entomophila harboring thioesterase gene. Metab Eng 2013; 17: 23–29. 10.1016/j.ymben.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 2. Gao X, Chen JC, Wu Q, Chen GQ. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol 2011; 22: 768–774. 10.1016/j.copbio.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 3. Gumel AM, Annuar MSM, Chisti Y. Recent advances in the production, recovery and applications of polyhydroxyalkanoates. J Polym Environ 2013; 21: 580–605. [Google Scholar]
- 4. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006; 7: 2249–2258. [DOI] [PubMed] [Google Scholar]
- 5. Tanadchangsaeng N, Tsuge T, Abe H. Comonomer compositional distribution, physical properties, and enzymatic degradability of bacterial poly(3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate) copolyesters. Biomacromolecules 2010; 11: 1615–1622. 10.1021/bm100267k [DOI] [PubMed] [Google Scholar]
- 6. Chee J, Yoga S, Lau N, Ling S, Abed R, Sudesh K. Bacterially Produced Polyhydroxyalkanoate (PHA): Converting Renewable Resources into Bioplastics. Current Research, Technology and education Topics in Applied microbiology and applied Biotechnology 2010; 2: 1395–1404. [Google Scholar]
- 7. Le Meur S, Zinn M, Egli T, Thony-Meyer L, Ren Q. Improved productivity of poly (4-hydroxybutyrate) (P4HB) in recombinant Escherichia coli using glycerol as the growth substrate with fed-batch culture. Microb Cell Fact 2014; 13: 131 10.1186/s12934-014-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsumoto K, Ishiyama A, Sakai K, Shiba T, Taguchi S. Biosynthesis of glycolate-based polyesters containing medium-chain-length 3-hydroxyalkanoates in recombinant Escherichia coli expressing engineered polyhydroxyalkanoate synthase. J Biotechnol 2011; 156: 214–217. 10.1016/j.jbiotec.2011.07.040 [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto K, Terai S, Ishiyama A, Sun J, Kabe T, Song Y, et al. One-pot microbial production, mechanical properties, and enzymatic degradation of isotactic P[(R)-2-hydroxybutyrate] and its copolymer with (R)-lactate. Biomacromolecules 2013; 14: 1913–1918. 10.1021/bm400278j [DOI] [PubMed] [Google Scholar]
- 10. Jo SJ, Matsumoto K, Leong CR, Ooi T, Taguchi S. Improvement of poly(3-hydroxybutyrate) [P(3HB)] production in Corynebacterium glutamicum by codon optimization, point mutation and gene dosage of P(3HB) biosynthetic genes. J Biosci Bioeng 2007; 104: 457–463. 10.1263/jbb.104.457 [DOI] [PubMed] [Google Scholar]
- 11. Maehara A, Ikai K, Ueda S, Yamane T. Gene dosage effects on polyhydroxyalkanoates synthesis from n-alcohols in Paracoccus denitrificans . Biotechnol Bioeng 1998; 60: 61–69. [PubMed] [Google Scholar]
- 12. Ren Q, van Beilen JB, Sierro N, Zinn M, Kessler B, Witholt B. Expression of PHA polymerase genes of Pseudomonas putida in Escherichia coli and its effect on PHA formation. Antonie Van Leeuwenhoek 2005; 87: 91–100. [DOI] [PubMed] [Google Scholar]
- 13. Matsumoto K, Tanaka Y, Watanabe T, Motohashi R, Ikeda K, Tobitani K, et al. Directed evolution and structural analysis of NADPH-dependent Acetoacetyl Coenzyme A (Acetoacetyl-CoA) reductase from Ralstonia eutropha reveals two mutations responsible for enhanced kinetics. Appl Environ Microbiol 2013; 79: 6134–6139. 10.1128/AEM.01768-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shozui F, Sun J, Song Y, Yamada M, Sakai K, Matsumoto K, et al. A new beneficial mutation in Pseudomonas sp. 61–3 polyhydroxyalkanoate (PHA) synthase for enhanced cellular content of 3-hydroxybutyrate-based PHA explored using its enzyme homolog as a mutation template. Biosci Biotechnol Biochem 2010; 74: 1710–1712. [DOI] [PubMed] [Google Scholar]
- 15. Taguchi S, Doi Y. Evolution of polyhydroxyalkanoate (PHA) production system by "enzyme evolution": successful case studies of directed evolution. Macromol Biosci 2004; 4: 146–156. [DOI] [PubMed] [Google Scholar]
- 16. Yamada M, Matsumoto K, Shimizu K, Uramoto S, Nakai T, Shozui F, et al. Adjustable mutations in lactate (LA)-polymerizing enzyme for the microbial production of LA-based polyesters with tailor-made monomer composition. Biomacromolecules 2010; 11: 815–819. 10.1021/bm901437z [DOI] [PubMed] [Google Scholar]
- 17. Nduko JM, Matsumoto K, Ooi T, Taguchi S. Effectiveness of xylose utilization for high yield production of lactate-enriched P(lactate-co-3-hydroxybutyrate) using a lactate-overproducing strain of Escherichia coli and an evolved lactate-polymerizing enzyme. Metab Eng 2013; 15: 159–166. 10.1016/j.ymben.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 18. Lee TH, Chang YK, Chung BH, Park YH. Correlation of redox potential with state variables in cultures under controlled dissolved oxygen concentration and pH. Biotechnol Prog 1998; 14: 959–962. [DOI] [PubMed] [Google Scholar]
- 19. Zinn M, Durner R, Zinn H, Ren Q, Egli T, Witholt B. Growth and accumulation dynamics of poly(3-hydroxyalkanoate) (PHA) in Pseudomonas putida GPo1 cultivated in continuous culture under transient feed conditions. Biotechnol J 2011; 6: 1240–1252. 10.1002/biot.201100219 [DOI] [PubMed] [Google Scholar]
- 20.Kadoya R, Kodama Y, Matsumoto K, Taguchi S. Enhanced cellular content and lactate fraction of the poly(lactate-co-3-hydroxybutyrate) polyester produced in recombinant Escherichia coli by the deletion of sigma factor RpoN. J Biosci Bioeng 2014. [DOI] [PubMed]
- 21. Zhao K, Liu M, Burgess RR. Promoter and regulon analysis of nitrogen assimilation factor, sigma54, reveal alternative strategy for E. coli MG1655 flagellar biosynthesis. Nucleic Acids Res 2010; 38: 1273–1283. 10.1093/nar/gkp1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derouaux A, Wolf B, Fraipont C, Breukink E, Nguyen-Disteche M, Terrak M. The monofunctional glycosyltransferase of Escherichia coli localizes to the cell division site and interacts with penicillin-binding protein 3, FtsW, and FtsN. J Bacteriol 2008; 190: 1831–1834. 10.1128/JB.01377-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Berardino M, Dijkstra A, Stuber D, Keck W, Gubler M. The monofunctional glycosyltransferase of Escherichia coli is a member of a new class of peptidoglycan-synthesising enzymes. FEBS Lett 1996; 392: 184–188. [DOI] [PubMed] [Google Scholar]
- 24. Hara H, Suzuki H. A novel glycan polymerase that synthesizes uncross-linked peptidoglycan in Escherichia coli . FEBS Lett 1984; 168: 155–160. [DOI] [PubMed] [Google Scholar]
- 25. Elsden SR, Gilchrist FM, Lewis D, Volcani BE. Properties of a fatty acid forming organism isolated from the rumen of sheep. J Bacteriol 1956; 72: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsusaki H, Manji S, Taguchi K, Kato M, Fukui T, Doi Y. Cloning and molecular analysis of the Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61–3. J Bacteriol 1998; 180: 6459–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peoples OP, Sinskey AJ. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem 1989; 264: 15293–15297. [PubMed] [Google Scholar]
- 28. Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci U S A 2008; 105: 17323–17327. 10.1073/pnas.0805653105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Som T, Tomizawa J. Origin of replication of Escherichia coli plasmid RSF 1030. Mol Gen Genet 1982; 187: 375–383. [DOI] [PubMed] [Google Scholar]
- 30. Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, et al. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 2005; 12: 291–299. [DOI] [PubMed] [Google Scholar]
- 31. de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol 1990; 172: 6568–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nduko JM, Matsumoto K, Ooi T, Taguchi S. Enhanced production of poly(lactate-co-3-hydroxybutyrate) from xylose in engineered Escherichia coli overexpressing a galactitol transporter. Appl Microbiol Biotechnol 2014; 98: 2453–2460. 10.1007/s00253-013-5401-0 [DOI] [PubMed] [Google Scholar]
- 33. Kichise T, Taguchi S, Doi Y. Enhanced accumulation and changed monomer composition in polyhydroxyalkanoate (PHA) copolyester by in vitro evolution of Aeromonas caviae PHA synthase. Appl Environ Microbiol 2002; 68: 2411–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbüchel A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol 1999; 171: 73–80. [DOI] [PubMed] [Google Scholar]
- 35. Matsumoto K, Okei T, Honma I, Ooi T, Aoki H, Taguchi S. Efficient (R)-3-hydroxybutyrate production using acetyl CoA-regenerating pathway catalyzed by coenzyme A transferase. Appl Microbiol Biotechnol 2013; 97: 205–210. 10.1007/s00253-012-4104-2 [DOI] [PubMed] [Google Scholar]
- 36. Sambrook JRD. Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 37. Washio K, Lim SP, Roongsawang N, Morikawa M. Identification and characterization of the genes responsible for the production of the cyclic lipopeptide arthrofactin by Pseudomonas sp. MIS38. Biosci Biotechnol Biochem 2010; 74: 992–999. [DOI] [PubMed] [Google Scholar]
- 38. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2006; 2: 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 2000; 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vollmer W, Bertsche U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli . Biochim Biophys Acta 2008; 1778: 1714–1734. [DOI] [PubMed] [Google Scholar]
- 41. Popham DL, Young KD. Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr Opin Microbiol 2003; 6: 594–599. [DOI] [PubMed] [Google Scholar]
- 42. Schiffer G, Holtje JV. Cloning and characterization of PBP 1C, a third member of the multimodular class A penicillin-binding proteins of Escherichia coli . J Biol Chem 1999; 274: 32031–32039. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Wu H, Jiang X, Chen GQ. Engineering Escherichia coli for enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metab Eng 2014; 25: 183–193. 10.1016/j.ymben.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 44. Dmitriev B, Toukach F, Ehlers S. Towards a comprehensive view of the bacterial cell wall. Trends Microbiol 2005; 13: 569–574. [DOI] [PubMed] [Google Scholar]
- 45. Eraso JM, Margolin W. Bacterial cell wall: thinking globally, actin locally. Curr Biol 2011; 21: R628–630. 10.1016/j.cub.2011.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang H, Si F, Margolin W, Sun SX. Mechanical control of bacterial cell shape. Biophys J 2011; 101: 327–335. 10.1016/j.bpj.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meroueh SO, Bencze KZ, Hesek D, Lee M, Fisher JF, Stemmler TL, et al. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc Natl Acad Sci U S A 2006; 103: 4404–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kadoya R, Baek JH, Sarker A, Chattoraj DK. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J Bacteriol 2011; 193: 1504–1514. 10.1128/JB.01067-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 1985; 33: 103–119. [DOI] [PubMed] [Google Scholar]
- 50. Takase K, Taguchi S, Doi Y. Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the type II polyhydroxyalkanoate synthase gene. J Biochem 2003; 133: 139–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were grown on LB medium at 30°C for 48 h. Proteins in culture medium was concentrated with acetone and applied to SDS-PAGE. M, size marker. 1, BW25113. 2. JW3175. There was no significant difference in protein level in the medium, indicating that no cell lysis was promoted by mtgA deletion.
(EPS)
E. coli BW25113 (wild-type) harboring pGEMphaC1 Ps(ST/QK)AB grown on LB medium containing glucose (A). E. coli JW3725 (ΔmtgA) harboring pGEMphaC1 Ps(ST/QK)AB grown on LB medium containing glucose (B). The cells produced P(3HB) under the presence of glucose. Scale bar = 5 μm.
(EPS)
All strains were grown in 1.7 ml of LB medium containing 20 g/l of glucose at 30°C for 48 h with reciprocal shaking at 180 rpm. The data represent the average ± standard deviation of three independent trials.
(DOCX)
E. coli BW25113 (wild type) and JW3175 (ΔmtgA) harboring pGEMphaC1 Ps(ST/QK)AB [50] were grown on LB medium containing 20 g/l of glucose at 30°C for 48 h with reciprocal shaking at 180 rpm. The data represent the average ± standard deviation of three independent trials.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.