Abstract
Background
Evidence suggests an etiologic role for inflammation in ovarian carcinogenesis and heterogeneity between tumor subtypes and anthropometric indices. Prospective studies on circulating inflammatory markers and epithelial invasive ovarian cancer (EOC) have predominantly investigated overall risk; data characterizing risk by tumor characteristics (histology, grade, stage, dualistic model of ovarian carcinogenesis) and anthropometric indices are sparse.
Methods
We conducted a nested case-control study in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort to evaluate C-reactive protein (CRP), interleukin-6 (IL-6), and EOC risk by tumor characteristics. A total of 754 eligible EOC cases were identified; two controls (n=1,497) were matched per case. We used multivariable conditional logistic regression to assess associations.
Results
CRP and IL-6 were not associated with overall EOC risk. However, consistent with prior research, CRP >10 vs. CRP ≤1 mg/L was associated with higher overall EOC risk (OR=1.67 [1.03 - 2.70]). We did not observe significant associations or heterogeneity in analyses by tumor characteristics. In analyses stratified by waist circumference, inflammatory markers were associated with higher risk among women with higher waist circumference; no association was observed for women with normal waist circumference: (e.g., IL-6: waist ≤80: ORlog2=0.97 [0.81 - 1.16]; waist >88: ORlog2=1.78 [1.28 - 2.48], pheterogeneity ≤0.01).
Conclusions
Our data suggest that high CRP is associated with increased risk of overall EOC, and that IL-6 and CRP may be associated with EOC risk among women with higher adiposity.
Impact
Our data add to global evidence that ovarian carcinogenesis may be promoted by an inflammatory milieu.
Keywords: ovarian cancer, histological subtypes, obesity, inflammation, type I / type II model
Introduction
A role for inflammation in ovarian carcinogenesis was first proposed in the ‘incessant ovulation theory’ (1). The rupture of the ovarian surface epithelium induces an inflammatory reaction (1) leading to cell damage and proliferation, and enhanced potential for aberrant DNA repair, inactivation of tumor-suppressor genes, and subsequent mutagenesis (2). Chronic diseases of the female reproductive tract, including endometriosis (3), polycystic ovary syndrome (4) and pelvic inflammatory disease (5) are associated with inflammation and have been suggested as epithelial invasive ovarian cancer (EOC) risk factors. Adiposity contributes to chronic inflammation (6-10), and abdominal adiposity (11, 12), has been associated with increased EOC risk, though prior findings are not entirely consistent [(13, 14)].
C-reactive protein (CRP) is a systemic marker of inflammation, and epidemiological evidence consistently supports an association between elevated CRP and risk of epithelial cancers [e.g., breast, endometrium, liver, lung, colon; (15-18)]. It is unclear whether this association is causal, or due to factors related to both circulating CRP concentrations and cancer risk (19). CRP synthesis in the liver is triggered by interleukin-6 (IL-6), a pro-inflammatory cytokine (20). IL-6 increases cell proliferation and hinders apoptosis in human epithelial breast, colon and prostate cell lines (20). In human ovarian cancer cells IL-6 signaling was shown to regulate proliferation, adhesion and invasion (21).
EOC is a heterogeneous disease and associations of inflammatory markers with disease risk may differ between (1) histological subtypes of EOC (serous, mucinous, endometrioid and clear cell carcinomas) and (2) tumors defined by the dualistic model of carcinogenesis [type I and type II tumors; (22-24)]. Under the dualistic model, type I tumors are defined as low-grade serous and endometrioid, mucinous, malignant Brenner and clear cell tumors, while type II tumors include high-grade serous and endometrioid tumors, undifferentiated or mesodermal mixed tumors (23). We hypothesized that inflammation may be most strongly associated with tumors of serous histology, which may arise in the fimbriae of the fallopian tube (25) and may be induced by chronic intra-tubal inflammation (23, 26). Additionally, we hypothesized these associations may differ between low- and high-grade serous tumors, and, thus, between type I and II tumors, as marked biological differences between these subtypes have been reported (26) and high-grade serous tumors have been linked to inflammatory agents [e.g., menstrual cytokines; (26)]. Finally, prior epidemiological studies have reported a positive association between inflammatory markers and breast cancer risk among women with excess adiposity (18). We hypothesized similar results for EOC, and that higher circulating inflammatory markers among women with higher adiposity at blood donation would be at increased risk.
Data on the association between inflammatory markers and EOC are sparse and previous studies had limited sample size [range: 149 cases (27) - 376 cases (28)]. Circulating CRP concentrations have been consistently associated with increased risk of EOC (27, 29-31); while prior prospective studies on IL-6 and EOC risk provide conflicting results (27, 28, 32). To our knowledge, one single study has investigated inflammatory markers and EOC risk by serous vs. non-serous histology (27) and no study has investigated inflammation and EOC across tumor characteristics; the dualistic model of ovarian carcinogenesis or by anthropometric indices. Thus, we evaluated associations between CRP and IL-6 with risk of EOC in the largest single study to date including 754 cases and 1,497 controls nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
Materials and Methods
The EPIC cohort
EPIC is a multicenter prospective cohort study. Descriptions of study design, population and baseline data collection have been reported in detail (33). In brief, 521,330 participants (367,903 women) aged 25 to 70 years were enrolled from 1992 - 2000 in 23 centers in 10 European countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
Data on diet, reproductive and menstrual factors, past and current use of exogenous hormones (oral contraceptives (OC) and hormone replacement therapy (HRT)), disease history, smoking, and anthropometric measures were collected at baseline.
Anthropometric indices (height (cm), weight (kg), as well as waist and hip circumferences (cm)) and body mass index (BMI; kg/m2) were measured according to standardized procedures, except for the Oxford cohort and part of the French cohort, where height, weight, and body circumferences were predominantly self-reported. For participants from the Oxford cohort where only self-reported data were available, linear regression models were used to recalibrate values using age-specific measurements from subjects with both measured and self-reported body measures. Waist circumference was measured either at the midpoint between the lower ribs and iliac crest or at the narrowest torso circumference or a combination of the methods. Hip circumference (HC) was measured over the buttocks. In Umeå (Sweden) and Norway waist and HC data is not available. The waist-to-hip ratio (WHR) was calculated by dividing waist circumference by HC.
All subjects gave informed consent. The Ethical Review Board of the International Agency for Research on Cancer and the Institutional Review Board of each center approved the study.
Blood sample collection and storage
A total of 226,673 women provided a baseline blood sample. Details of blood sample processing have been published previously (33). Briefly, for each participant 30 mL of blood was drawn, and after centrifugation blood fractions (serum, plasma, buffy coat, and red blood cells) were aliquoted in 28 plastic straws, which were heat sealed and stored. For all centers except Sweden and Denmark, samples are stored under liquid nitrogen (−196 C). Samples from Sweden are stored locally at −70 C; samples from Denmark are locally stored in 1 mL tubes in liquid nitrogen vapor (−150 C).
Determination of menopausal status and phase of menstrual cycle at blood donation
Women were considered premenopausal if they reported regular menstrual cycles over the 12 months prior to blood donation. If this information was missing, women were considered to be premenopausal if they were less than 42 years at recruitment. Women were classified as postmenopausal if they reported not having any menses over the past 12 months or at >55 years of age. Women were classified as peri-menopausal/having unknown menopausal status, if they were between 42 and 55 years of age and had missing or incomplete questionnaire data, or reported irregular menstrual cycles in the past 12 months or a previous hysterectomy (without oophorectomy).
Determination of menstrual cycle phase in premenopausal women in EPIC has been reported (34). Two different dating methods were used: ‘forward’ dating counted forward from the woman’s reported date of the start of her last menses, whereas ‘backward’ dating counted backward from the date of the start of her next menses after blood donation. Backward dating method was used to determine menstrual cycle phase when available, as it is more accurate (35).
Follow-up for cancer incidence and vital status
In all countries except of France, Germany and Greece, follow-up was based on record linkage with cancer and pathology registries and the end of follow-up was the date of last complete follow-up for both cancer incidence and vital status, which ranged between 2003 and 2006, depending on center. In France, Germany and Greece, participant follow-up and cancer outcome was verified with health insurance records, cancer and pathology registries and active follow-up with study participants and their next of kin. Vital status was collected from mortality registries at the regional/national level, which was combined with health insurance data or data collected by active follow-up. End of follow-up for these centers was the last contact, date of diagnosis, or date of death, whichever occurred first. The end of follow-up for these centers ranged from 2005 (France) to 2008 (Germany).
Selection of case and control subjects
We excluded women with cancer prior to recruitment (n=19,707), incomplete follow-up data (n=2,209), lifestyle (n=526), or diet (n=2,713), and women with bilateral oophorectomy at baseline (n=10,500). A total of 344,754 women were evaluated for eligibility. Participants who donated a blood sample and had data on exogenous hormone use at blood donation were eligible (n=183,257). Cases were identified using the International Classification of Diseases for Oncology (ICD) 0-3 codes. Eligible cases were women diagnosed with incident invasive epithelial ovarian (ICD-O-3: C569), fallopian tube (C570) or primary peritoneal (C480, C481, C482, C488) cancer, and with data on tumor histology.
Up to 2 controls for each case were randomly selected among appropriate risk sets including all female cohort members with a blood sample, alive and free of cancer at the time of diagnosis of the index case. An incidence density sampling protocol was used, such that controls could include subjects who became a case later in time and each control could be sampled more than once. Cases and controls were matched on: center, age at blood donation (+/-6 months), time of the day of blood collection (+/− 1 hour), fasting status (<3 hours, 3–6 hours, >6 hours); exogenous hormone use at blood donation (no/yes), and menstrual cycle phase for premenopausal women (‘early follicular’ (days 0-7 of the cycle), ‘late follicular’ (days 8-11), ‘periovulatory’ (days 12-16), ‘midluteal’ (days 20-24), and ‘other luteal’ (days 17-19 or days 25-40)). Cases missing data on phase of menstrual cycle were matched to controls missing information on menstrual cycle phase.
A total of 754 eligible incident invasive cases were identified (699 ovarian, 31 fallopian tube and 24 primary peritoneal tumors) with 1,497 matched controls (743 complete sets: 1 case, 2 controls). Information on tumor characteristics was available from pathology reports and from cancer registries. A total of 56% of tumors were of serous histology (n=423), 17% not otherwise specified (NOS) (n=128), 12% endometrioid (n=87), 7% mucinous (n=51), 4% clear cell (n=33) and 4% other (malignant neoplasms, carcinoma, mixed Mullerian, mixed mesodermal or malignant Brenner tumors; n=32). Information on grade was 58% complete; stage data was 88% complete.
We additionally classified tumors based on the dualistic pathway of ovarian carcinogenesis as defined by Kurman et al. (23). Type I tumors (n=81) include low-grade (well-differentiated) serous (n=16) and endometrioid (n=11), mucinous (n=51) or malignant Brenner (n=3) tumors. Type II tumors (n=316) include high-grade (moderately, poorly or undifferentiated tumors) serous (n=260) and endometrioid (n=52), malignant mixed mesodermal (carcinosarcoma, n=2 and undifferentiated, n=2) tumors. Serous and endometrioid cases missing information on grade (serous n=147, endometrioid n=24) were excluded from type I/II analyses, as were clear cell carcinomas (n=33), as they demonstrate features of both type I and type II tumors (36).
Laboratory assays
Pre-diagnostic circulating concentrations of CRP (mg/L) and IL-6 (pg/ml) for cases and matched controls were analyzed within the same analytical batch. Laboratory technicians were blinded to case-control and quality control status.
Laboratory assays were conducted at the laboratory of the Division of Cancer Epidemiology at the German Cancer Research Center. CRP concentrations were quantified using a high sensitivity immunoassay and IL-6 concentrations were quantified using a high sensitivity quantitative sandwich enzyme immunoassay (R&D Systems Inc., Minneapolis, USA). The intra-assay coefficient of variations (CV) from duplicate quality control samples were 10.9% for CRP and 3.9% for IL-6. Inter-assay CVs were 19.2% for CRP and 10.4% for IL-6.
Statistical analyses
Biomarker concentrations were log2 transformed; a one-unit increase in log2-transformed biomarker corresponds to a doubling of concentration. Case and control differences across baseline characteristics were assessed using conditional logistic regression. We used Spearman coefficients (r) adjusted for EPIC recruitment center, age at blood donation and menopausal status at blood donation to assess correlations between inflammatory markers, BMI, waist circumference, and WHR among controls.
Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated using conditional logistic regression models. The risk associated with biomarker concentrations was examined in tertiles, based on the distribution in study controls. Tests for trend were assessed using the tertile medians. A meta-analysis of previous nested-case control studies showed significantly increased risk among women with CRP concentrations above 10 mg/L (28); thus, we additionally evaluated risk using this cut point.
Covariates changing the OR by more than 10% (i.e., by a factor 1.10 or its reciprocal) were included in the final multivariable models (37); ever full-term pregnancy (never/ever), age at first birth (continuous) and BMI (continuous) met this criteria. Missing values (≤3.7 %) were accounted for by creating a separate category for categorical variables. Age at first birth was centered at the median for parous women and a value of zero was assigned for nulliparous women.
Potential confounders evaluated but not included in the final models were: age at menarche (continuous), age at menopause (continuous), age at first pregnancy (continuous), number of full-term pregnancies (0,1,2,≥ 3), OC use (never / past / current) duration of OC use (<5, 5-10, >10 years), HRT use (never / past / current), duration of HRT use (<5, 5-10, >10 years), smoking (never / past / current), average alcohol consumption at baseline (0, ≤3, 4-19, and >19 g/d), physical activity (active, moderately active, moderately inactive, and inactive) and height (cm, continuous).
We conducted analyses stratified by BMI (<25/25-30/>30); waist circumference (<80/80-88/>88) and waist-hip ratio (<0.85/≥0.85) using World Health Organization (WHO) cut off points. We additionally controlled for BMI as a continuous variable in these models. We did not additionally adjust for waist circumference, as results were similar when waist circumference was included in the final models with BMI. We conducted analyses stratified by menopausal status and HRT use at blood donation and age at diagnosis (age ≤55 and >55 years). In analyses stratified by menopausal status at blood collection we combined postmenopausal and perimenopausal women as circulating concentrations of CRP and IL-6 do not vary by menopausal status (38). Heterogeneity was assessed using likelihood-ratio tests for the comparison of the model fit for logistic regression models with and without corresponding interaction terms (39).
Sensitivity analyses included mutual adjustment (i.e., CRP adjusted for IL-6, and vice versa), and exclusion of women providing a blood sample <2 years prior to diagnosis, women with hysterectomy (n=210) or tubal ligation (n=58).
Inflammatory marker concentrations above the upper limit of detection (ULD) were set to the ULD (CRP n=42; 13.52 mg/L; IL-6: n=15, 10.00 pg/ml; highest value from the assay’s standard curve)). We assigned a value equal to the midpoint between zero and the lower limit of detection (LLD) for CRP and IL-6 (CRP n=84; 1.789 mg/L; IL-6: n=35, 0.078 pg/ml), as <5% were below the LLD. Outlying values, as detected with the extreme studentized deviate test (CRP: n=11; IL-6: n=5 (40)), were retained as risk estimates were comparable after excluding these values.
All statistical tests were two-tailed and significant at the p<0.05 level. SAS v. 9.2 (SAS Institute Inc., Cary, NC, USA) was utilized for all statistical analyses.
Results
We observed expected differences between cases and controls for parity (p≤0.01), ever OC use (p≤0.01) and duration of OC use (p=0.03) (Table 1). Mean age at blood donation for cases was 56.6 years (range: 33.2 – 80.7 years) with a median age at diagnosis of 63.1 years (range: 34.6 – 86.5 years) and an average of 6.4 years (range: 0.1 - 16 years) between blood collection and diagnosis. We observed no case-control differences for circulating CRP overall or by tumor characteristics (data not shown). Geometric means of IL-6 concentrations were higher in cases relative to their matched controls (p≤0.01). The correlation of circulating CRP with IL-6 concentrations in controls (r= 0.38; p≤ 0.01). BMI correlated most strongly with CRP (r= 0.33; p≤ 0.01), while the strongest correlation for IL-6 was observed with waist circumference (r = 0.30; p≤ 0.01; data not shown). Case characteristics at diagnosis are presented in Table 2.
Table 1.
Baseline characteristics of EOC cases and matched controls at enrolment in the EPIC study [median (range) or number (percentage)]*
| cases (n = 754) | controls (n=1,497) | Pdifference | |
|---|---|---|---|
| Age at blood donationa | 56.6 (33.2-80.7) | 56.5 (33.1-79.3) | |
| Age at diagnosis | 63.1 (34.6-86.5) | ||
| Lagtime | 6.4 (0-16) | ||
| Menopausal statusa | |||
| Pre | 118 (16%) | 234 (16%) | |
| Post | 636 (84%) | 1,263 (84%) | |
| Age at menarche | 13 (9-20) | 13 (8-20) | 0.39 |
| Age at menopauseb | 50 (32-63) | 50 (30-63) | 0.05 |
| Ever fullterm pregnancy | ≤0.01 | ||
| No | 124 (17%) | 163 (11%) | |
| Yes | 603 (83%) | 1,277 (89%) | |
| Age at first full-term pregnancy | 24 (14-40) | 24 (16-45) | 0.51 |
| Number of full-term pregnancies | ≤0.01 | ||
| 0 | 124 (17%) | 163 (11%) | |
| 1 | 111 (16%) | 221 (16%) | |
| 2 | 290 (41%) | 609 (43%) | |
| ≥ 3 | 188 (26%) | 420 (30%) | |
| Ever OC use | ≤0.01 | ||
| Never | 421 (56%) | 748 (50%) | |
| Past | 323 (43%) | 710 (47%) | |
| Current | 10 (1%) | 32 (2%) | |
| Duration of OC use (years) | 6 (1-25) | 5 (1-25) | 0.03 |
| Ever HRT use | 0.19 | ||
| Never | 523 (69%) | 1,024 (69%) | |
| Past | 72 (10%) | 140 (9%) | |
| Current | 158 (21%) | 326 (22%) | |
| Duration of HRT use (years) | 3.0 (0.1-20) | 4.0 (0.1-27) | 0.10 |
| BMI (kg/m2) | 25.1 (17.2-45.4) | 25.0 (14.9-50.6) | 0.09 |
| Waist circumference (cm) | 80.5 (59.0 – 136.0) | 79.0 (54.5-126.00) | 0.05 |
| WHR | 0.80 (0.60-1.10) | 0.80 (0.50-1.40) | 0.76 |
| Smoking | 0.15 | ||
| Never | 408 (55%) | 854 (58%) | |
| Past | 174 (23%) | 342 (23%) | |
| Current | 164 (22%) | 289 (19%) | |
| Alcohol (g/day) | 0.89 | ||
| 0 | 125 (17%) | 275 (18%) | |
| ≤ 3 | 259 (34%) | 432 (29%) | |
| 4-19 | 269 (36%) | 628 (42%) | |
| > 19 | 101 (13%) | 161 (11%) | |
| Physical activity | 0.78 | ||
| ..Inactive | 184 (26%) | 377 (26%) | |
| Moderately inactive | 260 (36%) | 510 (36%) | |
| Moderately active | 151 (21%) | 295 (21%) | |
| Active | 120 (17%) | 241 (17%) | |
| Height (cm) | 161.5 (144-184) | 161.5 (137-186) | 0.14 |
| Biomarkers | |||
| CRP (mg/L) c | 6.63 (5.28-8.32) | 5.76 (4.89-6.79) | 0.66 |
| IL-6 (pg/ml) c | 3.44 (3.18-3.71) | 2.94 (2.79-3.11) | ≤0.01 |
Differences between cases and matched controls are based on conditional logistic regression.
Matching factor.
Among postmenopausal women only.
Biomarker concentrations between cases and matched controls are presented as geometric means (95% confidence intervals).
Table 2.
Selected tumor characteristics of EOC cases [number (percentage)] in the EPIC cohort
| Tumor Characteristics | |
|---|---|
| Histology | |
| Serous | 423 (56%) |
| Mucinous | 51 (7%) |
| Endometrioid | 87 (12%) |
| Clear cell | 33 (4%) |
| NOS | 128 (17%) |
| Other | 32 (4%) |
| Grade | |
| Low grade (G1) | 41 (9%) |
| High grade (G2/G3) | 399 (91%) |
| Stage | |
| Low stage (S1) | 103 (15%) |
| High stage (S2/S3) | 563 (85%) |
| Type I / Type II | |
| Type I | 81 (20%) |
| Type II | 316 (80%) |
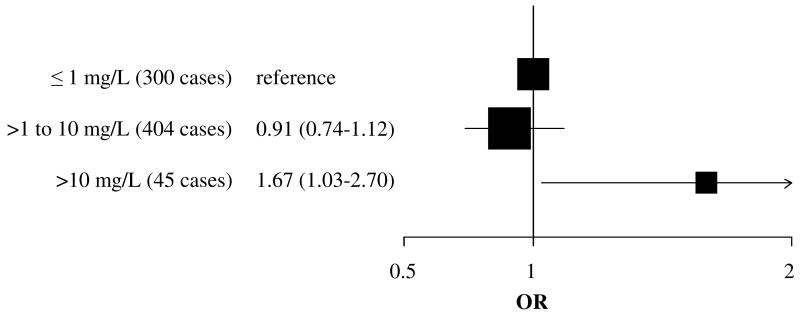
We did not observe an association between CRP examined continuously or in tertiles and overall EOC risk (e.g., CRP: ORlog2=0.99 (95% confidence interval) [0.93 - 1.05] Table 3). We did not observe significant heterogeneity in analyses by tumor histology (across histologic subtypes, phet=0.05; serous vs. non-serous, phet=0.08 [data not shown]) or tumor characteristics (e.g., grade phet=0.57; Table 3). However, higher circulating CRP was inversely associated with risk of type II EOC (ORQ3-Q1=0.66 [0.45-0.97], ptrend=0.06; case n=312; type I vs II phet=0.45). CRP concentrations >10 versus ≤1 mg/L were associated with significantly increased risk of EOC (OR=1.67 [1.03–2.70]; case n =41; Figure 1). Results were similar in exploratory analyses by subgroups (e.g., serous (>10 mg/L case n=16), OR=1.45 [0.71-2.99]; non-serous (>10 mg/L case n=25), OR=1.92 [0.98-3.75]; phet=0.11).
Table 3.
Odds ratios (95% CI) for ovarian cancer by tertile concentrations and for doubling in CRP by tumor characteristics
| Tertiles | ORlog2 (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Ptrend1 | phet2 | |||
| Overall | (738 sets) | ||||||
| Unadjusted* | ref. | 0.92 (0.74-1.14) | 0.96 (0.77-1.21) | 0.74 | 1.01 (0.96 - 1.07) | ||
| Multivariable** | ref. | 0.88 (0.70-1.10) | 0.87 (0.68-1.11) | 0.26 | 0.99 (0.93 - 1.05) | ||
| Histology | |||||||
| Serous | (411 sets) | ||||||
| Unadjusted* | ref. | 0.87 (0.65-1.16) | 0.79 (0.58-1.08) | 0.14 | 0.97 (0.90 - 1.05) | ||
| Multivariable** | ref. | 0.85 (0.63-1.13) | 0.76 (0.55-1.06) | 0.10 | 0.97 (0.89 - 1.05) | ||
| Mucinous | (48 sets) | ||||||
| Unadjusted* | ref. | 0.50 (0.18-1.35) | 0.98 (0.43-2.25) | 0.99 | 1.02 (0.84 - 1.25) | ||
| Multivariable** | ref. | 0.53 (0.19-1.50) | 0.84 (0.34-2.08) | 0.70 | 0.99 (0.80 - 1.21) | ||
| Endometrioid | (87 sets) | ||||||
| Unadjusted* | ref. | 0.81 (0.43-1.55) | 1.26 (0.66-2.41) | 0.49 | 1.09 (0.93 - 1.28) | ||
| Multivariable** | ref. | 0.78 (0.40-1.56) | 1.07 (0.52-2.20) | 0.83 | 1.07 (0.88 - 1.29) | ||
| Clear cell | (33 sets) | ||||||
| Unadjusted* | ref. | 1.63 (0.53-5.00) | 0.99 (0.36-2.68) | 0.93 | 1.00 (0.79 - 1.26) | ||
| Multivariable** | ref. | 1.69 (0.46-6.20) | 0.82 (0.25-2.69) | 0.71 | 0.91 (0.68 - 1.23) | ||
| NOS | (127 sets) | ||||||
| Unadjusted* | ref. | 1.45 (0.83-2.55) | 1.67 (0.95-2.92) | 0.08 | 1.09 (0.95 - 1.25) | ||
| Multivariable** | ref. | 1.29 (0.72-2.33) | 1.39 (0.75-2.55) | 0.31 | 1.03 (0.88 - 1.19) | ||
| Other | (32 sets) | ||||||
| Unadjusted* | ref. | 0.67 (0.23-1.92) | 0.54 (0.16-1.75) | 0.30 | 0.99 (0.69 - 1.42) | 0.04* | |
| Multivariable** | ref. | 0.66 (0.22-2.02) | 0.59 (0.16-2.15) | 0.40 | 1.06 (0.71 - 1.58) | 0.05** | |
| Grade | |||||||
| Low Grade | (40 sets) | ||||||
| Unadjusted* | ref. | 0.43 (0.16-1.22) | 1.18 (0.47-2.97) | 0.68 | 1.09 (0.84 - 1.41) | ||
| Multivariable** | ref. | 0.41 (0.14-1.19) | 0.97 (0.35-2.69) | 0.93 | 1.01 (0.75 - 1.36) | ||
| High Grade | (390 sets) | ||||||
| Unadjusted* | ref. | 0.80 (0.60-1.07) | 0.82 (0.60-1.12) | 0.20 | 0.97 (0.90 - 1.04) | 0.53* | |
| Multivariable** | ref. | 0.76 (0.56-1.03) | 0.74 (0.53-1.04) | 0.11 | 0.95 (0.87 - 1.03) | 0.57** | |
| Stage | |||||||
| Low Stage | (100 sets) | ||||||
| Unadjusted* | ref. | 1.16 (0.66-2.05) | 1.25 (0.68-2.27) | 0.46 | 1.09 (0.94 - 1.28) | ||
| Multivariable** | ref. | 1.00 (0.54-1.84) | 1.03 (0.53-1.98) | 0.94 | 1.06 (0.90 - 1.25) | ||
| High Stage | (553 sets) | ||||||
| Unadjusted* | ref. | 0.91 (0.71-1.18) | 0.95 (0.73-1.24) | 0.49 | 1.00 (0.94 - 1.07) | 0.33* | |
| Multivariable** | ref. | 0.88 (0.68-1.15) | 0.87 (0.66-1.15) | 0.90 | 0.98 (0.91 - 1.05) | 0.30** | |
| Type I / Type II | |||||||
| Type I | (77 sets) | ||||||
| Unadjusted* | ref. | 0.50 (0.24-1.04) | 0.98 (0.51-1.92) | 0.81 | 1.02 (0.86 - 1.20) | ||
| Multivariable** | ref. | 0.52 (0.25-1.10) | 0.89 (0.43-1.84) | 0.51 | 0.99 (0.83 - 1.18) | ||
| Type II | (308 sets) | ||||||
| Unadjusted* | ref. | 0.76 (0.55-1.05) | 0.73 (0.51-1.04) | 0.09 | 0.95 (0.87 - 1.03) | 0.46* | |
| Multivariable** | ref. | 0.72 (0.51-1.01) | 0.66 (0.45-0.97) | 0.06 | 0.93 (0.85 - 1.02) | 0.45** | |
Matched for study center, age at blood donation, menopausal status, time of the day of blood collection, fasting status, exogenous hormone use at blood donation and phase of the menstrual cycle
Additionally adjusted for BMI (continuous scale), ever full-term pregnancy (never/ever), age at first birth (continuous scale).
Linear trends based on the median CRP values for tertiles.
Statistical tests for heterogeneity were based on the likelihood-ratio test, comparing the model fit for logistic regression models with and without corresponding interaction term.
Tertile cut-offs: CRP (mg/L): first tertile 0.53 – 1.47; second tertile 1.48-4.01 third tertile: >4.01
Figure 1.
Odds ratios (95% CI) for epithelial invasive ovarian cancer among women with circulating CRP concentration >10 mg/L compared to women with CRP concentration ≤1mg/L at blood donation.
IL-6 was not associated with overall risk of EOC (e.g., IL-6: ORlog2=1.09 [0.97-1.21], Table 4), and we did not observe heterogeneity in the association across histology (across histological subtypes phet=0.07; serous vs. non-serous histology, phet=0.08 [data not shown]) or other tumor characteristics (e.g., grade phet=0.17). IL-6 was not associated with any EOC subgroup, with the exception of an inverse association with risk of low-grade tumors (ORQ3-Q1=0.22 [0.05-0.87], ptrend=0.22; case n=40; low vs. high-grade tumors phet=0.17).
Table 4.
Odds ratios (95% CI) for ovarian cancer by tertile concentrations and for doubling in IL-6 by tumor characteristics
| Tertiles | ORlog2 (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Ptrend1 | phet2 | |||
| Overall | (741 sets) | ||||||
| Unadjusted* | ref. | 0.94 (0.75-1.17) | 1.12 (0.89-1.41) | 0.34 | 1.11 (1.00 - 1.24) | ||
| Multivariable** | ref. | 0.90 (0.71-1.13) | 1.03 (0.81-1.32) | 0.79 | 1.09 (0.97 - 1.21) | ||
| Histology | |||||||
| Serous | (414 sets) | ||||||
| Unadjusted* | ref. | 0.78 (0.58-1.05) | 0.95 (0.69-1.31) | 0.71 | 1.01 (0.87 - 1.17) | ||
| Multivariable** | ref. | 0.75 (0.56-1.02) | 0.93 (0.67-1.30) | 0.62 | 1.02 (0.87 - 1.19) | ||
| Mucinous | (51 sets) | ||||||
| Unadjusted* | ref. | 1.12 (0.46-2.73) | 1.38 (0.58-3.27) | 0.46 | 1.29 (0.83 - 2.00) | ||
| Multivariable** | ref. | 0.92 (0.35-2.42) | 0.89 (0.33-2.44) | 0.82 | 1.01 (0.60 - 1.69) | ||
| Endometrioid | (86 sets) | ||||||
| Unadjusted* | ref. | 1.44 (0.73-2.83) | 1.58 (0.83-3.02) | 0.17 | 1.24 (0.90 - 1.70) | ||
| Multivariable** | ref. | 1.38 (0.69-2.75) | 1.44 (0.72-2.90) | 0.31 | 1.21 (0.86 - 1.70) | ||
| Clear cell | (33 sets) | ||||||
| Unadjusted* | ref. | 2.18 (0.65-7.24) | 1.39 (0.45-4.33) | 0.65 | 1.31 (0.81 - 2.12) | ||
| Multivariable** | ref. | 1.62 (0.46-5.64) | 0.99 (0.28-3.50) | 0.96 | 1.11 (0.64 - 1.90) | ||
| NOS | (126 sets) | ||||||
| Unadjusted* | ref. | 1.00 (0.57-1.77) | 1.15 (0.67-1.95) | 0.60 | 1.24 (0.98 - 1.57) | ||
| Multivariable** | ref. | 0.91 (0.51-1.64) | 0.92 (0.52-1.63) | 0.80 | 1.15 (0.90 - 1.47) | ||
| Other | (31 sets) | ||||||
| Unadjusted* | ref. | 1.05 (0.29-3.86) | 1.59 (0.50-5.08) | 0.35 | 1.15 (0.67 - 1.95) | 0.06* | |
| Multivariable** | ref. | 1.04 (0.23-4.73) | 1.37 (0.38-4.99) | 0.57 | 1.08 (0.58 - 2.02) | 0.07** | |
| Grade | |||||||
| Low Grade | (40 sets) | ||||||
| Unadjusted* | ref. | 0.47 (0.17-1.32) | 0.30 (0.09-1.04) | 0.36 | 0.64 (0.36 - 1.15) | ||
| Multivariable** | ref. | 0.39 (0.13-1.15) | 0.22 (0.05-0.87) | 0.22 | 0.52 (0.26 - 1.02) | ||
| High Grade | (391 sets) | ||||||
| Unadjusted* | ref. | 0.89 (0.65-1.21) | 1.10 (0.81-1.51) | 0.58 | 1.03 (0.89 - 1.21) | 0.17* | |
| Multivariable** | ref. | 0.85 (0.62-1.17) | 1.04 (0.75-1.44) | 0.67 | 1.01 (0.86 - 1.19) | 0.17** | |
| Stage | |||||||
| Low Stage | (103 sets) | ||||||
| Unadjusted* | ref. | 1.37 (0.75-2.52) | 1.15 (0.63-2.07) | 0.62 | 1.23 (0.93 - 1.63) | ||
| Multivariable** | ref. | 1.35 (0.72-2.54) | 1.07 (0.56-2.03) | 0.81 | 1.21 (0.89 - 1.64) | ||
| High Stage | (551 sets) | ||||||
| Unadjusted* | ref. | 0.87 (0.67-1.12) | 1.10 (0.84-1.44) | 0.49 | 1.07 (0.94 - 1.21) | 0.39* | |
| Multivariable** | ref. | 0.82 (0.63-1.07) | 1.02 (0.76-1.36) | 0.90 | 1.04 (0.91 - 1.19) | 0.39** | |
| Type I / Type II | |||||||
| Type I | (80 sets) | ||||||
| Unadjusted* | ref. | 0.85 (0.42-1.71) | 0.82 (0.40-1.67) | 0.88 | 0.94 (0.66 - 1.35) | ||
| Multivariable** | ref. | 0.68 (0.32-1.44) | 0.62 (0.28-1.39) | 0.47 | 0.79 (0.52 - 1.19) | ||
| Type II | (309 sets) | ||||||
| Unadjusted* | ref. | 0.85 (0.61-1.20) | 0.99 (0.69-1.41) | 0.91 | 0.95 (0.80 - 1.14) | 0.96* | |
| Multivariable** | ref. | 0.81 (0.57-1.15) | 0.95 (0.65-1.39) | 0.99 | 0.95 (0.78 - 1.14) | 0.94** | |
Matched for study center, age at blood donation, menopausal status, time of the day of blood collection, fasting status, exogenous hormone use at blood donation and phase of the menstrual cycle.
Additionally adjusted for BMI (continuous scale), ever full-term pregnancy (never/ever), age at first birth (continuous scale).
Linear trends based on the median IL-6 values for tertiles.
Statistical tests for heterogeneity were based on the likelihood-ratio test, comparing the model fit for logistic regression models with and without corresponding interaction term.
Tertile cut-offs: IL-6 (pg/mL): first tertile 0.78 – 1.26; second tertile 1.27-2.17 third tertile: >2.17.
We observed significant heterogeneity in the association between inflammatory markers and EOC risk in analyses stratified by anthropometric indices, with positive associations between CRP and IL-6 and EOC observed only among women with higher adiposity (Table 5). Higher CRP was associated with suggestively higher risk among obese women (BMI>30 kg/m2, 1.25 (0.96-1.62)), and not associated with risk among leaner women (BMI<25 kg/m2, 0.98 (0.88-1.08), phet=0.04). The same pattern was observed for IL-6 in results stratified by waist circumference (e.g., >88 cm: ORlog2=1.78 [1.28–2.48]; 80-88 cm: ORlog2=0.85 [0.66–1.11]; ≤80 cm: ORlog2=0.97 [0.81–1.16]; phet<0.01). We additionally assessed risk of serous, nonserous, and type II tumors in analyses stratified by anthropometric measures. Heterogeneity by anthropometric factors was observed consistently observed for serous tumors for both CRP and IL-6 (i.e., serous: BMI <25 vs ≥25: CRP: phet <0.01; IL-6: phet <0.01)).
Table 5.
Odds ratios (95% CI) for ovarian cancer for doubling in CRP and IL-6 by BMI categories and waist circumference*
| CRP | IL-6 | ||||
|---|---|---|---|---|---|
| ORlog2 (95% CI) | phet1 | ORlog2 (95% CI) | phet1 | ||
| BMI <25 | |||||
| All Sets | (345 sets) | 0.98 (0.88 - 1.08) | 1.06 (0.88 - 1.28) | ||
| Serous tumors | (216 sets) | 0.97 (0.84 - 1.11) | 0.89 (0.70 - 1.14) | ||
| Non-serous tumors | (135 sets) | 0.98 (0.84 - 1.13) | 1.36 (1.01 - 1.82) | ||
| Type II tumors | (151 sets) | 0.98 (0.83 - 1.15) | 0.72 (0.51 - 1.00) | ||
| BMI ≥25 | |||||
| All Sets | (363 sets) | 1.09 (0.98 - 1.20) | 0.12 | 1.12 (0.92 - 1.35) | 0.05 |
| Serous tumors | (187 sets) | 1.10 (0.96 - 1.26) | <0.01 | 1.13 (0.86 - 1.48) | <0.01 |
| Non-serous tumors | (182 sets) | 1.07 (0.93 - 1.24) | 0.26 | 1.10 (0.84 - 1.44) | 0.18 |
| Type II tumors | (146 sets) | 1.00 (0.86 - 1.16) | 0.26 | 1.15 (0.85 - 1.56) | 0.06 |
| BMI <25 | |||||
| All Sets | (345 sets) | 0.98 (0.88 - 1.08) | 1.06 (0.88 - 1.28) | ||
| Serous tumors | (216 sets) | 0.97 (0.84 - 1.11) | 0.89 (0.70 - 1.14) | ||
| Non-serous tumors | (135 sets) | 1.07 (0.93 - 1.24) | 1.36 (1.01 - 1.82) | ||
| Type II tumors | (151 sets) | 0.98 (0.83 - 1.15) | 0.72 (0.51 - 1.00) | ||
| BMI 25-30 | |||||
| All Sets | (232 sets) | 1.06 (0.94 - 1.19) | 1.02 (0.81 - 1.27) | ||
| Serous tumors | (114 sets) | 1.01 (0.86 - 1.19) | 0.98 (0.70 - 1.38) | ||
| Non-serous tumors | (122 sets) | 1.10 (0.93 - 1.31) | 1.04 (0.77 - 1.40) | ||
| Type II tumors | (94 sets) | 0.93 (0.78 - 1.10) | 1.06 (0.73 - 1.55) | ||
| BMI ≥30 | |||||
| All Sets | (131 sets) | 1.25 (0.96 - 1.62) | 0.04 | 1.35 (0.82 - 2.23) | 0.74 |
| Serous tumors | (73 sets) | 1.25 (0.85 - 1.84) | 0.05 | 1.28 (0.68 - 2.41) | 0.03 |
| Non-serous tumors | (60 sets) | 1.26 (0.87 - 1.81) | 0.81 | 1.50 (0.67 - 3.35) | 0.28 |
| Type II tumors | (52 sets) | 1.18 (0.78 - 1.77) | 0.33 | 1.30 (0.66 - 2.57) | 0.07 |
| Waist ≤88 | |||||
| All Sets | (507 sets) | 0.94 (0.87 - 1.01) | 0.94 (0.82 - 1.09) | ||
| Serous tumors | (293 sets) | 0.92 (0.83 - 1.02) | 0.84 (0.69 - 1.02) | ||
| Non-serous tumors | (222 sets) | 0.97 (0.86 - 1.08) | 1.08 (0.88 - 1.33) | ||
| Type II tumors | (216 sets) | 0.88 (0.78 - 0.99) | 0.77 (0.60 - 0.98) | ||
| Waist >88 | |||||
| All Sets | (179 sets) | 1.26 (1.06 - 1.49) | 0.19 | 1.78 (1.28 - 2.48) | <0.01 |
| Serous tumors | (97 sets) | 1.38 (1.07 - 1.79) | 0.05 | 2.14 (1.33 - 3.45) | <0.01 |
| Non-serous tumors | (86 sets) | 1.16 (0.92 - 1.47) | 0.63 | 1.46 (0.91 - 2.34) | 0.51 |
| Type II tumors | (67 sets) | 1.26 (0.95 - 1.66) | 0.44 | 2.10 (1.20 - 3.67) | 0.04 |
| Waist ≤80 | |||||
| All Sets | (341 sets) | 0.93 (0.85 - 1.03) | 0.97 (0.81 - 1.16) | ||
| Serous tumors | (205 sets) | 0.92 (0.81 - 1.06) | 0.87 (0.68 - 1.11) | ||
| Non-serous tumors | (142 sets) | 0.94 (0.82 - 1.08) | 1.11 (0.85 - 1.43) | ||
| Type II tumors | (154 sets) | 0.91 (0.79 - 1.05) | 0.84 (0.62 - 1.13) | ||
| Waist 80-88 | |||||
| All Sets | (167 sets) | 0.99 (0.87 - 1.14) | 0.85 (0.66 - 1.11) | ||
| Serous tumors | (88 sets) | 0.93 (0.78 - 1.11) | 0.73 (0.49 - 1.08) | ||
| Non-serous tumors | (80 sets) | 1.11 (0.90 - 1.36 | 0.97 (0.67 - 1.42) | ||
| Type II tumors | (62 sets) | 0.87 (0.71 - 1.08) | 0.58 (0.35 - 0.96) | ||
| Waist >88 | |||||
| All Sets | (179 sets) | 1.26 (1.06 - 1.49) | 0.05 | 1.78 (1.28 - 2.48) | <0.01 |
| Serous tumors | (97 sets) | 1.38 (1.07 - 1.79) | 0.02 | 2.14 (1.33 - 3.45) | <0.01 |
| Non-serous tumors | (86 sets) | 1.16 (0.92 - 1.47) | 0.36 | 1.46 (0.91 - 2.34) | 0.14 |
| Type II tumors | (67 sets) | 1.26 (0.95 - 1.66) | 0.12 | 2.10 (1.20 - 3.67) | 0.03 |
| WHR <0.85 | |||||
| All Sets | (541 sets) | 0.96 (0.90 - 1.03) | 0.98 (0.86 - 1.13) | ||
| Serous tumors | (312 sets) | 0.94 (0.85 - 1.03) | 0.90 (0.74 - 1.08) | ||
| Non-serous tumors | (238 sets) | 0.98 (0.88 - 1.09) | 1.08 (0.89 - 1.32) | ||
| Type II tumors | (229 sets) | 0.86 (0.69 - 1.08) | 0.86 (0.69 - 1.08) | ||
| WHR ≥0.85 | |||||
| All Sets | (145 sets) | 1.18 (1.00 - 1.38) | 0.47 | 1.47 (1.09 - 1.99) | 0.03 |
| Serous tumors | (78 sets) | 1.22 (0.97 - 1.54) | 0.09 | 1.61 (1.04 - 2.51) | 0.01 |
| Non-serous tumors | (70 sets) | 1.12 (0.90 - 1.40) | 0.40 | 1.36 (0.91 - 2.04) | 0.52 |
| Type II tumors | (54 sets) | 1.21 (0.89 - 1.63) | 0.63 | 1.16 (0.66 - 2.04) | 0.34 |
All analyses conducted using conditional logistic regression retaining the matched sets; Cases and controls were matched for study center, age at blood donation, menopausal status, time of the day of blood collection, fasting status, exogenous hormone use at blood donation and menstrual cycle phase. Models adjust for BMI (continuous scale), ever full-term pregnancy (never/ever), age at first birth (continuous scale).
Statistical tests for heterogeneity were based on the likelihood-ratio test, comparing the model fit for logistic regression models with and without corresponding interaction term for the unadjusted and the multivariable model.
Associations for both biomarkers were similar in analyses stratified by menopausal status, HRT use at blood collection, or age at cancer diagnosis (e.g., IL-6: premenopausal women: ORlog2=1.06 [0.79–1.43]; postmenopausal women: ORlog2=1.09 [0.96-1.23]; phet=0.66; Supplementary Table 1). Results were similar after excluding women with hysterectomy, reporting tubal ligation, diagnosed with fallopian tube or primary peritoneal cancer, or diagnosed within 2 years of blood donation (data not shown).
Results were similar after mutual adjustment, with the exception of a strengthening of the association between IL-6 with overall risk of EOC (without adjusting for CRP: ORlog2=1.09 [0.97–1.21]; adjusted for CRP: ORlog2=1.15 [1.02–1.30]; data not shown). In analyses stratified by anthropometric factors, mutual adjustment resulted in strengthened associations for both biomarkers for women in the highest BMI categories; however, the heterogeneity was no longer statistically significant (Supplementary Table 2).
Discussion
With a total of 754 cases and 1,497 controls, this is the largest prospective study to date on the relationship between pre-diagnostic inflammatory markers and EOC, and the first to examine this relationship by tumor characteristics (histology (beyond serous vs. non-serous), grade, stage, type I/II) and anthropometric indices. We found no overall association between pre-diagnostic CRP and IL-6 and risk of EOC on the continuous scale or comparing top to bottom tertiles. However, we observed an increased risk of EOC in women with high CRP concentrations (>10 mg/L), consistent with previous studies. We observed no significant heterogeneity in associations by tumor characteristics (e.g., histology, grade, stage or type I/type II model). However, heterogeneity in associations between both biomarkers and EOC risk was observed in analyses stratified by anthropometric indices (e.g., BMI, waist circumference, WHR); higher concentrations were associated with an increased risk of EOC in women with higher adiposity at blood donation.
Prior prospective studies on the association between CRP and EOC have consistently shown an increased risk with higher CRP concentrations (27-29, 31), particularly for women with relatively high circulating CRP. In a recent meta-analysis, circulating CRP of >10 vs. ≤1 mg/L was associated with a 2.5-fold increased risk of EOC [OR=2.47 [1.53-4.01]; (28)]. Consistent with prior studies, we observed a significant increase in risk with CRP >10 vs. ≤1 mg/L. A similar increase in risk was observed for the serous and non-serous tumor subgroups, however these analyses were exploratory as sample size was limited. Recent data from one large prospective study (n=3,300) suggest that CRP concentrations above the 10 mg/L threshold may be a useful surrogate biomarker to distinguish between acute (≤10 mg/L) and chronic inflammation (>10 mg/L), especially among obese women (41). However, data on the usefulness of CRP in differentiating acute vs. chronic inflammation is limited.
Three prospective studies have evaluated the association between pre-diagnostic IL-6 and EOC (27, 28, 32), yielding conflicting results. While one study observed a positive association between IL-6 and ovarian cancer risk (case n= 230 (32); ORQ4-Q1=1.63 [1.03-2.58]), the two others found no association (case n= 376; ORQ4-Q1= 0.85 [0.52-1.40) (28); case n= 149; ORabove vs below LLD= 1.41 [0.81-2.46) (27)). Consistent with the most recent prior studies (27, 28), we did not observe an association between IL-6 and EOC risk.
Only one prior study has considered heterogeneity between histological subtypes (serous vs. non-serous; (27)). This study observed significant positive associations between CRP >9.8 mg/L in serous tumors (cases n= 37; OR= 3.96 [1.41-11.14]) and no association in nonserous tumors (cases n= 26; OR= 2.13 [0.75-6.05]). IL-6 was not associated with either histological subtype (27). To our knowledge, no prior study has evaluated circulating concentrations of CRP and IL-6 with EOC risk in the context of the dualistic model of ovarian carcinogenesis (type I vs. type II; (23)). This may be of importance as higher expression of COX-2, an enzyme involved in inflammatory response, has been observed in the fimbria of women diagnosed with high-grade (type II) versus low-grade serous tumors (type I). Further, inflammatory exposures (e.g., menstrual cytokines, retrograde menstruation) have been linked to high-grade serous tumors (26, 42). However, we did not observe significant heterogeneity in the strength of association by the dualistic model, and despite biological plausibility, we did not observe the hypothesized association between inflammatory markers and serous tumors.
We observed a positive association between CRP and IL-6 and EOC risk, among women with higher adiposity. However, heterogeneity for IL-6 was significant only for waist circumference, while heterogeneity for CRP was limited to analyses stratified by BMI. We observed consistent heterogeneity by both BMI and waist circumference for both inflammatory markers in analyses limited to serous tumors, and no associations for nonserous tumors. There was no heterogeneity in the associations between CRP and IL-6 and EOC risk after stratifying by BMI in a prior study on inflammation and EOC (28). However, a recent prospective study on inflammation and postmenopausal breast cancer (case n= 549) observed increased risk with circulating CRP concentrations among women with excess adiposity (18). We extend these findings to EOC.
BMI and waist circumference are associated with low-grade, chronic inflammation (6) and women with higher adiposity may be exposed to higher concentrations of inflammatory markers (43). Human omental adipocytes induce ovarian cancer cell proliferation and invasion in vivo and secrete cytokines (44), including IL-6, which may promote carcinogenesis via IL-6-induced p53 downregulation (7, 45). Ovarian cancer grows in the anatomical vicinity of visceral adipose tissue (44) and central adiposity may be more directly associated with an inflammatory milieu (46). Obesity is associated with higher concentrations of estrogens, testosterone and androstenedione (47), and overexpression of inflammatory cytokines (7, 48). Further, IL-6 stimulates aromatase in adipose tissue, enhancing estrogen synthesis (49), with estrogens proposed to increase EOC risk (50). It is plausible that inflammation is more strongly associated with overall ovarian cancer risk in the context of the altered hormonal milieu of obesity.
Although this is the largest prospective study to date on inflammatory markers and EOC risk, and the first to evaluate risk by tumor characteristics, case numbers were small for many subgroups and this study was restricted to invasive cases. A limitation of this and previous studies is that participants provided a single blood sample. CRP concentrations are relatively stable with high correlations over more than a decade (r=0.92; CI 0.88-0.95) (51). However, the within person stability of IL-6 measurements is modest, (ICC over 4 years: IL-6=0.47; (52)) and a single measurement may not accurately reflect a woman’s average IL-6 concentration over longer time periods. Circulating concentrations may also not reflect paracrine concentrations; this may be of importance for ovarian or fallopian tube derived cancers because the surface epithelium is not vascular (50). However, in women with endometriomas and benign or malignant cystic ovarian tumors, serum and cyst fluid levels of IL-6 were modestly correlated (r=0.63; (53)). To our knowledge, there are no data on the association between circulating and intra-ovarian or intra-tubal cytokines in healthy women. Finally, while our analyses were hypothesis driven, the possibility of chance findings cannot be excluded.
Our data support a limited role of CRP and IL-6 in ovarian carcinogenesis; this role may be driven by adiposity. In conclusion, our data add to the evidence that an inflammatory milieu may contribute to an increased risk of epithelial invasive ovarian cancer, specifically in the subgroup of serous tumors. Larger pooled studies are needed to confirm our results and to explore associations in smaller subgroups.
Supplementary Material
Acknowledgements
We thank all the EPIC participants for their invaluable contribution to the study.
Financial support: The German Cancer Research Center, Division of Cancer Epidemiology (Principal Investigator: Rudolf Kaaks) funded the analysis for this study. The coordination of the European Prospective Investigation into Cancer and Nutrition is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); Hellenic Health Foundation (Greece) and the Stavros Niarchos Foundation; Italian Association for Research on Cancer (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health. (Norway); Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country, Murcia (no. 6236) and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK (no. 14136 (KTK), no. C570/A16491 (RCT)), Medical Research Council (United Kingdom; no. G1000143).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Molecular and cellular endocrinology. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. Journal of the National Cancer Institute. 1999;91:1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. British journal of cancer. 2014;110:1878–90. doi: 10.1038/bjc.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human reproduction update. 2014 doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. The lancet oncology. 2011;12:900–4. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN oncology. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delort L, Kwiatkowski F, Chalabi N, Satih S, Bignon YJ, Bernard-Gallon DJ. Central adiposity as a major risk factor of ovarian cancer. Anticancer research. 2009;29:5229–34. [PubMed] [Google Scholar]
- 9.Lahmann PH, Cust AE, Friedenreich CM, Schulz M, Lukanova A, Kaaks R, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer Journal international du cancer. 2010;126:2404–15. doi: 10.1002/ijc.24952. [DOI] [PubMed] [Google Scholar]
- 10.Olsen CM, Nagle C, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocrine-related cancer. 2013 doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canchola AJ, Chang ET, Bernstein L, Largent JA, Reynolds P, Deapen D, et al. Body size and the risk of ovarian cancer by hormone therapy use in the California Teachers Study cohort. Cancer causes & control : CCC. 2010;21:2241–8. doi: 10.1007/s10552-010-9647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chionh F, Baglietto L, Krishnan K, English DR, MacInnis RJ, Gertig DM, et al. Physical activity, body size and composition, and risk of ovarian cancer. Cancer causes & control : CCC. 2010;21:2183–94. doi: 10.1007/s10552-010-9638-y. [DOI] [PubMed] [Google Scholar]
- 13.Brandstedt J, Nodin B, Manjer J, Jirstrom K. Anthropometric factors and ovarian cancer risk in the Malmo Diet and Cancer Study. Cancer epidemiology. 2011;35:432–7. doi: 10.1016/j.canep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kotsopoulos J, Baer HJ, Tworoger SS. Anthropometric measures and risk of epithelial ovarian cancer: results from the nurses’ health study. Obesity (Silver Spring) 2010;18:1625–31. doi: 10.1038/oby.2009.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:381–4. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 16.Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer causes & control : CCC. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 17.Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocrine-related cancer. 2010;17:1007–19. doi: 10.1677/ERC-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dossus L, Jimenez-Corona A, Romieu I, Boutron-Ruault MC, Boutten A, Dupre T, et al. C-reactive protein and postmenopausal breast cancer risk: results from the E3N cohort study. Cancer causes & control : CCC. 2014;25:533–9. doi: 10.1007/s10552-014-0355-9. [DOI] [PubMed] [Google Scholar]
- 19.Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. Journal of the National Cancer Institute. 2010;102:202–6. doi: 10.1093/jnci/djp459. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Ashcraft K. An IL-6 link between obesity and cancer. Front Biosci (Elite Ed) 2013;5:461–78. doi: 10.2741/e628. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X, et al. Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine. 2012;59:228–36. doi: 10.1016/j.cyto.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 23.Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Human pathology. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. The American journal of surgical pathology. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vang R, Shih Ie M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62:44–58. doi: 10.1111/his.12046. [DOI] [PubMed] [Google Scholar]
- 26.Salvador S, Gilks B, Kobel M, Huntsman D, Rosen B, Miller D. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009;19:58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 27.Trabert B, Pinto L, Hartge P, Kemp T, Black A, Sherman ME, et al. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecologic oncology. 2014 doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A Prospective Study of Circulating C-Reactive Protein, Interleukin-6, and Tumor Necrosis Factor alpha Receptor 2 Levels and Risk of Ovarian Cancer. American journal of epidemiology. 2013;178:1256–64. doi: 10.1093/aje/kwt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstetrics and gynecology. 2007;109:933–41. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 30.Lundin E, Dossus L, Clendenen T, Krogh V, Grankvist K, Wulff M, et al. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer causes & control : CCC. 2009;20:1151–9. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toriola AT, Grankvist K, Agborsangaya CB, Lukanova A, Lehtinen M, Surcel HM. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: a longitudinal study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1916–21. doi: 10.1093/annonc/mdq694. [DOI] [PubMed] [Google Scholar]
- 32.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, Koenig KL, Berrino F, Lukanova A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public health nutrition. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 34.Kaaks R, Tikk K, Sookthai D, Schock H, Johnson T, Tjonneland A, et al. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status-Results from the EPIC cohort. International journal of cancer Journal international du cancer. 2013 doi: 10.1002/ijc.28528. [DOI] [PubMed] [Google Scholar]
- 35.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. American journal of epidemiology. 1998;147:1071–80. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- 36.Zannoni GF, Morassi F, Prisco MG, De Stefano I, Vellone VG, Arena V, et al. Clinicopathologic and immunohistochemical features of ovarian clear cell carcinomas in comparison with type I and type II tumors. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2012;31:507–16. doi: 10.1097/PGP.0b013e3182518557. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Sites CK, Toth MJ, Cushman M, L’Hommedieu GD, Tchernof A, Tracy RP, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertility and sterility. 2002;77:128–35. doi: 10.1016/s0015-0282(01)02934-x. [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2008 [Google Scholar]
- 40.Rosner B. PERCENTAGE POINTS FOR A GENERALIZED ESD MANY-OUTLIER PROCEDURE. Technometrics. 1983;25:165–72. [Google Scholar]
- 41.Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DR, Jr., Cho HJ, et al. Gender, obesity and repeated elevation of C-reactive protein: data from the CARDIA cohort. PloS one. 2012;7:e36062. doi: 10.1371/journal.pone.0036062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reade CJ, McVey RM, Tone AA, Finlayson SJ, McAlpine JN, Fung-Kee-Fung M, et al. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2014;36:133–40. doi: 10.1016/S1701-2163(15)30659-9. [DOI] [PubMed] [Google Scholar]
- 43.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:993–7. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 44.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et biophysica acta. 2013;1831:1533–41. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brighenti E, Calabrese C, Liguori G, Giannone FA, Trere D, Montanaro L, et al. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: a new pathway connecting inflammation to cancer. Oncogene. 2014 doi: 10.1038/onc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermsdorff HH, Zulet MA, Puchau B, Martinez JA. Central adiposity rather than total adiposity measurements are specifically involved in the inflammatory status from healthy young adults. Inflammation. 2011;34:161–70. doi: 10.1007/s10753-010-9219-y. [DOI] [PubMed] [Google Scholar]
- 47.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. European journal of endocrinology / European Federation of Endocrine Societies. 2004;150:161–71. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. International journal of endocrinology. 2013;2013:678159. doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:98–107. [PubMed] [Google Scholar]
- 51.Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kario K, Ito Y, et al. Comparison of C-reactive protein levels between serum and plasma samples on long-term frozen storage after a 13. 8 year interval: the JMS Cohort Study. Journal of epidemiology / Japan Epidemiological Association. 2007;17:120–4. doi: 10.2188/jea.17.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obesity research. 2003;11:1055–64. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 53.Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL) -6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–5. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.