Abstract
The complex, interacting influences on eating behavior and energy expenditure prevent elucidation of the causal role of any single factor in the current obesity epidemic. However, greater variety in the food supply, particularly in the form of highly palatable, energy-dense foods, has likely made a contribution. This study was undertaken to test the hypothesis that greater dietary variety is associated with greater caloric intake within individual meals consumed by free-feeding, socially-housed female rhesus monkeys. Meal patterns were assessed during two, two-week dietary phases. One phase consisted of a choice between a standard chow diet and a highly palatable diet (HPD). The other phase consisted of access to the chow only. Food intake for each subject was recorded continuously using previously validated, automated feeders, and a meal was defined based on a minimum kilocalorie requirement and a minimum inter-meal interval. During the choice condition, animals electively consumed mixed meals that incorporated both diets as well as other meals that consisted exclusively of a single diet – chow-only or HPD-only. Animals consumed the most calories per meal when the meal was comprised of both the chow and HPD, which differed in caloric density, flavor, and texture. Interestingly, however, there was no significant difference in the amount of calories consumed as HPD-only meals in the choice condition compared to meals in the chow-only, no choice condition, suggesting consumption of a single food during a meal, regardless of palatability, provides a constant sensory experience that may lead to more rapid habituation and subsequent meal cessation. Additionally, during the dietary choice condition, animals consumed fewer calories in the form of chow-only meals. Thus, the present results suggest that limiting dietary variety, regardless of palatability, may be a useful strategy for weight loss in overweight and obese individuals by reducing caloric intake within individual meals.
Introduction
Appetitive behaviors are shaped by the interaction between the external, physical environment and an individual’s internal, physiologic processes. While the physical environment encompasses a host of influential factors including presentation, accessibility, and social contexts 1, internal processes refer to homeostatic and hedonic mechanisms governed by the central nervous system 2. Homeostatic control of appetite, regulated, in part, by the arcuate nucleus of the hypothalamus and the brain stem, is driven by the biological need to maintain energy stores. Both long-term (e.g., leptin, insulin) and short-term (e.g., ghrelin, cholecystokinin, peptide YY) hunger and satiety signals provide feedback to ensure that the body acquires the necessary nutrients for survival. Hedonic regulation, by comparison, is regulated by corticolimbic neural networks and is mediated by reward. These reward-based pathways, activated by highly palatable food, influence the cognitive, motivational, and emotional aspects of food intake and offer one explanation for eating in the absence of hunger or nutrient depletion 3.
The complex, interacting influences on eating behavior prevent elucidation of the causal role of any single factor in the current obesity epidemic, which affects approximately 36% of U.S. adults 4. However, greater variety in the food supply, particularly in the form of highly palatable, energy-dense foods, is likely a contributing factor 5. The basis for the hypothesis linking greater dietary variety with increased caloric intake is sensory-specific satiety, a phenomenon in which hedonic ratings of a food eaten to satiation decrease to a greater degree than hedonic ratings of foods not eaten to satiation. In other words, when a meal or diet is composed of foods that differ in at least one sensory characteristic (e.g., flavor, texture, color), differential experiences with the sensory characteristics of foods may enhance food intake. Conversely, consuming a single food during a meal provides a constant sensory experience that may lead to habituation, a phenomenon in which repeated presentation of a stimulus results in a decrease in response to that stimulus 6.
The present study was undertaken to assess the impact of dietary variety on caloric intake within individual meals consumed by free-feeding, socially-housed female rhesus monkeys. This model adds significant value to the well-established literature on animal models of appetite regulation 7 by providing a means to better define the socio-environmental factors that affect the neurobiology of food intake among pre- and postmenopausal women. To test the hypothesis that greater dietary variety is associated with greater caloric intake within a meal, a meal was defined based on a minimum kilocalorie requirement and a minimum inter-meal interval. Caloric intake was monitored during two dietary conditions – a “choice” condition in which both a standard chow diet and a more palatable high-fat, sugary diet were offered simultaneously and a “no choice” condition when animals only had access to the chow diet.
Methods
Study subjects were ovariectomized, adult female rhesus monkeys (n=38) that were socially housed in groups consisting of five or six animals each (4 to 5 females and 1 male) in adjacent indoor-outdoor enclosures measuring 3.8 m by 3.8 m by 3.8 m at the Yerkes National Primate Research Center. Indoor light cycles were maintained on a 12h: 12h schedule; however, access to outdoor caging allowed the natural, autumnal photoperiod to prevail. Social groups were established approximately three years prior to the study using previously described methods 8. The animals formerly served as subjects in NIH-funded studies to determine the effects of psychosocial stress, induced by social subordination, on a number of behavioral, metabolic and reproductive outcomes 8–13 that required brief treatment with estradiol and/or progesterone. For the present study, females were not receiving hormone replacement. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals.”
Food intake for each subject was recorded continuously using previously validated automated feeders 14. Two feeders were placed on each housing unit. Crystal Tag RF microchips were implanted subcutaneously in both wrists of each animal. When an animal placed its hand in a feeder, a Datamars reader surrounding the food dispenser detected the microchip and sent a signal to a remote computer that identified the monkey and triggered the delivery of a single food pellet. This system allowed for continual quantification of caloric intake of individual monkeys embedded in social groups. As previously described 13, females were studied for two, two-week dietary phases separated by a three-week washout period when animals were maintained on standard laboratory chow and food intake was not monitored. One phase consisted of a choice between the standard chow diet (3.45 kcal/g, 12% fat, 18% protein, and 4.14% sugar carbohydrate and 65.9% fiber carbohydrate; Purina #5038) and a highly palatable diet (HPD; 3.72 kcal/g, 36% fat, 18% protein, 16.4% sugar carbohydrate and 29.6% fiber-starch carbohydrate; Purina Typical American Diet #5L0P). The other phase consisted of access to the chow only. The order of dietary phases was counterbalanced so that half of the females received the choice condition first while the other half received the no choice, chow-only condition first. For the present analysis, diet order was not considered. During the choice condition, one feeder contained chow while the other dispensed HPD. At the midpoint of the choice trial, the diets in the feeders were switched to control for feeder-specific preferences.
Feeding data were processed using BioDAQ Data Viewer© (Research Diets, New Brunswick, NJ). For the purposes of this investigation, a meal was defined as any feeding bout that resulted in ingestion of a minimum of 70 kilocalories (kcal), separated from a previous feeding bout by a minimum time interval of 1300 seconds. This definition was based on empirically defined parameters described previously15 and validated using statistical methods from previous meal pattern analyses 16. All statistical analyses were performed using SPSS. The caloric content of meals was determined in the chow-only condition and the choice condition. Meals in the choice condition were further categorized as chow-only meals, HPD-only meals, and mixed meals. Differences in the frequency of occurrence and caloric content of these meal categories were assessed using multivariate ANOVA. Post-hoc pairwise comparisons were generated to assess all main effects. Weekly body weights were also recorded to the nearest hundredth of a kilogram throughout the study, and variation in body weight across the two dietary phases was assessed using univariate ANOVA. To determine whether body weights predicted parameters of specific meals, Pearson correlations were calculated between body weight and meal size, and meal frequency among each individual meal category (no choice: chow-only; choice: mixed, HPD-only, and chow-only). Results are presented as the mean ± standard error. P-values < 0.05 were considered significant.
Results
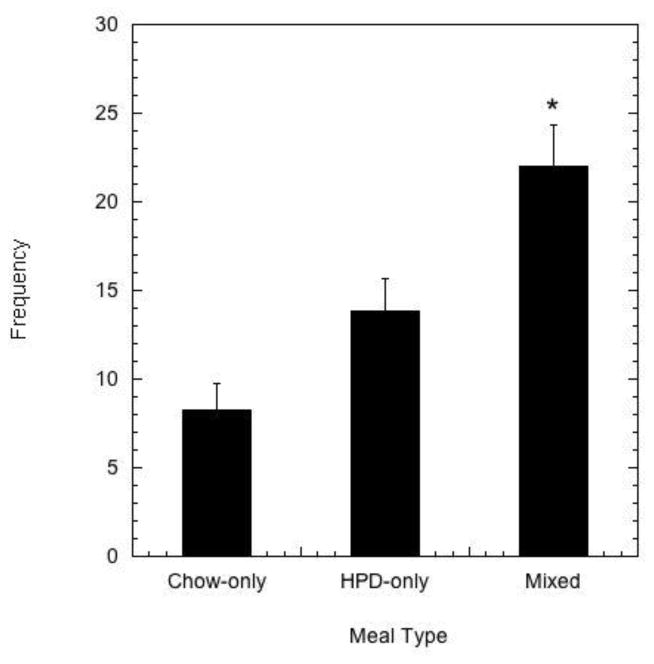
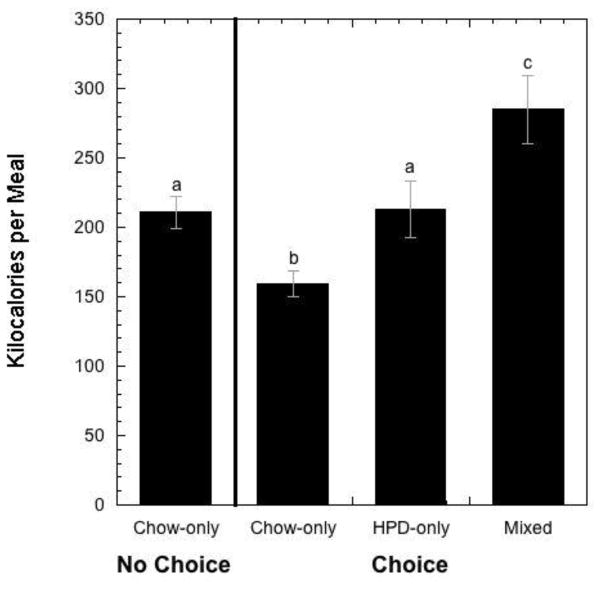
During the choice condition, animals consumed mixed meals most frequently (Figure 1; F 2,37 = 11.970, p < 0.001) compared to HPD-only meals (p = 0.002) and chow-only meals (p < 0.001). Although the frequency of HPD-only meals was higher than chow-only meals in this choice condition, the difference was not significant (p = 0.07). As illustrated in Figure 2, the number of calories ingested within a meal differed significantly depending upon diet condition and meal composition (F 3,27 = 10.337, p < 0.001). Post-hoc, pairwise comparisons revealed that the caloric content of mixed meals (285.200 ± 24.614 kcals) was significantly greater than the caloric content of all other meal types: meals in the chow-only, no choice condition (211 ± 11.731 kcal, p = 0.005), chow-only meals in the choice condition (159.355 ± 9.578 kcal, p < 0.001), and HPD-only meals in the choice condition (213.086 ± 20.171 kcals, p = 0.026). Importantly, there was no significant difference in caloric content of HPD-only meals in the choice condition and chow-only meals in the no choice condition (p = 0.96). However, the caloric content of LCD-only meals in the choice condition was significantly lower than meals in the chow-only, no choice condition (p = 0.001) and HPD-only meals in the choice condition (p = 0.022). Body weights at the conclusion of the choice condition (8.278 ± 0.238 kg) did not differ (F 1,37 = 0.879, p = 0.355) from body weights at the end of the chow-only condition (8.223 ± 0.245 kg).
Figure 1.
Mean meal frequency ± SEM during the two-week choice condition when both chow and HPD were available based on meal type: chow-only, HPD-only, or mixed. An asterisk indicates significant difference in the average frequency of consumption for a particular meal type.
Figure 2.
Mean kilocalories ± SEM consumed in meals during the chow-only condition as well as chow-only, HPD-only, and mixed meals during the choice condition when both chow and HPD were available. Differing alphabetic symbols indicate statistically significant differences in average caloric content among meal categories.
In order to evaluate whether body weights predicted meal parameters, Pearson correlation analyses were performed between body weight, meal size, and meal frequency among each individual meal category (no choice: chow-only; choice: mixed, HPD-only, and chow-only). As illustrated in Table 1, body weight was significantly correlated with mixed meal frequency (p = 0.024) but not the frequency of other meal types or the sizes of specific meals (p > 0.05). Additionally, animals that had a higher frequency of mixed meals also had a higher frequency of all meal types regardless of diet condition (p < 0.05). These animals also consumed larger mixed meals in the choice condition and larger LCD meals in the no choice condition (p < 0.05).
Table 1.
Pearson correlations between body weight, meal size, and meal frequency among each individual meal category in the no choice (chow-only) and choice (Mixed, HPD-only, and chow-only) diet conditions. Bold typeface indicates statistically significant associations.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Body Weight | - | |||||||
| 2. Mixed Meal Frequency | .361* | - | ||||||
| 3. HPD-only Meal Frequency | .180 | .991** | - | |||||
| 4. Chow-only Meal Frequency | −.142 | .976** | .965** | - | ||||
| 5. No Choice Meal Frequency | −.144 | .476** | −.092 | .114 | - | |||
| 6. Mixed Meal Size | −.035 | .460** | .230 | −.007 | .327* | - | ||
| 7. HPD-only Meal Size | .150 | .323 | .249 | −.135 | −.033 | .126 | - | |
| 8. Chow-only Meal Size | −.237 | −.035 | .087 | .210 | −.053 | .533** | −.003 | - |
| 9. No Choice Meal Size | −.198 | .428** | −.216 | .326* | .718** | .332* | .227 | −.045 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Discussion
Findings from this study indicate that female rhesus monkeys consumed significantly more calories per meal when the meal was comprised of two separate diets that differed in energy density, flavor, and texture. Further, there was no significant difference in the amount of calories in meals comprised entirely of the HPD in the choice condition compared to meals in the chow-only, no choice condition. Not surprisingly, animals consumed fewer calories in the form of chow-only meals when given a choice between chow and HPD, indicating an expected shift in preference to the more palatable food when this dietary option was available. Despite these variations in meal size across the two dietary conditions, there was no significant change in body weights, which was likely a result of the short study duration. Nonetheless, Pearson correlation analyses revealed that higher body weight was associated with greater mixed meal frequency, and animals that consumed more frequent mixed meals consumed all other meal types (HPD-only, chow-only, no choice) more frequently. Additionally, greater mixed meal frequency was associated with larger mixed meals (kcals) in the choice condition and larger meals during the no choice, chow-only condition (kcals).
The finding that greater dietary variety was associated with greater caloric intake is corroborated by a number of investigations with animals and humans 6,17. Studies with rats have consistently demonstrated that flavor variety enhances food intake within the context of a single meal 18 even when the macronutrient composition of the foods is identical 19–21. Conversely, rodent studies that have not found a significant effect of variety on meal size have introduced variety successively across days rather than simultaneously or sequentially within meals 22. Human investigations have also consistently shown that sensory variety among available foods promotes greater intake within the context of single meals 18,23,24, particularly when multiple sensory properties are varied among available options17.
Although the evaluation of feeding microstructure does not permit causal conclusions related to total caloric intake and weight status, the aforementioned finding that animals consumed fewer calories within meals comprised only of HPD relative to mixed meals potentially challenges the idea that simply adding foods of lower energy density to one’s diet will displace more energy dense foods resulting in reduced energy intake and weight loss. While subjects demonstrated a clear preference for the HPD, it appears that within the context of individual feeding bouts, animals terminated meals earlier when the meal was comprised of a single, palatable food compared to meals that incorporated both a palatable food and a less palatable chow. Additionally, animals consumed essentially the same number of calories within meals consisting of a single diet regardless of whether the diet was chow-only (in the no choice condition) or HPD-only (in the choice condition), suggesting that sensory specific satiety may operate independently of palatability. In agreement with these findings, an investigation measuring weight gain among rats maintained on different diets concluded that restricting rats to just one palatable food reduces the likelihood that animals will become obese compared to animals with a more varied diet, including both chow and palatable food 18. Further, a review of the literature assessing dietary strategies for weight management reported that advising subjects to increase fruit and vegetable intake without emphasizing strategies to decrease total caloric intake and/or increase energy expenditure was associated with no change or an increase in body weight, suggesting that these lower energy density foods either displaced other foods without changing overall caloric intake or simply added extra calories 25. Thus, these findings combined with the present results suggest that limiting dietary variety, perhaps through dietary restriction to a small subset of highly preferred foods, may be a useful strategy for weight loss in overweight and obese individuals.
Whereas many previous experiments, both human and animal, have assessed the impact of offering a variety of highly palatable foods or varying flavors of a standard chow diet, this study took the approach of offering a standard chow diet alongside a more palatable, slightly more calorically dense diet (HPD). Indeed, the present design more adequately models the usual dietary environment of people consuming Western diets, which consist of foods that vary in both sensory qualities and nutrient composition17. However, the task of disentangling the effects of sensory variety from energy density is complicated by the use of these two diets. Further, the HPD-only meals that were evaluated during this study were electively consumed within the context of a varied dietary environment, and the present analysis is limited strictly to the assessment of caloric intake within individual meals. When attempting to project the broader implications of these findings with regard to obesity risk, it is critical to realize that excess caloric intake may occur via several avenues, and both meal frequency and between meal snacking are equally as important as meal size in influencing total caloric intake. A recently published companion analysis revealed that animals in this study consumed more calories in the form of snacks during the dietary choice condition, and the majority of snack calories (57.9% ± 3.1%) were derived from HPD 15. Thus, it is possible that animals may compensate for the reduced meal size observed within HPD-only meals with greater or more frequent snacking between meals leading to greater overall caloric intake. Future studies are planned to assess how a no variety, HPD-only condition would affect not only meal patterning but also snacking and total caloric intake using this female monkey model.
Additionally, while the feeders and the ad libitum availability of food captured actual intake, we cannot assume that caloric intake during the two-week study period is fully representative of long-term dietary patterns. Thus, monitoring food intake for a period longer than two weeks is critical to assess causal relationships and longitudinal changes in weight status that are induced via changes in caloric intake and energy expenditure. Of final note, animals utilized for this study were ovariectomized and were not receiving hormone replacement during these experiments. Both estradiol and progesterone are known to affect appetite and meal size 26–28. Thus, the effects of female hormones on feeding patterns were eliminated and must be considered when evaluating the external validity of our results. Importantly, however, the present design does provide an ethologically relevant model to explore socio-environmental factors that affect the neurobiology of food intake among postmenopausal women.
In closing, it is critical to note that strategies to reduce total caloric intake by limiting the variety of foods in the diet may not necessarily be favorable to promote adequate intake of non-caloric but vital micronutrients 5, and monotonous dietary regimens may hinder long-term compliance resulting in regression to former eating habits and regain of any weight lost 29. Nonetheless, investigations that have directly assessed the association of dietary variety with body weight have generally found positive associations between dietary variety and adiposity 17. While more research is needed in this area, the present results refute the idea that simply adding foods of lower energy density to one’s diet will displace the consumption of more energy dense foods. Rather, the data provide additional support for the notion that limiting dietary variety may assist with reducing energy intake and promoting weight loss in overweight and obese individuals by reducing caloric intake within individual meals.
Highlights.
The caloric content of meals was assessed in choice and no choice dietary environments.
Animals consumed the most calories per meal when the meal was comprised of two separate diets.
Caloric content of meals comprised of a single palatable diet did not differ from chow meals.
Consumption of a single food may lead to more rapid habituation and subsequent meal cessation.
Limiting dietary variety may be a useful strategy for weight loss among overweight individuals.
Acknowledgments
The study was conducted with the invaluable, expert technical assistance of Jennifer Whitley, Shannon Bounar, Jodi Godfrey, Christine Marstellar, Jonathan Lowe, Natalie Brutto, Angela Tripp, and Kathy Reding. This work was supported by NIH HD 046501 (MW), MH081816 (DT), F31MH085445 (VM), and RR00165. Authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr. 2004;24:455–479. doi: 10.1146/annurev.nutr.24.012003.132140. [DOI] [PubMed] [Google Scholar]
- 2.Harrold JA, Dovey TM, Blundell JE, Halford JC. CNS regulation of appetite. Neuropharmacology. 2012 Jan 30; doi: 10.1016/j.neuropharm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Seagle HM, Strain GW, Makris A, Reeves RS. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009 Feb;109(2):330–346. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.McCrory MA, Fuss PJ, McCallum JE, et al. Dietary variety within food groups: association with energy intake and body fatness in men and women. The American journal of clinical nutrition. 1999 Mar;69(3):440–447. doi: 10.1093/ajcn/69.3.440. [DOI] [PubMed] [Google Scholar]
- 6.Raynor HA, Jeffery RW, Tate DF, Wing RR. Relationship between changes in food group variety, dietary intake, and weight during obesity treatment. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004 Jun;28(6):813–820. doi: 10.1038/sj.ijo.0802612. [DOI] [PubMed] [Google Scholar]
- 7.Avena NM. Animal models of eating disorders. Totowa N.J: Humana Press; 2013. [Google Scholar]
- 8.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008 Mar 18;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009 Dec;81(6):1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and Behavior. 2011 Apr;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiology & Behavior. 2011 Mar 1;102(3–4):382–388. doi: 10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 Oct 24; doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- 13.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012 Feb 27; doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav. 2008 Jul 5;94(4):586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore CJ, Lowe J, Michopoulos V, et al. Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite. 2012 Nov 30;62C:60–69. doi: 10.1016/j.appet.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2005 Jun;288(6):R1450–1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 17.McCrory MA, Burke A, Roberts SB. Dietary (sensory) variety and energy balance. Physiol Behav. 2012 Jun 21; doi: 10.1016/j.physbeh.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Rolls BJ, Van Duijvenvoorde PM, Rowe EA. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol Behav. 1983 Jul;31(1):21–27. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Le Magnen J. Effect of a multiplicity of food stimuli on the amount eaten by the rat (first published in French in 1960) Appetite. 1999 Aug;33(1):36–39. doi: 10.1006/appe.1999.0258. [DOI] [PubMed] [Google Scholar]
- 20.Morrison GR. Alterations in palatability of nutrients for the rat as a result of prior tasting. J Comp Physiol Psychol. 1974 Jan;86(1):56–61. doi: 10.1037/h0035961. [DOI] [PubMed] [Google Scholar]
- 21.Treit D, Spetch ML, Deutsch JA. Variety in the flavor of food enhances eating in the rat: a controlled demonstration. Physiol Behav. 1983 Feb;30(2):207–211. doi: 10.1016/0031-9384(83)90007-0. [DOI] [PubMed] [Google Scholar]
- 22.Warwick ZS, Schiffman SS. Flavor-calorie relationships: effect on weight gain in rats. Physiol Behav. 1991 Sep;50(3):465–470. doi: 10.1016/0031-9384(91)90531-r. [DOI] [PubMed] [Google Scholar]
- 23.Norton GN, Anderson AS, Hetherington MM. Volume and variety: relative effects on food intake. Physiol Behav. 2006 Apr 15;87(4):714–722. doi: 10.1016/j.physbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Rolls BJ, Rowe EA, Rolls ET. How sensory properties of foods affect human feeding behavior. Physiol Behav. 1982 Sep;29(3):409–417. doi: 10.1016/0031-9384(82)90259-1. [DOI] [PubMed] [Google Scholar]
- 25.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004 Jan;62(1):1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 26.Gray JM, Greenwood MR. Time course of effects of ovarian hormones on food intake and metabolism. The American journal of physiology. 1982 Nov;243(5):E407–412. doi: 10.1152/ajpendo.1982.243.5.E407. [DOI] [PubMed] [Google Scholar]
- 27.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2006 Jul 29;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995 Dec;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 29.Hill JO, Thompson H, Wyatt H. Weight maintenance: what’s missing? J Am Diet Assoc. 2005 May;105(5 Suppl 1):S63–66. doi: 10.1016/j.jada.2005.02.016. [DOI] [PubMed] [Google Scholar]