Abstract
Objectives
This study aims to validate a modified dried blood spot (DBS)-based glycosylated hemoglobin (HbA1c) assay protocol, after a pretest in India showed poor correlation between the original DBS-based protocol and venous results.
Methods
The original protocol was tested on different chemistry analyzers and then simplified at the University of Washington (UW). A second pretest was conducted in India to validate the modified assay protocol, using 44 quality control specimens.
Results
Data from UW indicated that, using the original protocol, the correlation coefficients between DBS and venous results were above 0.98 on both Bio-Rad and Olympus chemistry analyzers. The protocol worked equally well on filter paper, with or without pre-treatment, and when the recommended amount of blood spot material, or less, was used. A second pretest of the modified protocol confirmed that DBS-based levels from both Olympus and Roche chemistry analyzers were well correlated with DBS results from UW (correlation coefficients were above 0.96), as well as with venous values (correlation coefficients were above 0.94).
Conclusions
The DBS-based HbA1c values are highly correlated with venous results. The pre-treatment of filter paper does not appear to be necessary. The poor results from the first pretest are probably due to factors unrelated to the protocol, such as problems with the chemistry analyzer or assay reagents.
Keywords: dried blood spot based assay, glycosylated hemoglobin, assay validation, India
BACKGROUND
Glycosylated hemoglobin (HbA1c) measures average plasma glucose concentration over the previous three months and is used for the diagnosis of diabetes (American Diabetes Association, 2010). Among diabetic patients, higher levels of HbA1c indicate poorer glycemic control and are associated with increased risk of cardiovascular diseases, nephropathy, and retinopathy (Jun, et al., 2011; Holman, et al., 2008). Lack of fasting or biased reporting of fasting status often introduces error to blood glucose results. In contrast, an HbA1c measurement does not require a fasting specimen—an important advantage in community-based surveys, for which obtaining fasting specimens may not always be possible or may result in non-representative samples.
The measurement of HbA1c in a field setting has been made easier by the development of dried blood spot (DBS)-based assays, which have been adopted by several large studies, such as the Health and Retirement Study (HRS) and the National Social Life, Health, and Aging Project (NSHAP) (Heisler, et al., 2007; Williams and McDade, 2009; Crimmins, et al., 2014). However, the DBS-based HbA1c test was initially proprietary and available only in the United States. Therefore, the Study of Global AGEing and Adult Health (SAGE), organized by the World Health Organization (WHO), developed a DBS-based HbA1c assay protocol for use in other countries. The SAGE protocol uses a citrate-based solution to pre-treat filter paper to prevent potential further glycosylation of hemoglobin after blood spot collection.
The Longitudinal Aging Study in India (LASI) team tested this SAGE protocol at the National AIDS Research Institute (NARI) in Pune, India, in 2012. The USC/UCLA Center on Biodemography and Population Health prepared quality control (QC) DBS samples from 33 volunteers who also had simultaneous venous HbA1c measurements on a Primus Ultra2 analyzer at UCLA Clinical Laboratory. These QC DBS specimens were shipped to NARI, maintaining a temperature of under −40° Celsius while in transit. The pretest data showed a poor correlation between venous values and DBS results, using an Olympus AU400 chemistry analyzer at NARI. In this brief report, we summarize LASI’s effort to further modify and validate the original SAGE HbA1c protocol to enhance correspondence between its DBS results and the “gold standard” venous results.
METHODS
Validation and modification of DBS-based HbA1c assay protocol at the University of Washington (UW)
As the first step in the validation process, the UW laboratory, which had established a validated DBS-based HbA1c assay using a Bio-Rad system, tested its HbA1c protocol and the SAGE protocol on both Bio-Rad Variant II and Olympus AU400 chemistry analyzers. UW also explored whether pre-treatment of filter paper would affect HbA1c results. Table 1 shows the correlation coefficients and mean differences between DBS-based and venous HbA1c values by different assay protocols, type of chemistry analyzer, and pre-treatment of filter paper. All correlation coefficients were above 0.984. However, the absolute values from DBS were generally lower than venous results with BioRad system, but higher than venous results with Olympus system.
Table 1.
Correlation coefficients and mean differences between dried blood spot (DBS)-based and venous-based glycosylated hemoglobin values, by assay protocols, type of chemistry analyzer, and pre-treatment of filter paper (N=40 for each of the 6 categories).
| Assay protocol | Type of chemistry analyzer | Pre-treatment of filter paper | |||
|---|---|---|---|---|---|
| Yes | No | ||||
|
| |||||
| Correlation coefficient | Mean difference (%) | Correlation coefficient | Mean difference (%) | ||
| University of Washington | Bio-Rad | 0.996 | −2.2 * | 0.998 | −0.9 |
| Olympus | 0.991 | 1.4 | 0.989 | 1.6 | |
| SAGE † | Olympus | 0.984 | 1.2 | 0.985 | 1.4 |
The mean difference is negative when DBS values are lower than the corresponding venous results and positive when DBS values are higher than venous results.
The Study of Global AGEing and Adult Health
The UW laboratory also modified the original SAGE protocol by 1) reducing the amount of DBS material from two 3.2 mm punches to one; 2) reducing the volume of hemolyzing reagent by 50%; and 3) decreasing sample elution time. Subsequent testing indicated that the revised protocol worked equally well. Details of this revised DBS protocol have been summarized in Table 2.
Table 2.
Revised dried blood spot (DBS) based glycosylated hemoglobin (HbA1c) assay protocol for Olympus AU400 chemistry analyzer.
| Method: | Total hemoglobin concentration is measured by a colorimetric method monitoring the change in absorbance at 600 nm. HbA1c concentration is measured by a turbidmetric immunoinhibition method monitoring the change in absorbance at 700 nm. HbA1c concentration is expressed as a percentage of total hemoglobin. |
| Filter paper | Whatman 903 protein saver cards without additional pre-treatment with a citrate-based solution |
| DBS punch size | One 3.2 mm (1/8 inch) punch |
| Materials |
|
| Procedure |
|
Second HbA1c pretest at NARI
Because NARI had purchased a Roche Cobas Integra 400 chemistry analyzer after the first pretest, both Olympus and Roche HbA1c kits were purchased to test the modified protocol. NARI had the Olympus chemistry analyzer serviced prior to the second pretest, during which only untreated blood spots were used.
UW provided NARI with 24 DBS QC samples with known DBS-based HbA1c values. The HbA1c levels from these UW specimens ranged from 4.1% to 11.8%. NARI also tested 20 frozen LASI DBS validation samples with known venous values from UCLA, ranging from 4.3% to 8.2%. These specimens were also used during the first pretest.
Statistical analysis
Pearson correlation coefficients were calculated to examine the relationship between the results from different assay methods and different laboratories. We also reported summary statistics and coefficients of variation (CVs) for repeated measurements. For comparison between DBS and venous results, we performed Bland-Altman analysis, plotting both the differences and ratios of DBS to venous results on the y-axis (McDade, 2014).
RESULTS
Results from the Olympus AU400 system
The NARI laboratory measured HbA1c levels twice on the 24 UW DBS QC samples with known HbA1c values from Bio-Rad Variant II. The correlation coefficient between the average of two NARI measurements and UW values was 0.961, with NARI recording higher absolute HbA1c values. The mean difference was 1.4% (standard deviation: 0.7%). The CVs for NARI’s repeated measurements ranged from 0% to 7.16%.
For the 20 LASI QC samples, the correlation coefficient between DBS and venous values was 0.939. The DBS values were higher than the venous values (mean absolute difference: 1.5%; standard deviation: 0.5%). Bland-Altman analysis revealed no evidence of change in variability across the assay range.
Results from the Roche Cobas Integra 400 system
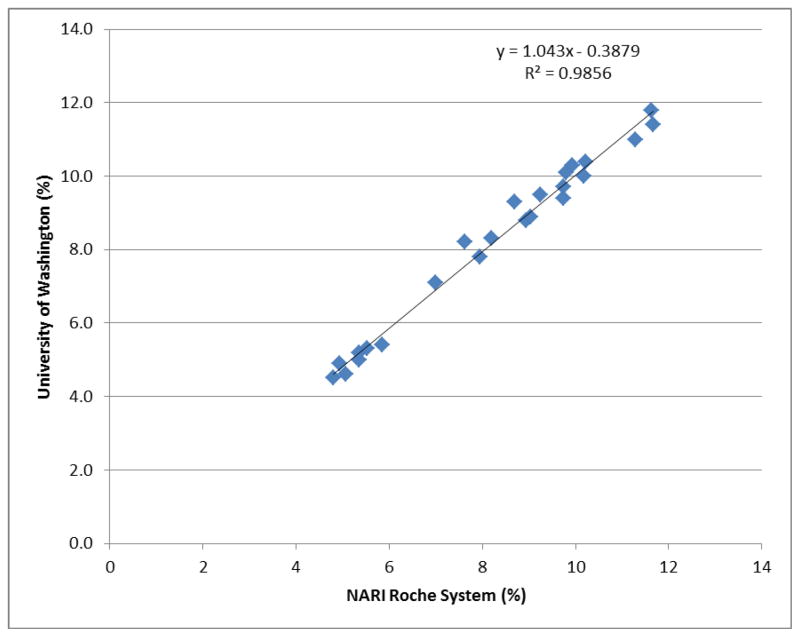
The initial validation attempt on the Roche analyzer encountered several problems, including suboptimal calibration curves and out-of-range control sample results. Further investigation identified that 1) the local vendor supplied the incorrect type of hemolyzing reagent; and 2) the Roche chemistry analyzer at NARI had not received maintenance service for over five months. After correcting these problems, NARI re-measured the 24 UW QC samples. Figure 1 shows the scatter plot of the DBS HbA1c values from NARI’s Roche system and UW’s Bio-Rad Variant II. The correlation coefficient was 0.993. The average difference in absolute numbers was 0.03% (standard deviation: 0.3%). The between-laboratory CVs ranged from 0.29% to 5.54%.
Fig. 1.
Comparison of dried blood spot-based glycosylated hemoglobin results from the National AIDS Research Institute (NARI) Roche analyzer system and the University of Washington Bio-Rad Variant II system
For the 20 LASI QC DBS samples with known venous HbA1c levels, the correlation coefficient between DBS and venous results was 0.960. NARI’s DBS values were again higher than the venous values (mean absolute difference: 0.5%; standard deviation: 0.4%). Bland-Altman analysis showed no variability across the assay range.
Discussion
Diabetes mellitus and metabolic syndrome have become major public health problems in many developing countries experiencing rapid economic growth (Cheema, et al., 2014; Yan, et al., 2012). Incorporating biomarkers into community-based studies improves measurement of an overall health profile, especially in populations for whom self-reported status is often biased due to lower education levels and limited access to health care services (Johnston, et al., 2009). Measuring biomarkers also affords a better understanding of the biological pathways through which socioeconomic factors influence population health (Seeman, et al., 2004). Because of the cost, burden, and logistics associated with venous blood collection, DBS serves as a low-cost, less invasive, and field-friendly alternative (McDade, et al., 2007; McDade, 2014).
To our knowledge, this is the first study that has systematically evaluated the validity of DBS-based HbA1c assays on multiple chemistry analyzer platforms both in and outside the United States. The data show that DBS-based HbA1c results at NARI are highly correlated with DBS values at UW and the venous values. The correlation coefficients for the Bio-Rad, Olympus, and Roche platforms are all above 0.94. These findings suggest that, with proper personnel training and ongoing quality assessment, a DBS-based HbA1c assay can have a validity that approximates venous-based testing and may be successfully implemented in community-based surveys in less-developed countries, realizing benefits of cost and flexibility for such studies.
In addition to working with different chemistry analyzers, our team has explored ways to simplify the original SAGE protocol, including the amount of blood spot material, elution time, and pre-treatment of filter paper. The results indicate the DBS-based HbA1c protocol is robust, and pre-treatment of filter paper does not improve assay results. The latter finding is particularly important, because the revised protocol will reduce the complexity of field operations and offer more flexibility in the use of DBS for different assays.
Some limitations of our QC work should be considered. First, our sample size for QC specimens is relatively small, and all samples had HbA1c levels below 11.8%. While this range covers non-diabetic individuals and most patients with diabetes, we are not able to evaluate how well the assay protocol works when the HbA1c level is very high. Second, although we have identified non-protocol-related factors that may affect assay results, such as issues related to the chemistry analyzer and assay reagents, we cannot isolate the precise reasons the HbA1c results were so poor during our first pretest.
Despite these limitations, our study produced the following findings: the DBS-based HbA1c protocol appears to be robust and works well on different chemistry analyzer systems. DBS-based HbA1c values have a very highly correlation with venous measurements. Pre-treatment of filter paper for this test does not appear to be necessary. During the assay, it is essential to devote attention to other technical aspects beyond the protocol itself, such as the regular servicing of chemistry analyzers, the proper training of laboratory personnel, and the condition of assay reagents.
Acknowledgments
Funding for the LASI pilot study was provided by the National Institute on Aging (NIA), grant 1R21 AG034443. Funding for the USC/UCLA Center on Biodemography and Population Health (CBPH) was provided by the NIA, grant P30 AG017265.
Footnotes
Conflict of Interest
The authors have no conflict of interest or any financial disclosure.
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Supplement 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema A, Adeloye D, Sidhu S, Sridhar D, Chan KY. Urbanization and prevalence of type 2 diabetes in Southern Asia: A systematic analysis. J Glob Health. 2014;4(1):010404. doi: 10.7189/jogh.04.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography Soc Biol. 2014;60(1):38–48. doi: 10.1080/19485565.2014.901885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853–60. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: Evidence from hypertension for the income/health gradient. J Health Econ. 2009;28(3):540–52. doi: 10.1016/j.jhealeco.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Jun M, Perkovic V, Cass A. Intensive glycemic control and renal outcome. Contrib Nephrol. 2011;170:196–208. doi: 10.1159/000325664. [DOI] [PubMed] [Google Scholar]
- McDade TW. Development and validation of assay protocols for use with dried blood spot samples. Am J Hum Biol. 2014;26(1):1–9. doi: 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: Dried blood spots as a minimally-invasive method for integrating biomarkers into population-based research. Demography. 2007;44:889–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58(10):1985–97. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Williams SR, McDade TW. The use of dried blood spot sampling in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i131–6. doi: 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Li J, Li S, Zhang B, Du S, Gordon-Larsen P, Adair L, Popkin B. The expanding burden of cardiometabolic risk in China: the China Health and Nutrition Survey. Obes Rev. 2012;13(9):810–21. doi: 10.1111/j.1467-789X.2012.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]