Summary
Obstructive sleep apnoea syndrome (OSAS) is common in children. Craniofacial anomalies such as cleft palate are among the most common congenital conditions. Children with a variety of craniofacial conditions, including cleft palate, micrognathia, craniosynostosis, and midface hypoplasia are at increased risk for OSAS. Available evidence, which is largely limited to surgical case series and retrospective studies, suggests that OSAS can be successfully managed in these children through both surgical and non-surgical techniques. Prospective studies using larger cohorts of patients and including polysomnograms are needed to better understand the risk factors for this patient population and the efficacy of treatment options for OSAS and their underlying conditions.
Keywords: Obstructive sleep apnea, craniofacial abnormalities, Pierre Robin Syndrome, micrognathia, cleft palate, craniosynostoses, child, infant
Introduction
Obstructive sleep apnoea syndrome (OSAS) is defined in children as a ‘disorder of breathing during sleep characterized by prolonged partial upper airway obstruction and/or intermittent complete obstruction…that disrupts normal ventilation during sleep and normal sleep patterns.’ 1 OSAS is common in children, with the prevalence ranging between 1.2 and 5.8% of the general paediatric population, depending on the criteria used to define OSAS. Most otherwise-healthy children with OSAS have adenotonsillar hypertrophy and/or obesity as risk factors. However, specific paediatric populations with underlying diseases are also at risk for OSAS. These include children with a history of prematurity, asthma, Down syndrome, sickle cell disease, achondroplasia, neuromuscular disease and craniofacial anomalies, among others. Children with craniofacial conditions are generally at increased risk for development of OSAS 2 but this population is complex because of its heterogeneity. Thus, individual conditions must be considered in the diagnosis and treatment. OSAS is important to identify in children, as untreated OSAS can result in significant sequelae, including cognitive and behavioral deficits, cardiac ventricular remodeling and hypertension, and inflammation 2.
Types of craniofacial conditions
Craniofacial conditions are highly variable, and may occur in isolation or as part of a syndrome. The Whitaker classification separates craniofacial anomalies into four groups, including clefts, craniosynostoses, hypoplasia, and neoplasia 3, but there is no comprehensive system to classify all craniofacial conditions.
Craniofacial clefts may include a cleft lip, cleft palate or both cleft lip and palate (CLP). Over 200 syndromes include cleft lip and/or palate as a feature, but approximately 85% of clefts occur in isolation 4. Orofacial clefts are one of the most common congenital conditions, with approximately one in 700 live births being affected. Cleft palate may be unilateral or bilateral, affecting either the soft palate alone or both the hard and soft palate. There are many genetic mutations that cause cleft palate and although some clefts occur as a result of familial inheritance, most are the result of a de novo mutation. Syndromes that commonly include cleft palate include Pierre Robin sequence, Stickler syndrome, Treacher Collins syndrome, Goldenhar syndrome, and Nager syndrome.
Craniosynostoses are congenital conditions that include premature fusion of one or more of the cranial sutures, resulting in abnormal growth of the skull in the direction parallel to the fused suture. Craniosynostoses affect approximately one in 2500 live births and can be either syndromic or nonsyndromic. Common syndromes that include craniosynostosis include Apert, Cruzon, Pfeiffer, Nuenke, and Saerthe-Chozen syndromes.
Complications of craniofacial conditions
The burden of disease in children with craniofacial conditions is highly variable, but can be substantial. These children are often followed by multidisciplinary teams that include speech therapists, otolaryngologists, audiologists, plastic surgeons, and orthodontists, among others. Children with CLP and Pierre Robin sequence may have cognitive impairment across a range of domains, including both verbal and non-verbal domains. The etiology of cognitive delay is likely multifactorial, and may include hearing dysfunction and frequent hospitalizations. Children with craniosynostosis are also at risk for intellectual disability, with one recent study finding that school age children with syndromic craniosynostosis have an increased risk for having cognitive, motor, and language delays 5. Cognitive impairment is also seen in children with significant OSAS, but the link between reduced performance and OSAS in the craniofacial population has not been established. In children with syndromic craniosynostosis, OSAS has been shown to be related to poorer quality of life 6.
One of the most common impairments in children with craniofacial conditions is feeding. The causes can be variable depending on the patient’s underlying condition, but usually result in an uncoordinated suck-swallow-breathe pattern or an inability to maintain a good seal during sucking. This usually results in longer feeding times, and can cause a host of other medical problems, including dehydration and laryngeal penetration or aspiration. In some children with Pierre Robin sequence, early airway surgery to relieve upper airway obstruction is associated with less need for a feeding tube 7. Infants with craniofacial conditions are at risk for poor growth, especially early in life 8.
Otitis media with effusion is nearly universal in infants born with cleft palate, and without intervention, can result in a conductive hearing loss that can affect language development. In many of the craniosynostoses, mutations in fibroblast growth factor receptor genes affect inner ear morphogenesis, causing hearing loss 9.
OSAS in craniofacial conditions
Children with a variety of craniofacial conditions have been shown to be at increased risk for upper airway obstruction, but the lack of prospective studies makes the prevalence of OSAS and the causes of OSAS in this population difficult to determine.
Screening for OSAS in children with craniofacial conditions can be challenging for a number of reasons. Screening tools for OSAS are unreliable in otherwise healthy children and have not been validated in other populations that may have cognitive and hearing deficits that would alter the results of screening tools 10. One study of children with Treacher Collins syndrome found that the Brouillette questionnaire correlated poorly with polysomnographic findings 11. Another study found that the Pediatric Sleep Questionnaire was a poor predictor of OSAS in a general craniofacial population 12.
OSAS is the result of both structural factors that reduce the size of the airway as well as neuromotor deficits that impair the ability of the patient to maintain airway patency during sleep. Imaging has been shown to be a powerful tool to assess the structural differences in children with a variety of craniofacial conditions. In children with micrognathia, those who go on to experience clinical episodes of apnoea have been shown to have smaller airway measures on cephalometry than those who remain asymptomatic. 13 Early reports of mandibular distraction osteogenesis (MDO) used cephalometric imaging to demonstrate improvement in glossoptosis and more recent studies have used computed tomography to demonstrate increased oropharyngeal airway volume following distraction. 14
In addition to the structural features that predispose children with craniofacial conditions to OSAS, there is evidence that neuromotor differences also contribute. Craniofacial conditions such as cleft palate and micrognathia cause oropharyngeal muscular dysfunction, which affects swallowing, speech, and breathing. In studies of upper airway muscle function, EMG response during swallowing is reduced in children with cleft palate compared to controls. 15 In other children, micrognathia changes the normal length-tension relationship of upper airway muscles, possibly preventing them from working efficiently. In children with syndromic micrognathia, the upper airway may have increased collapsibility secondary to upper airway muscle fatigue, as a result of the upper airway muscles constantly working against an increased mechanical load with each breath. This is consistent with data from adults, showing that upper airway muscles work near their maximal range during wakefulness in patients with OSAS. 16
Cleft lip/ palate
In children with cleft palate, OSAS is thought to develop from morphologic changes that result in a small midface and retruded mandible. Children with CLP often also have nasal deformities as well, but how these affect upper airway obstruction is not clear (Figure 1). Retrospective studies have shown high rates of OSAS in a referred population with CLP, but these studies are limited by selection bias 17. A recent prospective study of 50 infants found a mean apnoea hypopnoea index (AHI) of 7.6/hour in those with non-syndromic CLP 18. Prospective studies of larger cohorts are needed to determine the true prevalence of OSAS in this population and to better understand how risk factors differ from the general pediatric population. One of the difficulties in understanding the prevalence of and risk factors for OSAS in the CLP population is the heterogeneity of the phenotype, with a range from unilateral cleft lip to a cleft that extends through both the soft and hard palate.
Figure 1. 1-week old girl with cleft lip and palate.
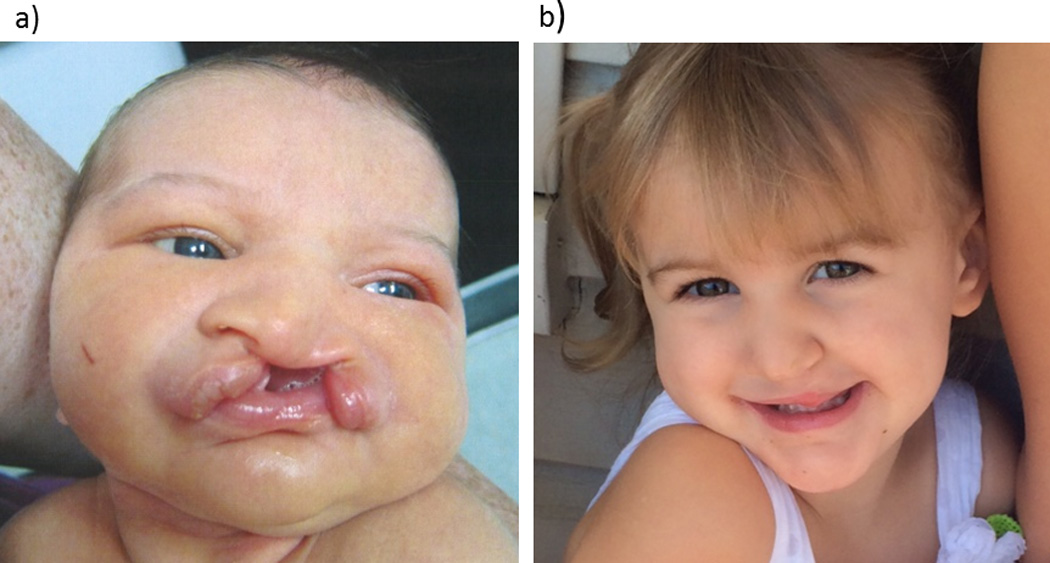
a) Despite having a complete bilateral cleft lip and palate and substantial nasal deformity, this patient did not have significant OSAS.
b) The same patient at age 2, after cleft lip and palate repair
In developed countries, children with cleft palate are treated with primary palatoplasty before or around the child’s first birthday. The effect of primary palatoplasty on upper airway obstruction has not been well studied, but there is some evidence that children who have OSAS pre-operatively are at risk for developing perioperative airway complications 19.
As many as 13% of children with cleft palate develop velopharygneal insufficiency as a result of primary palatoplasty, causing hypernasal voice and swallowing dysfunction. Correcting velopharyngeal insufficiency usually involves additional surgery, which could include posterior pharyngeal flap, dynamic sphincter pharyngoplasty, or revision Furlow palatoplasty. Obstructive sleep apnoea occurring after posterior pharyngeal flap surgery is well documented in children with cleft palate20 and there are case reports of post-operative death21 from upper airway obstruction, although the prevalence and duration of this post-operative OSAS is somewhat controversial 22. Many surgical case series report little or no OSAS following surgery but do not include PSG. There is some evidence that dynamic sphincter pharyngoplasty may result in an increased incidence of post-operative OSAS 23 and that Furlow palatoplasty may cause a mild increase in upper airway obstruction immediately following surgery 24. Velopharyngeal insufficiency can be very bothersome to patients and a balance must be struck between correcting it and the consequences of OSAS that may result. Many centers advocate evaluation for OSAS in children undergoing secondary palatoplasty.
Conditions with micrognathia
Conditions that include micrognathia are thought to cause OSAS due to obstruction at the base of the tongue from glossoptosis and reduced oropharyngeal airway size 25.
Pierre Robin sequence, a clinically-identified anomaly containing mandibular hypoplasia, glossoptosis, and a U-shaped cleft palate, is the most common cause of syndromic micrognathia. Retrospective studies have demonstrated high rates of severe OSAS in Pierre Robin sequence, but these series are limited to patients clinically referred for intervention due to upper airway obstruction 26. In one recent retrospective series, OSAS was identified in 11 of 13 infants with Pierre Robin sequence, with a mean AHI of 33.5/hour (range 0 to 85.7/hour) 27.
Similar to other conditions with underdevelopment of the mandible, patients with hemifacial microsomia have the potential for increased risk for OSAS (figure 2). One study found this population to have increased symptoms of breathing difficulties during sleep, sleepiness, and night awakenings compared to controls 28. Another study found that patients with hemifacial microsomia who have more severe mandibular deformities were at higher risk for OSAS 29.
Figure 2. Two young boys with syndromic micrognathia.
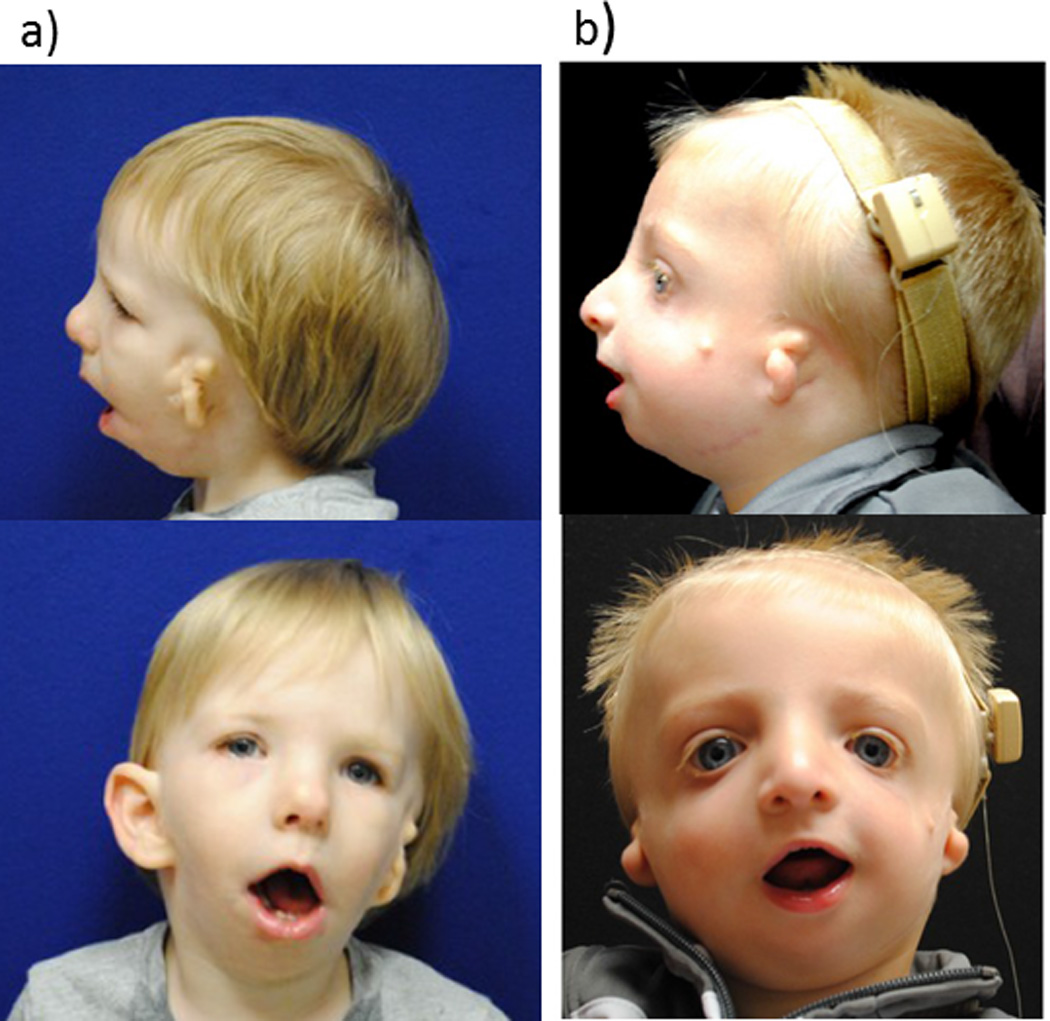
Both had severe OSAS during infancy and had mandibular distraction osteogenesis.
a) 22 month old with hemifacial microsomia. Note mandibular hypoplasia and unilateral microtia.
b) 13 month old with Treacher Collins syndrome. Note down-slanting palpebral fissures, bilateral microtia, and mandibular hypoplasia.
Children with Treacher Collins syndrome are also at high risk for OSAS due to micrognathia. Compared with children who have Pierre Robin sequence, those with Treacher Collins syndrome have longer mandibular body length and shorter mandibular ramus length . A study of 35 adults and children found the prevalence of OSAS to be 46% 30. In this series, many of the patients who had airway surgery, including MDO, had persistent OSAS post-operatively, which the authors attribute to multiple sites of obstruction identified with flexible and rigid bronchoscopy. OSAS has been shown to be linked with reduced quality of life in this population.
Craniosynostosis
Children with syndromic craniosynostosis are at increased risk for OSAS mainly due to midface hypoplasia, but other factors such as adenotonsillar hypertrophy and choanal atresia are also risk factors. Between 40 and 68 percent of children with syndromic craniosynostosis will have OSAS but the means of making this diagnosis vary between studies 31, 32. One longitudinal study found that prevalence seems to reduce with age, and children less than three years old seem to be at the highest risk and those with midface hypoplasia are more likely to have persistent OSAS. 31
Down syndrome
OSAS is highly prevalent in children with Down syndrome, with studies finding between 31 and 100 percent of children having gas exchange abnormalities on polysomnograms. Midface and mandibular hypoplasia result in narrowing of the hypopharynx and relative macroglossia, predisposing children with Down syndrome to OSAS. In addition to structural abnormalities, low muscle tone also contributes to OSAS in this population, with dynamic MRI demonstrating dynamic upper airway collapse. Higher rates of lingual tonsilar hypertrophy and obesity also contribute to the high prevalence of OSAS 33.
Achondroplasia
Achondroplasia causes short stature and craniofacial hypoplasia, including maxillary hypoplasia and depressed nasal bridge. Snoring is very common in children with achondroplasia and studies have identified between 10 and 35 percent of patients with OSAS 34. One large series found that nearly half of children referred for polysomnograms had an abnormal finding, but that hypoxaemia was the most common abnormality 35. Compared with children who have OSAS due to adenoidal hypertrophy, children with achondroplasia have radiographic evidence of upper airway narrowing and retrognathia. There are neurologic complications associated with achondroplasia, and these patients are also at risk for central sleep apnoea.
Therapies for OSAS in the paediatric craniofacial population
Multiple therapies for OSAS in children with craniofacial conditions are available and options depend on the underlying condition. While tracheostomy remains an option for those with the most severe upper airway obstruction, alternative medical and surgical therapies exist, making the need for tracheostomy rare in this patient population today.
Surgical therapies
Adenotonsillectomy is first-line therapy for many otherwise healthy children with OSAS but has not been well-studied as a therapy in the craniofacial population. However, even in the craniofacial population, adenotonsillectomy may be helpful by widening the upper airway. Adenotonsillar hypertrophy should be suspected as an exacerbating factor in children with craniofacial anomalies who present with symptoms of OSAS in later childhood rather than at birth. With adenoidectomy, there is the potential risk for exacerbating velopharyngeal insufficiency, so some surgeons favor a procedure that involves removing the superior and leaving the inferior rim of the adenoid tissue to maintain speech. One study found that in children with cleft palate who had primary palatoplasty, tonsillectomy and/or partial adenoidectomy was generally effective, but the cleft status and surgical techniques varied considerably among the 17 patients 36. In children with Down syndrome, adenotonsillectomy should be considered as a first-line therapy in children with OSAS, but it has a much lower success rate than in otherwise healthy children 33. In children with achondroplasia, there is a high rate of recurrence of OSAS after adenoidectomy but adenotonsillectomy may be more successful 37. More studies are needed to better understand which populations would benefit most by adenotonsillectomy. The impact of adenotonsillectomy on OSA in children with craniosynostosis is largely unknown; one recent study of five patients showed improvement in OSAS in two following surgery 38
MDO is a surgical procedure that involves bilateral corticotomy of the mandible, insertion of either internal or external metal distractors, and gradual distraction of the mandible while new bone forms in the gap. There is some variability in technique in terms of the time and length of distraction. Mandibular lengthening with MDO causes advancement of the tongue base at the site of the attachment of the genioglossus to the mandible, increasing the size of the upper airway. Complications from MDO include infection, jaw malocclusion, hypertrophic scarring, and injury to the surrounding nerves or teeth in as many as 23% 39. The effect on mandibular growth plates is not entirely known. MDO is an effective alternative to tracheostomy in children with micrognathia and severe obstructive apnoea. One meta-analysis found that 84% of children who were tracheostomy-dependent were able to be decannulated following MDO 39. There are reports of MDO being used successfully to treat OSAS in age groups ranging from infants to adults. Early studies of MDO in young children reported high rates of clinical resolution of symptoms 40 and subsequent studies involving PSG have shown substantial improvement in OSAS in the majority of patients. 41 MDO has been shown to increase not only the length of the mandible ramus and body but also the airway volume in the hypopharynx. 14 Still, there are no large prospective studies of MDO in children with micrognathia.
Tongue-lip adhesion is a surgical procedure that attempts to correct glossoptosis causing upper airway obstruction in children with Pierre Robin sequence and other similar conditions. This procedure involves securing the tongue to the mucosa and muscle of the lower lip, and does not address any skeletal malformations. This procedure was first proposed in 1946 and for many years was the only surgical alternative to tracheostomy for severe upper airway obstruction. The impact of tongue-lip adhesion on OSA in children with micrognathia has not been carefully studied, but surgical case series suggest that it offers improvement in OSAS but incomplete resolution in most patients 42. Recent studies have compared surgical outcomes of tongue-lip adhesion with MDO. Although no large prospective studies have been performed, available data suggests that tongue-lip adhesion results in a shorter hospital stay but MDO results in improved OSAS and earlier return to oral feeding 43, at least in non-syndromic infants with micrognathia. Infants undergoing MDO may be less likely to require a second surgery to correct persistent upper airway obstruction 41.
In patients with craniosynostosis who have substantial midface hypoplasia, surgical advancement of the midface can be used to enlarge the upper airway, often using a Le Fort II or Le Fort III procedure that involves internal or external distraction devices. These surgeries involve osteotomies and advancement of the maxilla and midface. Other methods exist, including using an external transfacial pin for distraction. One study found that midface distraction substantially reduced the AHI and improved the oxygen saturation in a cohort of 11 young children with Pfeiffer, Cruzon, or Apert syndrome and severe OSAS 44.
Non-surgical therapies
Positive airway pressure is first-line therapy for many adults with OSAS and for otherwise healthy children where adenotonsillectomy is not curative. Continuous positive airway pressure (CPAP) may be challenging to use in children with craniofacial conditions for several reasons. In infants, there is a lack of available interfaces to fit children with normal craniofacial anatomy, and infants with craniofacial syndromes will be even harder to accommodate. The same may be true for older children, particularly those with significant nasal deformities. Some children with craniofacial conditions will have had multiple facial surgeries and may have an increased sensitivity to a mask on their face. CPAP has been described as successful therapy for children who developed upper airway obstruction following secondary palatoplasty 45 and in several patients with micrognathia 46. One study found that children with craniofacial conditions did not require higher CPAP pressures than other children to achieve control of OSAS 47. More research is needed to understand whether CPAP is an effective treatment for OSAS in patients with velopharyngeal insufficiency, and in other populations such as those with micrognathia.
When OSAS is only present when the patient is supine, sleeping in different position could provide effective therapy. Lateral or prone positioning in infants with micrognathia or glossoptosis has been used since the days of Pierre Robin and has been reported to be successful in treating upper airway obstruction 48, but polysomnographic evidence of success has not been demonstrated. Studies that include polysomnographic recording in multiple positions are needed to evaluate the efficacy of positional therapy in this population. This therapy is difficult to maintain once an infant is able to roll over.
A nasopharyngeal airway is a tube inserted through the naris that extends to the oropharynx and acts as a stent of the upper airway, physically preventing collapse. One study of young children with syndromic craniosynostosis found that using a nasopharyngeal airway for long-term management of upper airway obstruction both successfully treated OSAS and improved quality of life 49. More studies are needed to evaluate the long-term safety and efficacy of this modality. Frequent suctioning or replacement of the stents may be needed to maintain patency.
In craniofacial conditions where there are structural abnormalities, it is unclear how growth affects the degree of upper airway obstruction as the child develops from infancy to adulthood. There are case reports of spontaneous resolution of OSAS with growth in children with Pierre Robin sequence 50 but the long-term effect of growth on upper airway obstruction both on those who have had surgery and those who have not remains unknown. There is some evidence that as children with craniosynostosis grow, there is reduction in AHI 31. However, waiting for children to ‘outgrow’ OSAS could result in significant morbidity and is not recommended.
Future directions
Despite the foundation of knowledge that exists regarding OSAS in the craniofacial population, there are substantial gaps that need further exploration. Specifically, there is a need for clinical studies that are larger, include longer-term follow up, and include PSG. Additionally, more mechanistic studies are needed to better understand the structural and neuromotor risk factors associated with OSAS in this population and the benefits of interventions, including surgery. Most of the available evidence concerning OSAS in the craniofacial population, not surprisingly, comes from retrospective studies of surgical cohorts. Unfortunately, by the nature of these studies, many of the patients with less severe OSAS will be excluded. Not including patients with micrognathia deemed not severe enough to require corrective surgery make the true prevalence of OSAS in this population difficult to estimate. In cleft palate studies, often the only patients to undergo PSG are those where there is a clinical concern for OSAS.
There have been no randomized controlled trials comparing surgical techniques in the craniofacial population. Such studies are needed to help surgeons better understand the impact of different procedures for correction of velopharyngeal insufficiency and micrognathia, among others.
Until recently, respiratory assessment for craniofacial patients has largely been clinical and somewhat subjective. In many studies, post-operative determination of OSAS was based on parental report. Unfortunately, studies have shown that clinical assessment is often unreliable for detecting OSAS in children, especially in complex populations like those with craniofacial syndromes. With PSG widely available, even for infant populations, and considered the gold standard for assessment of pediatric OSAS 1, it should be included in the evaluation of craniofacial patients when OSAS is being assessed.
In addition to a lack of prospective studies, the literature on OSAS in children with craniofacial conditions is limited by the size of the studies. Multicenter studies or multi-institutional databases would be useful in pooling data, particularly for rare syndromes. For example, patients with micrognathia and glossoptosis who have different underlying syndromes may not respond to mandibular distraction in the same way because of a variety of factors, including airway tone and midface hypoplasia.
Another difficulty in interpreting the literature on OSAS in this population is the lack of long-term studies. Since children with cleft palate and craniofacial syndromes are often followed lifelong by craniofacial centers, the opportunity for studies assessing OSAS as these children grow following surgery exists. This would allow us to answer questions such as whether OSAS following secondary palatoplasty persists, and whether OSAS reoccurs after MDO due to poor post-operative mandibular growth. Other longitudinal outcomes that need to be assessed include swallowing, cognition, and growth.
Understanding which children with craniofacial conditions are at risk for OSAS, what puts them at risk, and what the best therapies are for OSAS in this population will help improve the care of these complex patients. Better understanding of OSAS in this population will lead to a better understanding of which patients to evaluate, when to evaluate them, and by what means. This type of research can help improve the standard of care for these patients.
Conclusions
OSAS is common in children, and children with a variety of craniofacial conditions are at increased risk for OSAS due to abnormalities in both their facial structure as well as likely upper airway neuromotor deficits. Cleft lip and palate may predispose children to having OSAS; secondary palatoplasty for velopharyngeal insufficiency seems to substantially increase the risk for OSAS. Conditions with micrognathia, including Pierre Robin sequence, result in a high rate of OSAS. Similarly, children with syndromic craniosynostosis have high rates of OSAS. A variety of treatment options, both surgical and more conservative, exist for OSAS in this population. The current evidence is based mostly on retrospective reports; more prospective studies are needed to better understand this population.
Table 1.
mechanisms of OSAS in children with select craniofacial conditions
| Condition | Potential causes of OSAS |
|---|---|
| Craniofacial cleft | Short mandible, retrognathia ± nasal deformity |
| Syndromic micrognathia (i.e. Pierre Robin Sequence, Stickler syndrome, etc) | Micrognathia, glossoptosis, ± midface hypoplasia |
| Craniosynostosis | Midface hypoplasia |
| Down syndrome | Relative macroglossia, midface hypoplasia, reduced muscle tone |
| Achondroplasia | Midface hypoplasia, retrognathia |
Table 2.
Surgical therapies for OSAS in children with craniofacial conditions
| Condition | Potential surgical therapies |
|---|---|
| Craniofacial cleft | Adenotonsillectomy |
| Syndromic micrognathia (i.e. Pierre Robin Sequence, Stickler syndrome, etc) | First line: mandibular distraction osteogenesis Second line: tongue-lip adhesion |
| Craniosynostosis | Midface advancement |
| Down syndrome | Adenotonsillectomy |
| Achondroplasia | Adenotonsillectomy, midface advancement |
Educational aims
To understand how obstructive sleep apnoea syndrome (OSAS) affects children with different craniofacial conditions
To become familiar with treatment options available to children with craniofacial conditions who have OSAS
To identify limitations in available data and the areas that require additional research
Practice Points
Children with craniofacial conditions may be at increased risk for OSAS although the mechanisms are not entirely clear
Children with cleft palate should be carefully monitored for OSAS, especially after secondary palatoplasty
Therapy for OSAS in children with craniofacial conditions includes both surgical and non-surgical options and should be customized for each individual patient
The current evidence base for evaluation and treatment of OSAS in these patients is mostly based on retrospective series.
Research Directions
Longitudinal studies including PSG of OSAS in children with craniofacial conditions
Mechanistic studies to better understand the causes of OSAS in these patients
Randomized controlled trials of surgical techniques for correction of OSAS
Multicenter studies of rare conditions
Educational Questions.
1. Which of the following is true regarding children with craniofacial syndromes?
The Pediatric Sleep Questionnaire is a good tool to screen for OSAS.
Children with craniofacial syndromes should have annual screening polysomnograms.
The presence of a craniofacial syndrome is a risk factor for OSAS.
The prevalence of OSAS in children with craniofacial syndromes increases with age
None of the above.
2. With regard to children with micrognathia:
Tongue-lip adhesion has been shown to be superior to mandibular distraction osteogenesis in treating severe OSAS
OSAS is relatively uncommon in infants with Pierre Robin sequence
Adenotonsillectomy is effective treatment for most infants with severe OSAS and syndromic micrognathia
CPAP is the preferred first-line therapy for OSAS in infants with micrognathia.
None of the above
3. The following is true in children with cleft palate:
Secondary palatoplasty for velopharyngeal insufficiency generally cures OSAS
Primary palatoplasty results in OSAS in many children with cleft palate
Most occur as part of an underlying syndrome
The morphologic cause of OSAS in children with cleft lip and palate is not well understood
None of the above
4. With regard to craniosynostoses:
They result in abnormal growth of the skull in the direction parallel to the fused suture(s).
Mandibular hypoplasia is the primary cause of OSAS in this population
Mandibular distraction osteogenesis is the first-line surgical therapy
Children over 3 years old are at the greatest risk for OSAS
None of the above.
5. All of the following are true regarding OSAS in children EXCEPT:
Prevalence of OSAS in the general pediatrics population is between 1.2 and 5.8%
Children with Down syndrome are at increased risk of OSAS
Untreated OSAS can result in difficulty behavioral deficits and ventricular remodeling
Unlike adults, obesity is not a risk factor for OSAS in children
First line therapy for otherwise healthy children with OSAS is adenotonsillectomy
Acknowledgements
Dr. Cielo is supported by KL2 TR000139.
Conflict of interest statement
CLM has research support from Philips Respironics and Ventus, unrelated to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher M. Cielo, 9NW50, Main Hospital, The Children’s Hospital of Philadelphia, 34th and Civic Center Boulevard, Philadelphia, PA 19104, cieloc@email.chop.edu.
Carole L. Marcus, 9NW50, Main Hospital, The Children’s Hospital of Philadelphia, 34th and Civic Center Boulevard, Philadelphia, PA 19104, marcus@email.chop.edu.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):1–9. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker LA, Pashayan H, Reichman J. A proposed new classification of craniofacial anomalies. Cleft Palate J. 1981 Jul;18(3):161–176. [PubMed] [Google Scholar]
- 4.Christensen K, Mitchell LE. Familial recurrence-pattern analysis of nonsyndromic isolated cleft palate--a Danish Registry study. Am J Hum Genet. 1996 Jan;58(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- 5.Maliepaard M, Mathijssen IM, Oosterlaan J, Okkerse JM. Intellectual, behavioral, and emotional functioning in children with syndromic craniosynostosis. Pediatrics. 2014 Jun;133(6):e1608–e1615. doi: 10.1542/peds.2013-3077. [DOI] [PubMed] [Google Scholar]
- 6.Bannink N, Maliepaard M, Raat H, Joosten KF, Mathijssen IM. Obstructive sleep apneaspecific quality of life and behavioral problems in children with syndromic craniosynostosis. Journal of developmental and behavioral pediatrics : JDBP. 2011 Apr;32(3):233–238. doi: 10.1097/DBP.0b013e318206d5e3. [DOI] [PubMed] [Google Scholar]
- 7.Lidsky ME, Lander TA, Sidman JD. Resolving feeding difficulties with early airway intervention in Pierre Robin Sequence. Laryngoscope. 2008 Jan;118(1):120–123. doi: 10.1097/MLG.0b013e31815667f3. [DOI] [PubMed] [Google Scholar]
- 8.Daniel M, Bailey S, Walker K, Hensley R, Kol-Castro C, Badawi N, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol. 2013 Apr;77(4):499–503. doi: 10.1016/j.ijporl.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Agochukwu NB, Solomon BD, Muenke M. Hearing loss in syndromic craniosynostoses: introduction and consideration of mechanisms. Am J Audiol. 2014 Jun 1;23(2):135–141. doi: 10.1044/2014_AJA-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011 Feb;15(1):19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plomp RG, Joosten KF, Wolvius EB, Hoeve HL, Poublon RM, van Montfort KA, et al. Screening for obstructive sleep apnea in Treacher-Collins syndrome. Laryngoscope. 2012 Apr;122(4):930–934. doi: 10.1002/lary.23187. [DOI] [PubMed] [Google Scholar]
- 12.Cielo CM, Silvestre J, Paliga JT, Maguire M, Gallagher PR, Marcus CL, et al. Utility of screening for obstructive sleep apnea syndrome in children with craniofacial disorders. Plast Reconstr Surg. 2014 Sep;134(3):434e–441e. doi: 10.1097/PRS.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn TR, Tonkin SL, Hadden W, Davis SL, Gunn AJ. Neonatal micrognathia is associated with small upper airways on radiographic measurement. Acta Paediatr. 2000 Jan;89(1):82–87. [PubMed] [Google Scholar]
- 14.Roy S, Munson PD, Zhao L, Holinger LD, Patel PK. CT analysis after distraction osteogenesis in Pierre Robin Sequence. Laryngoscope. 2009 Feb;119(2):380–386. doi: 10.1002/lary.20011. [DOI] [PubMed] [Google Scholar]
- 15.Nagaoka K, Tanne K. Activities of the muscles involved in swallowing in patients with cleft lip and palate. Dysphagia. 2007 Apr;22(2):140–144. doi: 10.1007/s00455-006-9067-y. [DOI] [PubMed] [Google Scholar]
- 16.Series F, Straus C, Demoule A, Attali V, Arnulf I, Derenne JP, et al. Assessment of upper airway dynamics in awake patients with sleep apnea using phrenic nerve stimulation. Am J Respir Crit Care Med. 2000 Sep;162(3 Pt 1):795–800. doi: 10.1164/ajrccm.162.3.9906135. [DOI] [PubMed] [Google Scholar]
- 17.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008 Feb;118(2):348–353. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 18.Maclean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012 Dec;97(12):1058–1063. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 19.Smith D, Abdullah SE, Moores A, Wynne DM. Post-operative respiratory distress following primary cleft palate repair. J Laryngol Otol. 2013 Jan;127(1):65–66. doi: 10.1017/S0022215112002563. [DOI] [PubMed] [Google Scholar]
- 20.Liao YF, Noordhoff MS, Huang CS, Chen PK, Chen NH, Yun C, et al. Comparison of obstructive sleep apnea syndrome in children with cleft palate following Furlow palatoplasty or pharyngeal flap for velopharyngeal insufficiency. Cleft Palate Craniofac J. 2004 Mar;41(2):152–156. doi: 10.1597/02-162. [DOI] [PubMed] [Google Scholar]
- 21.Kravath RE, Pollak CP, Borowiecki B, Weitzman ED. Obstructive sleep apnea and death associated with surgical correction of velopharyngeal incompetence. J Pediatr. 1980 Apr;96(4):645–648. doi: 10.1016/s0022-3476(80)80730-x. [DOI] [PubMed] [Google Scholar]
- 22.Griner D, Sargent LA, Overmeyer CL. Changes in airflow dynamics after creation of pharyngeal flaps in nonsyndromic children. Ann Plast Surg. 2013 May;70(5):517–520. doi: 10.1097/SAP.0b013e31827f52eb. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger RE, Oppenheimer AJ, Lau D, Hassan F, Newman MH, Buchman SR, et al. Obstructive sleep apnea after dynamic sphincter pharyngoplasty. J Craniofac Surg. 2012 Nov;23(7 Suppl 1):1974–1976. doi: 10.1097/SCS.0b013e31825b3ba9. [DOI] [PubMed] [Google Scholar]
- 24.Liao YF, Yun C, Huang CS, Chen PK, Chen NH, Hung KF, et al. Longitudinal follow-up of obstructive sleep apnea following Furlow palatoplasty in children with cleft palate: a preliminary report. Cleft Palate Craniofac J. 2003 May;40(3):269–273. doi: 10.1597/1545-1569_2003_040_0269_lfoosa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 25.Bookman LB, Melton KR, Pan BS, Bender PL, Chini BA, Greenberg JM, et al. Neonates with tongue-based airway obstruction: a systematic review. Otolaryngol Head Neck Surg. 2012 Jan;146(1):8–18. doi: 10.1177/0194599811421598. [DOI] [PubMed] [Google Scholar]
- 26.Wilson AC, Moore DJ, Moore MH, Martin AJ, Staugas RE, Kennedy JD. Late presentation of upper airway obstruction in Pierre Robin sequence. Arch Dis Child. 2000 Nov;83(5):435–438. doi: 10.1136/adc.83.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011 Sep;48(5):614–618. doi: 10.1597/10-100. [DOI] [PubMed] [Google Scholar]
- 28.Cloonan YK, Kifle Y, Davis S, Speltz ML, Werler MM, Starr JR. Sleep outcomes in children with hemifacial microsomia and controls: a follow-up study. Pediatrics. 2009 Aug;124(2):e313–e321. doi: 10.1542/peds.2008-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SR, Levitt CA, Simms C, Burstein FD. Airway disorders in hemifacial microsomia. Plast Reconstr Surg. 1999 Jan;103(1):27–33. doi: 10.1097/00006534-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Plomp RG, Bredero-Boelhouwer HH, Joosten KF, Wolvius EB, Hoeve HL, Poublon RM, et al. Obstructive sleep apnoea in Treacher Collins syndrome: prevalence, severity and cause. Int J Oral Maxillofac Surg. 2012 Jun;41(6):696–701. doi: 10.1016/j.ijom.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Driessen C, Joosten KF, Bannink N, Bredero-Boelhouwer HH, Hoeve HL, Wolvius EB, et al. How does obstructive sleep apnoea evolve in syndromic craniosynostosis? A prospective cohort study. Arch Dis Child. 2013 Jul;98(7):538–543. doi: 10.1136/archdischild-2012-302745. [DOI] [PubMed] [Google Scholar]
- 32.Lo LJ, Chen YR. Airway obstruction in severe syndromic craniosynostosis. Ann Plast Surg. 1999 Sep;43(3):258–264. doi: 10.1097/00000637-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Rosen D. Management of obstructive sleep apnea associated with Down syndrome and other craniofacial dysmorphologies. Curr Opin Pulm Med. 2011 Nov;17(6):431–436. doi: 10.1097/MCP.0b013e32834ba9c0. [DOI] [PubMed] [Google Scholar]
- 34.Afsharpaiman S, Saburi A, Waters KA. Respiratory difficulties and breathing disorders in achondroplasia. Paediatr Respir Rev. 2013 Dec;14(4):250–255. doi: 10.1016/j.prrv.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Mogayzel PJ, Jr, Carroll JL, Loughlin GM, Hurko O, Francomano CA, Marcus CL. Sleep-disordered breathing in children with achondroplasia. J Pediatr. 1998 Apr;132(4):667–671. doi: 10.1016/s0022-3476(98)70358-0. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Aziz M. The effectiveness of tonsillectomy and partial adenoidectomy on obstructive sleep apnea in cleft palate patients. Laryngoscope. 2012 Nov;122(11):2563–2567. doi: 10.1002/lary.23507. [DOI] [PubMed] [Google Scholar]
- 37.Sisk EA, Heatley DG, Borowski BJ, Leverson GE, Pauli RM. Obstructive sleep apnea in children with achondroplasia: surgical and anesthetic considerations. Otolaryngol Head Neck Surg. 1999 Feb;120(2):248–254. doi: 10.1016/S0194-5998(99)70414-6. [DOI] [PubMed] [Google Scholar]
- 38.Willington AJ, Ramsden JD. Adenotonsillectomy for the management of obstructive sleep apnea in children with congenital craniosynostosis syndromes. J Craniofac Surg. 2012 Jul;23(4):1020–1022. doi: 10.1097/SCS.0b013e31824e6cf8. [DOI] [PubMed] [Google Scholar]
- 39.Tahiri Y, Viezel-Mathieu A, Aldekhayel S, Lee J, Gilardino M. The effectiveness of mandibular distraction in improving airway obstruction in the pediatric population. Plast Reconstr Surg. 2014 Mar;133(3):352e–359e. doi: 10.1097/01.prs.0000438049.29258.a8. [DOI] [PubMed] [Google Scholar]
- 40.Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001 Aug;108(2):302–311. doi: 10.1097/00006534-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Flores RL, Tholpady SS, Sati S, Fairbanks G, Socas J, Choi M, et al. The surgical correction of Pierre Robin sequence: mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg. 2014 Jun;133(6):1433–1439. doi: 10.1097/PRS.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 42.Sedaghat AR, Anderson IC, McGinley BM, Rossberg MI, Redett RJ, Ishman SL. Characterization of obstructive sleep apnea before and after tongue-lip adhesion in children with micrognathia. Cleft Palate Craniofac J. 2012 Jan;49(1):21–26. doi: 10.1597/10-240. [DOI] [PubMed] [Google Scholar]
- 43.Papoff P, Guelfi G, Cicchetti R, Caresta E, Cozzi DA, Moretti C, et al. Outcomes after tongue-lip adhesion or mandibular distraction osteogenesis in infants with Pierre Robin sequence and severe airway obstruction. Int J Oral Maxillofac Surg. 2013 Nov;42(11):1418–1423. doi: 10.1016/j.ijom.2013.07.747. [DOI] [PubMed] [Google Scholar]
- 44.Mitsukawa N, Kaneko T, Saiga A, Akita S, Satoh K. Early midfacial distraction for syndromic craniosynostotic patients with obstructive sleep apnoea. J Plast Reconstr Aesthet Surg. 2013 Sep;66(9):1206–1211. doi: 10.1016/j.bjps.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 45.Witt PD, Marsh JL, Muntz HR, Marty-Grames L, Watchmaker GP. Acute obstructive sleep apnea as a complication of sphincter pharyngoplasty. Cleft Palate Craniofac J. 1996 May;33(3):183–189. doi: 10.1597/1545-1569_1996_033_0183_aosaaa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 46.Miller SD, Glynn SF, Kiely JL, McNicholas WT. The role of nasal CPAP in obstructive sleep apnoea syndrome due to mandibular hypoplasia. Respirology. 2010 Feb;15(2):377–379. doi: 10.1111/j.1440-1843.2009.01681.x. [DOI] [PubMed] [Google Scholar]
- 47.Marcus CL, Ward SLD, Mallory GB, Rosen CL, Beckerman RC, Weesemayer DE, et al. Use of Nasal Continuous Positive Airway Pressure as Treatment of Childhood Obstructive Sleep-Apnea. Journal of Pediatrics. 1995 Jul;127(1):88–94. doi: 10.1016/s0022-3476(95)70262-8. [DOI] [PubMed] [Google Scholar]
- 48.Meyer AC, Lidsky ME, Sampson DE, Lander TA, Liu M, Sidman JD. Airway interventions in children with Pierre Robin Sequence. Otolaryngol Head Neck Surg. 2008 Jun;138(6):782–787. doi: 10.1016/j.otohns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Randhawa PS, Ahmed J, Nouraei SR, Wyatt ME. Impact of long-term nasopharyngeal airway on health-related quality of life of children with obstructive sleep apnea caused by syndromic craniosynostosis. J Craniofac Surg. 2011 Jan;22(1):125–128. doi: 10.1097/SCS.0b013e3181f6f82c. [DOI] [PubMed] [Google Scholar]
- 50.Kiely JL, Deegan PC, McNicholas WT. Resolution of obstructive sleep apnoea with growth in the Robin sequence. Eur Respir J. 1998 Aug;12(2):499–501. doi: 10.1183/09031936.98.12020499. [DOI] [PubMed] [Google Scholar]


