Abstract
Allergic asthma is a chronic, inflammatory lung disease. Some forms of allergic asthma are characterized by Th2-driven eosinophilia while others are distinguished by Th17-driven neutrophilia. Stimulation of Toll-like receptor 4 (TLR4) on hematopoietic and airway epithelial cells (AECs) contributes to the inflammatory response to lipopolysaccharide (LPS) and allergens, but the specific contribution of TLR4 in these cell compartments to airway inflammatory responses remains poorly understood. We used novel, conditionally mutant Tlr4fl/fl mice to define the relative contributions of AEC and hematopoietic cell Tlr4 expression to LPS- and allergen-induced airway inflammation. We found that Tlr4 expression by hematopoietic cells is critical for neutrophilic airway inflammation following LPS exposure and for Th17-driven neutrophilic responses to the house dust mite (HDM) lysates and ovalbumin (OVA). Conversely, Tlr4 expression by AECs was found to be important for robust eosinophilic airway inflammation following sensitization and challenge with these same allergens. Thus, Tlr4 expression by hematopoietic and airway epithelial cells controls distinct arms of the immune response to inhaled allergens.
Introduction
Allergic asthma is an increasingly prevalent disease that is characterized by chronic inflammation of the airway and reversible airway obstruction. Asthma is widely considered to stem from allergen-specific T helper (Th)2 responses that result in eosinophilic inflammation, but, in many individuals, neutrophils are the predominant leukocytes in the airway (1). Several studies have suggested that neutrophilic forms of asthma arise from allergen-specific Th17 responses (2, 3), and are characterized by airway hyper-responsiveness and resistance to glucocorticoid therapy (4–6). These findings have been replicated in mouse models of experimental allergic asthma (4, 7). Defining the cellular and molecular events that promote Th2 and Th17 responses to inhaled allergens will likely be critical for developing novel, effective strategies targeting specific subtypes of asthma.
A large body of evidence suggests that many allergens trigger maladaptive immune responses by direct interactions with innate immune receptors such as TLRs (8). The best-characterized TLR in this regard is TLR4, which signals in response to LPS, a membrane component of Gram negative bacteria (9). However, the relationship between environmental LPS and asthma is complex. Whereas some epidemiologic studies have linked LPS exposure to an increased prevalence of asthma (10, 11), other studies have suggested that exposure to LPS decreases the risk of developing allergic asthma (12). Mouse models of asthma have confirmed a role for LPS during allergic sensitization to experimental allergens, with very high doses of inhaled LPS triggering Th1 responses to OVA, and lower doses promoting Th2 and Th17 responses (13, 14). In addition to LPS, some bona fide allergens display structural and functional homology to components of the TLR4 receptor complex, and can directly trigger TLR4 signaling and consequent allergic sensitization (15). Despite this wealth of evidence supporting an important role for TLR4 in the development of allergic responses, the specific function of this receptor during allergic sensitization remains unclear. In particular, studies employing bone marrow chimera techniques mice have led to diverse conclusions regarding the relative contribution of different Tlr4-expressing cell populations to LPS or allergen responsiveness (16–18). Unfortunately, experiments of this type are confounded by the persistence of large numbers of host macrophages and dendritic cells (DCs) after irradiation (19). Furthermore, although AECs are widely regarded as radio-resistant, radiation induces profound transcriptional changes in these cells that have unknown effects on their function (20). Thus, while studies of irradiated bone marrow chimeric mice are useful, they have inherent limitations.
Here, we describe the creation of conditionally mutant Tlr4 mice that can be used to delete Tlr4 in distinct cell compartments without the complications associated with irradiation. Our studies revealed that Tlr4 expression by hematopoietic cells controls neutrophilic responses to inhaled LPS and allergens, whereas expression of this receptor by AECs is important for eosinophilic responses to inhaled allergens.
Materials and Methods
Mice
Animals were housed under specific pathogen-free conditions at the National Institute of Environmental Health Science or Cincinnati Children’s Research Foundation and used between 5 and 12 weeks of age. All animal experiments were conducted in accordance with the Institutional Animal Care and Use Committee of the respective institutions.
Mice expressing Cre recombinase in the Shh locus (B6.Cg-Shhtm1(EGFP/cre)Cjt/J) or from the Vav1 promoter B6.Cg-Tg(Vav1-Cre)A2Kio/J, as well as C57BL/6 J, Tlr4−/− (B6.B10ScN-Tlr4lps-del/JthJ), B6(Cg)-Tyrc-2J/J, B6.Cg-Tg(ACTFLPe)9205Dym/J mice were purchased from Jackson Laboratories, Bar Harbor, ME. Cre-expressing mice were bred to conditionally mutant, Tlr4fl/fl mice generated in collaboration with the Gene Targeted Mouse Service Core at the University of Cincinnati. The Tlr4 targeting vector included two LoxP sites flanking exon 3, an FRT-flanked neomycin resistance gene (Neo) for positive selection, a thymidine kinase gene for negative selection, and 1.9 kb and 3.4 kb arms of genome-derived DNA homologous with the Tlr4 locus. The targeting vector was introduced by electroporation into C57BL/6 mouse embryonic stem (ES) cells, and drug-resistant clones were screened by a PCR assay specific for the targeted Tlr4 gene. Two cell lines whose correctly targeted Tlr4fl locus had been confirmed by Southern blot analysis were used to generate chimeras by injection into blastocysts from albino C57BL/6 (B6(Cg)-Tyrc-2J/J) mice. Two such chimeras were chosen to breed with B6(Cg)-Tyrc-2J/J mice, and ES cell-derived offspring were identified by their black coat color. Black mice bearing the targeted Tlr4 gene were then bred to FlpE recombinase-encoding B6.Cg-Tg(ACTFLPe)9205Dym/J mice to delete neo from the Tlr4 locus. Offspring with the target Tlr4 locus, but lacking neo, were crossed to B6.Cg-Tg(Vav1-cre)A2Kio/J and B6.Cg-Shhtm1(EGFP/cre)Cjt/J mice and the offspring interbred to obtain homozygous Tlr4fl/fl mice with the desired Cre-expressing transgene.
Reciprocal bone marrow chimeric animals were generated using WT C57BL/6J and Tlr4−/− donors and recipient mice. Recipient animals were irradiated at 6 to 8 weeks of age with 900 rads over 12 minutes and injected 1 day later with 107 donor bone marrow cells. Mice were given acidified water, supplemented with 500 μg/ml neomycin for 2 weeks post-irradiation. The animals were used in experiments after allowing for a minimum of 10 weeks for hematopoietic reconstitution, as confirmed by flow cytometry (data not shown).
Models of airway disease
Mice were anesthetized with inhaled isoflurane and all reagents were administered via intratracheal aspiration (21). To measure innate immune responses, mice were administered a single dose of 100 μg of Dermatophagoides pteronyssinus (Der p) extracts (Greer Laboratories, Lenoir, NC) or 1 μg of TLR4-specific ultrapure LPS (Invivogen, San Diego, CA) in a total volume of 40 μl, and bronchoalveolar lavage (BAL) was performed at 2, 4, 6, 8 or 24 hours after challenge. For the model of HDM-mediated asthma, mice were given 100 μg Der p extracts on days 0, 7, 14, and BAL performed on day 17. For the OVA/LPS model of asthma, mice were given 100 μg LPS-depleted OVA (BioVendor Inc. Candler, NC) together with 100 ng LPS from E. coli (Sigma, St. Louis, MO) on days 0, 7, and 14 and BAL was performed on day 16. Cell differential analysis was carried out as described previously (21). Lungs were cultured for 24 hours in cRPMI (10% FBS, 0.1% 2-mercaptoethanol, 1000U/L Penicillin/Streptomycin, 10–30 μg/mL HDM or 10 μg/mL OVA), and cytokines in lung culture supernatants and BAL fluid (BALF) were quantified using Luminex or ELISA.
In vivo cytokine capture assay
Systemic cytokines were quantified using the In Vivo Cytokine Capture Assay (IVCCA) as previously described (22). All antibodies and standards were from eBioscience, San Diego, CA.
Airway epithelial cell cultures
Airway epithelial cells (AECs) were isolated and cultured as previously described (23). Briefly, 5 week-old tracheas were excised and disaggregated by 0.1% pronase (Roche) and DNase I (Sigma) digestion, followed by fibroblast removal by plastic adherence. MTECs were submersion cultured on type I collagen-coated, 0.4 μm pore transwell inserts (BD Pharmingen) until formation of tight junctions (R ≥ 1,000Ω; 7–14 days). An air-liquid interphase was established and maintained with daily basolateral media changes for 7 days before the AECs were stimulated apically with 10–100 ng/mL TLR4-specific ultrapure E. coli K12 LPS, 100 ng/mL Pam3CSK4 (Invivogen), 30 mg/mL HDM (Greer Laboratories) or vehicle control. Basolateral chemokine and cytokine production was quantified by commercially available Luminex or ELISA assays.
Bone marrow-derived dendritic cell culture
Dendritic cells were generated from bone marrow using standard protocols as previously described (24). Cells were stimulated with LPS or Pam3Cys at the indicated concentrations for 24hrs and cytokine levels in supernatants were quantified by commercially available ELISA.
Cytokines
Cytokine concentrations in sample supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) using commercially available ELISA kits from R&D Systems and BD Biosciences, or using Milliplex™ Multiplex kits (Millipore, Billerica, MA) according to the manufacturer’s protocol and read using luminex technology on the Bio-Plex™ (Bio-Rad, Hercules, CA). Concentrations were calculated from standard curves using recombinant proteins and expressed in pg(ng)/ml.
Gene expression analysis by qPCR
RNA was extracted using TRIzol Reagent (Invitrogen) according to manufacturer instructions. cDNA was generated as previously described (25) and subjected to qPCR analysis using Light Cycler 480 II (Roche). Sybr Green I Master mix (Roche) and the following primers pairs were used: TLR4: 5′-CATCCAGGAAGGCTTCCACA-3′ and 5′-GGCGATACAATTCCACCTGC-3′; β-actin: 5′-GCCCCAGAGCAAGAGAGGTA-3′ and 5′-GGTTGGCCTTAGGTTTCAGG-3′.
Genomic DNA analysis
DNA was isolated from cells and tissues using the QIAamp DNA Mini Kit (Qiagen) according to manufacturer instructions and analyzed by PCR using the following primers: TLR4: 5′-CATCCAGGAAGGCTTCCACA-3′ and 5′-GGCGATACAATTCCACCTGC-3′; β-actin: 5′-CGCTCAGGAGGAGCAATGAT-3′ and 5′-TCCTTAGCTTGGTGAGGGTG-3′. Products were run on a 2% agarose gel and imaged.
Statistics
Data were analyzed by One-Way ANOVA, followed by Tukey’s correction for multiple comparisons, or by unpaired Student’s t-test, as appropriate.
Results
Generation of mice lacking Tlr4 expression by airway epithelial or hematopoietic cells
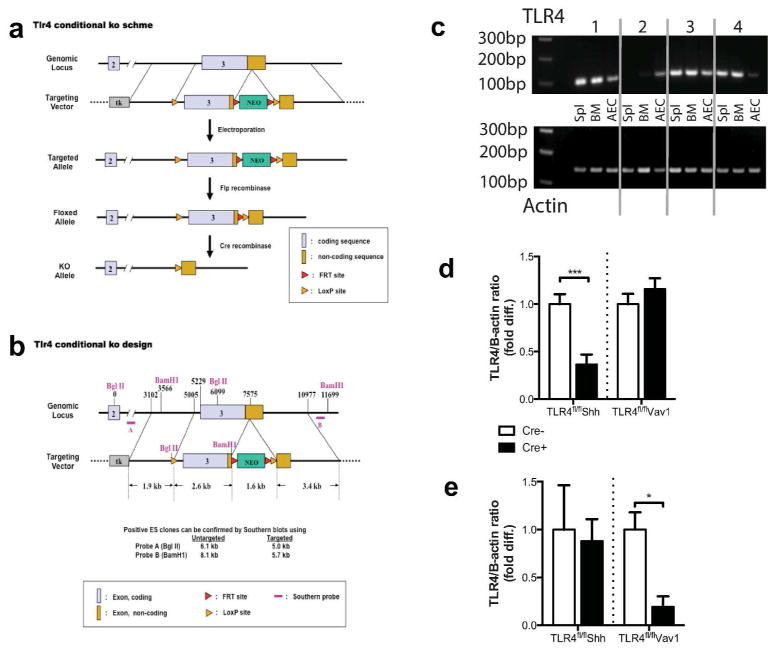
We generated conditionally mutant Tlr4 mice by flanking the third exon of Tlr4 with LoxP sites (Tlr4fl/fl) (Fig. 1a, b). This strategy was chosen because germline mutant mice lacking the third exon are unresponsive to LPS (9). To inactivate Tlr4 in the airway epithelium, Tlr4fl/fl mice were bred to animals in which expression of Cre recombinase is controlled by the Shh locus (Shh-Cre mice) (26). Shh expression by the lung epithelium arises at E9.5 with full expression throughout the lung epithelium by E12.5 (27, 28). Thus, Cre-mediated genetic excisions are maintained in the progeny of these cells, which include all pulmonary epithelial cell lineages (29). Further, to inactivate TLR4 in the hematopoietic compartment, Tlr4fl/fl mice were bred to transgenic animals expressing Cre under control of the HS21/45-vav1 oncogene promoter (Vav1-Cre mice) (30).
Figure 1. Generation of a Tlr4fl/fl mouse.
(a) Structure of the targeting vector and (b) strategy used to create Tlr4fl/fl mice. (c) PCR-based assay confirming selective deletion of Tlr4 from genomic DNA from the indicated cell types; total splenocytes, Spl; bone marrow, BM; and airway epithelial cells, AECs. Section 1, Tlr4fl/fl; Section 2, Tlr4fl/flVav1-Cre; Section 3, Tlr4fl/fl; Section 4, Tlr4fl/flShh-Cre. Tlr4 mRNA levels in (d) AECs and (e) total splenocytes. Data shown represent mean values ±SEM, and are from one experiment, representative of 2–3. N = 4 to 6 mice per group. ***P<0.001.
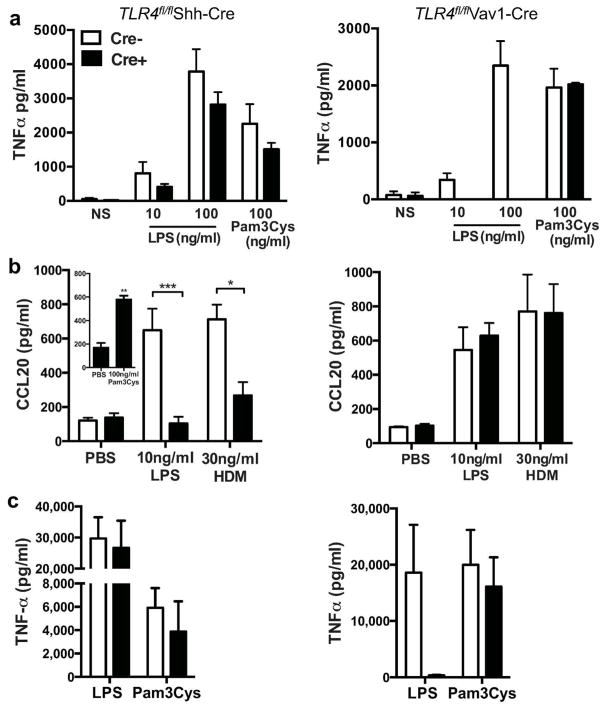
Selective Tlr4 deletion in AECs of Tlr4fl/flShh-Cre mice, and in hematopoietic cells of Tlr4fl/flVav1-Cre mice, was confirmed by PCR analysis of genomic DNA (Fig. 1c) and by analysis of Tlr4-specific RNA prepared from AECs (Fig. 1d) and total splenocytes (Fig 1e). Inactivation of Tlr4 was also confirmed by in vitro and in vivo functional studies. Bone marrow derived DCs (BMDCs) from Tlr4fl/flShh-Cre mice and their Cre-negative Tlr4fl/fl littermates produced comparable amounts of TNF-α after treatment with LPS, whereas BMDCs from Tlr4fl/flVav1-Cre mice failed to produce this cytokine following LPS stimulation, although they responded strongly to the TLR2 ligand, Pam3Cys (Fig. 2a). Conversely, AECs from Tlr4fl/flVav1-Cre and Tlr4fl/fl mice produced comparable levels of the epithelium-derived chemokine, CCL20, in response LPS or HDM challenge, whereas AECs from Tlr4fl/flShh-Cre mice had dramatically reduced responses to these stimuli (Fig. 2b). Lastly, Tlr4fl/flShh-Cre mice, but not Tlr4fl/flVav1-Cre, exhibited high levels of serum TNF-α after intraperitoneal injection of LPS mice (Fig. 2c). Taken together, these data indicate efficient functional deletion of TLR4 in AECs of Tlr4fl/flShh-Cre mice and in hematopoietic cells of Tlr4fl/flVav1-Cre mice.
Figure 2. LPS-responsiveness of cells from TLR4fl/flShh-Cre and TLR4fl/flVav1-Cre mice.
(a) TNFα production by LPS- and Pam3Cys-treated BMDCs (n=4–6 mice). (b) CCL20 production by LPS-, HDM- and Pam3Cys-treated AECs (n=6–10 mice/group). (c) Serum TNFα in mice 24 h after i.p. injection of LPS (n=6–8 mice/group). Data represent mean values ±SEM from one of 2–3 experiments yielding similar results. *P<0.05; **P<0.01; ***P<0.001.
Tlr4 expression by hematopoietic cells promotes LPS-mediated airway inflammation
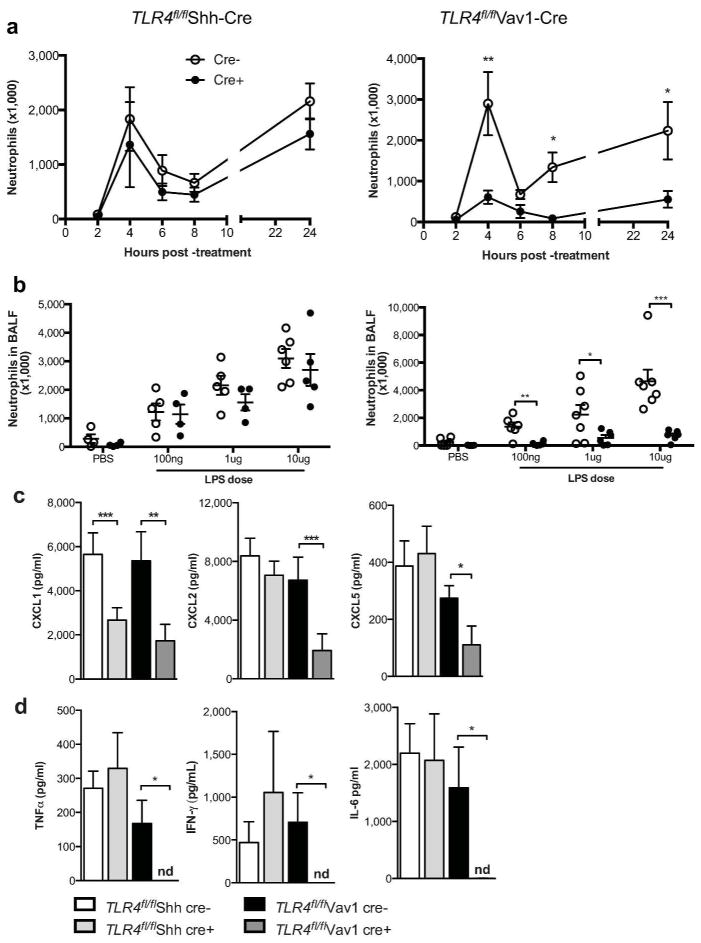
Previous studies employing bone marrow chimeric mice to address the relative contribution of different Tlr4-expressing cell populations to LPS responsiveness have led to diverse conclusions (16–18). To address this issue without the complications associated with irradiation, we administered ultra-pure LPS to Tlr4fl/flShh-Cre mice and Tlr4fl/flVav1-Cre mice by intratracheal aspiration. Two peaks of neutrophil accumulation were seen in the airways of Tlr4fl/fl and Tlr4fl/flShh-Cre mice; at 4 and 24 hours after LPS treatment (Fig. 3a). However, at both of these time points, Tlr4fl/flVav1-Cre mice had significantly reduced numbers of these neutrophils, regardless of the amount of LPS used (Fig. 3b). Thus, in non-irradiated mice, hematopoietic cell-specific Tlr4 expression is critical to LPS-mediated neutrophil recruitment to the airway and AEC-specific expression of this gene is dispensable. Tlr4fl/flShh- and Vav1-cre mice also had a trend towards reduced neutrophil recruitment to the lung following PBS treatment when compared to PBS-treated littermate controls, although these differences did not reach statistical significance. This trend might have been due to a role of Tlr4 in neutrophilic responses to minor injury caused by repeated fluid installation into the lungs (31).
Figure 3. Tlr4-expressing hematopoietic cells promote LPS-mediated neutrophilic inflammation.
(a) Time course of neutrophil recruitment following inhalation of 1 μg LPS. (b) LPS dose-response for neutrophils in mice harvested 24 h after treatment. Chemokine and cytokine levels in the BAL fluid at (c) 2 h and (d) 24 h after treatment with 1 μg LPS (n.d., not detectable). Data represent mean values ±SEM from one experiment, representative of three, with 4–12 mice per group. *P<0.05; **P<0.01; ***P<0.001.
The markedly reduced neutrophil accumulation seen in Tlr4fl/flVav1-cre mice following LPS instillation suggested that neutrophil-attracting chemokines might also be reduced in these animals. To test this, we instilled LPS into the airways of mice and assayed BAL fluid for CXCL1, CXCL2, and CXCL5. Each of these chemokines were significantly reduced in the airways of Tlr4fl/flVav1-cre mice compared with Tlr4fl/fl littermates, whereas concentrations of these chemokines in Tlr4fl/flShh-cre mice were generally similar to their non-transgenic littermates (Fig. 3c). An exception was the reduction in CXCL1 seen in Tlr4fl/flShh-cre mice, although this reduction was apparently insufficient to significantly affect the number of neutrophils recruited to the airways. In addition to the reduction in amounts of neutrophil-attracting chemokines in the airway, Tlr4fl/flVav1-cre mice also had significantly reduced amounts of IFN-γ, TNF-α and IL-6 in BAL fluid 24 hours after challenge (Fig. 3d), cytokines whose levels were unaffected in Tlr4fl/flShh-cre mice. Collectively, these data demonstrate that hematopoietic cell expression of Tlr4 is critical for LPS-driven recruitment of neutrophil the airway.
Tlr4 expression by hematopoietic cells regulates neutrophilic responses to HDM
HDM represent a ubiquitous source of household allergens to which many patients with allergic asthma are sensitized. To determine the roles of Tlr4-expressing AECs and hematopoietic cells in acute immune responses to HDM, we challenged the airways of mice with HDM and evaluated airway inflammation 24 hours later. As observed after LPS treatment, HDM-treated Tlr4fl/flVav1-Cre mice had significantly fewer airway neutrophils than did their Tlr4fl/fl littermate controls, whereas Tlr4fl/flShh-Cre mice and control Tlr4fl/fl littermates had similar numbers of neutrophils (Fig. 4a). To determine a possible cause for the decreased number of neutrophils recruited to the airways of Tlr4fl/flVav1-cre mice, we quantified CXCL1 and CXCL2 in the BAL fluid. Both chemokines were reduced in Tlr4fl/flVav1-cre mice when compared to non-transgenic littermates (Fig. 4b), whereas these chemokines were similar in Tlr4fl/flShh-cre mice and non-transgenic Tlr4fl/fl controls. Because IL-25 and IL-33 have been implicated in allergic sensitization through the airway (32–37), we also quantified these cytokines in HDM-treated mice. Tlr4fl/flShh-cre mice had reduced levels of IL-25 and IL-33 (Fig. 4c) in the BAL fluid, compared with Tlr4fl/fl littermate controls, whereas Tlr4fl/flVav1-cre mice and their Tlr4fl/fl littermates had similar amounts of these cytokines. Thus, Tlr4-expressing hematopoietic cells produce neutrophil-attracting chemokines in response to LPS, whereas Tlr4-expressing AECs produce Th2-promoting cytokines.
Figure 4. Role of Tlr4-expressing AECs and hematopoietic cells in innate immune responses to HDM.
(a) Neutrophils in BAL fluid of mice 24 h after a single instillation of HDM. (b) CXCL1 and CXCL2 and (c) IL-25 and IL-33 levels in the BAL fluid. Data represent means ±SEM from one experiment, representative of two, with 6–8 mice per group. *P<0.05; **P<0.01.
TLR4-expressing airway epithelial and hematopoietic cells differentially regulate eosinophilic and neutrophilic allergic airway inflammation
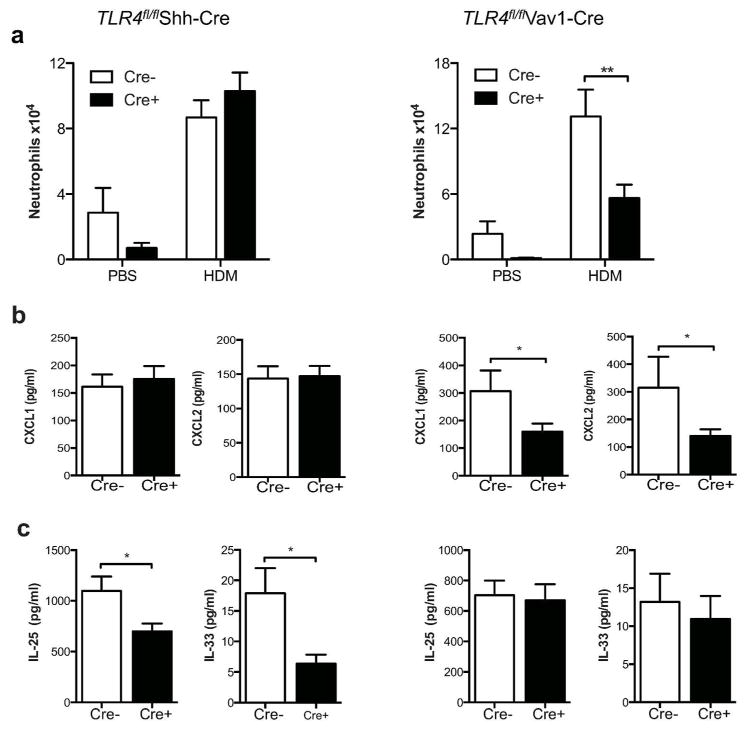
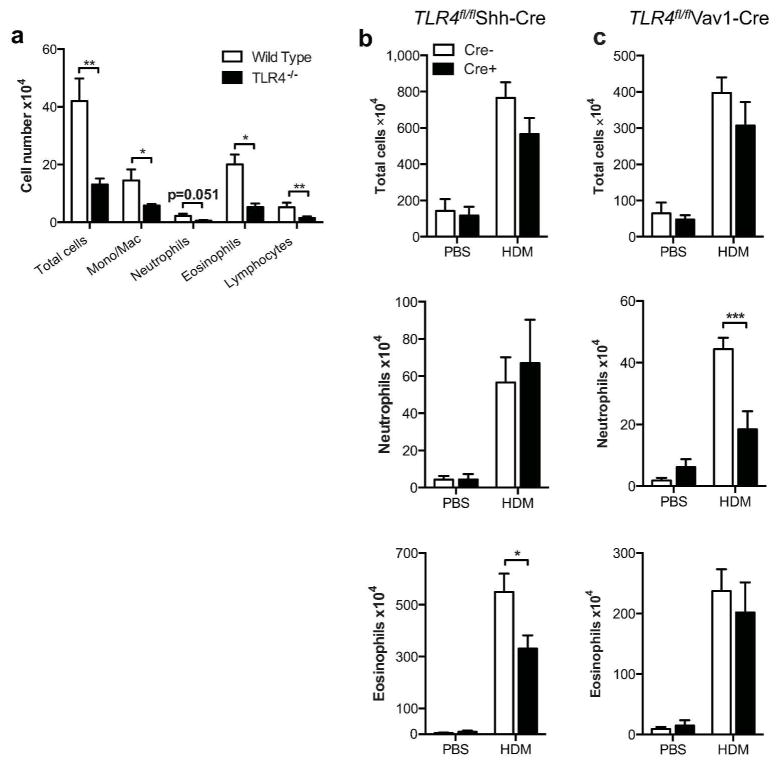
To determine the role of TLR4 expression by airway epithelial and hematopoietic cells in a clinically relevant mouse model of asthma, we sensitized Tlr4fl/flShh-cre mice and Tlr4fl/flVav1-cre mice with HDM on days 0 and 7, followed by challenge with this same allergen preparation on day 14. The commercial preparation of HDM we used contains approximately 100 ηg LPS per 100 μg of HDM, which is sufficient to promote adaptive immune responses to bystander allergens and drive Tlr4-dependent eosinophilia and neutrophilia in several mouse strains (13, 14, 18, 38) and (Fig. 5a). No differences between either of the present strains and their Tlr4fl/fl littermates were seen when total leukocytes in the airway were quantified (Fig. 5b, c). However, cell differential analysis of the leukocytes in BAL revealed marked differences among these strains. Tlr4fl/flVav1-cre mice had significantly fewer neutrophils in their airways than did their Tlr4fl/fl littermates, whereas Tlr4fl/flShh-cre mice had similar numbers of neutrophils compared with their non-transgenic littermates. Conversely, eosinophil numbers were significantly reduced in Tlr4fl/flShh-cre mice compared with their Tlr4fl/fl littermates, whereas the numbers of these cells were similar in Tlr4fl/flVav1-cre and Tlr4fl/fl mice. Thus, Tlr4 expression in specific cell compartments impacts distinct arms of the airway immune response, with expression in hematopoietic cells promoting neutrophilic inflammation and expression in epithelial cells promoting eosinophilic inflammation.
Figure 5. Tlr4-expressing AECs and hematopoietic cells control distinct aspects of HDM-driven allergic airway inflammation.
Airway inflammation in the HDM model of asthma. (a) Mean cell numbers ±SEM are shown for the indicated cell types in BAL fluid from WT and Tlr4−/− mice. Data are from one experiment, representative of two. (b) Tlr4fl/flShh-cre and (c) Tlr4fl/flVav1-cre mice sensitized and challenged with HDM extracts. Values for the indicated cell types represent mean cell numbers ±SEM from one experiment, representative of three. N = 6 –12 mice/group. *P<0.05; **P<0.01; ***P<0.001.
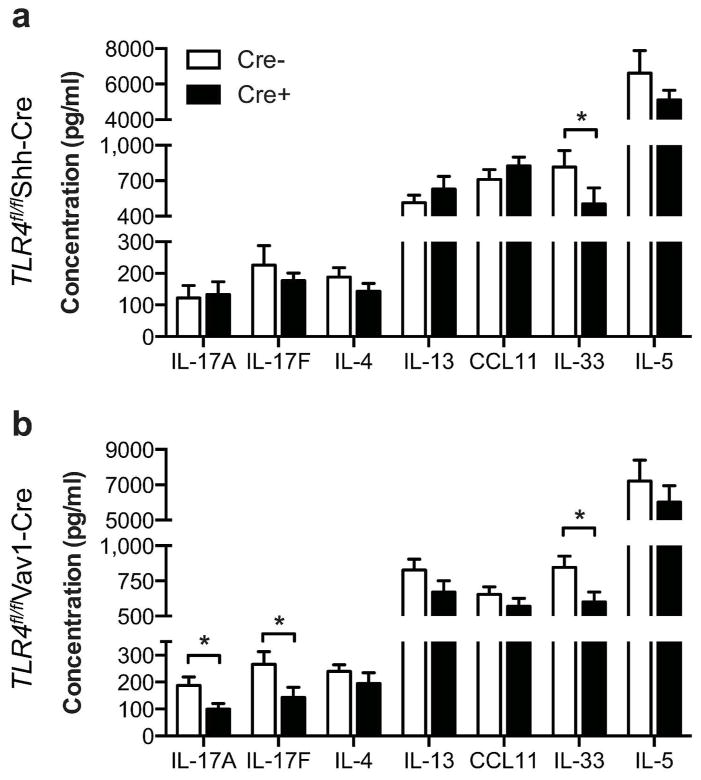
To examine whether differences in cytokine and chemokine levels might have led to qualitative differences in airway inflammation, whole lungs from HDM-sensitized and - challenged mice were restimulated in vitro with HDM. Surprisingly, IL-33 was the only cytokine we found to be significantly reduced in the lungs of Tlr4fl/flShh-Cre mice compared to Tlr4fl/fl littermate controls (Fig. 6a). Lungs from Tlr4fl/flVav1-Cre mice also had reduced amounts of IL-33 (Fig. 6b), and produced significantly less of the neutrophilia promoting cytokines, IL-17A and IL-17F.
Figure 6. Effect of Tlr4-expressing cell types on cytokine and chemokine production in the lung.
Cytokines and chemokines in the supernatants of whole lungs restimulated ex vivo for 5 days with HDM from (a) Tlr4fl/flShh-cre and (b) Tlr4fl/flVav1-cre mice. Data represent mean concentrations ±SEM from one experiment, representative of two. N = 6 to 12 mice/group. *P<0.05.
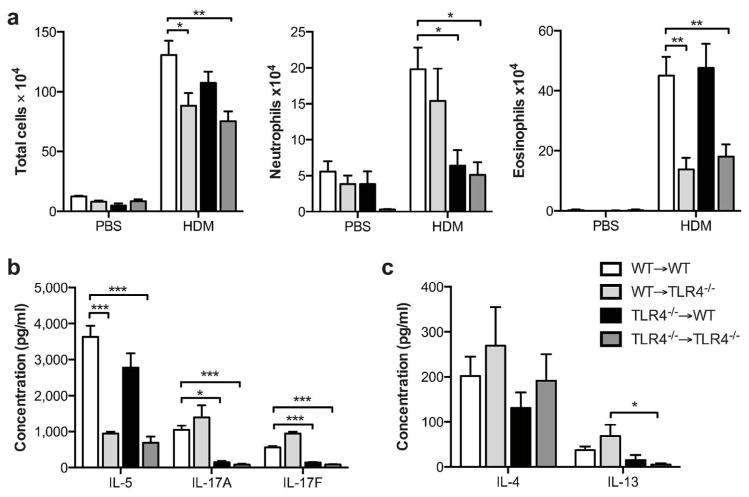
To compare our study of Tlr4fl/fl mice to previous studies using bone marrow chimeric mice, we used WT and Tlr4−/− animals to generate reciprocal bone marrow chimeras and studied them in the HDM-driven model of allergic asthma. As expected, when WT marrow was transferred into irradiated WT recipients (WT→WT), HDM-treated mice developed both eosinophilic and neutrophilic airway inflammation (Fig. 7a). However, WT→ Tlr4−/− mice displayed very little eosinophilia, in agreement with our finding of reduced eosinophilia in Tlr4fl/flShh-Cre mice and confirming an important role of Tlr4 expression in airway structural cells for eosinophilic inflammation. Conversely, neutrophilic airway inflammation was not significantly affected in the WT→Tlr4−/− mice, but was completely abrogated in Tlr4−/−→WT mice, whereas eosinophils were unaffected. This finding was also consistent with our observation that neutrophilia is reduced in Tlr4fl/flVav1-Cre mice, confirming that Tlr4 expression in hematopoietic cells is critical for the neutrophilic component of airway inflammation. Analysis of cytokines in lungs prepared from bone marrow chimeric mice revealed that, as seen in the Tlr4fl/fl model, Tlr4 expression in hematopoietic cells is critical for production of IL-17A and IL-17F (Fig. 7b).
Figure 7. Allergic responses to HDM sensitization and challenge in bone marrow chimeric mice.
(a) Airway inflammation and (b, c) cytokine production in the HDM model of asthma. Cytokines are from cultures of whole lungs restimulated ex vivo for 5 days with HDM. Data represent mean values ±SEM from one experiment, representative of three. *P<0.05; **P<0.01; ***P<0.001.
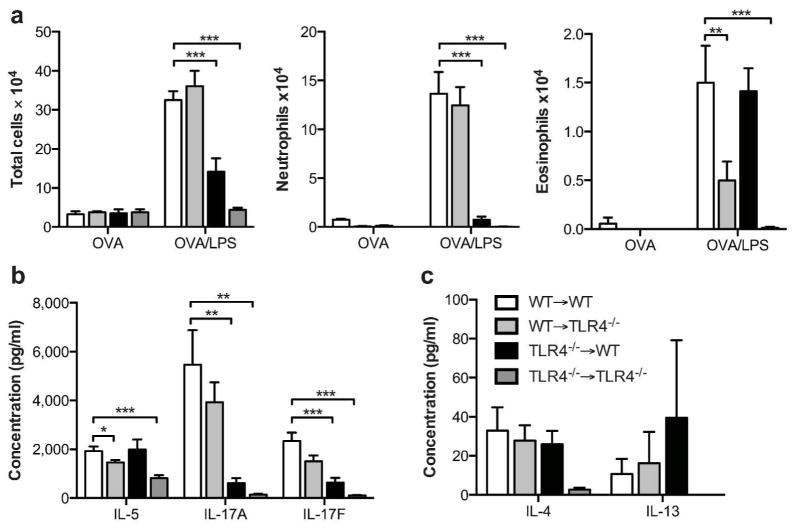
To confirm that our results were not unique to the HDM model of allergic inflammation, we carried out experiments in a different established mouse model of asthma in which inhaled LPS promotes Th2 and Th17 responses to inhaled ovalbumin (OVA). These experiments confirmed that, as we had seen with the HDM model, Tlr4 expression in hematopoietic cells is critical for production of IL-17A and IL-17F and associated neutrophilic inflammation, whereas Tlr4 expression in hematopoietic cells is critical for production of IL-5 and eosinophilic inflammation (Fig. 8).
Figure 8. Allergic responses to OVA/LPS sensitization and challenge in bone marrow chimeric mice.
(a) Airway inflammation and (b, c) cytokine production in the OVA/LPS model of asthma. Cytokines are from cultures of whole lungs restimulated ex vivo for 5 days with OVA. Data shown represent mean values ±SEM, and are from one experiment, representative of three. N = 2 to 4 mice/per group. *P<0.05; **P<0.01; ***P<0.001
Discussion
Previous studies employing bone marrow chimeric mice have led to diverse conclusions regarding the contribution of Tlr4-expressing cell compartments to leukocyte recruitment in animal models of acute inflammation and allergic airway inflammation. Some of this diversity is likely due to variable effects of radiation on structural and hematopoietic cell function (20). In the present study, we circumvented this complication by generating Tlr4fl/fl mice in which deletion of Tlr4 signaling can be achieved in specific cell types without irradiation. With regard to acute immune responses to inhaled LPS, Tlr4fl/flVav1-cre mice displayed significant reductions in airway neutrophilia (and the neutrophil chemokines CXCL1 and CXCL2), indicating that Tlr4 expression in by hematopoietic cells is important for airway neutrophilic immune responses. However, neutrophilic inflammation was not completely absent in Tlr4fl/flVav1-cre mice, and Tlr4fl/flShh-cre mice lacking functional Tlr4 in airway AECs had only modest reductions in neutrophilia and CXCL1. Thus, in these experiments, Tlr4 expression by hematopoietic cells was primarily responsible for neutrophil recruitment to airways in both genetic and radiation chimera models of hematopoietic cell deletion of TLR4 expression. This conclusion is concordant with a previous study of bone marrow chimeric mice by Hollingsworth et al. (16), but in opposition to the results of a bone marrow chimera study by Hammad et al. (17) that reported airway structural cells were necessary and sufficient for airway neutrophilia. The reasons underlying the latter, discrepant, results remain unclear.
A novel finding of our study is that in mouse models of allergic asthma, Tlr4 expression by hematopoietic cells and AECs drives distinct arms of the airway immune responses. Specifically, whereas neutrophil recruitment following allergen sensitization and challenge was primarily dependent on hematopoietic cell expression of Tlr4, eosinophilic inflammation was chiefly dependent on Tlr4 expression by AECs. Moreover, this dichotomy was observed in two different models of allergic asthma, suggesting that TLR4 signaling in these two cell types leads to fundamentally distinct outcomes. The deficit in neutrophil recruitment after HDM sensitization and challenge was more pronounced in Tlr4−/−→WT bone marrow chimeras than in Tlr4fl/flVav1-cre mice, possibly because structural cells other than AECs are damaged by the radiation and therefore less responsive to LPS in the chimeric mice. The decreased airway neutrophilia observed in mice lacking TLR4 in hematopoietic cells was associated with decreased concentrations of IL-17A and IL-17F, cytokines that are known to promote neutrophil accumulation in the lungs (14, 39, 40). This finding is consistent with a previous report showing that with high doses of LPS (10 – 15 μg), Tlr4 expression by hematopoietic cells is primarily responsible for the production of IL-17 in an OVA/LPS model of asthma (18). A similar effect was not seen when the lower dose of 100 ng LPS was used, possibly because the timing of the OVA/LPS sensitizations used in that study leads to a weaker Th17-mediated neutrophilic response to OVA challenge than does the protocol employed in our study (Whitehead et al, unpublished observations). IL-17A and IL-17F induce neutrophil recruitment to the airways by inducing chemokine production, primarily by AECs (41). Taken together with these previous observations, our current findings suggest that chemokine production by AECs is at least partially dependent on TLR4 responsiveness in hematopoietic cells. The concept of cross-talk between AECs and hematopoietic cells has been proposed previously (42, 43), but there is only limited understanding of the specific pathways that might be involved. The conditionally targeted Tlr4fl/fl mice provide a novel resource to study how functional deletion of Tlr4 in one cell type can alter the function of other cell types.
Studies of Tlr4fl/fl mice and bone marrow chimeric mice support the previous findings that Tlr4 expression by AECs strongly contributes to the eosinophilic component of allergen-driven airway eosinophilia. This finding is consistent with previous work with bone marrow chimeric mice in the HDM (17) and OVA/LPS (18) model of allergic asthma. However, the reduction in eosinophils was less marked in the Tlr4fl/flShh-cre mice than in the WT→Tlr4−/− chimeric mice, suggesting that structural cells other than AECs also contribute to the HDM-mediated eosinophilic response. Our present study also confirms previous results suggesting that the absence of Tlr4 expression in hematopoietic cells does not significantly affect HDM-driven airway eosinophilia (17).
A previous study attributed decreased eosinophilia in WT→Tlr4−/− bone marrow chimeric mice to reduced MHCII+CD11b+DC activation and reduced G-CSF, TSLP, IL-25, and IL-33 production after HDM sensitization. Here, we also found that selective deletion of functional Tlr4 from AECs led to reduced IL-25 and IL-33 in the BAL fluid 24 hours after a single HDM instillation. Thus, reductions in these cytokines might have contributed to the decreased eosinophilia seen after allergen challenge because they are reported to promote development of Th2-type immune responses that drive eosinophilia in the lung (44, 45). However, the amounts of IL-4, IL-13, and IL-5 were comparable in lungs of HDM-challenged Tlr4fl/flShh-cre mice and their non-transgenic TLR4fl/fl littermates, suggesting that Th2 cell development might not be affected by the lack of Tlr4 expression in AECs. Unlike in Tlr4fl/flShh-cre mice, IL-5 was significantly reduced in lungs of HDM-sensitized and challenged WT→Tlr4−/− bone marrow chimeric mice compared to WT→WT control animals, while IL-4 and IL-13 were not significantly affected. This suggests that Tlr4-expressing radio-resistant cells in the lung - other than AECs - regulate IL-5 production. These data extend previous studies by showing that TLR4 expression in AECs is necessary for allergen-driven lung eosinophilia, although the molecular mechanisms that drive this response in the present model remain unclear.
Our novel finding that Tlr4 expression in hematopoietic cells and AECs direct distinct arms of the airway immune response has important implications for the therapeutic treatment of individuals with distinct forms of asthma. Global blockade of TLR4 signaling in the airway has shown promise for diminishing allergic sensitization (17), but this approach might impair the function of cells that are important for host defense against pathogens. Selectively targeting TLR4 signaling in specific cell types that promote either neutrophilic or eosinophilic forms of asthma might reduce the severity of this disease while minimizing effects on host defense. Such a targeted approach might be particularly important for neutrophilic forms of asthma that are resistant to glucocorticoid therapy (4–6). We have not yet identified the specific hematopoietic cell type(s) expressing Tlr4 that are necessary for the induction of neutrophilic inflammation. Dendritic cells represent a compelling hypothesis in this regard–responding to LPS by increasing co-stimulatory molecule expression and the production of cytokines such as IL-1 and IL-6 that promote the development of allergen-specific Th17 cells, in turn promoting neutrophilic asthma. However, macrophages, innate lymphoid and/or α/β T cells might also play a role. Defining roles for Tlr4 expression in each of these cell types awaits the further generation of appropriate cell-specific Cre TLR4-floxed crosses. Identification of the specific hematopoietic cell type(s) requiring Tlr4 expression for the induction of neutrophilic asthma will be critical for a deeper understanding of asthma and for designing novel strategies for therapeutic intervention.
Acknowledgments
We thank Matt Edin and Michael Fessler (NIEHS) for critical reading of the manuscript and Traci Stankiewicz and Elizabeth Donelan (CCHMC) for technical assistance. This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) and the NIEHS (D.N.C), NIH R01 AI088372 and R01 HL094576 (C.L.K) and F32 HL110497 (J.W.M.).
Footnotes
Disclosure: None to report
References
- 1.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–9. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respiratory medicine. 2003;97(6):726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 3.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respiratory research. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–9. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119(5):1043–52. doi: 10.1016/j.jaci.2007.02.042. quiz 53–4. [DOI] [PubMed] [Google Scholar]
- 7.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11(10):928–35. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karp CL. Guilt by intimate association: what makes an allergen an allergen? J Allergy Clin Immunol. 2010;125(5):955–60. doi: 10.1016/j.jaci.2010.03.002. quiz 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–52. [PubMed] [Google Scholar]
- 10.Ross MA, Curtis L, Scheff PA, Hryhorczuk DO, Ramakrishnan V, Wadden RA, et al. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000;55(8):705–11. doi: 10.1034/j.1398-9995.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 11.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172(11):1371–7. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, et al. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med. 2005;171(8):806–13. doi: 10.1164/rccm.200407-953OC. [DOI] [PubMed] [Google Scholar]
- 17.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 2010;184(7):3535–44. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- 19.Hahn I, Klaus A, Maus R, Christman JW, Welte T, Maus UA. Dendritic cell depletion and repopulation in the lung after irradiation and bone marrow transplantation in mice. Am J Respir Cell Mol Biol. 2011;45(3):534–41. doi: 10.1165/rcmb.2010-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citrin DE, Shankavaram U, Horton JA, Shield W, 3rd, Zhao S, Asano H, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. Journal of the National Cancer Institute. 2013;105(19):1474–84. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 22.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 6. Chapter 6. 2003. p. 28. [DOI] [PubMed] [Google Scholar]
- 23.Lewkowich IP, Lajoie S, Stoffers SL, Suzuki Y, Richgels PK, Dienger K, et al. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal immunology. 2013;6(4):728–39. doi: 10.1038/mi.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6(6):571–8. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley IT, Giles DA, Pfluger PT, Burgess SL, Walters S, Hembree J, et al. Differential colonization with segmented filamentous bacteria and Lactobacillus murinus do not drive divergent development of diet-induced obesity in C57BL/6 mice. Molecular metabolism. 2013;2(3):171–83. doi: 10.1016/j.molmet.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118(4):517–28. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Developmental biology. 1995;172(1):126–38. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 28.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103(7):2208–13. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology. 2003;33(2):314–25. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 31.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 32.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clinical immunology. 2012;143(3):222–35. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 34.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118(3):606–14. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 35.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120(6):1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181(7):4780–90. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 37.Ramaprakash H, Shibata T, Duffy KE, Ismailoglu UB, Bredernitz RM, Moreira AP, et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. The American journal of pathology. 2011;179(1):104–15. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead GS, Thomas SY, Cook DN. Modulation of Distinct Asthmatic Phenotypes in Mice by Dose-Dependent Inhalation of Microbial Products. Environmental health perspectives. 2013 doi: 10.1289/ehp.1307280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogli LK, Sundrud MS, Goel S, Bajwa S, Jensen K, Derudder E, et al. T cell-derived IL-17 mediates epithelial changes in the airway and drives pulmonary neutrophilia. J Immunol. 2013;191(6):3100–11. doi: 10.4049/jimmunol.1301360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170(9):4665–72. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 41.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–9. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 42.Upham JW, Stick SM. Interactions between airway epithelial cells and dendritic cells: implications for the regulation of airway inflammation. Current drug targets. 2006;7(5):541–5. doi: 10.2174/138945006776818647. [DOI] [PubMed] [Google Scholar]
- 43.Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376(9743):835–43. doi: 10.1016/S0140-6736(10)61226-3. [DOI] [PubMed] [Google Scholar]
- 44.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106(32):13463–8. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204(7):1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]