Abstract
Interleukin-10 (IL-10) is arguably the most potent anti-inflammatory cytokine. It is produced by almost all the innate and adaptive immune cells. These cells also serve as its targets, indicating that IL-10 secretion and action is highly regulated and perhaps compartmentalized. Consistent with this notion, various efforts directed at systemic administration of IL-10 to modulate autoimmune diseases (type 1 diabetes, multiple sclerosis, rheumatoid arthritis, psoriasis) have produced conflicting and largely inconsequential effects. On the other hand, IL-10 can promote humoral immune responses, enhancing class II expression on B cells and inducing immunoglobulin (Ig) production. Consequently, the high IL-10 level in systemic lupus erythematosus (SLE) patients is considered pathogenic and its blockade ameliorates the disease. In this perspective, we review preclinical findings and results of recent clinical studies using exogenous IL-10 to treat the aforementioned autoimmune diseases. In addition, given the limited success of IL-10 supplementation, we suggest that future studies should be expanded beyond modulating the delivery modes to include developing new strategies to protect and replenish the endogenous sources of IL-10. As an example, we provide evidence that aberrant Fas-mediated deletion of IL-10-producing B cells subverts the immunoregulatory role of IL-10 in autoimmune diabetes and that modulation of the Fas pathway preserves the IL-10-producing B cells and completely protects NOD mice from developing the disease.
Keywords: Interleukin-10, Autoimmunity, Fas pathway, lpr, gld, B-cells
1. Introduction
Interleukin 10 (IL-10) is a potent anti-inflammatory cytokine that was originally labelled CSIF or - cytokine synthesis inhibitory factor – due to its ability to inhibit production of proinflammatory (IFN-γ and TNFα) cytokines by T helper l (Th1) cells [1]. Subsequent studies showed that multiple cell types are targets of IL-10 action and that through its inhibitory effects on macrophages and DCs, IL-10 restrains immune responses to pathogens and microbial flora and prevents their pathologies [2]. These properties prompted early and extensive efforts to utilize IL-10 to modulate inflammatory and autoimmune diseases both in mice and humans [3–9]. However, reaching this goal has been challenging as indicated by the limited success of varied strategies to immunomodulate autoimmune diseases using recombinant IL-10. The difficulties are related to the complexity of the mechanisms controlling IL-10 production and suppressive function. These include multiple sources and targets, and feed-forward conditions that modulate its production. This intricate regulatory network enables IL-10 signalling to be tightly and locally controlled, hence hard to recapitulate through simple provision of exogenous IL-10. New approaches based on modulating endogenous sources of IL-10 may prove more effective than simple provision of IL-10. In this perspective, we review preclinical findings and results of recent clinical studies using exogenous IL-10 to treat the aforementioned autoimmune diseases. In addition, given the limited success of IL-10 supplementation, we suggest that future studies should be directed towards developing new strategies to protect and replenish the endogenous sources of IL-10. As an example, we will provide evidence that aberrant Fas-mediated deletion of IL-10-producing B cells subverts the immunoregulatory role of IL-10 in autoimmune diabetes and that modulation of the Fas pathway preserves the IL-10-producing B cells and completely protects NOD mice from developing the disease.
2. Cellular sources of IL-10
Almost all leukocytes, including T and B cells, dendritic cells, γδ T cells, NK cells, mast cells, neutrophils, eosinophils, and keratinocytes produce IL-10 [10–16]. The reasons underlining the evolution of ubiquitous sources of IL-10 are poorly understood, but clearly underscores its physiologic significance and the complexity of its regulation. IL-10 has a short half-life and short range of activity. Thus, endowment of so many cell types with the ability to produce IL-10 could be necessary to ensure its rapid availability at different locales when needed. Also, it could be important to compartmentalize IL-10 action. Additionally, special roles for different cell types in mediating IL-10 function has not been ruled out. For example, regulatory T cells are particularly known for utilizing IL-10 to suppress inflammation and autoimmunity. This was initially demonstrated in a colitis model [17] and subsequently in other disease models [18–20]. Likewise, regulatory B cells (Bregs) are increasingly being investigated for their roles in maintaining self-tolerance via secretion of IL-10 [19]. These B cells differ in their phenotypes, yet they commonly use IL-10 to suppress excessive inflammatory responses in various disease models and to support generation of Tr1 cells [19]. Further support for specialized roles of various cell types in delivering IL-10 is indicated by the studies that implicated altered homeostasis of Breg cells in the pathogenesis of several autoimmune diseases, including contact hypersensitivity (CHS), inflammatory bowel disease (IBD), experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA), and type I diabetes (T1D) [20–22]. Antigen-presenting cells (APCs) and innate immune cells are also important and rapid sources of IL-10 that can serve, in autocrine feedback fashion, to constrain activation of APCs and the development of adaptive immune responses. On the other hand, natural killer (NK) cells have also been described as another innate source of IL-10 [23]. This multitude of cell types that produce IL-10 is symbolic of a complex function that is yet to be successfully recapitulated through provision of exogenous IL-10.
3. Cellular and molecular mechanisms of IL-10 action
As a potent immunosuppressive cytokine, IL-10 blocks immune responses at different levels by acting directly and indirectly on both the innate and adaptive arms of the immune system. Consequently, IL-10 can inhibit production of proinflammatory cytokines, antigen presentation, and cell proliferation [24–27]. IL-10 performs these regulatory functions by binding to a specific cell surface receptor (IL-10R) that is made of two chains, IL-10R1 and IL-10R2. Both chains are transmembrane glycoproteins whose intracellular domains differ in length and amino acid sequence [28]. The IL-10R1 is located on human chromosome 11 and the IL-10R2 on chromosome 21 [29–30]. The IL-10R2, which is widely expressed [31–32], binds IL-10 only after IL-10 binds to the IL-10R1 [33–35]. On the other hand, IL-10R1 expression is restricted mainly to the immune cells [36–37] and particularly highly on monocytes and macrophages [38]. It is also expressed on placental cytotrophoblasts [39] and colonic epithelium [40]. Among T cells, the expression level of IL-10R is higher on memory than on naïve CD4 T cells [41]. Binding of IL-10 to IL-10R1 leads to conformational changes in IL-10 that allows its association with the IL-10R2 and the generation of IL-10/IL-10R complexes. These complexes can suppress immune responses by multiple mechanisms, but inhibiting nuclear translocation of the NF-κb and its DNA-binding activity is considered the main one [42]. In addition, IL-10 inhibits TLR-triggered production of proinflammatory mediators via inhibition of MyD88 translation [43] and ubiquitination [44]. Furthermore, IL-10 inhibits IFN-α and -γ induced-gene transcription (e.g. CXCL10, ISG-54) and STAT1 phosphorylation [26, 45]. IL-10 also inhibits major histocompatibility complex class II expression, limiting costimulation, and reducing proinflammatory cytokine production by antigen-presenting cells (APC), particularly DCs and macrophages [24, 46]. Besides its effects on APCs, IL-10 can directly inhibit activation and proliferation of T cells by suppression of IL-2 production and CD28 signaling [27, 47–48]. The effects of IL-10 on humoral immune responses, however, is considered stimulatory and depend on several factors that regulate generation, maintenance, and propagation of B cells [49]. For example, IL-10 promotes B cell differentiation, proliferation, survival, and antibody production. A stimulatory role for IL-10 in antibody production has been implicated in the pathogenesis of multiple sclerosis and SLE [50–52]. IL-10 is also reported to promote proliferation of mast cells [53] and thymocytes [54]. For natural killer (NK) cells, contradictory effects have been described for IL-10, depending on the cellular context. IL-10 inhibits interferon-γ (IFN-γ) production by NK cells in the presence of APCs, partially as a result of a decrease of IFN-γ-inducing cytokines [25, 55]. Thus, consistent with its multiple sources, IL-10 engages multiple cellular and molecular pathways to suppress and in certain instances to stimulate immune responses.
4. IL-10 and pathogenesis of autoimmune diseases
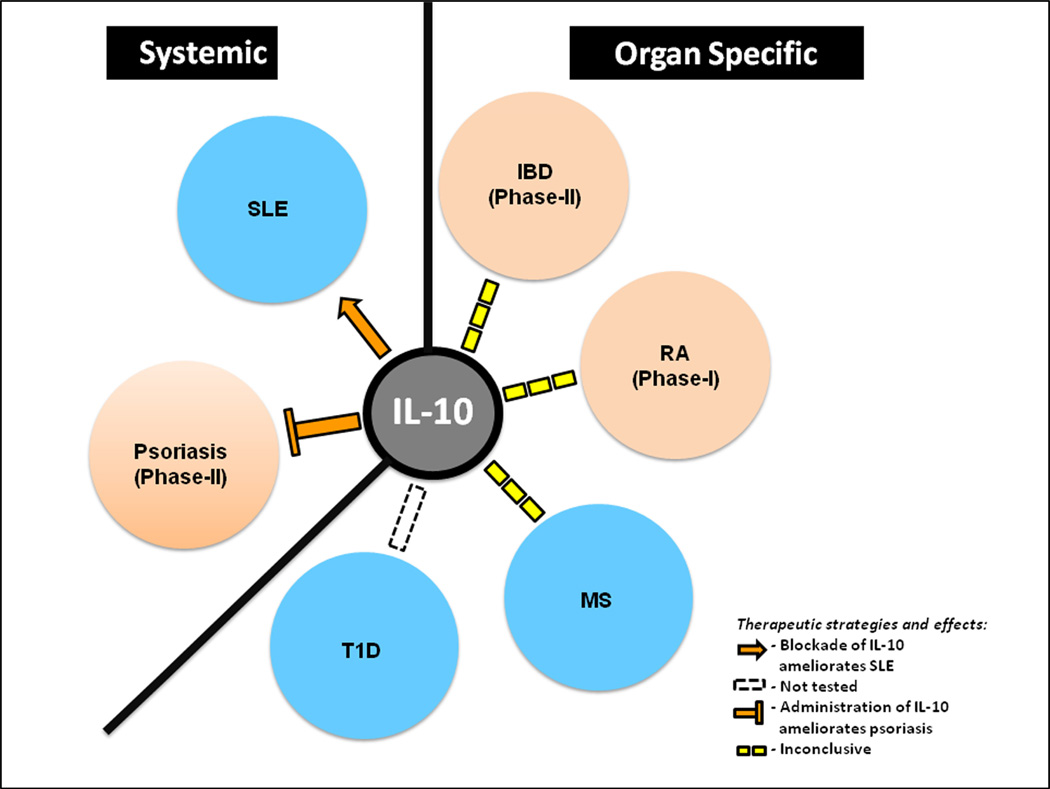
Given the broad anti-inflammatory effects of IL-10, a variety of clinical studies were undertaken to assess efficacy of recombinant IL-10 to treat autoimmune diseases. Unfortunately, despite initial high hopes, results of most clinical trials were less than encouraging. In hind sight, however, these poor results should not have been unexpected. One evolutionary indicator is the existence of a complex network of cells that act as sources and subjects to IL-10 action, indicating highly regulated and perhaps compartmentalized effects of IL-10 that is unlikely to be recapitulated by simple infusion of recombinant IL-10. This notion is reinforced by the lack of clear patterns of IL-10 serum level with disease development or activity in most autoimmune diseases. The notable exceptions, however, are SLE and psoriasis, where most published studies indicated high serum levels in SLE and low levels in psoriasis [56–58]. Consistently, blockade of IL-10 appears to be a promising therapeutic strategy against SLE, whereas direct injection of IL-10 in lesions appears effective against psoriasis. Below we will review major autoimmune diseases where IL-10 directed therapies have been assessed and results published (Fig. 1). In addition, we will discuss our suggestion that future studies should be expanded beyond modulating the delivery modes to include developing new strategies to protect and replenish the endogenous sources of IL-10.
Figure 1. Effects of IL-10 on pathogenesis of the indicated systemic- and organ-specific autoimmune diseases.
The solid arrow shows promotion of autoimmunity, T line indicates inhibition, whereas broken lines indicate inconclusive effect. Clinical trials for IBD and Psoriasis are completed and for RA are still ongoing. No reported clinical trials for other diseases [59]
4.1 Type-1 diabetes (T1D)
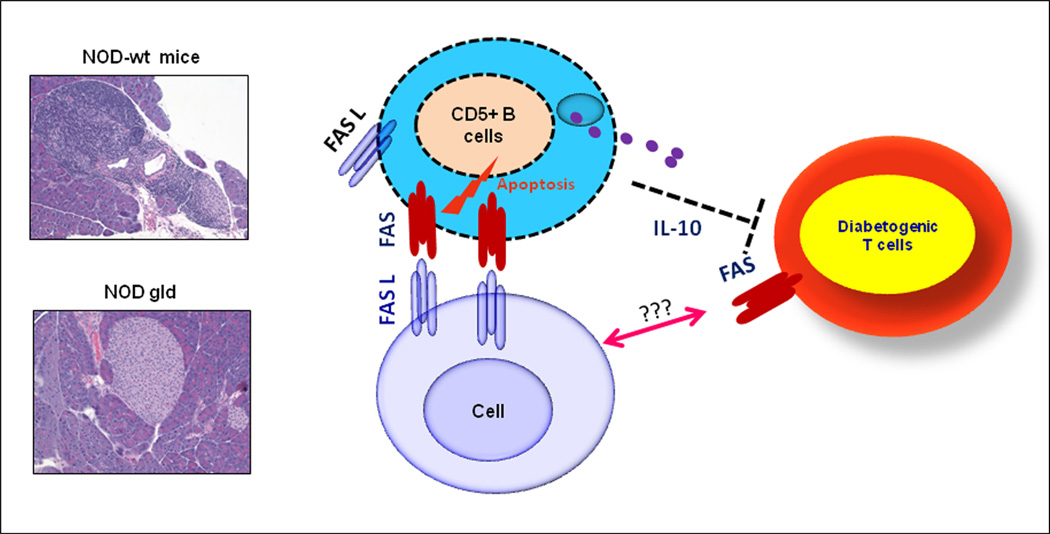
This is a chronic organ-specific autoimmune disease that is caused by autoreactive T cells that infiltrate and destroy insulin-producing pancreatic beta-cells [60], leading to insulin deficiency and hyperglycemia [61]. In healthy individuals, such autoreactive T cells are kept in check by peripheral tolerance mechanisms [62–63]. Analysis of the role of IL-10 in the pathogenesis of T1D, using the NOD mouse model, produced conflicting results. Early studies in 1990s showed that injection of NOD mice with IL-10 delayed [8] or resulted in a long lasting protection [64–65]. By contrast, transgenic expression of IL-10 in pancreatic islets of NOD mice accelerated the disease [66–67]. Furthermore, neutralization of endogenous IL-10 at the age of three weeks inhibited the development of insulitis [68], whereas treatment of in NOD mice at later ages had no significant effects on the disease development [8]. Likewise, genetic deletion of IL-10 produced no detectable effects on the disease pathogenesis [69]. However, clues that the immunoregulatory effect of IL-10 might be pathogenically subverted in NOD mice were revealed by our finding that inactivation of Fas-mediated apoptosis restores a potent role for IL-10 in protecting pancreatic islets from infiltration by diabetogenic T cells [70]. The results also pointed to CD5+ B cells as the major local pancreatic source of protective IL-10 in NOD mice [70]. Thus, aberrant deletion of IL-10-producing B cells appears to be involved in breaking of self-tolerance against islet autoantigens and that rectifying of the underlying cause of their deficiency prevents the disease.
A role for IL-10-producing B cells in T1D patients has recently been reported by Thompson et al.[71]. Furthermore, CD4+ T cells from T1D patients with later onset of disease were reported to maintain IL-10 production, as compared to CD4+ T cells from patients with rapid disease onset and progression [55]. Serum levels of IL-10 and the frequency of IL-10+ CD4+ T cells were increased in recent-onset T1D patients treated with an anti-CD3 mAb [72]. To our knowledge, there are currently, no ongoing clinical trials focusing on IL-10 in T1D. Taken together, these findings indicate a complex role for IL-10 in autoimmune diabetes that is dynamic and likely sensitive to spatial and temporal changes and level / duration of exposure. However, our findings that aberrant deletion of IL-10-producing B cells could be involved in breaking of self-tolerance against islet autoantigens may lead to new strategies to augment IL-10 sources via protecting its sources from aberrant death.
4.2 Rheumatoid arthritis (RA)
This disease is characterized by synovitis, systemic inflammation, high level of the rheumatoid factor, and autoantibodies particularly against citrullinated peptides [73]. The disease is driven mainly by overproduction of TNFα by T and B cells, synovial-like fibroblasts, and macrophages [74]. Elevated levels of TNFα fuel secretion of other cytokines, including IL-6, leading to a persistent inflammation and joint destruction [75]. IL-10 was also elevated in sera and synovial fluids of RA patients and could be involved in diminishing the disease activity [76–78]. However, consistent with the IL-10 stimulatory effect on B cells, its high serum levels are suggested to drive autoantibody production in RA [79–80], while suppressing serum levels of IL-6 and acute phase reactants [81]. Thus, IL-10 seems to play a dual role in RA by simultaneously suppressing proinflammatory cytokines and enhancing humoral autoimmune response. These opposing roles are reflected in the results of a trial was conducted to assessed safety and therapeutic efficiency of IL-10 in RA. Whereas a single dose of IL-10 (25 µg/kg i.v. bolus injection) was found non-toxic, clinical complications, including neutrophilia, monocytosis, and lymphopenia, were observed after serial administration of higher doses [82]. In another Phase I study performed with subcutaneously administered IL-10 for 28 days, a limited efficacy was observed, but no serious complications were noted [83]. An ongoing clinical trial is still ongoing as posted on clinicaltrials.gov.
4.3 Psoriasis
This is a common chronic inflammatory disease of the skin where polymorphonuclear leukocytes migrate from dermal vessels into the epidermis where they form spongiform pustules [84]. The proinflammatory cytokines IFN-γ, IL-6, IL-8, and TNFα are found at high levels in psoriatic lesions, which are also infiltrated by activated CD4 and CD8 T cells [85]. Analysis of IL-10 shows that its level is lower in psoriatic lesions than in non-inflammatory dermatoses [86–88]. These findings led to the assessment of IL-10 efficacy as a therapy for psoriasis. In a proof-of-principle study, Assadullha et al [88] demonstrated that daily injection of 8 µg/Kg body weight of IL-10 for 24 days, directly under the psoriatic plaque, suppressed the inflammation in one of two patients and skewed T cell response from a Th1 to Th2 type. A more recent study showed positive responses of patients with psoriasis vulgaris to the treatment with a combination of IL-4, IL-10 and IL-11[89]. Thus, psoriasis might emerge as a rare example among the autoimmune diseases where IL-10 immunotherapy could prove effective. A likely reason is the direct access and injection of IL-10 into the target organ, the skin, thereby bypassing to some extent the complex regulatory network that controls IL-10 biology. Determination of definitive clinical efficacy of IL-10 in psoriasis, however, awaits the results of phase III studies [90–91].
4.4 Inflammatory bowel disease (IBD)
IBD represents a group of chronic inflammatory disorders that affect both the small and large intestines [92]. The two known major forms are Crohn’s disease (CD) and ulcerative colitis (UC). The disease pathogenesis is complex and it involves genetic, environmental, microbial, and immunologic alterations. Immunologically, there are dysregulated responses of both innate and adaptive immune cells due to a loss of self-tolerance to commensal bacteria in susceptible individuals [93]. IL-10 plays a particularly critical role in maintaining intestinal homeostasis as indicated by the chronic enterocolitis that develops spontaneously in IL-10 knockout mice, housed under conventional conditions [9]. In humans, a GWAS study uncovered a significant association between a single nucleotide polymorphism (SNP) in the IL-10 gene and UC [94]. In addition, impaired IL-10 production has been detected in severe cases of UC and CD [95–96] . Furthermore, another study suggested that a low ileal IL-10 concentration predicts endoscopic recurrence of Crohn's disease [97]. However, no a specific pattern or clear relationship between the serum level of IL-10 and IBD has been detected [98–101]. Beside the documented beneficial role for IL-10 in murine colitis, a potentially beneficial role for IL-10 treatment against IBD is suggested by a dose-dependent inhibition of IL-1β in organ cultures of intestinal biopsies from UC patients [102]. Subsequent clinical trials, however, produced largely negative results. In one hand, treatment with recombinant IL-10 (Tenovil) failed to prevent endoscopic recurrence of Crohn's disease [103]. On the other hand, a multicenter study showed that subcutaneous administration of IL-10 in patients with mild to moderately active Crohn's disease was safe and resulted in clinical and endoscopic improvement [104]. Furthermore, a computer-assisted search of the Cochrane Central Register of controlled Trials and the Cochrane IBD/FBD Review group Specialized Trials Register concluded that IL-10 administration does not appear to provide significant benefit against active Crohn's disease. Worrisome, the analysis uncovered an increased rate of participants’ withdrawal due to adverse events that were associated with the treatment [105]. Thus, the clinical trials using IL-10 immunotherapy for Crohn’s disease have largely been negative, despite the well-documented role of IL-10 in suppressing intestinal inflammation in mouse models [106–107] . The only reasonable way to explain this dichotomy, in our opinion, is to relate it to the highly complex mechanisms that regulate physiological production and responses to IL-10 and the apparent technical difficulties that hamper effective delivery of exogenous IL-10 to the target ogran.
4.5 Multiple sclerosis (MS)
This is an inflammatory disorder of the central nervous system (CNS) in which focal lymphocytic infiltration leads to damage of myelin and axons [108]. Both autoreactive T cells [109–110] and B cells are implicated in promoting the disease pathogenesis [111–112], whereas IL-10-secreting B cells are implicated in reducing the proinflammatory response by suppressing proliferation and cytokine production by CD4 T cells [113–114]. However, B cells from MS patients are reported to have diminished capacity to secrete IL-10 [115–116] and that enhanced IL-10 production correlated with successful treatment of MS patients with IFN-β [117–120]. Furthermore, an increase in IL-10-producing B cells was detected in the CNS of both patients and mice undergoing remission in EAE) model [121–123]. Nonetheless, earlier attempts to treat MS with systemic IL-10 yielded inconsistent results perhaps due to issues related to experimental variations in timing and dosages and counter-regulation [7, 124]. Likewise, intravenous IL-10 did not show any promising results and was found, at least in some studies, to exacerbate disease [125]. Thus, as in T1D, IBD, simple administration of IL-10 appears ineffective in ameliorating MS.
4.6 Systemic lupus erythematosus (SLE)
This is a multi-organ systemic autoimmune disease with a wide range of clinical features. Immunological abnormalities in SLE include impaired apoptotic cell clearance and hyperactivity of T and B cells that result in autoantibody production, immune complex formation as well as direct antibody-mediated cytotoxicity [126]. Contrary to most other autoimmune diseases where IL-10 deficiency is implicated in the disease pathogenesis, overproduction of IL-10 is being clearly implicated in the pathogenic mechanism of SLE. Increased IL-10 production by PBMCs from untreated SLE patients than in healthy controls was first reported in 1993 [127]. Since then several studies have reported high IL-10 production in SLE with the serum levels positively correlating with the disease severity. Most of IL-10 in SLE patients is derived from monocytes and B cells, with a minor contribution from T cells [128–132]. IL-10 appears to promote the disease by enhancing B cell proliferation, differentiation, and autoantibody production [50, 133]. Blocking IL-10 led to decreased autoantibody production and inhibited in vitro cellular immune responses of PBMCs from SLE patients [134]. In a small, open-label trial, anti-IL-10 treatment of SLE patients using a 3-week regimen improved the clinical conditions in five of six patients within 6 months [135]. Thus, a pathogenic role for IL-10 appears to predominate and affect many facets of SLE and its blockade is likely to prove effective therapeutic strategy.
5. Challenges facing effective therapeutic utilization of IL-10 against autoimmune diseases
As summarized above, provision of exogenous IL-10 turned out to be a largely ineffective therapy against most autoimmune diseases. This raises the question of why administration of this undisputedly powerful antiinflammatory cytokine fails to treat autoimmune diseases. In our opinion, immunomodulatory properties of IL-10 are too complex and intricate to be recapitulated by simple offering of exogenous IL-10. Consistent with this notion is the multitude of cell types that produce IL-10 and existence of feed-forward and feedback pathways that temporally and spatially regulate endogenous IL-10 secretion and function. In addition, IL-10 has a short change and short life; the mean terminal phase half-life of recombinant human IL-10 is 2.7 to 4.5 h [136]. Access, timing, duration, and local concentrations are factors that are likely to play direct and critical roles in influencing the ability of IL-10 to ameliorate or exacerbate local inflammation and autoimmune responses. Thus, effective delivery is apparently a major obstacle hampering IL-10 immunotherapies. Emphasizing this notion are the encouraging outcomes of the psoriasis studies where IL-10 was injected directly into the psoriatic lesions [90, 135]. We believe that strategies to improve delivery via using innovative time course, multiple injections and/or various dosage, alternate mode and site of administration of IL-10, co-stimulation blockade along with IL-10 therapy, use of small molecule mimics of IL-10 are unlikely to solve this complex problem. Instead, more efforts should be directed toward fortressing and protecting the endogenous IL-10 sources. Case in point, we found that aberrant Fas-mediated apoptosis compromises the protective role of IL-10 in autoimmune diseases by inadvertently depleting IL-10-producing B cells and that genetic or antibody blockade of FasL restores the IL-10 role in protecting pancreatic islets from diabetogenic T cell cells [70]. Thus, identifying pathogenic mechanisms that work to subvert the immunomodulatory function of IL-10 in susceptible individuals and animal models may lead to novel and effective therapeutic strategies.
6. Concluding remarks
IL-10 is a pluripotent cytokine with potent immunoregulatory effects on both the innate and adaptive arms of the immune system and thus holds a great promise as an immunotherapeutic agent. On the other hand, a complex network of immunoregulatory mechanisms have evolved to control IL-10 availability and action. As indicated in various clinical trials, provision of exogenous IL-10, appears to be a too simplistic approach to recapitulate the immunosuppressive function of IL-10. Directing more efforts towards bolstering and protecting the endogenous sources of IL-10 could prove more effective in exploiting the immunomodulatory function of IL-10.
Figure 2. Inactivating Fas pathway restores a protective role for IL-10 against insulitis in NOD mice.
Images show that the gld mutation of FasL protects pancreatic islets against insulitis. The model depicts how interaction of IL-10-secreting CD5+ B cells with local FasL sources could lead to their deletion and depriving of the host of a critical source of protective IL-10 [70].
-
➢
IL-10 is one of the most potent anti-inflammatory cytokine
-
➢
IL-10 therapeutic potential have been hard to harness
-
➢
Most of the therapeutic strategies are based on provision of exogenous IL-10
-
➢
Modifications of delivery modes of IL-10 might yield only incremental advances
-
➢
New therapeutic strategies that protect natural IL-10 sources may prove efficacious
Acknowledgements
This work is supported by a 1R01AI099027-01A1 (AH) and a generous gift by Dr. Julius Edlow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflict of interest.
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spits H, de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99:8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 3.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 4.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson S, Mikulowska A, Narula S, O'Garra A, Holmdahl R. Interleukin-10 suppresses the development of collagen type II-induced arthritis and ameliorates sustained arthritis in rats. Scand J Immunol. 1996;44:607–614. doi: 10.1046/j.1365-3083.1996.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 6.Reich K, Garbe C, Blaschke V, Maurer C, Middel P, Westphal G, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319–329. doi: 10.1046/j.1523-1747.2001.01248.x. [DOI] [PubMed] [Google Scholar]
- 7.Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- 8.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 10.Gasim S, Elhassan AM, Khalil EA, Ismail A, Kadaru AM, Kharazmi A, et al. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin Exp Immunol. 1998;111:64–69. doi: 10.1046/j.1365-2249.1998.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomarat P, Rissoan MC, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993;177:523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra PT, Donnelly RP, Wong S, Kanegane H, Geremew A, Mostowski HS, et al. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol. 1998;160:2637–2644. [PubMed] [Google Scholar]
- 15.Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur J Immunol. 2008;38:2274–2283. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- 16.Speiran K, Bailey DP, Fernando J, Macey M, Barnstein B, Kolawole M, et al. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J Leukoc Biol. 2009;85:826–836. doi: 10.1189/jlb.0708448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 18.Guichelaar T, ten Brink CB, van Kooten PJ, Berlo SE, Broeren CP, van Eden W, et al. Autoantigen-specific IL-10-transduced T cells suppress chronic arthritis by promoting the endogenous regulatory IL-10 response. J Immunol. 2008;180:1373–1381. doi: 10.4049/jimmunol.180.3.1373. [DOI] [PubMed] [Google Scholar]
- 19.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 21.Lampropoulou V, Calderon-Gomez E, Roch T, Neves P, Shen P, Stervbo U, et al. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol Rev. 2010;233:146–161. doi: 10.1111/j.0105-2896.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 22.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 23.Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6:503–512. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 25.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. [PubMed] [Google Scholar]
- 27.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 costimulation pathway. Eur J Immunol. 2000;30:1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 29.Taniyama T, Takai S, Miyazaki E, Fukumura R, Sato J, Kobayashi Y, et al. The human interleukin-10 receptor gene maps to chromosome 11q23.3. Hum Genet. 1995;95:99–101. doi: 10.1007/BF00225083. [DOI] [PubMed] [Google Scholar]
- 30.Lutfalla G, Gardiner K, Uze G. A new member of the cytokine receptor gene family maps on chromosome 21 at less than 35 kb from IFNAR. Genomics. 1993;16:366–373. doi: 10.1006/geno.1993.1199. [DOI] [PubMed] [Google Scholar]
- 31.Wolk K, Witte E, Reineke U, Witte K, Friedrich M, Sterry W, et al. Is there an interaction between interleukin-10 and interleukin-22? Genes Immun. 2005;6:8–18. doi: 10.1038/sj.gene.6364144. [DOI] [PubMed] [Google Scholar]
- 32.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Reineke U, Schneider-Mergener J, Glaser RW, Stigler RD, Seifert M, Volk HD, et al. Evidence for conformationally different states of interleukin-10: binding of a neutralizing antibody enhances accessibility of a hidden epitope. J Mol Recognit. 1999;12:242–248. doi: 10.1002/(SICI)1099-1352(199907/08)12:4<242::AID-JMR461>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Yoon SI, Logsdon NJ, Sheikh F, Donnelly RP, Walter MR. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex. J Biol Chem. 2006;281:35088–35096. doi: 10.1074/jbc.M606791200. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SI, Jones BC, Logsdon NJ, Harris BD, Deshpande A, Radaeva S, et al. Structure and mechanism of receptor sharing by the IL-10R2 common chain. Structure. 2010;18:638–648. doi: 10.1016/j.str.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. [PubMed] [Google Scholar]
- 37.Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC. Characterization of interleukin-10 receptors on human and mouse cells. J Biol Chem. 1993;268:21053–21059. [PubMed] [Google Scholar]
- 38.Tan JC, Braun S, Rong H, DiGiacomo R, Dolphin E, Baldwin S, et al. Characterization of recombinant extracellular domain of human interleukin-10 receptor. J Biol Chem. 1995;270:12906–12911. doi: 10.1074/jbc.270.21.12906. [DOI] [PubMed] [Google Scholar]
- 39.Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol. 1999;205:194–204. doi: 10.1006/dbio.1998.9122. [DOI] [PubMed] [Google Scholar]
- 40.Denning TL, Campbell NA, Song F, Garofalo RP, Klimpel GR, Reyes VE, et al. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. Int Immunol. 2000;12:133–139. doi: 10.1093/intimm/12.2.133. [DOI] [PubMed] [Google Scholar]
- 41.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 43.Dagvadorj J, Naiki Y, Tumurkhuu G, Hassan F, Islam S, Koide N, et al. Interleukin-10 inhibits tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW 264.7 cells through reduced MyD88 expression. Innate Immun. 2008;14:109–115. doi: 10.1177/1753425908089618. [DOI] [PubMed] [Google Scholar]
- 44.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci U S A. 2009;106:18327–18332. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaoka K, Otsuka T, Niiro H, Nakashima H, Tanaka Y, Nagano S, et al. Selective DNA-binding activity of interleukin-10-stimulated STAT molecules in human monocytes. J Interferon Cytokine Res. 1999;19:679–685. doi: 10.1089/107999099313839. [DOI] [PubMed] [Google Scholar]
- 46.Thibodeau J, Bourgeois-Daigneault MC, Huppe G, Tremblay J, Aumont A, Houde M, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–1230. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 48.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–1668. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 49.Go NF, Castle BE, Barrett R, Kastelein R, Dang W, Mosmann TR, et al. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragheb S, Li Y, Simon K, VanHaerents S, Galimberti D, De Riz M, et al. Multiple sclerosis: BAFF and CXCL13 in cerebrospinal fluid. Mult Scler. 2011;17:819–829. doi: 10.1177/1352458511398887. [DOI] [PubMed] [Google Scholar]
- 52.Kalechman Y, Gafter U, Da JP, Albeck M, Alarcon-Segovia D, Sredni B. Delay in the onset of systemic lupus erythematosus following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-alpha levels. J Immunol. 1997;159:2658–2667. [PubMed] [Google Scholar]
- 53.Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
- 55.Schroder M, Meisel C, Buhl K, Profanter N, Sievert N, Volk HD, et al. Different modes of IL-10 and TGF-beta to inhibit cytokine-dependent IFN-gamma production: consequences for reversal of lipopolysaccharide desensitization. J Immunol. 2003;170:5260–5267. doi: 10.4049/jimmunol.170.10.5260. [DOI] [PubMed] [Google Scholar]
- 56.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–570. [PubMed] [Google Scholar]
- 57.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer JP, Renauld JC. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 58.Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediators Inflamm. 2003;12:309–313. doi: 10.1080/09629350310001619753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. http://clinicaltrials.gov/ct2/home.
- 60.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 61.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 63.Tindle RW. Peripheral T-cell tolerance defined through transgenic mouse studies. Autoimmunity. 2001;33:135–149. doi: 10.3109/08916930108995998. [DOI] [PubMed] [Google Scholar]
- 64.Zheng XX, Steele AW, Hancock WW, Stevens AC, Nickerson PW, Roy-Chaudhury P, et al. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J Immunol. 1997;158:4507–4513. [PubMed] [Google Scholar]
- 65.Moritani M, Yoshimoto K, Ii S, Kondo M, Iwahana H, Yamaoka T, et al. Prevention of adoptively transferred diabetes in nonobese diabetic mice with IL-10-transduced islet-specific Th1 lymphocytes. A gene therapy model for autoimmune diabetes. J Clin Invest. 1996;98:1851–1859. doi: 10.1172/JCI118986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee MS, Wogensen L, Shizuru J, Oldstone MB, Sarvetnick N. Pancreatic islet production of murine interleukin-10 does not inhibit immune-mediated tissue destruction. J Clin Invest. 1994;93:1332–1338. doi: 10.1172/JCI117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee MS, Mueller R, Wicker LS, Peterson LB, Sarvetnick N. IL-10 is necessary and sufficient for autoimmune diabetes in conjunction with NOD MHC homozygosity. J Exp Med. 1996;183:2663–2668. doi: 10.1084/jem.183.6.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balasa B, Van Gunst K, Jung N, Katz JD, Sarvetnick N. IL-10 deficiency does not inhibit insulitis and accelerates cyclophosphamide-induced diabetes in the nonobese diabetic mouse. Cell Immunol. 2000;202:97–102. doi: 10.1006/cimm.2000.1658. [DOI] [PubMed] [Google Scholar]
- 70.Xiao Z, Mohamood AS, Uddin S, Gutfreund R, Nakata C, Marshall A, et al. Inhibition of Fas ligand in NOD mice unmasks a protective role for IL-10 against insulitis development. Am J Pathol. 2011;179:725–732. doi: 10.1016/j.ajpath.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson WS, Pekalski ML, Simons HZ, Smyth DJ, Castro-Dopico X, Guo H, et al. Multi-parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin Exp Immunol. 2014;177:571–585. doi: 10.1111/cei.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 73.Song YW, Kang EH. Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM. 2010;103:139–146. doi: 10.1093/qjmed/hcp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 75.Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46:3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 76.Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, et al. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- 78.al-Janadi M, al-Dalaan A, al-Balla S, al-Humaidi M, Raziuddin S. Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J Clin Immunol. 1996;16:198–207. doi: 10.1007/BF01541225. [DOI] [PubMed] [Google Scholar]
- 79.Bober LA, Rojas-Triana A, Jackson JV, Leach MW, Manfra D, Narula SK, et al. Regulatory effects of interleukin-4 and interleukin-10 on human neutrophil function ex vivo and on neutrophil influx in a rat model of arthritis. Arthritis Rheum. 2000;43:2660–2667. doi: 10.1002/1529-0131(200012)43:12<2660::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 80.van Roon J, Wijngaarden S, Lafeber FP, Damen C, van de Winkel J, Bijlsma JW. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol. 2003;30:648–651. [PubMed] [Google Scholar]
- 81.Lacki JK, Klama K, Mackiewicz SH, Mackiewicz U, Muller W. Circulating interleukin 10 and interleukin-6 serum levels in rheumatoid arthritis patients treated with methotrexate or gold salts: preliminary report. Inflamm Res. 1995;44:24–26. doi: 10.1007/BF01630483. [DOI] [PubMed] [Google Scholar]
- 82.Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, et al. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995;154:5492–5499. [PubMed] [Google Scholar]
- 83.Maini RNPH, Breedveld PC. Hu lL-10 in subjects with active rheumatoid arthritis (I&A): phase I and cytokine response study. Arthritis Rheum; Abstract Supplement of National Scientific Meeting; Washington. 1997. p. 224. [Google Scholar]
- 84.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 85.Stern RS. Psoriasis. Lancet. 1997;350:349–353. doi: 10.1016/S0140-6736(97)05257-4. [DOI] [PubMed] [Google Scholar]
- 86.Nickoloff BJ, Fivenson DP, Kunkel SL, Strieter RM, Turka LA. Keratinocyte interleukin-10 expression is upregulated in tape-stripped skin, poison ivy dermatitis, and Sezary syndrome, but not in psoriatic plaques. Clin Immunol Immunopathol. 1994;73:63–68. doi: 10.1006/clin.1994.1170. [DOI] [PubMed] [Google Scholar]
- 87.Mussi A, Bonifati C, Carducci M, Viola M, Tomaselli R, Sacerdoti G, et al. IL-10 levels are decreased in psoriatic lesional skin as compared to the psoriatic lesion-free and normal skin suction blister fluids. J Biol Regul Homeost Agents. 1994;8:117–120. [PubMed] [Google Scholar]
- 88.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberti ML, Ricottini L, Capponi A, Sclauzero E, Vicenti P, Fiorentini E, et al. Immunomodulating treatment with low dose interleukin-4, interleukin-10 and interleukin-11 in psoriasis vulgaris. J Biol Regul Homeost Agents. 2014;28:133–139. [PubMed] [Google Scholar]
- 90.Asadullah K, Docke WD, Ebeling M, Friedrich M, Belbe G, Audring H, et al. Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial. Arch Dermatol. 1999;135:187–192. doi: 10.1001/archderm.135.2.187. [DOI] [PubMed] [Google Scholar]
- 91. http://clinicaltrials.gov/ct2/show/study/NCT00001797?term=IL-10&rank=7.
- 92.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 93.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 94.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 95.Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 96.Correa I, Veny M, Esteller M, Pique JM, Yague J, Panes J, et al. Defective IL-10 production in severe phenotypes of Crohn's disease. J Leukoc Biol. 2009;85:896–903. doi: 10.1189/jlb.1108698. [DOI] [PubMed] [Google Scholar]
- 97.Meresse B, Rutgeerts P, Malchow H, Dubucquoi S, Dessaint JP, Cohard M, et al. Low ileal interleukin 10 concentrations are predictive of endoscopic recurrence in patients with Crohn's disease. Gut. 2002;50:25–28. doi: 10.1136/gut.50.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang AH, Lam WJ, Han DY, Ding Y, Hu R, Fraser AG, et al. The effect of IL-10 genetic variation and interleukin 10 serum levels on Crohn's disease susceptibility in a New Zealand population. Hum Immunol. 2011;72:431–435. doi: 10.1016/j.humimm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 99.Kucharzik T, Stoll R, Lugering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;100:452–456. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitsuyama K, Tomiyasu N, Takaki K, Masuda J, Yamasaki H, Kuwaki K, et al. Interleukin-10 in the pathophysiology of inflammatory bowel disease: increased serum concentrations during the recovery phase. Mediators Inflamm. 2006;2006:26875. doi: 10.1155/MI/2006/26875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ljuca F, Gegic A, Salkic NN, Pavlovic-Calic N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn's disease. Dig Dis Sci. 2010;55:2316–2326. doi: 10.1007/s10620-009-1016-9. [DOI] [PubMed] [Google Scholar]
- 102.Ishizuka K, Sugimura K, Homma T, Matsuzawa J, Mochizuki T, Kobayashi M, et al. Influence of interleukin-10 on the interleukin-1 receptor antagonist/interleukin-1 beta ratio in the colonic mucosa of ulcerative colitis. Digestion. 2001;63(Suppl 1):22–27. doi: 10.1159/000051906. [DOI] [PubMed] [Google Scholar]
- 103.Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut. 2001;49:42–46. doi: 10.1136/gut.49.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473–1482. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 105.Buruiana FE, Sola I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010:CD005109. doi: 10.1002/14651858.CD005109.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sattler S, Ling GS, Xu D, Hussaarts L, Romaine A, Zhao H, et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50:107–122. doi: 10.1016/j.jaut.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 109.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 112.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 113.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 115.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 116.Hirotani M, Niino M, Fukazawa T, Kikuchi S, Yabe I, Hamada S, et al. Decreased IL-10 production mediated by Toll-like receptor 9 in B cells in multiple sclerosis. J Neuroimmunol. 2010;221:95–100. doi: 10.1016/j.jneuroim.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 117.Porrini AM, Gambi D, Reder AT. Interferon effects on interleukin-10 secretion. Mononuclear cell response to interleukin-10 is normal in multiple sclerosis patients. J Neuroimmunol. 1995;61:27–34. doi: 10.1016/0165-5728(95)00070-i. [DOI] [PubMed] [Google Scholar]
- 118.Rudick RA, Ransohoff RM, Peppler R, VanderBrug Medendorp S, Lehmann P, Alam J. Interferon beta induces interleukin-10 expression: relevance to multiple sclerosis. Ann Neurol. 1996;40:618–627. doi: 10.1002/ana.410400412. [DOI] [PubMed] [Google Scholar]
- 119.Chabot S, Yong VW. Interferon beta-1b increases interleukin-10 in a model of T cell-microglia interaction: relevance to MS. Neurology. 2000;55:1497–1505. doi: 10.1212/wnl.55.10.1497. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, Chen M, Wandinger KP, Williams G, Dhib-Jalbut S. IFN-beta-1b inhibits IL-12 production in peripheral blood mononuclear cells in an IL-10-dependent mechanism: relevance to IFN-beta-1b therapeutic effects in multiple sclerosis. J Immunol. 2000;165:548–557. doi: 10.4049/jimmunol.165.1.548. [DOI] [PubMed] [Google Scholar]
- 121.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 122.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Issazadeh S, Mustafa M, Ljungdahl A, Hojeberg B, Dagerlind A, Elde R, et al. Interferon gamma, interleukin 4 and transforming growth factor beta in experimental autoimmune encephalomyelitis in Lewis rats: dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J Neurosci Res. 1995;40:579–590. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- 124.Nagelkerken L, Blauw B, Tielemans M. IL-4 abrogates the inhibitory effect of IL-10 on the development of experimental allergic encephalomyelitis in SJL mice. Int Immunol. 1997;9:1243–1251. doi: 10.1093/intimm/9.9.1243. [DOI] [PubMed] [Google Scholar]
- 125.Cannella B, Gao YL, Brosnan C, Raine CS. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J Neurosci Res. 1996;45:735–746. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 126.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014 doi: 10.1016/S0140-6736(14)60128-8. In Press. [DOI] [PubMed] [Google Scholar]
- 127.Llorente L, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Maillot MC, Durand-Gasselin I, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw. 1993;4:421–427. [PubMed] [Google Scholar]
- 128.Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Fourrier BM, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–1655. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 129.Llorente L, Richaud-Patin Y, Couderc J, Alarcon-Segovia D, Ruiz-Soto R, Alcocer-Castillejos N, et al. Dysregulation of interleukin-10 production in relatives of patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1429–1435. doi: 10.1002/art.1780400810. [DOI] [PubMed] [Google Scholar]
- 130.Horwitz DA, Gray JD, Behrendsen SC, Kubin M, Rengaraju M, Ohtsuka K, et al. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–844. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 131.Mongan AE, Ramdahin S, Warrington RJ. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997;46:406–412. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 132.Hagiwara E, Gourley MF, Lee S, Klinman DK. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 133.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Interleukin-10 blockade corrects impaired in vitro cellular immune responses of systemic lupus erythematosus patients. Arthritis Rheum. 2000;43:1976–1981. doi: 10.1002/1529-0131(200009)43:9<1976::AID-ANR8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 135.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 136.Huhn RD, Radwanski E, Gallo J, Affrime MB, Sabo R, Gonyo G, et al. Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin Pharmacol Ther. 1997;62:171–180. doi: 10.1016/S0009-9236(97)90065-5. [DOI] [PubMed] [Google Scholar]