Abstract
Assessing the severity of emerging infections is challenging because of potential biases in case ascertainment. In the second epidemic of human infections with avian influenza A(H7N9) virus in China in 2013–14, we estimated that the risk of death among hospitalized H7N9 cases was 48% (95% credibility interval: 42%–54%). Using data on symptomatic cases identified through national sentinel influenza-like illness surveillance, we estimated that the risk of death among symptomatic H7N9 cases was 0.10% (95% credibility interval: 0.029%–3.6%). These estimates of severity were quite similar to previous estimates for the first epidemic wave of human infections with H7N9 in 2013.
Keywords: avian influenza A(H7N9) virus, clinical severity, epidemiology, public health
INTRODUCTION
Since the first human case of infection with novel avian influenza A(H7N9) virus was identified in China in March 2013, there have been two major epidemics of human infections to date. The first epidemic, in the spring of 2013, waned during the late spring and summer [1–3], while a second major epidemic occurred during the winter of 2013–14 and had waned by the end of the spring of 2014 while sporadic cases have continued to be reported (as of 9 October 2014). A small number of clusters of laboratory-confirmed cases have been identified in both epidemics, but the virus does not yet appear to have the capacity for sustained human-to-human transmission [1]. Whereas confirmed H7N9 cases have generally been identified in hospitalized patients with pneumonia [4], identification of a small number of confirmed cases through routine sentinel influenza-like illness (ILI) surveillance indicates a potential larger number of mild H7N9 virus infections [5, 6]. This has implications for determination of the clinical severity of H7N9 virus infections, because the confirmed cases may not fully reflect the clinical spectrum of infections, and consequently changes in case ascertainment could lead to artefactual variation in risk of severe outcomes.
In previous work, we demonstrated that the fatality risk among confirmed cases of H1N1pdm09 was very heterogeneous and difficult to interpret [7], and we characterized the severity of H7N9 virus infections via the risk of fatality among hospitalized cases (the “hospitalization fatality risk”, HFR) and the risk of fatality among symptomatic cases (the “symptomatic case fatality risk”, CFR) [3]. In the first epidemic wave of H7N9 in spring 2013, we estimated the HFR at approximately 36%, and the CFR at 0.16% to 2.8% [3]. The objective of the present study is to estimate the HFR and symptomatic CFR in the second wave, and to determine whether the severity of human infections with H7N9 virus has changed over time.
METHODS
Sources of data
All laboratory-confirmed human cases of avian influenza A(H7N9) virus infection are reported to the Chinese Center for Disease Control and Prevention (China CDC) through a national surveillance system. Case definitions, surveillance for identification of cases, and laboratory assays have been previously described [1]. Demographic, epidemiological, and basic clinical data were obtained from each confirmed case with standardised forms. An integrated database was constructed by China CDC, with detailed epidemiological information about each confirmed H7N9 case reported by 9 October, 2014. We used information about age, sex, place of residence, dates of illness onset, hospital admission, ICU admission, mechanical ventilation, death, and recovery or discharge.
Statistical analysis
Cases were determined to be hospitalized for medical reasons (rather than solely for isolation purposes) based on routine clinical judgment, e.g. those presenting with complications such as pneumonia. A small number of mild cases presented with respiratory symptoms but did not have any complications throughout the clinical course and were hospitalized only for the purpose of isolation. Among the confirmed H7N9 cases hospitalized for medical reasons, i.e. excluding these mild cases, we estimated the risks of intensive care unit (ICU) admission, mechanical ventilation, and death. To allow for the uncertain outcomes of cases that remained in hospital on the date of analysis (9 October 2014), we used the method proposed by Garske et al., which inflates the observed fatality risk based on the time to death distribution [8]. We constructed 95% confidence intervals (CIs) using a bootstrap approach with 1000 resamples.
To estimate the symptomatic CFR, we inferred the number of symptomatic cases based on the detection of symptomatic cases through sentinel ILI surveillance in urban areas [3]. We searched for urban areas where (i) the number of confirmed H7N9 cases registered by local ILI sentinels and other hospitals are both larger than one, and (ii) the number of outpatient visits at local ILI sentinels and other hospitals are available. In the spring 2013 epidemic, Shanghai and Nanjing (Jiangsu province) met the criteria, and in the winter 2013–14 epidemic the city of Shaoxing (Zhejiang province) met the criteria. In these selected urban areas, we determined the daily number of all ILI cases reported and specimens tested by ILI surveillance in each location during the relevant period to infer the number of infected individuals who would have sought medical care at ILI sentinels (NILI). We assumed that health-care seeking behavior of individuals with ILI associated with H7N9 virus infection was the same as health-care seeking behavior of individuals with ILI associated with H1N1pdm09 virus infection in 2009–10 in the same area of China. We used data from a nationwide sero-survey and ILI surveillance of H1N1pdm09 in China from June 2009, to January 2010, to estimate the proportion of individuals with symptomatic infections who sought medical care at ILI sentinels. We divided NILI by this proportion. We then estimated the symptomatic CFR in each location using the number of confirmed deaths as the numerator and the estimated number of mild cases as the denominator. We used a Bayesian framework to estimate the symptomatic CFR, and presented the estimates with 95% credibility intervals (CrI) which have a similar interpretation to confidence intervals [9].
We examined epidemiologic time-to-event distributions using kernel density methods as previously described [2]. All statistical analyses were performed using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and Matlab (Mathworks Inc., Natick, MA).
RESULTS
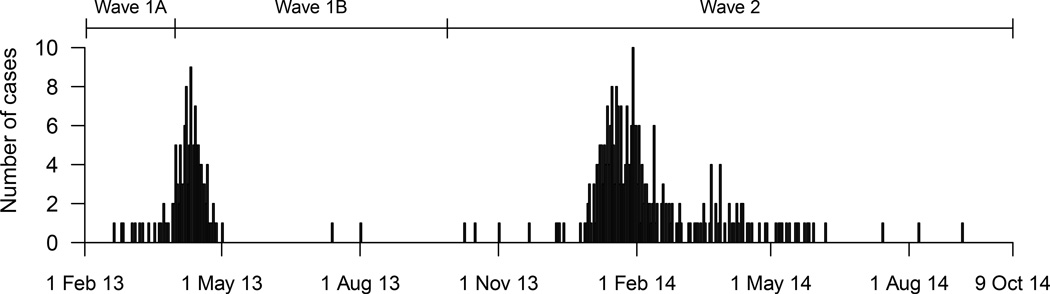
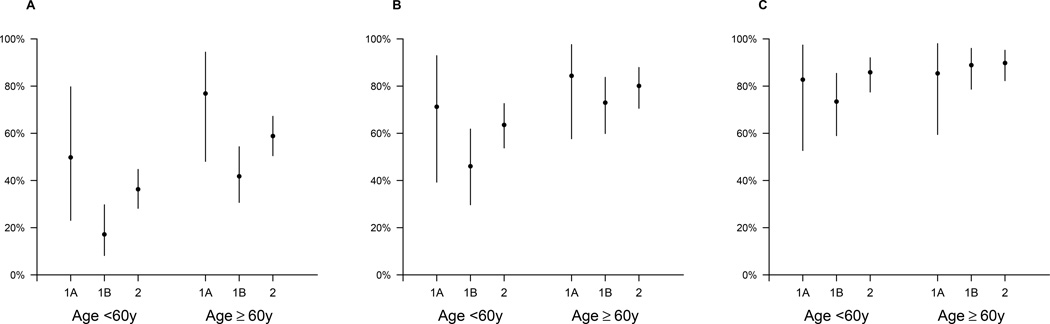
In the first wave of H7N9 cases in 2013, 134 confirmed cases were identified, of whom 124 required hospitalization for medical reasons. Among the hospitalized cases, the risk of serious outcomes was higher among older hospitalized cases. Furthermore, we identified higher risks of fatality among cases hospitalized before 31 March 2013, the date when the first confirmed human cases of H7N9 virus infection were officially announced in China (Table 1). We therefore divided the first wave into two parts: wave 1A for 18 cases hospitalized prior to April 1 2013, and wave 1B for 106 cases hospitalized from April 1 to September 30, 2013 (Figure 1). The median age was 60y in wave 1A and 61y in wave 1B. Among the cases <60y who required hospitalization for medical reasons, the HFR in wave 1A was 51% (95% CI: 21%, 79%), significantly higher (p=0.039) than the HFR of 17% (95% CI: 7.6%, 30%) in wave 1B. For cases ≥60y who required hospitalization for medical reasons, the HFR was also significantly higher (p=0.025) in wave 1A (77%; 95% CI: 48%, 94%) vs wave 1B (42%, 95% CI: 31%, 54%). We did not identify significant differences between wave 1A and 1B in the risk of death or ventilation, or in the risk of death/ventilation/ICU admission (Figure 2).
Table 1.
Estimates of the symptomatic case fatality risk
| Period considered in analysis |
Geographic location | Number of confirmed H7N9 deaths during period |
Estimated number of symptomatic H7N9 infections during period |
Estimated risk of fatality per 100,000 symptomatic cases |
|---|---|---|---|---|
| 1 Jan 2013 – 28 May 2013 | Shanghai | 14 | 3,020 (95% CI: 900–7,800) | 490 (95% CI: 170–1,800) |
| 1 Jan 2013 – 28 May 2013 | Nanjing (Jiangsu province) | 3 | 5,310 (95% CI: 880–17,300) | 69 (95% CI: 12–710) |
| 1 Jan 2014 – 21 Jan 2014 | Shaoxing (Zhejiang province) | 5 | 5,750 (95% CI: 1,960–12,730) | 100 (95% CI: 29–360) |
Figure 1.
Incidence of laboratory-confirmed human cases of avian influenza A(H7N9) virus infection in China, by date of hospitalization. The first wave of infections in 2013 is divided into two parts, before and after the announcement of human cases on March 31, 2013 because of the potential for under-ascertainment of less severe cases in the earlier period.
Figure 2.
Estimates and 95% credibility intervals of the risk of serious outcomes among confirmed H7N9 cases hospitalized for medical reasons, by age and wave. Panel A: the risk of death. Panel B: the risk of death or mechanical ventilation. Panel C: the risk of death or mechanical ventilation or ICU admission. The periods covered by waves 1A, 1B and 2 are shown in Figure 1.
In the second wave of H7N9, 273 of the 306 confirmed cases required hospitalization for medical reasons with onset dates between October 1, 2013 and October 9, 2014. The median age was 57y (range 2–88y). 69 percent of cases were male. Among the hospitalized cases, allowing for censoring of outcomes in 5 (2%) patients remain in hospital on 9 Oct 2014, we estimated HFRs of 36% (95% CI: 28%, 45%) in cases <60y, and 59% (95% CI: 51%, 67%) in cases ≥60y. These risks were significantly higher than in wave 1B (p=0.019 and p=0.025 respectively). There were no statistically significant differences between the age-specific risks of death or ventilation, or death/ventilation/ICU admission in wave 2 compared to either wave 1A or wave 1B while estimates of the risks of serious outcomes were generally lower across age groups in wave 1B compared to wave 2 (Figure 2). While the second epidemic wave occurred over a broader geographic area than the first wave, Zhejiang province was heavily affected in both epidemic waves. We therefore examined the risk of death among the subset of hospitalized cases in this province. Zhejiang province reported 40 cases in wave 1B and 88 cases in wave 2, and the risk of death among hospitalized cases <60y was significantly higher in wave 2 compared to wave 1B (risk ratio 7.1; 95% CI: 1.3, 292; p=0.017) and not significantly different in hospitalized cases ≥60y (risk ratio 1.5; 95% CI: 0.93, 2.8; p=0.099).
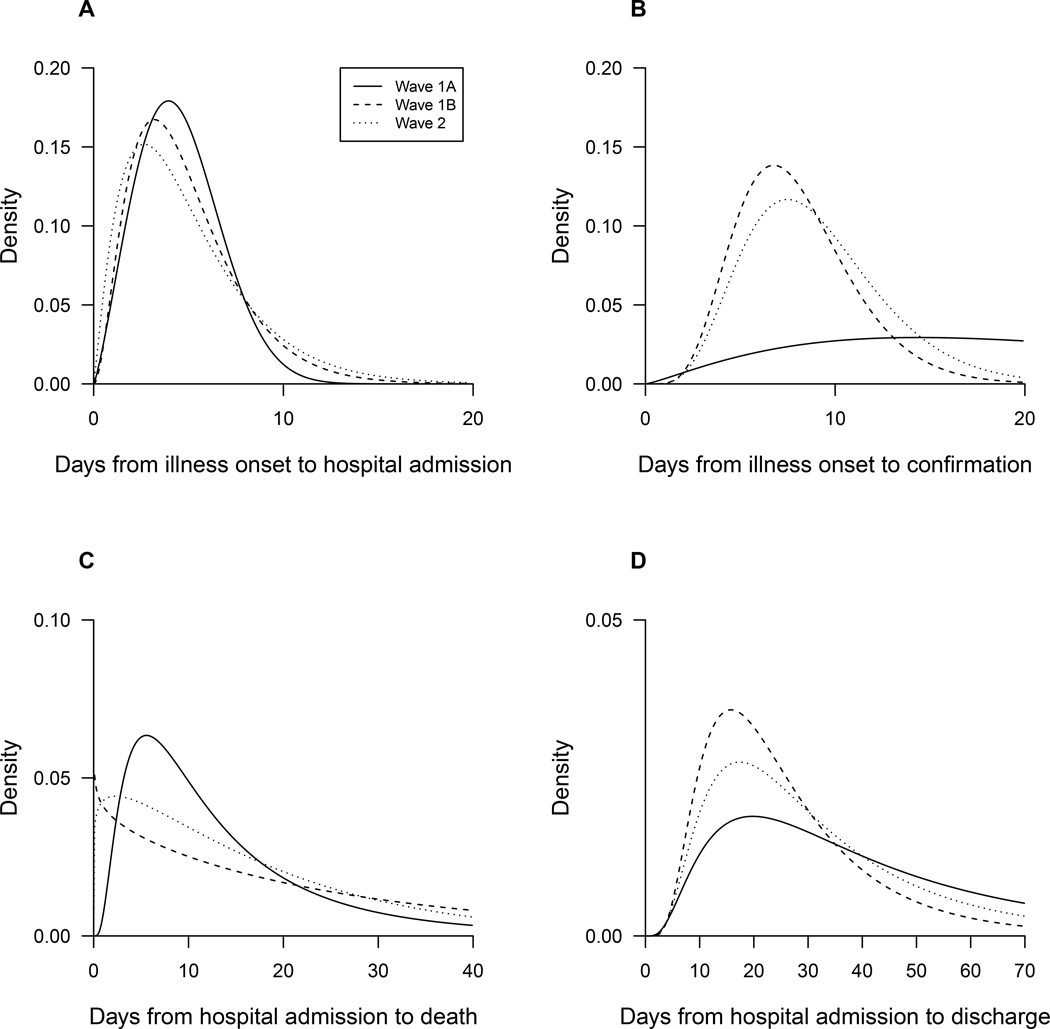
We examined the delays from onset to admission and identified similar patterns over calendar time, while the delay from onset to laboratory confirmation has shortened over time and in wave 2 the mean was 8.0 days (Figure 3). Distributions of time from admission to death and admission to discharge were similar over time (Figure 3).
Figure 3.
Comparisons of epidemiologic distributions between waves. Panel A: the time from illness onset to hospital admission. Panel B: the time from illness onset to laboratory confirmation. Panel C: the time from hospital admission to death. Panel D: the time from hospital admission to discharge. The periods covered by waves 1A, 1B and 2 are shown in Figure 1.
We previously used information on 3 confirmed H7N9 cases identified through ILI surveillance in Shanghai and Nanjing to estimate the number of symptomatic cases in the spring 2013 epidemic [3]. Here we also use information on 4 confirmed cases identified through ILI surveillance in Shaoxing in the winter 2013–14 epidemic, in the period from 1 January to 21 January 2014, prior to the closure of live poultry markets on 22 January. During the same period in Shaoxing, 9 hospitalized cases had onset of illness, of whom 5 died. Based on these observations, we estimated that there were 3,020 (95% CI: 900–7,800) and 5,310 (95% CI: 880–17,300) cases in the first epidemic wave in 2013 in Shanghai and Nanjing, respectively, and 5,750 (95% CI: 1,960–12,730) cases in Shaoxing in the second epidemic wave in 2013–14. These estimates correspond to symptomatic CFRs of 490 and 69 in Shanghai and Nanjing respectively in the first wave, and 100 per 100,000 symptomatic cases in Shaoxing in the second wave, with wide and overlapping credibility intervals (Table 1).
DISCUSSION
The resurgence of human infections with avian influenza A(H7N9) virus in a second epidemic in 2013–14 demonstrates the continued public health risk of this novel strain [10]. Control of the virus in animals is complicated, because the infections in poultry are asymptomatic [11]. Human-to-human transmissibility of the virus remains limited, as evidenced by the very small number of potential secondary infections identified through detailed contact tracing of confirmed cases [1, 2, 12–14].
We identified differences in the severity of illness of hospitalized cases in the earlier part of the first epidemic wave in 2013, with greater risk of mechanical ventilation, ICU admission and death among cases hospitalized prior to 31 March 2013 when the first confirmed human cases of H7N9 were officially announced (Table 1) [15]. One explanation for this is more timely antiviral treatment and more appropriate supportive care for cases hospitalized after 31 March 2013. Another possible explanation is detection bias in the early phase of the spring 2013 epidemic, where more severe cases were prioritized for repeated laboratory testing, and cases with prolonged virus shedding or higher virus shedding had a greater chance of confirmation.
In the second epidemic wave in 2013–14, we identified a significantly greater HFR compared to the latter part of the first epidemic wave in 2013 (Figure 2) and in persons <60y in Zhejiang province where cases occurred in both epidemics, but no difference in the symptomatic CFR (Table 1). It is possible that this significant difference in HFRs is due to ascertainment bias in cases in different locations at different times, even within the same province. Alternatively, the HFR could have increased, because hospitalized cases in the second epidemic in 2013–14 were less likely to be transferred to major advanced provincial hospitals (Dr Enfu Chen, Chief Epidemiologist in Zhejiang Provincial CDC, personal communication, June 2014), or because of seasonal changes in the prevalence of other pathogens that could cause secondary or co-infections and modify the severity of H7N9 virus infections [16]. Whereas ascertainment of infections in hospitalized cases may have changed over time due to changes in awareness and testing capacity, the ascertainment of H7N9 cases through the established sentinel ILI network should have remained more stable over time.
Large population-based serological studies in affected areas would permit assessment of severity with a denominator of infections, rather than cases of symptomatic disease or hospitalization, and infection-based severity measures could be less susceptible to biases due to differential health care seeking behaviors or diagnostic capacity [3, 7]. To date, few serological studies have been reported and such analyses are not yet possible [17–19].
Our estimates of the risks of serious outcomes in hospitalized cases are limited by the potential for under-ascertainment of cases, due to lack of access to laboratory testing in some areas, and the potential for imperfect sensitivity of laboratory testing for the H7N9 virus [20, 21]. While we accounted for unknown final status of cases that remain hospitalized in our analysis, the eventual estimates may change slightly once all outcomes are known. It is challenging to estimate the symptomatic CFR based on a small number of confirmed cases with milder disease identified through sentinel ILI surveillance, and our estimates are dependent on the assumptions that coverage of the sentinel system was similar in 2013–14 compared to 2009, and that health-care seeking behaviors for ILI were similar whether illness was caused by H7N9 or H1N1pdm09 [3]. In addition, the estimation of sCFR were based on data from geographic locations in which H7N9 virus infections were identified through sentinel ILI surveillance, and a more comprehensive analysis could also incorporate data on ILI surveillance in other areas.
In conclusion, it remains important to assess the severity of human infections with H7N9, as part of ongoing risk assessment of this virus. While the overall picture is that the severity of human infections has not substantially changed (Table 1), we found some evidence that the HFR was higher in the second epidemic wave in 2013–14 (Figure 2). Our results again highlight that many H7N9 virus infections can cause mild disease [3, 5, 6] and that the risk of death among laboratory-confirmed cases is a misleading measure of severity. If another epidemic of human infections with H7N9 virus occurs in the coming winter of 2014–15, proactive control measures on the poultry-human interface may be preferable to reactive measures [10, 22–24]. Comprehensive surveillance of avian influenza virus infections in animals and humans is essential in order to monitor risk and guide the use of control measures.
Acknowledgements
We thank staff members at county-, district-, prefecture-, and provincial-level CDCs at the provinces where human cases of influenza A(H7N9) virus infection occurred for providing assistance with field investigation, administration and data collection.
This study was funded by the United States National Institutes of Health (Comprehensive International Program for Research on AIDS grant U19 AI51915), the China-United States Collaborative Program on Emerging and Re-emerging Infectious Diseases, grants from the Ministry of Science and Technology, China (2012 ZX10004-201), the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06), and the Beijing Science and Technology Planning Project of Beijing Science and Technology Commission (Z131100005613048). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Conflicts of interest
GML has received consulting honoraria from Janssen Pharmaceuticals. BJC reports receipt of research funding from MedImmune Inc. and Sanofi Pasteur, and consults for Crucell NV.
Footnotes
Authors’ contributions
Hongjie Yu and Benjamin J Cowling designed the study. Luzhao Feng, Joseph T. Wu, Xiaoqing Liu, Peng Yang, Tim K. Tsang, Hui Jiang, Peng Wu, Juan Yang, Vicky J. Fang, Ying Qin, Eric H. Y. Lau, Ming Li, Jiandong Zheng, Zhibin Peng, Yun Xie, Quanyi Wang, Zhongjie Li, Gabriel M. Leung and George F. Gao collected data. Luzhao Feng, Joseph T. Wu, Tim K. Tsang, Peng Wu, Vicky J. Fang and Eric H. Y. Lau analysed data. Benjamin J Cowling wrote the first draft and all authors contributed to review and revision and have seen and approved the final version.
REFERENCES
- 1.Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370(6):520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowling BJ, Jin L, Lau EH, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382(9887):129–137. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H, Cowling BJ, Feng L, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382(9887):138–145. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang N, Havers F, Chen T, et al. Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004–2013. Emerg Infect Dis. 2013;19(11):1784–1790. doi: 10.3201/eid1911.130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip DK, Liao Q, Wu P, et al. Detection of mild to moderate influenza A/H7N9 infection by China's national sentinel surveillance system for influenza-like illness: case series. BMJ. 2013;346:f3693. doi: 10.1136/bmj.f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Havers F, Wang L, et al. Monitoring avian influenza A(H7N9) virus through national influenza-like illness surveillance, China. Emerg Infect Dis. 2013;19(8):1289–1292. doi: 10.3201/eid1908.130662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong JY, Kelly H, Ip DK, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013;24(6):830–841. doi: 10.1097/EDE.0b013e3182a67448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garske T, Legrand J, Donnelly CA, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 9.Sterne JA, Davey Smith G. Sifting the evidence-what's wrong with significance tests? BMJ. 2001;322(7280):226–231. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert M, Golding N, Zhou H, et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun. 2014;5:4116. doi: 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uyeki TM, Cox NJ. Global concerns regarding novel influenza A (H7N9) virus infections. N Engl J Med. 2013;368(20):1862–1864. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Zhu Y, Zhao B, et al. Limited human-to-human transmission of avian influenza A(H7N9) virus, Shanghai, China, March to April 2013. Euro Surveill. 2014;19(25) doi: 10.2807/1560-7917.es2014.19.25.20838. [DOI] [PubMed] [Google Scholar]
- 13.Qi X, Qian YH, Bao CJ, et al. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. BMJ. 2013;347:f4752. doi: 10.1136/bmj.f4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi L, Guan D, Kang M, et al. Family Clusters of Avian Influenza A H7N9 Infection in Guangdong Province, China. J Clin Microbiol. 2014 doi: 10.1128/JCM.02322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 16.Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS immunology and medical microbiology. 1999;26(3–4):189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Fang S, Lu X, et al. Seroprevalence to avian influenza A(H7N9) virus among poultry workers and the general population in southern China: a longitudinal study. Clin Infect Dis. 2014;59(6):e76–e83. doi: 10.1093/cid/ciu399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai T, Zhou J, Shu Y. Serologic study for influenza A (H7N9) among high-risk groups in China. N Engl J Med. 2013;368(24):2339–2340. doi: 10.1056/NEJMc1305865. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Chen Y, Cui D, et al. Avian-origin influenza A(H7N9) infection in influenza A(H7N9)-affected areas of China: a serological study. J Infect Dis. 2014;209(2):265–269. doi: 10.1093/infdis/jit430. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381(9881):1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Lu S, Song Z, et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381(9885):2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Wu JT, Cowling BJ, et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2014;383(9916):541–548. doi: 10.1016/S0140-6736(13)61904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu P, Jiang H, Wu JT, et al. Poultry market closures and human infection with influenza A(H7N9) virus, China, 2013–14. Emerging Infectious Diseases. 2014;20(11):1891–1894. doi: 10.3201/eid2011.140556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fournie G, Pfeiffer DU. Can closure of live poultry markets halt the spread of H7N9? Lancet. 2014;383(9916):496–497. doi: 10.1016/S0140-6736(13)62109-1. [DOI] [PubMed] [Google Scholar]