Abstract
Chronic mucosal inflammation is the hallmark of important and common airway diseases, such as allergic rhinitis and asthma. Lipoxin A4 (LXA4) is an endogenous pro-resolving mediator for mucosal inflammation that decreases allergic and asthmatic responses. Lipoxin B4 (LXB4) is a structurally distinct member of the lipoxin family that signals in a manner distinct from LXA4. LXB4 is generated by mucosal tissues, but its actions in allergic inflammation are unknown. Here, we used murine models of allergic rhinitis and asthma to investigate LXB4’s activity in mucosal inflammation. In the upper airway, LXB4 significantly decreased nasal mucosal leukocytes and degranulation of mast cells and eosinophils. In the lower airway, LXB4 significantly decreased airway inflammation, mucus metaplasia and hyper- responsiveness. Inhibition of mast cell degranulation in vivo by LXB4 was more potent than dexamethasone, and these agents displayed unique profiles for cytokine regulation; however, their overall anti-inflammatory actions were comparable. LXB4 decreased eotaxin-dependent eosinophil chemotaxis, IgE-mediated mast cell degranulation and expression of type 2 cytokine receptors. Together, these findings indicate that LXB4 carries cell type selective and mucosal protective actions that broaden the lipoxin family’s therapeutic potential for upper and lower airway catabasis.
INTRODUCTION
Allergic airway diseases, such as rhinitis and asthma, are common and adversely impact patients function and quality of life 1–2. Allergic rhinitis (AR) and atopic asthma can present with chronic mucosal inflammatory processes, and have been referred to as a “united airways” disease 3–4. Despite distinct clinical manifestations, these diseases have immunological similarities. For example, eosinophil tissue infiltration and IgE-mediated mast cell (MC) activation through FcεRI bound allergen are major factors in the pathogenesis of AR and atopic asthma5. Current treatments for AR and asthma principally target symptom relief and anti-inflammation with glucocorticosteroids (GC). Since GC are associated with heterogeneous therapeutic responses and can have serious side effects, there is still a need for new treatment strategies to control AR and asthma. Importantly, no curative therapy is available to resolve allergic airway inflammation.
Recently, research focused on the physiological termination of inflammatory responses has led to the identification of specialized mediators involved in the natural resolution of inflammation in health, including lipoxins (i.e., lipoxin A4 (LXA4), lipoxin B4 (LXB4))6. These specialized pro-resolving mediators are lipoxygenase-derived eicosanoids formed from arachidonic acid by transcellular metabolism during cell–cell interactions (reviewed in 6). Lipoxins are generated by the upper respiratory tract, including by nasal polyps 7, and are present in nasal lavage fluids from aspirin- challenged patients with aspirin-exacerbated respiratory disease 8. Lipoxins are also present in the lower airways in broncho-alveolar lavage fluid (BALF) from patients with asthma9. Of note, severe asthma is associated with decreased LXA4 generation9. Administration of a stable analog of LXA4 blocks allergic airway inflammation, mucus metaplasia and hyper-reactivity to methacholine10. LXA4 is a potent inhibitor of granulocyte recruitment and activation, including eosinophils10. LXB4 is a structurally distinct product of arachidonic acid metabolism. LXB4 regulates neutrophil activation 11; however, its actions in allergic inflammation and on the functional responses of mucosal effector cells (i.e. MC and eosinophils), has yet to be established. Here we provide the first evidence that LXB4 mediates anti- inflammatory and pro-resolving actions for allergic airway responses in murine models of upper and lower airway mucosal inflammation.
RESULTS
LXB4 reduces leukocyte infiltration and mucus secretion in the nasal mucosa
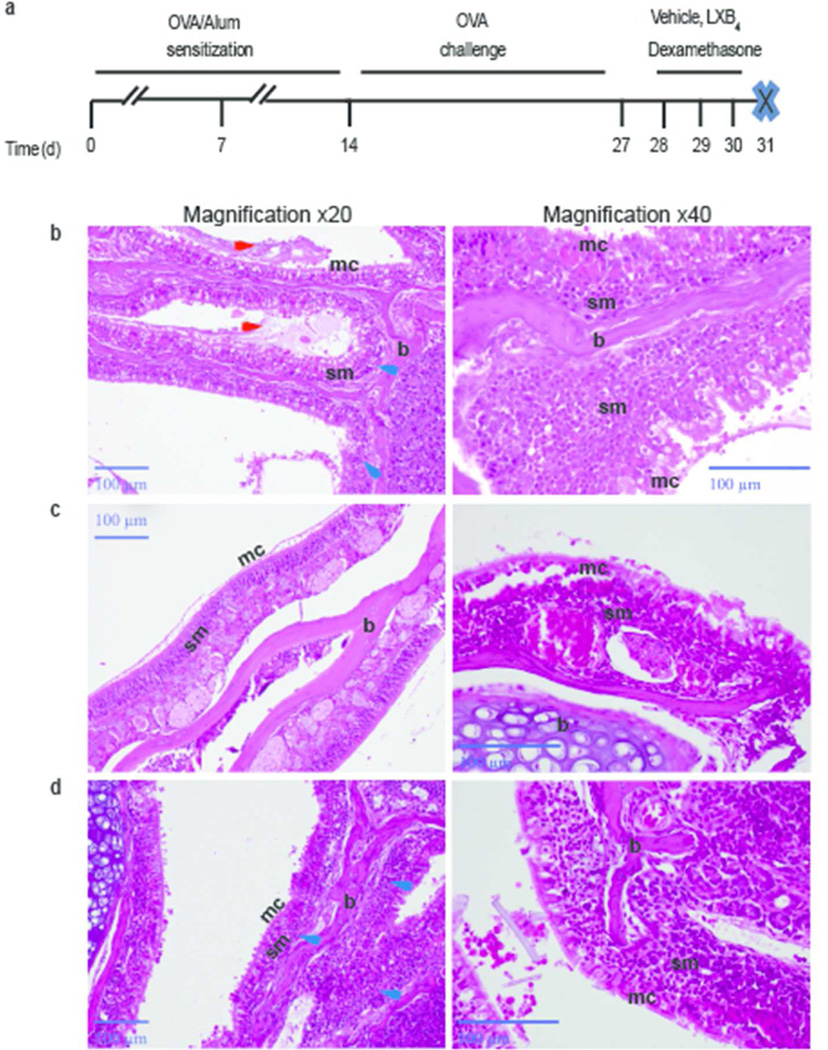
Mice (BALB/c) were systemically sensitized with OVA/Alum and then challenged with OVA intranasally to target the allergic responses to the nasal mucosa (Figure 1a, see Methods). Twenty four hours after the last nasal allergen challenge, mice were given LXB4 (as validated in Supplementary Figure S1) (100 ng, i.v), dexamethasone (100 ng) or vehicle control for 3 consecutive days (protocol day 28, 29, 30) (Figure 1a). Mice were sacrificed 24 hours later (protocol day 31). Histopathological assessment of the nasal cavity in mice exposed to vehicle, revealed mucosal allergic inflammation with mucin secretion and leukocyte infiltration (Figure 1b). Relative to vehicle, LXB4 reduced inflammatory infiltrates and mucin secretion in the lumen (Figure 1c). Equivalent amounts of dexamethasone also decreased the mucosal inflammation, but to a lesser degree than LXB4 (Figure 1d). BALF that was collected from these mice at the time of nasal tissue excision did not reveal significant increases in eosinophils or other leukocytes (data not shown).
Fig. 1. LXB4 reduces leukocyte infiltration and mucus secretion in the nasal mucosa.
(a) Days 0, 7: OVA/Alum i.p. sensitization, days 14–27: OVA i.n. challenge, days 29–30: vehicle (0.1%), LXB4 or dexamethasone (100ng) i.v. injection, day 30: sacrifice. (b-d) H&E stained mucosal sections of BALB/c mice injected with vehicle (b), LXB4 (c) or dexamethasone (d). Red arrows- mucus; blue arrows- leukocyte infiltration sm-submucosa; mc-mucosa; b-bone (×20 magnification- left panel; ×40 magnification- right panel).
LXB4 has pro-resolving actions on select nasal mucosa cell populations
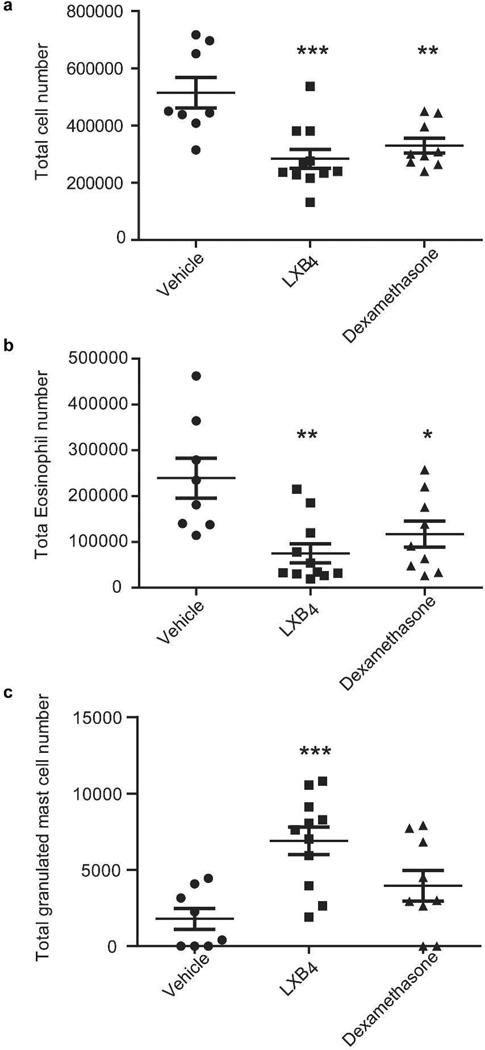
Leukocyte infiltration and total nasal mucosal cell numbers were significantly decreased by LXB4 compared to vehicle (Figure 2a). Differential cell count performed on Wright-Giemsa stained cytospins of nasal mucosa cells indicated a significant decrease in eosinophils numbers with LXB4 and dexamethasone (Figure 2b). The numbers of MC still containing granules significantly increased with LXB4 while dexamethasone gave no significant changes from vehicle (Figure 2c).
Fig. 2. LXB4 has pro-resolving effects on select nasal mucosa cell populations.
(a) Total cell number as evaluated in trypan blue exclusion test. (b-c) Differential counts of eosinophils (b) and granulated mast cells (c) in stained cytospins from BALB/c mice (n≥8).
Mast cell and eosinophil degranulation is decreased by LXB4
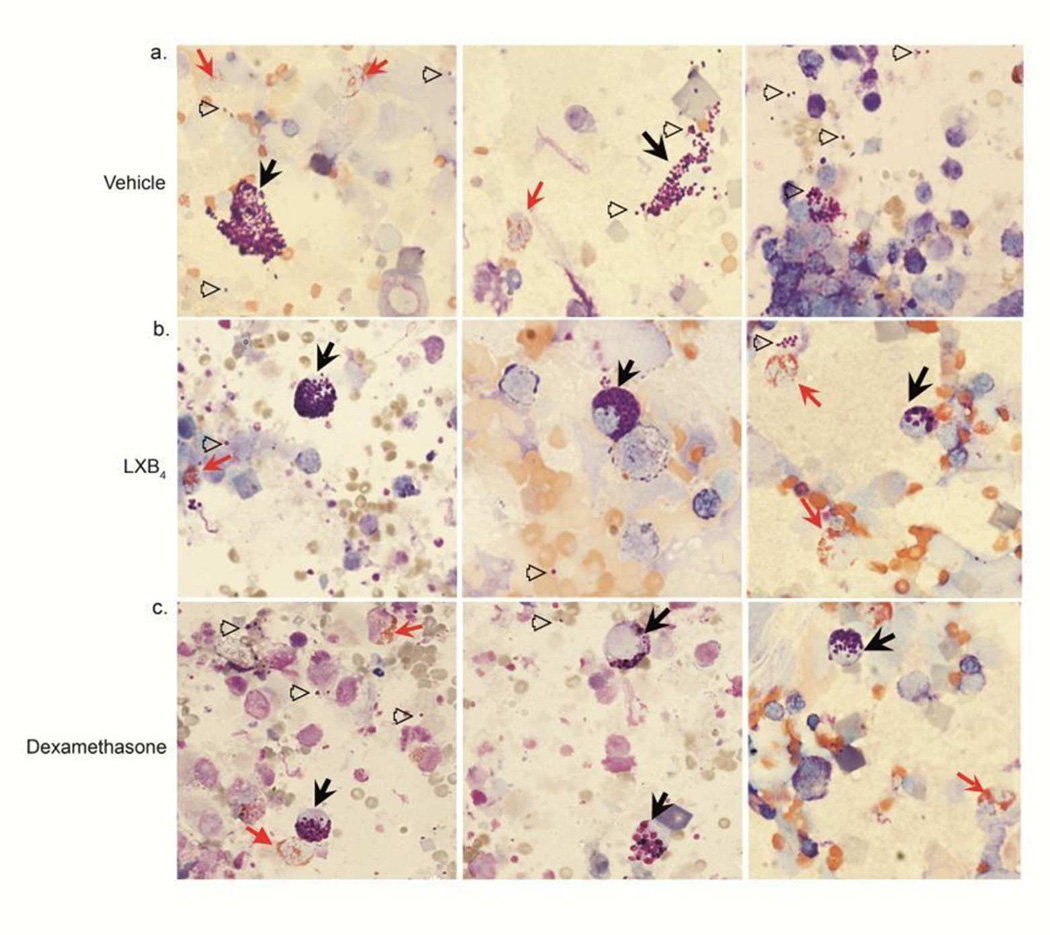
Examination of the cytospins following Wright-Giemsa staining revealed striking evidence of changes in leukocyte granule contents (Figure 3). Mice (BALB/c) exposed to vehicle had highly degranulated eosinophils and MC with evidence for released MC granules (Figure 3a). In sharp contrast, nasal mucosal MC from LXB4 treated mice were larger and retained most of their granule contents, indicative of a less activated state (Figure 3b). Eosinophils also appeared less degranulated (Figure 3b). Nasal mucosal MC following dexamethasone treatment showed cellular changes consistent with partial activation and degranulation with cells generally smaller in size than with LXB4 (Figure 3c). Next, the apparent decrease in eosinophils number and degranulation was validated by flow cytometry analysis where Siglec-F+ cells (Supplementary Figure S2a) and CD107a+ cells (Supplementary Figure S2b) were significantly decreased by LXB4 (3.50%±0.87 Siglec-F+ cells; 19.54%±1.74 CD107a+ cells, p<0.05) compared to vehicle (8.51%± 1.86 Siglec-F+ cells; 27.15%±1.706 CD107a+ cells). In comparison, dexamethasone decreased Siglec-F expressing cells (4.59%±0.79, p<0.05, Supplementary Figure S2a), but had no significant effect on cell degranulation (CD107a+, p=0.531, Supplementary Figure S2b).
Fig. 3. Fewer degranulated mast cells and eosinophils were evident in nasal mucosa with LXB4 treatment.
(a-c) Images of Wright-Giemsa stained cytospins of vehicle (a), LXB4 (b) and dexamethasone (c) injected BALB/c mice (×40). Representative images from 3 mice. Black arrows-mast cells; hollow arrowheads- mast cell granules; red arrows- eosinophils.
LXB4 decreases serum OVA-specific IgE levels and select cytokines/chemokines
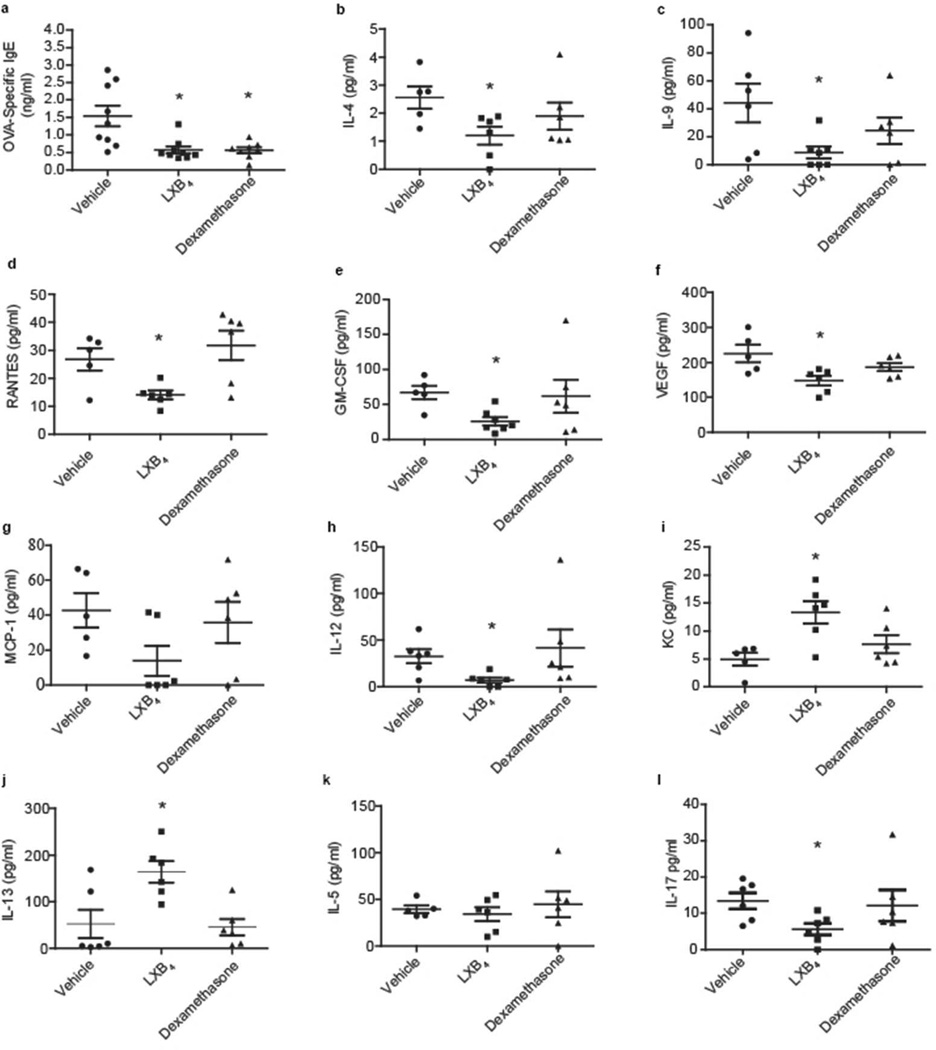
Serum titers of OVA-specific IgE (BALB/c mice) were significantly decreased with LXB4 (0.57±0.10 pg/ml, p<0.01) and dexamethasone (0.56±0.08 pg/ml, p<0.01) relative to vehicle (1.54±0.29 pg/ml) (Figure 4a). Additionally, LXB4 significantly decreased serum levels of several mediators associated with IgE production and allergic responses (Figure 4b-k), including IL-4 (Figure 4b), IL-9 (Figure 4c), RANTES (CCL5) (Figure 4d), granulocyte macrophage colony-stimulating factor (GM-CSF; Figure 4e), vascular endothelial growth factor (VEGF; Figure 4f) and IL- 12 (Figure 4h). Monocyte chemoattractant protein-1 (MCP-1) was also decreased by LXB4, but did not reach statistical significance with this sample size (Figure 4g; p=0.053). Dexamethasone did not share LXB4’s broad immunoregulatory actions at this time point, but did decrease serum IL-4, IL-9 and GM-CSF (Figure 4b, 4c, 4e). Of interest, LXB4 gave modest increases in keratinocyte chemoattractant (KC) (Figure 4i; p<0.01) and IL-13 (Figure 4j, p<0.05) levels. The increased serum KC level was not associated with increased tissue neutrophils in the nasal mucosa. Levels of IL-5 were not significantly influenced by either LXB4 or dexamethasone (Figure 4k). Serum levels of IL-17 were also detected and significantly down-regulated by LXB4 (Figure 4l, p<0.01), but not by dexamethasone (Figure 4l).
Fig. 4. LXB4 decreases OVA-specific IgE levels and modulates various cytokines in serum of AR mice.
(a) OVA-specific IgE levels in vehicle, LXB4 and dexamethasone injected BALB/c mice (n=9). (b-k) Cytokine expression in serum of vehicle, LXB4 and dexamethasone injected mice (n≥5), p<0.05; p<0.01 two-tailed Student's t-test.
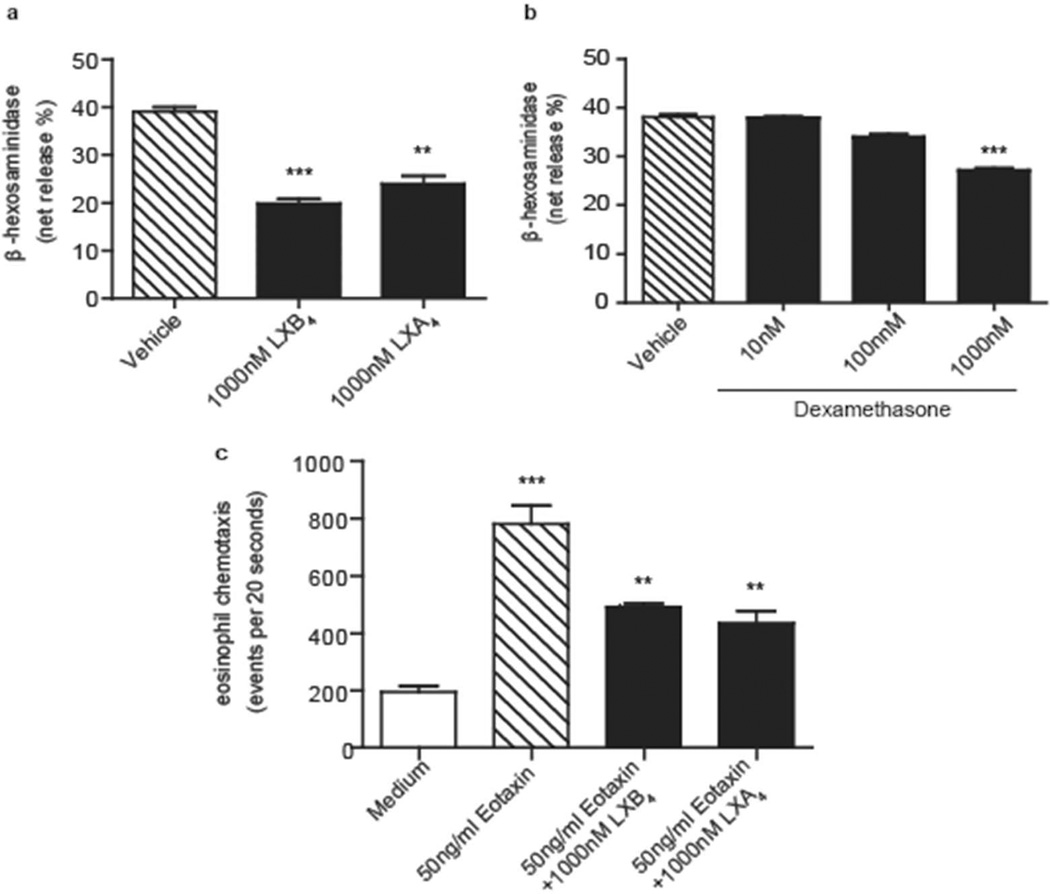
LXB4 decreases bone marrow-derived MC degranulation and bone marrow- derived eosinophil chemotaxis
To quantitatively determine if LXB4 carried direct actions for cell functional responses, IgE-sensitized bone marrow (BM)-derived mast cells (BMMCs) from naive mice were exposed to LXB4 and compared to LXA4, dexamethasone or vehicle. Cell activation for degranulation was initiated in a non-antigen specific manner with an IgG (Fab)2' antibody as in 12, see Methods. BMMC degranulation, monitored by the percentage of β-hexosaminidase release, was significantly decreased with LXB4 (19.90%±0.86 of total release relative to vehicle control cells: 39.11%±0.83, p<0.0001) (Figure 5a). LXA4 also decreased BMMC degranulation to 23.97%±1.64 of total release (p<0.01), and dexamethasone (10–1000nM) exhibited concentration-dependent inhibition of BMMC degranulation that relative to vehicle (38.07±0.4917) was similar in magnitude to LXB4 (100nM dexamethasone: 33.93±0.5301, p<0.01; 1000nM dexamethasone: 27.05±0.3910, p<0.0001) (Figure 5b). To determine if these cellular responses were strain-specific, BALB/c and FVB cell responses were determined. Both BALB/c (Supplementary Figure S3a-b) and FVB-derived (Supplementary Figure S3c-d) BMMC responded similarly to IgE- mediated activation and degranulation, which was inhibited by LXB4 and LXA4 in a concentration-dependent manner. For eosinophil responses, murine BM-derived eosinophils were exposed to LXB4, and for comparison LXA4 or vehicle, prior to eotaxin exposure, to determine their impact on chemotaxis (see Methods). Relative to vehicle, LXB4 and LXA4 significantly decreased eotaxin-mediated chemotaxis (Figure 5c).
Fig. 5. LXB4 decreases BMMC degranulation and BM eosinophils chemotaxis.
Cells were derived from BALB/c mice bone marrow as described in Methods. (a-b) BMMC IgE-dependent activation (β-hexosaminidase release) (c) BM eosinophil chemotaxis to eotaxin presented as number of events counted per 20 seconds in the flow cytometer (see Methods) (n=3).
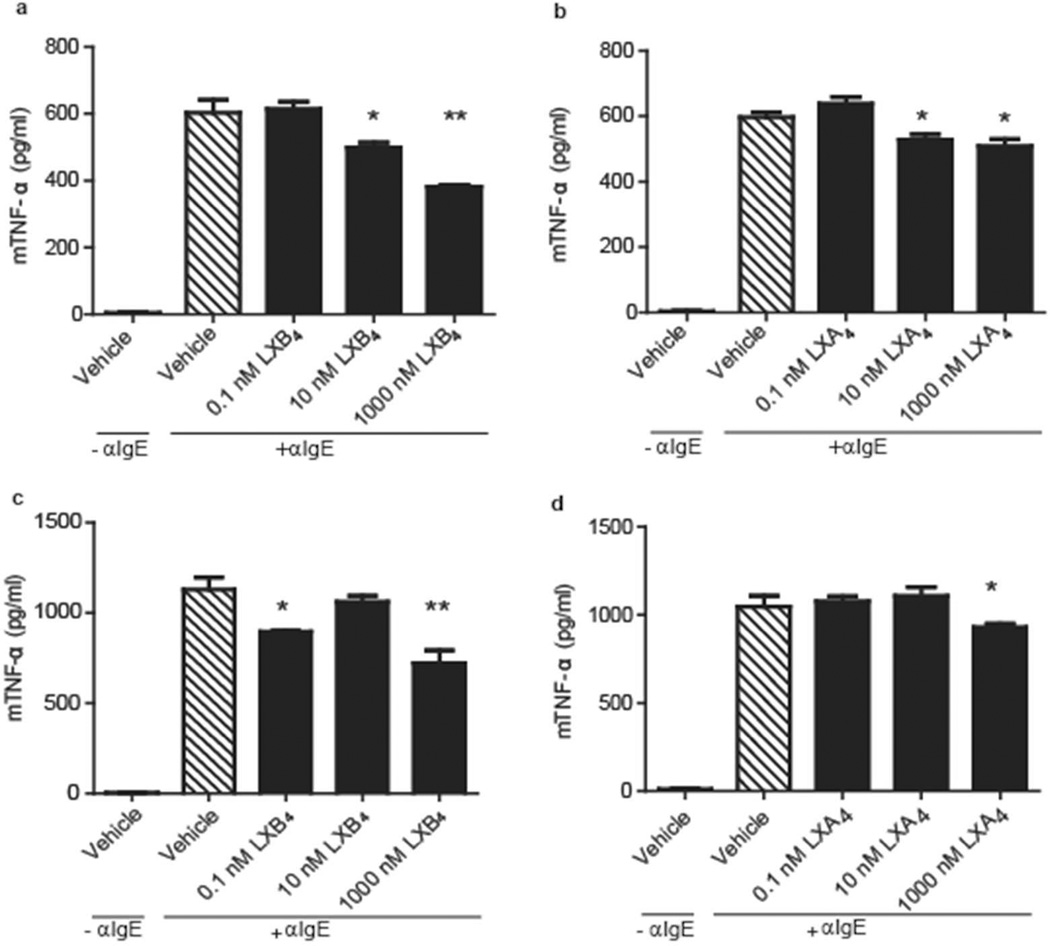
LXB4 and LXA4 decrease cytokine release from BMMC following IgE-mediated activation
Based on the serum cytokine regulation of AR mice by LXB4, the direct actions of lipoxins on BMMC cytokine release was next determined. BMMC were exposed to LXB4, LXA4 or vehicle prior to activation. After 18 hrs, IgE-mediated activation of BMMC led to a significant TNF-α release that was significantly inhibited by LXB4 and LXA4 in a concentration-dependent manner (Figure 6a-d). LXB4 also decreased FCεRI expression (Supplementary Figure S4a-b) without affecting cell viability (Supplementary Figure S4c).
Fig. 6. LXB4 and LXA4 decrease TNF-α release from BMMC following IgE- mediated activation.
BMMC were derived from BALB/c and FVB mice bone marrow and treated as described in Methods. IgE activation was carried out with anti-rat IgG (Fab)2' (10µg/ml) (a-b) TNF-α levels (pg/ml) in BALB/c cell supernatants following incubation with LXB4 (a) and LXA4 (b). (c-d) TNF-α levels (pg/ml) in FVB cell supernatants following incubation with LXB4 (c) and LXA4 (d)
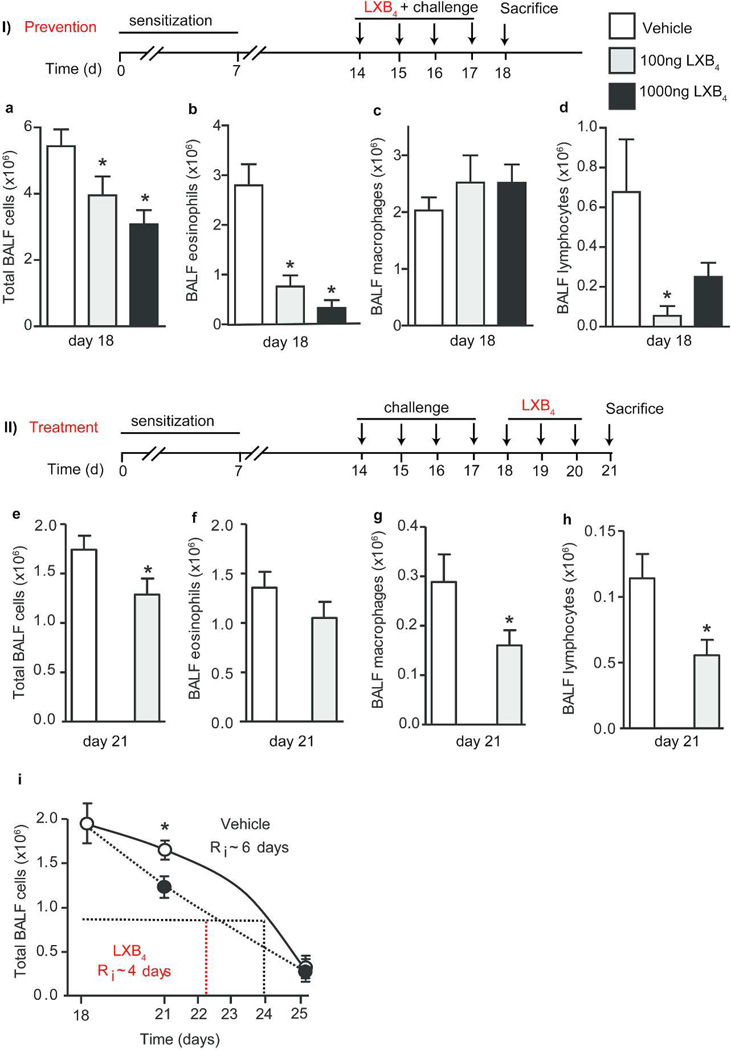
LXB4 regulates allergic lung inflammation
To determine if the actions of LXB4 in the lower airways were similar to the upper airways, we first investigated the anti-inflammatory activity of LXB4 (Figure 7I.Prevention). OVA sensitized animals (FVB) received LXB4 (100ng, 1000ng) or vehicle (i.v.) 30 minutes before OVA aerosol challenge on protocol days 14–17 and were then sacrificed 24 hours later on day 18– a time of peak allergic inflammation. LXB4 led to a significant dose-dependent decrease in total BALF cell numbers (Figure 7I. a), BALF eosinophils (Figure 7I.b) and BALF lymphocyte numbers (Figure 7I. d) but no significant change in macrophage numbers was seen (Figure 7I. c).
Fig. 7. LXB4 regulates allergic lung inflammation.
FVB Mice were sensitized and aerosol challenged with OVA. LXB4 (100, 1000ng) or vehicle were injected: I) prevention- 30 minutes before challenge (days 14–17). II) Treatment- after the last challenge (days 18–20). Total cells (a, e), eosinophils (b, f), macrophages (c, g) and lymphocytes (d, h) numbers in BALF. The resolution interval-Ri (i) for vehicle/LXB4 injected mice (n≥5), p<0.005 one-tailed Student’s t-test.
Given these anti-inflammatory actions, LXB4’s pro-resolving actions in the lower airways were next determined by administration after the allergic airways responses were fully established (Figure 7II. Treatment). Twenty four hours after the last OVA aerosol challenge, LXB4 (100ng, i.v.) was given (protocol day 18) and repeated on days 19 and 20. Mice were sacrificed 24 hours later (day 21) during the resolution phase of this model. Relative to vehicle, LXB4 significantly accelerated the resolution of the allergic lung inflammation with decreased BALF total cell number (Figure 7II. e) and decreased eosinophils, macrophage and lymphocyte numbers (Figure 7II. f-h). The resolution interval (Ri) was calculated for BALF total cells (approximately 6 days with vehicle) and LXB4 shortened the Ri by over 30% to ~4 days (Figure 7II. i).
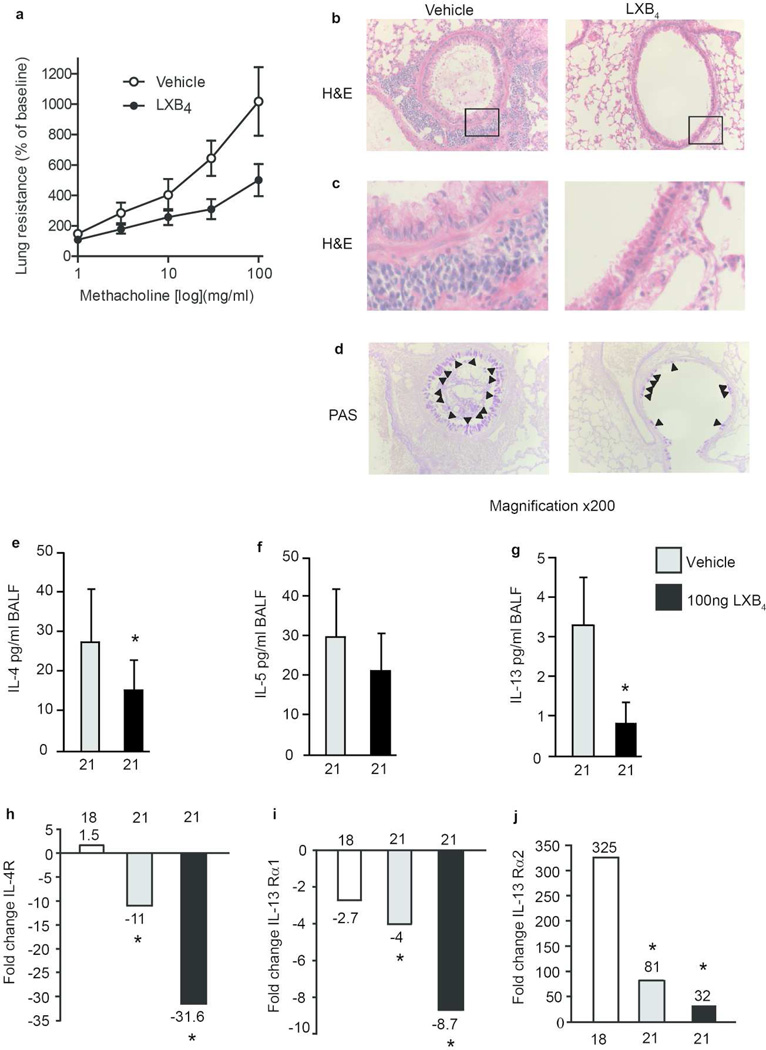
LXB4 promotes the resolution of Airway Hyper-responsiveness and lung inflammation
In addition to airway inflammation, airway hyperresponsiveness (AHR) is a clinical hallmark of asthma1, therefore the impact of LXB4 on methacholine-triggered increases in lung resistance was determined. Relative to vehicle, mice (FVB) exposed to 100ng LXB4 treatment (as in Figure 7II.) had significantly less AHR to methacholine (Figure 8a). In addition, histological analysis of lung sections from animals not subjected to AHR testing revealed that LXB4 led to significant reductions in leukocyte infiltration and epithelial cell activation (Figure 8b-c). Mice treated with LXB4 (100 ng) also exhibited a faster resolution of mucus metaplasia, as evidenced by decreased PAS positive airway epithelial cells compared to vehicle (Figure 8d). A significant decrease in BALF Th2 cytokines (Figure 8e-g) was observed, particularly for IL-4 (Figure 8e vehicle: 27.68±12.98; LXB4:15.51±7.40, p<0.05) and IL-13 (Figure 8f vehicle: 3.21±1.145; LXB4: 0.845±0.483, p<0.05). Given the importance of IL-13 signaling in airway mucus metaplasia and airway hyperreactivity to methacholine13–16, we next determined the influence of LXB4 on IL-13 receptors (Figure 8h-j). Lung expression of the shared IL-4 receptor decreased during resolution (day 21) relative to the peak of inflammation (day 18), and LXB4 further accelerated this decrease in lung IL-4 receptor expression (Figure 8h). LXB4 administration also markedly decreased the expression of IL-13 specific receptors IL-13Rα1 and IL-13Rα2 (Figure 8i-j).
Fig. 8. LXB4 promotes the resolution of Airway Hyper-responsiveness and lung inflammation.
(a) Determination of airway hyper-responsiveness to metacholine,(n≥5), p<0.005 one-tailed Student’s t-test (b, c) H&E and (d) PAS staining of FVB mice lungs. Arrow heads point to mucus (magenta positive) Goblet cells (×20). (e-g) IL-4, IL-5 and IL-13 cytokine levels in BALF, (n≥5) p<0.05 paired Students t-test. (h-j) mRNA levels of lung IL-4R, IL-13Rα1 and IL-13Rα2 (n≥5).
DISCUSSION
Allergic rhinitis and asthma are common allergic conditions with high morbidity. Allergen avoidance is often difficult and impractical and there is no curative therapy for the resultant allergic inflammation. Thus, new therapeutic approaches are needed. To this end, LXB4 (~5µg/kg) displayed pro-resolving actions for allergic mucosal inflammation in murine models of AR and asthma. LXB4 decreased mucosal leukocyte infiltration, OVA-specific IgE, MC and eosinophils degranulation, mucus metaplasia and AHR to methacholine.
LXB4 mediated direct actions on pivotal cellular effectors for allergy, namely MC and eosinophils (as shown on cell functional responses in vitro), as well as indirect actions on these cells (regulation of levels of inflammatory mediators). Distinct from their important role during the induction of allergic airway responses, the levels of type 2 cytokines during resolution and their response to LXB4 varied. In resolving upper and lower airways, LXB4 potently decreased serum IL-4 and IgE levels. Because significant changes were not present in IL-5 levels, LXB4 regulation of eosinophils function appears to have resulted from a direct action on these cells as well as from the reduced levels of GM-CSF. Surprisingly, LXB4 increased serum IL-13 as the upper airways resolved. In contrast, BALF IL-13 was decreased by LXB4, suggesting local and distinct regulatory mechanisms. Although IL-13 can initiate allergic inflammation, it can also play a role in limiting inflammation. Of note, IL-13 has the capacity to induce the expression of 15-lipoxygenase, which catalyzes the formation of both LXB4 and LXA4 6.
IL-4 plays important roles in isotype switching and IgE production 17–18, and LXB4 inhibited both IL-4 and serum IgE levels. Given the differential actions on IL-13 levels, expression of its receptors was determined. Lung expression of IL-4R, IL- 13Rα1 and IL-13Rα2 were down regulated during resolution and further decreased by LXB4. IL-4R deficient mice display weak Th2 responses 19. IL-13Rα1 deficiency has an established role in allergic airway responses 20. Thus, LXB4 regulation of IL-4R and IL-13Rα1 expression would serve to decrease mucus metaplasia and airway hyper-reactivity. No LXB4-mediated increases in expression of the IL-13 decoy receptor IL-13Rα2 were apparent. In asthma, regulation of IgE and IL-13 has beneficial actions in some asthmatic patients 21–22, so together these findings suggest therapeutic potential for LXB4 or its stable analogs 23.
The increase of serum KC with LXB4 was unexpected and distinct from LXA4 24. In addition to its well defined role as a neutrophil chemoattractant, KC can modulate ciliary function of murine sinonasal epithelial cells 25–26, suggesting that LXB4 might enhance mucociliary clearance via this mechanism. In contrast to KC, serum IL-17 levels were low and significantly decreased by LXB4. Compared to the GC dexamethasone, LXB4’s actions were of similar potency for decreasing inflammatory cell activation and more potent for regulating degranulation in vivo. Although consistently decreased by LXB4, there was donor-to-donor variability for the magnitude of stimulus-driven BMMC degranulation, suggesting context dependent responses for mast cells and their regulation by LXB4. Direct cellular actions for LXB4 were also identified in vitro for inhibition of BMMC TNF-α production and BM-derived eosinophil chemotaxis. Together, these lines of evidence support potent anti-allergic and pro-resolving actions for LXB4 on MC and eosinophils that were relevant for both upper and lower airways.
Notably, LXA4 blocks histamine release from MC during interactions with epithelial cells27 and decreases neutrophil degranulation of azurophilic granules28. In conjunction with LXB4’s actions here in vitro, the lipoxins (LXA4 and LXB4) carry MC and granulocyte stabilizing activity. Apart from its direct regulation of MC and eosinophils, LXB4 blocks T-cell activation, cytokine release29 and neutrophil chemotaxis 11. Indirect mechanisms are also likely in the accelerated resolution of allergic lung inflammation, as levels of several inflammatory mediators, including GM-CSF, a pro-survival cytokine for eosinophils, were decreased by LXB4. Of interest, LXA4 has a counter-regulatory effect on GM-CSF signaling in a human eosinophil cell line (EoL-1) and peripheral blood eosinophils 30. LXA4 also inhibits human eosinophils recruitment in vitro 31 and murine eosinophils in vivo 10, and can induce natural killer cells to promote human eosinophils apoptosis in asthma in order to enhance their non-phlogistic clearance from inflamed tissues 32. Together, these findings highlight several cellular mechanisms for lipoxin regulation of allergic airway inflammation.
LXB4 and LXA4 are generated by nasal polyps where a mucosal epithelial 15-lipoxygenase plays an important role in their biosynthesis. LXA4 is present in nasal secretions10 and airway samples from human asthmatic patients9,33. Eosinophilic donors generate LXA4 preferentially to LXB434, however, this is cell type specific as human platelet 12-lipoxygenase converts leukotriene A4 to LXA4 and LXB4 in an equimolar ratio35, and IL-4 exposed alveolar macrophage 15-lipoxygenase generates LXB4 preferentially to LXA4 36. The nanogram doses of LXB4 used here are within physiological concentrations generated during inflammatory responses and were very potent (~5µg/kg) in comparison to a similar dose of dexamethasone. LXA4 interacts with several receptors, including the G-protein coupled receptor ALX/FPR2. While LXB4 also displays pharmacological properties of a G-protein coupled receptor agonist, it binds to a receptor distinct from ALX/FPR2 that has yet to be identified. In conclusion, LXB4 displayed pro-resolving actions for mucosal allergic responses in the upper and lower airways. LXB4 is generated in the airway and the present results are the first demonstration of an airway protective effect for LXB4 or that any specialized pro-resolving mediator is active in the nasal mucosa. With the development of LXB4 stable analogs and the limited treatment options currently available, these findings suggest a potential new pro-resolving therapeutic strategy for allergic conditions.
Materials & Methods
Materials
Lipoxin A4 and Lipoxin B4 were from Cayman Chemical (Ann Arbor, Michigan, USA) and dexamethasone was from MP Biomedicals LLC (Solon-Ohio, USA). The lipoxins were stored at −80°C under a blanket of N2 and their integrity was monitored by UV-Vis spectrophotometry and HPLC (Supplementary Figure S1 Online).
Allergic upper and lower airway models
The allergic upper airway model was designed to reflect AR and was a modification of a previously published protocol 37 (Figure 1). Briefly, BALB/c female mice (Harlan Laboratories), aged 7–8 weeks (20–23gr) were sensitized on day 0 and day 7 by intraperitoneal injection (i.p.) of 200µl chicken ovalbumin (OVA; 1mg/ml) (Amresco, Ohio, USA) and aluminum hydroxide (alum; 50 mg/ml) (Acros Organics, New Jersey, USA) in PBS. Starting on day 14, awake mice were challenged twice a day by intranasal (i.n.) instillation of 20µl OVA (25mg/ml) daily until day 27. For the allergic lower airway model, a previously published protocol with OVA was used to reflect atopic asthma38. Briefly, FVB male mice aged 5–7 weeks (Charles River Laboratories) were housed in isolation cages under viral antibody-free conditions. Mice were fed a standard diet (Laboratory Rodent Diet 5001, PMI Nutrition International). Mice were sensitized with i.p. injections of OVA (10µg) and 1mg alum in 0.2ml saline on days 0 and 7. Mice were then challenged on days 14–17 with an aerosol of 6% OVA (25min).
Treatment protocols
Mice were given either intravenous (i.v.) LXB4 (100ng or 1000ng), vehicle (0.1% ethanol) or dexamethasone (100ng). To determine pro-resolving actions, treatments were given after the completion of allergen challenge at peak allergic airway inflammation time: days 28, 29, 30 in the upper airway model (Figure 1a) or days 18, 19 and 20 in the lower airway model (Figure 7II). Calculation of the resolution interval (Ri) (i.e. time interval for the maximum number of cells to decrease by 50%) was then evaluated as in 39. To determine anti-inflammatory actions, LXB4 (100ng) or vehicle were given i.v. on protocol days 14, 15, 16 and 17, 30 minutes prior to OVA aerosol challenge (Figure 7I). Twenty four hours after the last i.v. treatment, mice were euthanized for the collection of airway and systemic specimens. Mouse experiments were approved by the Animal Ethics committee, The Hebrew University, Jerusalem, Israel, and the Harvard Medical Area IRB, Boston, MA, USA (Protocol #03618).
Nasal tissue preparation of cell suspensions and histopathology
As in 37, extensive dissection was required to obtain the nasal mucosa cell suspensions (see supplemental methods in the Online Data Supplement). Total viable cell numbers were enumerated by Trypan blue exclusion. Nasal tissue sections were prepared and stained for H&E.
Flow cytometry
Single cell suspensions from nasal mucosa were resuspended in PBS/0.1% bovine serum albumin (BSA) seeded in a 96-well plate at 106 cells/ml, blocked with 5% goat serum and stained with Phycoerythrin conjugated anti-CD107a (eBioscience, San Diego, California, USA) and anti-Siglec-F (BD biosciences) for the detection of total degranulation40 and eosinophils, respectively. For in vitro assessment of BMMC expression of FCεRI, cells were stained with anti-FCεRI (eBioscience, San Diego, California, USA) and cell viability was determined by propidium iodide staining (Sigma Aldrich, Rehovot, Israel). Data were analyzed by a FACSCalibur and CellQuest software (Becton Dickinson, San Jose California, USA).
LXA4/LXB4 activity on mouse bone marrow derived mast cells (BMMC) and eosinophils
BMMC and BM eosinophils were obtained as previously described 41–42. BMMC from BALB/c and FVB mice (1×106/ml) were sensitized overnight with Rat Myeloma IgE (1µg/ml; Molecular Probes, Invitrogen, Carlsbad, California. USA). Cells were washed, resuspended in Tyrode's buffer and pre-incubated in a 96-well plate (2×105/well) with LXB4, LXA4 or vehicle at the indicated concentrations for 30 min at 37°C. Cells were then activated by the addition of a goat anti-rat IgG (Fab)2' antibody (10µg/ml)(Jackson Immunoreserach Laboratories Inc, PA, USA) (30 min, 37°C) in Tyrode's buffer. Degranulation was evaluated by a colorimetric enzymatic β- hexosaminidase release assay43. For TNF-α release, supernatants were collected 18 hrs following BMMC activation for evaluation of mTNF-α quantified by ELISA (Peprotech Asia, Israel). For chemotaxis, BM eosinophils were similarly pre-treated and then seeded into the upper chamber of a 24 transwell plate (2×105/well/100µl) (Corning) with a 5.0 µm pore size polycarbonate membrane. Cells were incubated (37°C, 3 hours) to allow migration across the membrane in response to medium only or medium with murine eotaxin (R&D biosystems, Minneapolis, Minnesota, USA) in the lower wells (50ng/ml/600µl). Migrated BM eosinophils were assessed by aspirating a fixed volume from the lower wells and counting the cells by flow cytometer (20 seconds, duplicate determinations).
Cytospin preparation
Nasal mucosa and BALF cells were resuspended in PBS/2% BSA, enumerated by a hemacytometer and cytocentrifuged (1000 rpm, 4 mins, r.t.) followed by staining with Modified Wright-Giemsa (Sigma Aldrich, Rehovot, Israel). Total cells, MC and eosinophils numbers were counted.
Evaluation of serum cytokines/chemokines and OVA specific IgE-levels
Blood samples were collected from the inferior vena cava and sera were stored (− 80°C) until measurement of OVA-specific IgE levels by ELISA (USCN life, China) and select cytokines/chemokines with Quantibody® Mouse cytokine array 1 (Ray Biotech, Inc. Norcross, Georgia, USA).
BALF and Lung sample preparation and evaluation
Bronchoalveolar lavage (BAL) was performed with 2 × 1 ml aliquots of PBS with 0.6 mM EDTA. Lungs were fixed at 25cm H2O in 10% buffered formalin and paraffin embedded for hematoxylin and eosin (H&E) and Periodic Acid Schiff (PAS) staining (Sigma). Cell-free BALF (centrifuged at 2000g for 10 min) were coded and select cytokines/chemokines measured by bead array (Pierce Biotechnology, Inc., Rockford, Illinois, USA).
RNA isolation and Real Time PCR
Lungs were obtained and snap frozen. RNA was extracted with TRIzol (Invitrogen) and reverse transcribed. The cDNA was used as a template for the amplification of selected genes. The difference between the Ct value for the gene of interest and the respective Ct value for the control gene was then calculated (ΔCt). The fold change was calculated as 2−ΔΔCt. Primers were purchased from Invitrogen: mouse IL- 13Rα1: right CGTGCAGAGATTTTCGACAG, left AGCTGTTGGTGCTGCTACTG, IL13Rα2: right GGCAAAGAAGTAACAAAAGGAAT ,left CGAATGGAGTGAAGAGGAATG, IL-4R: right AAGCACGCAGATCCAAAATC ,left GTGGAGCCTGAACTCGCA , GAPDH: right TTGATGGCAACAATCTCCAC, left CGTCCCGTAGACAAAATGGT, β-actin: right ATGGAGGGGAATACAGCCC, left TTCTTTGCAGCTCCTTCGTT.
Statistical analysis
All parameters were analyzed using GraphPad Prism 5 software (GraphPad Software, La Jolla, California). Data are expressed as means ±SEMs. Statistical comparisons were performed using 1-way ANOVA, followed by the Tukey post test. When only 2 groups were compared statistical differences were determined using the unpaired Student t-test. A p value of less than 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Professor Charles N. Serhan for helpful discussions on this project and critical appraisal of the manuscript, as well as Nour Karra and Jennifer Colby for assisting with sample preparations.
This research was supported in part by the US National Institutes of Health grants AI068084, HL68669 and P01-GM095467 (BDL) and by the Aimwell Charitable Fund, UK (FLS).
Footnotes
Disclosure/Conflict of Interest:
None of the authors declare any financial competing interests.
Supplementary Material is linked to the online version of the paper at http://www.nature.com/mi.
The PDF file contains additional explanation on nasal mucosa extraction as well as flow cytometry staining on nasal mucosal cells and LXB4 validation methodology.
AUTHOR CONTRIBUTIONS
L.K. planned and performed experiments, collected and analyzed data, and wrote the manuscript; O.H. planned and performed experiments, collected and analyzed data, and edited the manuscript; R.P. performed experiments, B.D.L and F.L.S co- designed the study, performed experiments, analyzed data, and wrote the manuscript.
References
- 1.Fanta CH. Asthma. N. Engl. J. Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 2.Plaut M, Valentine MD. Clinical practice. Allergic rhinitis. N. Engl J. Med. 2005;353:1934–1944. doi: 10.1056/NEJMcp044141. [DOI] [PubMed] [Google Scholar]
- 3.KleinJan A, et al. United airways: circulating Th2 effector cells in an allergic rhinitis model are responsible for promoting lower airways inflammation. Clin. Exp. Allergy. 2010;40:494–504. doi: 10.1111/j.1365-2222.2009.03417.x. [DOI] [PubMed] [Google Scholar]
- 4.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin. Chest. Med. 2012;33:559–570. doi: 10.1016/j.ccm.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edenius C, Kumlin M, Bjork T, Anggard A, Lindgren JA. Lipoxin formation in human nasal polyps and bronchial tissue. FEBS Lett. 1990;272:25–28. doi: 10.1016/0014-5793(90)80440-t. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, et al. Agonist-induced lipoxin A4 generation: detection by a novel lipoxin A4-ELISA. Lipids. 1993;28:1047–1053. doi: 10.1007/BF02537069. [DOI] [PubMed] [Google Scholar]
- 9.Planaguma A, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am. J. Respir. Crit. Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy BD, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat. Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 11.Lee TH, et al. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin. Sci. 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 12.Lu-Kuo JM, Joyal DM, Austen KF, Katz HR. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J. Biol. Chem. 1999;274:5791–5796. doi: 10.1074/jbc.274.9.5791. [DOI] [PubMed] [Google Scholar]
- 13.Fallon PG, Emson CL, Smith P, McKenzie AN. IL-13 overexpression predisposes to anaphylaxis following antigen sensitization. J. Immunol. 2001;166:2712–2716. doi: 10.4049/jimmunol.166.4.2712. [DOI] [PubMed] [Google Scholar]
- 14.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 15.Kuperman DA, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002;8:885, 889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmour J, Lavender P. Control of IL-4 expression in T helper 1 and 2 cells. Immunology. 2008;124:437–444. doi: 10.1111/j.1365-2567.2008.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwada M, et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc. Natl. Acad. Sci. USA. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopf M, et al. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 20.Ramalingam TR, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat. Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busse WW, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzel S, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 23.Maddox JF, et al. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- 24.Gronert K, et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 25.Shen JC, Chen B, Cohen NA. Keratinocyte chemoattractant (interleukin-8) regulation of sinonasal cilia function in a murine model. Int. Forum Allergy Rhinol. 2012;2:75–79. doi: 10.1002/alr.20087. [DOI] [PubMed] [Google Scholar]
- 26.Shen JC, Cope E, Chen B, Leid JG, Cohen NA. Regulation of murine sinonasal cilia function by microbial secreted factors. Int. Forum Allergy Rhinol. 2012;2:104–110. doi: 10.1002/alr.21002. [DOI] [PubMed] [Google Scholar]
- 27.Martin N, et al. Primary human airway epithelial cell-dependent inhibition of human lung mast cell degranulation. PLoS One. 2012;7:e43545. doi: 10.1371/journal.pone.0043545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gewirtz AT, Fokin VV, Petasis NA, Serhan CN, Madara JL. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am. J. Physiol. 1999;276:C988–C994. doi: 10.1152/ajpcell.1999.276.4.C988. [DOI] [PubMed] [Google Scholar]
- 29.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J. Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 30.Starosta V, Pazdrak K, Boldogh I, Svider T, Kurosky A. Lipoxin A4 counterregulates GM-CSF signaling in eosinophilic granulocytes. J. Immunol. 2008;181:8688–8699. doi: 10.4049/jimmunol.181.12.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soyombo O, Spur BW, Lee TH. Effects of lipoxin A4 on chemotaxis and degranulation of human eosinophils stimulated by platelet-activating factor and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Allergy. 1994;49:230–234. doi: 10.1111/j.1398-9995.1994.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 32.Barnig C, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3004812. 174ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazani S, et al. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J. Allergy Clin. Immunol. 2013;132:547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serhan CN, Hirsch U, Palmblad J, Samuelsson B. Formation of lipoxin A by granulocytes from eosinophilic donors. FEBS Lett. 1987;217:242–246. doi: 10.1016/0014-5793(87)80671-3. [DOI] [PubMed] [Google Scholar]
- 35.Romano M, et al. Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem. J. 1993;296:127–133. doi: 10.1042/bj2960127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy BD, et al. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J. Clin. Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyahara S, Miyahara N, Takeda K, Joetham A, Gelfand EW. Physiologic assessment of allergic rhinitis in mice: role of the high-affinity IgE receptor (FcepsilonRI) J. Allergy Clin. Immunol. 2005;116:1020–1027. doi: 10.1016/j.jaci.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Levy BD, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J. Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bannenberg GL, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 40.Grutzkau A, et al. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–68. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- 41.Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcepsilonRI-mediated signaling: coordinated suppression of mast cell activation. J. Pharmacol. Exp. Ther. 2008;324:128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dyer KD, et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blank U, Rivera J. Assays for regulated exocytosis of mast cell granules. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schawartz J, Yamada KM, editors. Current Protocols in Cell Biology. Wiley & Sons: New York; 2006. pp. 15.11.1–15.11.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.