Abstract
Exposure to cigarette smoke can initiate sterile inflammatory responses in the lung and activate myeloid dendritic cells (mDCs) that induce differentiation of T helper type 1 (Th1) and Th17 cells in the emphysematous lungs. Consumption of complement proteins increases in acute inflammation, but the contribution of complement protein 3 (C3) to chronic cigarette smoke-induced immune responses in the lung is not clear. Here we show that following chronic exposure to cigarette smoke, C3 deficient (C3−/−) mice develop less emphysema and have fewer CD11b+CD11c+ mDCs infiltrating the lungs as compared to wild type mice. Proteolytic cleavage of C3 by neutrophil elastase releases C3a, which in turn increases expression of its receptor (C3aR) on lung mDCs. Mice deficient in the C3aR (C3ar−/−) partially phenocopy the attenuated responses to chronic smoke observed in C3−/− mice. Consistent with a role for C3 in emphysema C3 and its active fragments are deposited on the lung tissue of smokers with emphysema, and smoke exposed mice. Together, these findings suggest a critical role for C3a through autocrine/paracrine induction of C3aR in the pathogenesis of cigarette smoke induced sterile inflammation and provide new therapeutic targets for the treatment of emphysema.
Keywords: Complement protein 3, Lung, Dendritic cells, Cigarette Smoke, emphysema, Th17 cells
Introduction
Exposure to cigarette smoke and/or other toxic environmental agents can initiate sterile inflammation in the lungs of asymptomatic smokers. Animal models have confirmed a causal role for smoke in lung destruction1,2. Activation of the immune system also occurs in atherosclerosis and lung cancer, two other diseases associated with cigarette smoking, suggesting that immune mediators that promote sterile inflammation may play a significant role in the pathophysiology of these diseases as well 3,4.
Acute exposure to cigarette smoke induces rapid recruitment of neutrophils into the airways, a process that requires signaling through TLR4, upregulation of interleukin 1 receptor 1 (IL-1R1), and activation of inflammasome pathways 5,6. Cigarette smoke-activated alveolar macrophages produce IL-1β that helps to perpetuate lung inflammation; with time the inflammation becomes independent of TLR4 signaling 7,5. Thus, although it is clear that acute cigarette smoke exposure activates early pro-inflammatory pathways, the additional stimuli that, even after smoke inhalation stops, cause this inflammatory response to persist and induce acquired immune responses to lung tissue elements remain poorly understood.
Recently, we and others have reported suggestive evidence that support how cigarette smoking may induce autoimmune responses in humans and animal models of emphysema2,8. Specifically, in addition to neutrophils and macrophages, cellular elements of the innate immune system, we and others have shown that adaptive immune responses involving T helper type 1 (Th1) and Th17 cells are a prominent feature of the pulmonary response to smoke in current and former smokers with emphysema 9-11; the presence of autoreactive T cells is associated with decline in lung function 12. Similarly chronic exposure to cigarette smoke increases the numbers of Th17 cells in the lungs and fosters emphysema in mice 10,13. Consistent with a pathogenic role for Th17 cells in emphysema, genetic ablation of IL-17A or IL-17R protects mice against smoke induced-emphysema 10,14,15. The mechanisms responsible for recruitment and activation of myeloid dendritic cells (mDC) in response to cigarette smoke are not well understood10,16. While both Th1 and Th17 cells are critical in emphysema pathogenesis, activated mDCs expressing high levels of MHC-II, CD11b, and CD11c drive the differentiation of these pathogenic T cells and can alone induce disease when transferred to naïve mice 10.
Collectively the complement proteins, among the most potent and heavily regulated innate immune factors, play key roles in acute and chronic inflammatory responses 17. In particular, complement protein 3 (C3), an essential component of the host defense system, has been shown to provide some of the most potent chemotactic responses in acute inflammation 18,19. C3a, an active cleaved product of C3, binds to its receptor (C3aR), a seven transmembrane signaling molecule expressed on mDCs, and is essential in development of allergic lung inflammation and in models of acute pneumonia 20-23. Whether C3 or its activation products play a role in the pathogenesis of sterile inflammation in response to cigarette smoke remains unclear.
In this report, we explored the role of complement C3 in a mouse model of cigarette smoke induced emphysema. We show that mice deficient in C3, develop attenuated responses to chronic cigarette smoke exposure characterized by reduced recruitment of CD11b+CD11c+ mDCs in the lungs and reduced emphysema. We further describe a new protease-dependent pathway for C3a generation in which neutrophil elastase, and to a lesser degree matrix metalloproteinase 12 (MMP12), cleave C3 to release C3a. We further show that C3a acts on mDCs to upregulate cell surface expression of the C3aR. Finally, we confirm the prominent deposition of C3 in the lungs of smokers with emphysema.
Results
C3 is required for development of smoke-induced emphysema
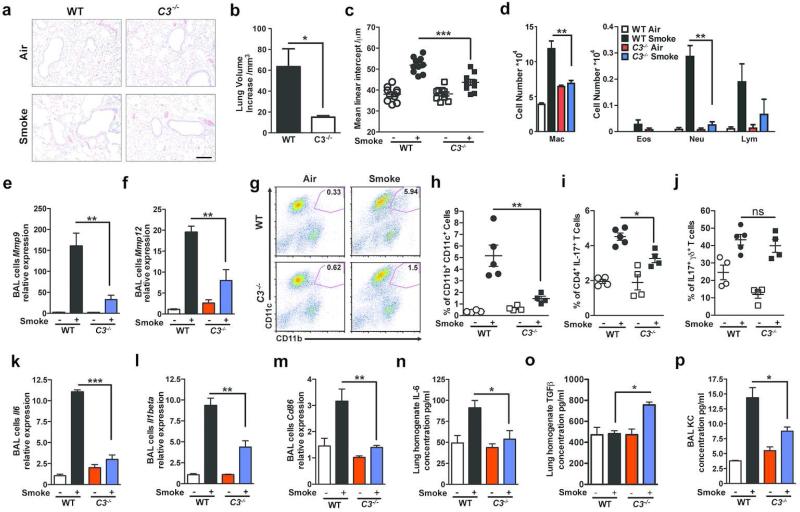
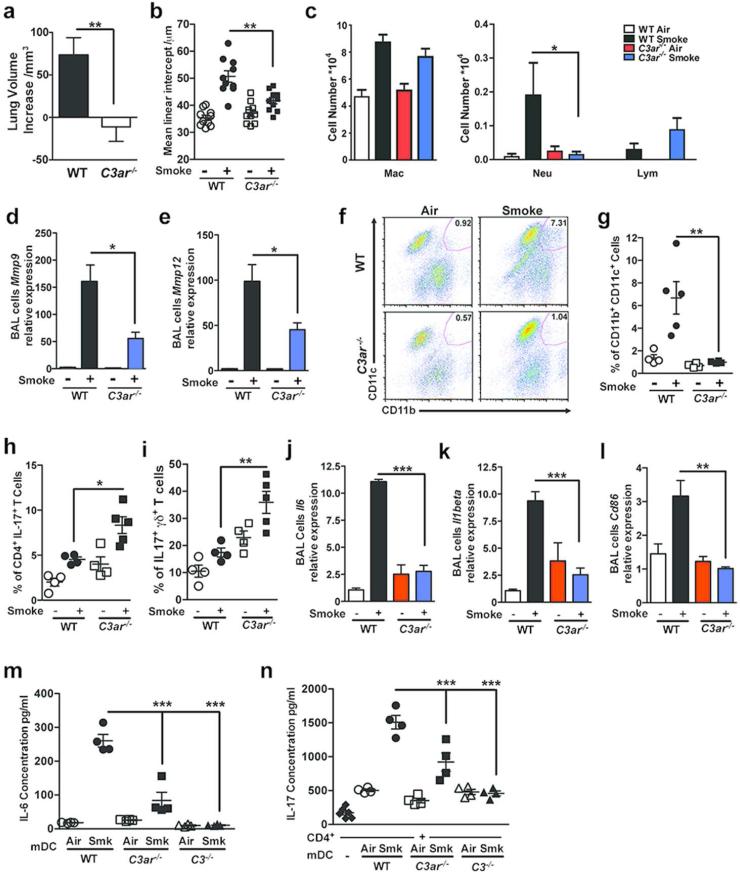
Complement is activated in human sera exposed to cigarette smoke; the chemotaxins generated include both C3a and C5a 24. To evaluate whether activation products of C3 play a role in the inflammatory response that results in emphysema, we exposed wild type (WT) and mice deficient in C3 (C3−/−) to cigarette smoke or air for six months 10,25. Hematoxylin and eosin (H&E) staining of lung sections, micro-computed tomography (microCT) quantification as well as unbiased stereologic morphometry 25 of the lungs following chronic cigarette smoke exposure showed less lung volume enlargement (emphysema) in C3−/− as compared to WT mice (>Figure 1a, b, c and Figure S1a).
Figure 1. Complement 3 is required in smoke-induced emphysema development.
Wild type (WT) or C3−/− mice were exposed to cigarette smoke or air for 6 months. (a) Representative images of hematoxylin and eosin (H&E)-stained lung sections from WT and C3−/− mice exposed to air or cigarette smoke. Representative of three independent studies (n=4-5/group). Scale bar: 100μm. (b) Micro-CT quantification of lung volume and (c) Mean Linear Intercept (MLI) using unbiased morphometry in the indicated groups of mice (n=10). *P<0.05, ***P<0.001, as determined by student t test and one-way ANOVA with Bonferroni's multiple comparison. (d) Bronchoalveolar Lavage (BAL) fluid analyses from the same group of mice (n=4 or 5 per group) showing macrophages (Mac), lymphocytes (Lymph) and neutrophils (Neu). **P<0.01, as determined by one-way ANOVA with Bonferroni's multiple comparison. Expression of Mmp9 (e) and (f) Mmp12 mRNA in BAL cells was measured by quantitative reverse transcription PCR (qPCR). **P<0.01, as determined by the one-way ANOVA with Bonferroni's multiple comparison. (g) Representative and (h) cumulative flow cytometry analysis of B220− CD11b+CD11c+ mDCs in the lung of the same group of mice (numbers in each quadrant indicate % positive cells for the indicated cytokines); right panel cumulative data (n=4 or 5 mice in each group). **P<0.01, as determined by the one-way ANOVA with Bonferroni's comparison. Cumulative intracellular cytokine staining of IL-17A in αβ (i), and γδ (j) CD3+/CD4+ T cells (n=4-5 in each group). *P<0.05, as determined by the one-way ANOVA with Bonferroni's comparison. (k) and (l) Expression of Il6 and Il1beta mRNA in BAL cells were measured by quantitative reverse transcription PCR (qPCR). **P<0.01, ***P<0.001, as determined by the one-way ANOVA with Bonferroni's comparison. (m) Expression of Cd86 mRNA in BAL cells was measured by quantitative reverse transcription PCR (qPCR). **P<0.01, as determined by the one-way ANOVA with Bonferroni's multiple comparison. IL-6 concentration (n) and (o) TGFβ concentration in mouse lung homogenate and (p) KC level in BAL fluid was measured by multiplex assay and ELISA (TGFβ). *P<0.05, as determined by the one-way ANOVA with Bonferroni's comparison. All mRNA gene expression was normalized to 18S expression and analyzed by Ct method. Results are represented as mean±s.e.m, from 3 independent experiments with 4-5 mice in each group.
We found significantly fewer macrophages and neutrophils in the bronchoalveolar lavage (BAL) fluid of C3−/− compared to WT mice treated the same way (Figure 1d). While Mmp9 and Mmp12 mRNA expression was increased in the BAL fluid cells of cigarette smoke exposed WT relative to air- exposed mice, we found significant less induction of these pro-inflammatory genes in smoke-exposed C3−/− mice (Figure 1e, f).
Given the relative B-cell mediated immune deficiency in C3−/− mice 26, we examined the possibility that these results could be explained by an occult infection or abnormal immunoglobulin G (IgG) levels. Serial culture of whole lung homogenates and BAL showed no evidence for bacterial infection in the experimental mice and while serum IgG concentrations were increased in response to smoke, they were not significantly different from those in WT mice (Figure S1b).
We have previously shown that exposure to cigarette smoke recruits mDCs marked by high expression of MHC-II, CD11b+, and CD11c+ into the lung.10. In concordance with the reduction in emphysema in smoke exposed C3−/− mice, there were fewer mDCs expressing CD11c and CD11b (Figure 1g, h), as well as decreased relative abundance of Th17 cells in the lungs of these mice (Figure 1i). In contrast, we found no significant differences in the relative abundance of IL-17A producing γδ T cells (Figure 1j).
Expression of IL-6 and IL-1β, two cytokines that are critical for Th17 cell differentiation 27, were significantly reduced in BAL fluid cells (e.g. macrophages, neutrophils, lymphocytes, and mDCs) of C3−/− mice exposed to cigarette smoke (Figure 1k-l). BAL fluid cells and isolated lung CD11c+CD11b+ cells recovered from C3-deficient mice exposed to cigarette smoke also showed reduced CD86 expression, a co-stimulatory molecule important for T cell activation, and C3aR expression (Figure 1m, Figure S1c, d). In whole lung homogenates, we found significantly reduced IL-6 protein and increased TGF-β in C3−/− mice exposed to cigarette smoke (Figure 1n, o). KC concentrations in the BAL fluid of C3−/− mice exposed to cigarette smoke was also reduced when compared with wild type mice (Figure 1p). Together, these findings suggest that C3 contributes significantly to both the recruitment and activation of immune cells into the lungs of smoke exposed mice and the induction of emphysema.
Neutrophil elastase cleaves and activates C3
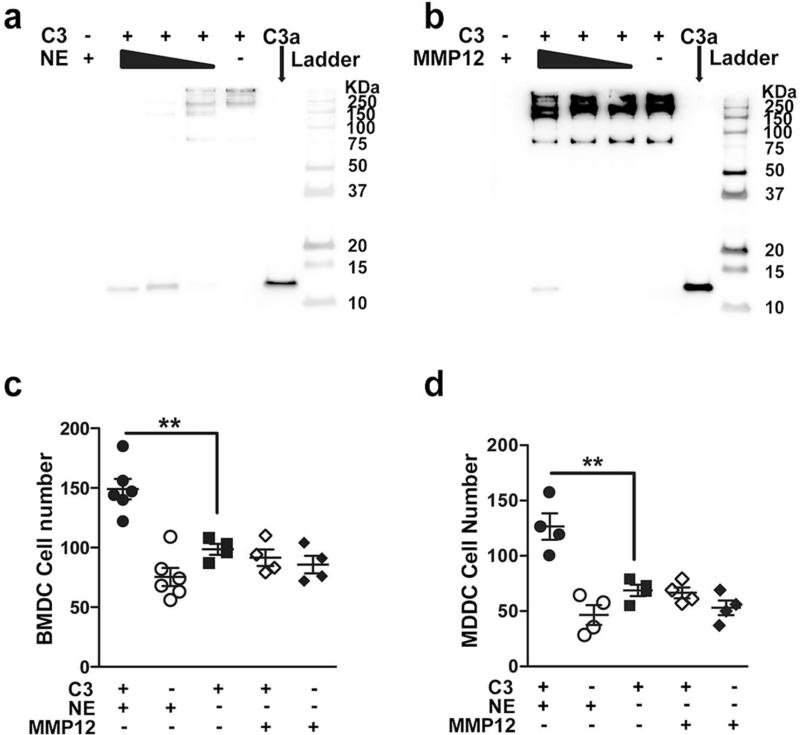
Neutrophil elastase (NE) a serine proteinase, has been shown to cleave C3 protein and generate C3d 28; binding of C3d to antigens may enhance antigen presentation and dendritic cell activation thereby facilitating an adaptive immune response to the C3d-linked antigen29. To test the hypothesis that NE and/or other pro-inflammatory proteinases associated with smoke-induced emphysema can generate activated C3 fragments, we examined the ability of activated recombinant (r)NE, MMP12, and MMP9 to cleave C3 in vitro. We found that rNE efficiently cleaved purified human plasma C3 and generated in a dose dependent manner a number of active cleavage products including C3a (Figure 2a) and C3d (Figure S2a). Human MMP12, and cathepsin G, but not proteinase 3 cleaved C3 and generated C3a, and C3c fragments (MMP12), albeit less efficiently, when compared to NE (Figure 2b, and Figure S2b-d). Although MMP9 is also highly upregulated in response to smoke and has been implicated in the pathophysiology of human emphysema, it failed to cleave C3 even at the highest concentration tested (10μg/ml; Figure S2b) indicating some serine proteinases (e.g NE, cathepsin G) and metalloproetinases (e.g. MMP12) can cleave C3 to generate several active fragments (e.g. C3a, C3c and C3d).
Figure 2. Neutrophil elastase (NE) and MMP12 cleave and activate complement C3.
Purified human C3 (100μg/ml) was cleaved with different concentrations of human (a) NE (10μg/ml, 1μg/ml, 0.1μg/ml) and (b) MMP12 (10μg/ml, 1μg/ml, 0.1μg/ml) for 4 hours at 37°C. Cleavage products were separated using 10% non-reducing Tricine gels, and detected by Western blot using anti-C3a antibody; purified C3a, NE and MMP12 were loaded as controls. (c) Bone marrow-derived dendritic cells (BMDCs; 5×104) and (d) myeloid-derived dendritic cells (MDDCs; 5×104) were suspended in media (RPMI-1640) and were placed on 48-well chemotaxis chambers for 1hr in the presence of intact or MMP12, NE cleaved C3 protein; control conditions included NE and MMP12. Transmigrating cells were detected in stained membranes visualized under microscope (20x) and reported as the average number of cells/field (n=4-6). **P<0.01, as determined by one-way ANOVA with Bonferroni's multiple comparison. Results are represented as mean±s.e.m, from 3 independent experiments.
To evaluate the functional significance of cleaved C3 fragments, we examined in vitro the migration of human and mouse mDCs in response to NE-, or MMP12-mediated cleavage of purified fragments of C3. Both mouse bone marrow derived dendritic cells (BMDCs) (Figure 2c) and human monocyte derived dendritic cells (MDDCs) (Figure 2d) migrated in response to NE-cleaved, but not to MMP12-cleaved C3 fragments suggesting that the efficient cleavage of C3 by NE likely plays a more important role in mDCs recruitment into the lungs.
C3a autocrine/paracrine responses increase C3a receptor (C3aR) expression
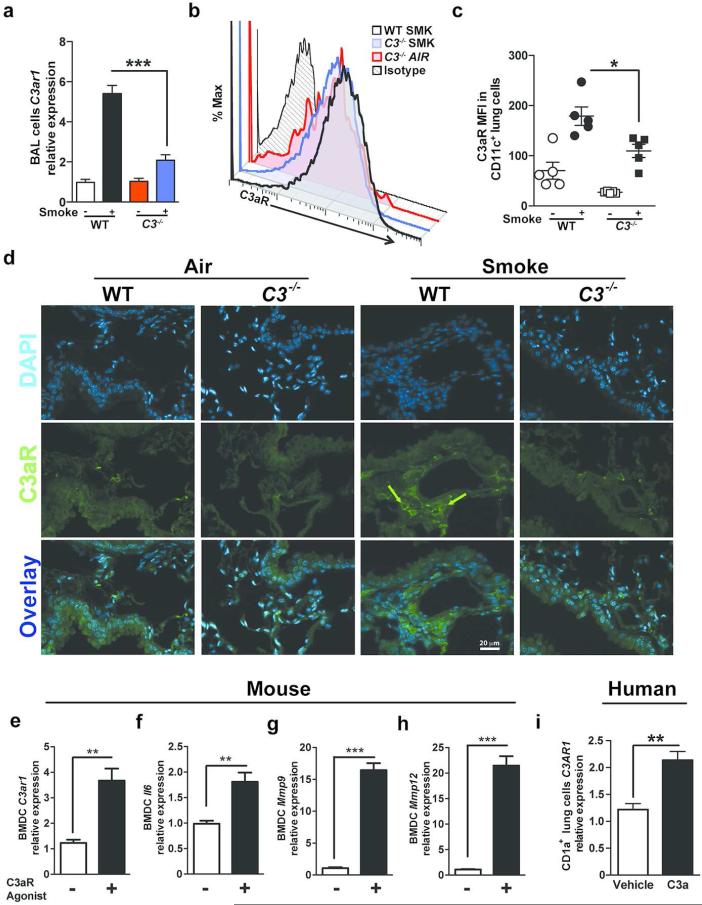
Given the importance of C3 in emphysema development and recruitment of lung mDCs, we next asked whether activated C3 fragments generated by NE and MMP12 could stimulate the expression of the C3a receptor. To address this question, we measured the expression of C3ar1 mRNA in BAL fluid inflammatory cells. We have previously shown an increase in CD11b+CD11c+ mDC population in BAL and lung parenchyma of mice exposed to smoke 10. Similarly we found increase in C3aR expression on CD11b+CD11c+ mDC population in BAL of WT mice exposed to smoke (Figure S3). Consistently, WT but not C3−/− BAL cells showed a five-fold increase in C3ar1 mRNA expression in response to cigarette smoke (Figure 3a). In agreement with the mRNA data, flow cytometry of CD11c+ lung APCs and immunofluorescent staining of lung tissue in mice exposed to smoke showed increased C3aR protein expression in WT compared to C3−/− mice treated in the same manner (Figure 3b-d). We next examined direct activation of C3aR in mouse bone marrow derived dendritic cells (BMDCs). BMDCs stimulated with C3aR agonist (CAS 944997-60-8) significantly increased the expression of C3ar1, as well as pro-inflammatory genes including Il6, Mmp9, and Mmp12 (Figure 3e-h). Since the only difference between WT and C3−/− mice is the inability to produce C3 in the latter, these results strongly suggest that the upregulation of C3aR in the former is a consequence of the stimulation provided by C3a, some other C3 breakdown product, or another complement activation fragment downstream of C3 produced during exposure to cigarette smoke. Consistent with this hypothesis, we found that CD1a+ mDCs, isolated from lung tissue of active or former smokers irrespective of emphysema significantly increased C3aR expression in response to C3a stimulation (Figure 3i and Figure S4a, b). Together our findings confirm an autocrine/paracrine C3a/C3aR axis in humans and in mice.
Figure 3. Expression of C3aR in mouse lung inflammatory cells and human mDCs.
WT and C3 deficient mice were exposed to cigarette smoke or air for 6 months. (a) Expression of C3ar1 mRNA in BAL cells isolated from WT or C3−/− mice exposed to air or cigarette smoke was measured by quantitative reverse transcription PCR (qPCR). ***P<0.001, as determined by the one-way ANOVA with Bonferroni's multiple comparison. Representative (b) and cumulative data (c) measuring C3aR mean fluorescent intensity (MFI) in single lung cells gated on B220− CD11c+ population using flow cytometry. *P<0.05, as determined by the one-way ANOVA with Bonferroni's multiple comparison. (d) Representative photomicrograph of WT and C3−/− mouse lung tissue exposed to six months of smoke or air immunostained for expression of C3aR (green) or nuclei (blue; DAPI). Scale bar: 20μm. Green arrows indicate C3aR+ cells. (e) to (h) Mouse bone marrow-derived dendritic cells (BMDCs; 2×105) were treated with C3aR Agonist (CAS 944997-60-8; 20ng/ml) or vehicle (2% DMSO) for 48 hours. Expression level of C3aR1, Il6, Mmp9 and Mmp12 mRNA were measured using qPCR (n=4 in each group; **P<0.01, ***P<0.001, as determined by student t test. (i) Human CD1a+ lung mDCs (2×105) were treated with purified human C3a (40ng/ml) for 24 hours or vehicle (media). Expression level of C3AR1 mRNA was measured by quantitative reverse transcription PCR (qPCR). n=3; **P<0.01, as determined by student t test. All gene expressions were normalized to 18S ribosomal RNA expression and analyzed by ΔΔCt. Results are represented as mean±s.e.m, from 3 independent experiments with 4-5 mice in each group (a-d).
Attenuated chemotactic responses to cigarette smoke in C3aR deficiency
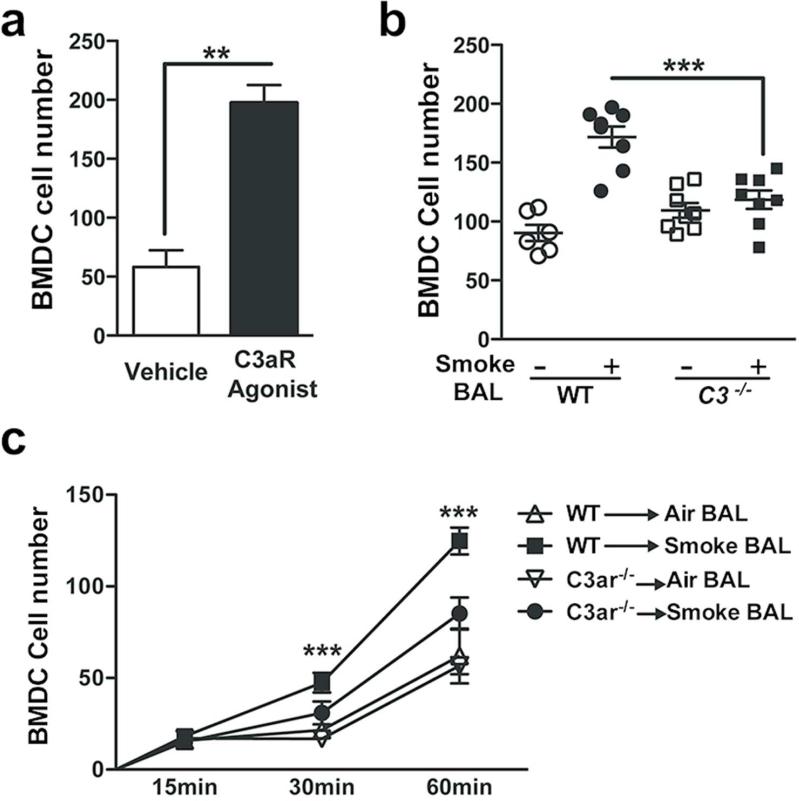
We have previously shown that CD11c+ mDCs are recruited into the lungs of mice exposed to chronic smoke. Given the robust C3 dependent increase in C3aR expression, we next examined whether direct activation of the C3aR could promote recruitment of lung mDCs. As expected, a C3aR agonist (CAS 944997-60-8) significantly increased migration of WT BMDCs when compared to vehicle control (Figure 4a). We found that BAL fluid from WT mice exposed to chronic smoke also resulted in significantly enhanced BMDC migration, when compared to the BAL fluid obtained from C3−/− mice treated the same way (Figure 4b). We next tested the kinetics of C3aR mediated migration by measuring BMDC chemotaxis towards BAL fluid over one hour. We found that although the migration of BMDCs lacking C3aR increased over time toward chemotaxins in BAL fluid from WT mice exposed to cigarette smoke, the response was significantly less than that of BMDCs isolated from WT mice (Figure 4c). Together these data suggest that the C3aR plays a critical role in the migration of DC to the lung in response to cigarette smoke.
Figure 4. C3aR is required for efficient chemotaxis.
(a) BMDCs (5×104) from WT mice were suspended in media (RPMI-1640) and were placed on 48-well chemotaxis chambers. After 1 hr. of incubation with C3aR agonist (CAS 944997-60-8; 100ng/ml) or vehicle (RPMI-1640) transmigrating BMDCs were detected in stained membranes, visualized under microscope and reported as the average number of cells/field. **P<0.01 as determined by the student t test. (b) BMDCs (5×104) from WT mice were placed on 48-well chemotaxis chambers. After 1 hr. of incubation with BAL fluid supernatant pooled from WT or C3−/− mice exposed to 6 month of air or smoke, transmigrating BMDCs were detected in stained membranes and were visualized under microscope and reported as the average number of cells/field. ***p<0.001, as determined by one-way ANOVA test with Bonferroni's multiple comparison. (c) BMDC (5×104) from WT and C3ar−/− mice were placed on 48-well chemotaxis chambers. After 15-, 30- and 60 min of incubation with BAL fluid supernatant pooled from WT mice exposed to 6 month of air or smoke, transmigrating BMDCs were detected in stained membranes, visualized under microscope and reported as the average number of cells/field. ***p<0.001 as determined by one-way ANOVA test with Bonferroni's multiple comparison. Results are represented as mean±s.e.m, from 3 independent experiments (n=6-8).
C3aR signaling is critical in smoke induced emphysema
We next examined the contribution of the C3aR in smoke induced emphysema. C3ar−/− mice exposed to chronic cigarette smoke had less emphysema, as measured by quantitative microCT, hematoxylin and eosin (H&E) staining of lung sections, unbiased stereologic morphometry of lung volumes as compared to WT mice exposed to cigarette smoke (Figure 5a, b). We found no differences in total number of lymphocytes or macrophages that we recovered in the BAL fluid, but neutrophil recovery was significantly less in BAL fluid of C3ar−/− mice as compared to WT mice (Figure 5c). Similar to our findings in C3−/− mice, the quantity of mRNA specific for Mmp9 and Mmp12 obtained from BAL fluid cells in C3ar−/− mice was significantly lower when compared to WT mice treated the same way (Figure 5d, e). Flow cytometry analysis of single cells isolated from whole lungs of mice showed a significant decrease in the relative abundance of CD11b+CD11c+ mDCs in C3ar−/− as compared to WT mice, both exposed to smoke (Figure 5f). Interestingly, the relative abundance of mDCs in whole lungs of C3ar−/− mice exposed to smoke was not significantly different from control mice exposed to air (Figure 5g).
Figure 5. C3aR is critical in cigarette smoke induced emphysema.
WT and C3ar−/− mice were exposed to cigarette smoke or air for 6 month. (a) Micro-CT quantification of lung volume in indicated groups of mice, and (b) Mean Linear Intercept (MLI) was measured using unbiased morphometry in the same groups of mice (n=10). **P<0.01 as determined by the student t test and one-way ANOVA with Bonferroni's multiple comparison. (c) Bronchoalveolar Lavage (BAL) fluid analyses from the same group of mice (n=4 or 5 per group) showing macrophages (Mac), lymphocytes (Lymph) and neutrophils (Neu). *P<0.05 as determined by the student t test. (d) Expression of Mmp9 and (e) Mmp12 mRNA in BAL cells was measured by quantitative reverse transcription PCR (qPCR). **P<0.01, as determined by one-way ANOVA with Bonferroni's multiple comparison. (f) Representatives and (g) cumulative of flow cytometry analysis of B220− CD11b+CD11c+ mDCs (enclosed population) in the lung of the same groups of mice (n=4 or 5 mice in each group). **P<0.01, as determined by one-way ANOVA with Bonferroni's multiple comparison. Cumulative intracellular cytokine staining of IL-17A in αβ (h), and γδ CD3+/CD4+ T cells (i) (n=4 or 5 in each group). Numbers in each quadrant indicate % positive cells for the indicated cytokines. *P<0.05, as determined by one-way ANOVA with Bonferroni's multiple comparison. (j) Expression of Il6 and (k) Il1beta mRNA in BAL cells were measured by quantitative reverse transcription PCR (qPCR). ***p<0.001 as determined by one-way ANOVA test with Bonferroni's multiple comparison. (l) Expression of Cd86 mRNA in BAL cells was measured by quantitative reverse transcription PCR (qPCR). **P<0.01, as determined by one-way ANOVA with Bonferroni's multiple comparison. (m) CD11c+ mDCs (2×104) isolated from whole lung homogenates isolated from WT, C3ar−/− and C3−/− mice exposed to 6 month of air or smoke, were cultured in complete media for 3 days, and the concentration of IL6 in the supernatant was measured by multiplex assay. ***p<0.001 as determined by one-way ANOVA test with Bonferroni's multiple comparison. (n) CD4+ splenic T cells isolated from naïve WT mice were co-cultured with CD11c+ mDCs shown in (m) in 10:1 ratio (CD4+ T cells and CD11c+ mDCs) for 3 days, and the concentration of IL17 in the supernatant was measured by multiplex assay. CD4+ T cells (2×105) without CD11c+ mDCs was cultured for 3 days as control. ***p<0.001 as determined by one-way ANOVA test with Bonferroni's multiple comparison. All mRNA gene expression was normalized to 18S ribosomal RNA expression and analyzed by ΔΔCt method. Results are expressed as mean±s.e.m, from 3 independent experiments with 4-5 mice in each group.
We detected a large of number of IL-17A producing cells in naïve C3ar−/− mice; the numbers of these cells paradoxically rose when these mice were exposed to cigarette smoke (Figure 5h). This increase in IL-17A producing cells was accompanied by a comparable increase in γδ T cells expressing IL-17 (Figure 5h, i), raising the possibility that signaling through C3aR negatively regulates this cell population which we previously showed play a protective role in smoke induced emphysema10.
Quantitative RT-PCR revealed that the mRNA level of IL6 and IL1β in BAL Fluid cells were reduced in C3ar−/− mice exposed to cigarette smoke when compared with WT counterparts, consistent with what was observed C3−/− mice (Figure 5j, k). Congruently there was no increase in CD86 mRNA in BAL cells of the smoke exposed C3ar−/− mice (Figure 5l). We next examined the function of CD11c+CD11b+ lung mDCs isolated from WT, C3−/− , and C3ar−/− mice exposed to air or smoke. As expected, lung mDCs isolated from the lungs of WT exposed to smoke express IL-6 (Figure 5m) and when co-cultured with naïve CD4 T cells induced the production of IL-6 and IL-17 cytokines (Figure 5n). In contrast, CD11c+CD11b+ lung mDCs isolated from C3−/− , and C3ar−/− failed to express IL-6 (Figure 5m) or drive naïve T cells to express the same cytokine (Figure 5n), suggesting that the mDCs from the C3 deficient mice are significantly less activated. These functional data provide further evidence that C3a-mediated signaling through the C3aR is necessary for the development of an inflammatory response to cigarette smoke that among other proinflammatory effects, involves the recruitment of CD11b+ CD11c+ mDCs to the lung that drive Th17 cell differentiation and emphysema.
Increased lung deposition, and plasma concentrations of C3 in smokers with emphysema
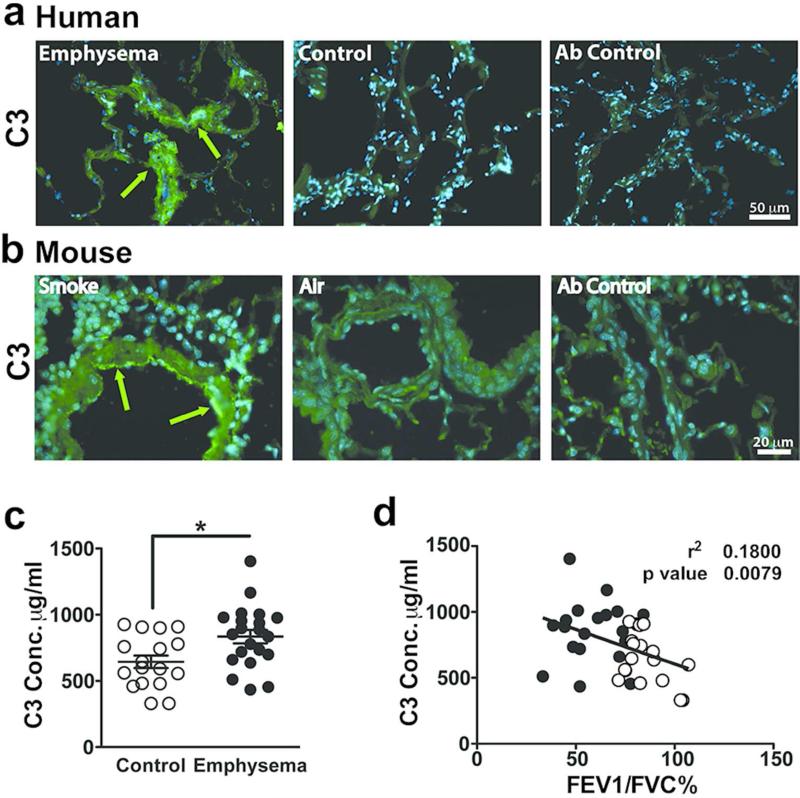
We next reasoned that if these same mechanisms were operative in humans with emphysema, we should expect to find abundant deposits of C3 and its fragments in lungs of smokers with emphysema. Immunohistological studies showed deposition of C3 in the airways and vascular endothelial cell of lungs removed from former smokers with emphysema, but not in those without emphysema (Figure 6a). Furthermore, extensive analysis of human surgical lung tissue failed to show any evidence for bacterial pathogens, indicating that these deposits developed in the absence of acute infection (data not shown). Lung tissue from mice exposed to cigarette smoke likewise showed airway deposits of C3 (Figure 6b).
Figure 6. Detection of complement 3 in human and mouse emphysematous lungs.
(a) Representative fresh frozen lung tissue obtained from emphysema or control subjects were stained with anti-C3 or non-immune control antibodies (far right panel). Green fluorescence was achieved by Alexa Fluor 488 (AF488) conjugated secondary antibody and blue fluorescence indicated 4',6-diamidino-2-phenylindole (DAPI) nucleus staining. Figures show overlay of green and blue fluorescence. Scale bar: 50 m. Green arrows indicate C3 deposition. (b) Representative paraffin embedded lung tissue from mice exposed to 6 months of cigarette smoke or air that were stained with anti-C3 or non-immune control antibodies (far right panel). Green fluorescence was achieved with AF488 conjugated secondary antibody and blue fluorescence resulted from DAPI nucleus staining. Figure show overlay of green and blue fluorescence. Scale bar: 20 m. Green arrows indicate C3 deposition. (c) Plasma from control (n=18) and emphysema patients (n=22) were used in an ELISA to measure C3 concentration. **P<0.01, as determined by the Student t test. (d) Correlation of plasma C3 concentration compared with FEV1/FVC%. Solid dots represent individual patients with emphysema and open dots represent individual control patients. P value and r2 were obtained by linear regression model. Results are represented as mean±s.e.m.
Quantification of C3 concentration in the plasma of a well-characterized cohort of smokers showed a significant increase in C3 in the smokers with emphysema, and was independent of active exposure to smoke (Figure 6c, Figure S4c, d). The plasma C3 concentration also negatively correlated with the extent of airway obstruction as determined by the decreased ratio of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), but not with different stages of COPD (Figure 6d, Figure S4e). In contrast, plasma C3a concentration in smokers with or without emphysema showed no significant differences, or correlation with airway obstruction (e.g. FEV1/FVC) (Figure S4f, g). The relatively rapid turnover of C3 during inflammation prompted us to search for a possible reduction in C3 clearance as a plausible mechanism for its increase plasma concentration and deposition in the lung tissue of smokers with emphysema. However, flow cytometry evaluation of lung mDCs in smokers with and without emphysema failed to show a significant difference in the expressional pattern of complement protein receptors such as CD46, CD55, CD95, or CD97 (Figure S5).
Discussion
Indirect evidence suggests that smoking-induced activation and/or consumption of complement is associated with development of emphysema 24,30,31. The current report links these observations mechanistically at the molecular level and demonstrates for the first time how complement derived C3a provides a means by which inflammatory cells may be recruited into lung parenchyma, and mDCs are activated following exposure to cigarette smoke. We show that in contrast to lung mDCs isolated from wild type exposed to chronic smoke, those from C3−/− and C3ar−/− mice fail to strongly induce IL-6, and IL-17 production in CD4 T cells. Together these findings indicate that C3 acts as an upstream mediator in cigarette smoke-induced model of emphysema.
We show that the chemotactic anaphylatoxin, C3a, could be released from complement C3 in the presence of activated serine proteinase (e.g. NE, cathepsin G, etc.) and to a lesser extent, MMP12 that are prominently present in innate lung immune cells (neutrophils and macrophages). The interaction of complement and the infiltrating innate immune cells could create a positive feedback loop that results in C3a mediated autocrine or paracrine signaling that upregulates C3aR expression. Specifically we show that C3a signaling upregulates C3aR on the surface of mDCs, rendering them more responsive to C3a in the lungs, while mice lacking C3aR have reduced expression of CD86 when exposed to smoke.
Our in vivo data supports a significant role for the generation of C3a that is upstream of the acquired immune responses to lung tissue elements found in end-stage emphysematous lungs of humans and in animal models of emphysema. The clinical significance of C3 activation in smoke induced emphysema was further underscored by our findings that C3 fragments deposit on the lung tissue of smokers with emphysema. Similarly, we detected C3 fragments on the lung vascular endothelial cell in mice exposed to chronic smoke, a finding that has not been previously appreciated, but may be of significant importance given the strong association between smoking and vascular diseases in humans 32,33. Although these findings merit further examination of the spatiotemporal regulation of C3 activation in response to smoke, further dissection of their effect on vascular endothelial cells are beyond the scope of the current report.
Several complement proteins are acute phase reactants, including C3; consequently while measurements of plasma complement levels are often elevated in the face of a prolonged inflammatory stimulus, they can also be depressed when sampling occurs before the acute phase response has started or if the disease causes such extensive catabolism that synthesis of new protein is not able to restore homeostasis. Although, previous studies have reported no change or a reduction in C3 serum concentration of smokers with COPD 30,31, here we found a significant increase in plasma levels of C3 that correlated with disease severity, but not with current smoking status. Furthermore, our data provide new insights into how C3a, cleaved from C3 by elastolytic proteinases (e.g. NE and MMP12), could drive a positive feedback loop that stimulates inflammation in lungs exposed to cigarette smoke.
Animal models of acute and chronic cigarette smoke induced lung disease share similar characteristics with the innate and acquired inflammatory changes seen in the lungs of human smokers 34. Specifically an acute three-day exposure to cigarette smoke results in sterile inflammation and activate the inflammasome pathways that facilitate recruitment of neutrophils, followed by macrophages 35, in the lungs. This finding was first described in humans 1. In contrast, we have shown that chronic smoke exposure results in a sterile inflammation that activates mDCs and induces a Th17 inflammatory response that results in autoimmune mediated destruction of the lungs 4. In this study we show that activation of mDCs as determined by increased expression of co-stimulatory molecules such as CD86, results from autocrine/paracrine signaling by C3a that stimulates C3aR expression and efficient induction of pro-inflammatory cytokines (e.g. IL-17 and IL-6). These findings provide a new insight into the link between innate immune activation of C3 molecule and smoke induced sterile inflammation that could propagate autoimmune inflammation and Th17 responses 9,12.
C3 plays a critical role in innate immune system activation, a task that is most often associated with protection against pathogenic insults 36. C3 deficiency, is associated with increased susceptibility to bacterial infection both in mice and humans 37,38,39. Given the long duration (6 months) of smoke exposure in our mice, we routinely monitored for occult bacterial infection. We found a slightly increased rate of bacterial infection in C3−/− mice (8/42) compared to WT (1/57) or C3ar−/− mice (1/41) irrespective of smoke exposure. We did not include mice with spontaneous pneumonia in our data analyses. It is interesting to note nevertheless that despite increased susceptibility to respiratory infection, C3 deficient mice were protected against smoke induced emphysema. Nonetheless, because of the important role of lung microbiota, future studies should examine the effects of relative immune deficiency in smoke induced emphysema in C3−/− mice that are concurrently infected with respiratory pathogens.
C3 and its activated downstream products play a role in activation of the acquired immune system during the development of several autoimmune inflammatory diseases. For instance, lupus-prone mice depleted of C3 are protected from the development of organ injury; also complement C3 inhibitor, acting upon sites of complement activation effectively ameliorates collagen-induced arthritis 40,41. Further, activation of C3 is required for effective trafficking of myeloid dendritic cells from the lung to the thoracic draining lymph node during influenza virus infection 42.
Our studies in C3−/− mice provide new insights into complement's role in activation of lung mDCs and the generation of increased numbers of Th17 cells following exposure to smoke. Specifically, we show here that both conventional CD4 T cells expressing the αβ chain of the T cell receptor and T cells with known regulatory function 43, express the γδ chain, produce IL-17 cytokine. Normally in the lungs of mice exposed to smoke there are a large number of γδT cells that express IL-17; mice deficient in γδT cells develop exaggerated emphysema when exposed to smoke 10. In C3ar−/− mice exposed to smoke, we found an increase in the relative abundance of Th17 cells compared to WT mice. Relatively large numbers of Th17 cells was associated with a reduction in emphysema. This suggests that in C3ar−/− mice, the presence of IL-17 producing γδT cells may protect against smoke induced emphysema. These findings were shown only in mice lacking C3aR, and not those lacking C3; the exact mechanisms that govern C3a signaling and recruitment of IL-17 producing γδT cell to the lungs of mice exposed to cigarette smoke remain to be determined.
Considering that symptomatic relief of airway obstruction is the only treatment available for management of emphysema, there is great need for better understanding of the pathogenesis of smoking induced emphysema. Now that we have shown that activation of the C3a/C3aR axis plays an important role in its pathophysiology, it will be appropriate to look for agents that block C3 cleavage and/or suppress production or the activity of C3 fragments that can be used to treat this disease.
Methods
Animals
Wild type C57BL/6 (WT) and C3−/− (C57BL/6 background) mice were purchased from the Jackson Laboratory. C3ar−/− mice were backcrossed (greater than ten generations) to C57BL/6 as previously described 44, and were bred in the transgenic animal facility at Baylor College of Medicine. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and followed the National Research Council Guide for the Care and Use of Laboratory Animals.
Characterization of human subjects
Plasma sample and lung tissues were collected from a total of 40 non-atopic current or former smokers, serially entered into the study; all smoker subjects had significant (>20 pack-years) history of smoking (Table S1). COPD was diagnosed according to the criteria recommended by the National Institutes of Health–World Health Organization workshop summary and emphysema was detected on chest CT scan as previously described 45. Smoking one pack of cigarettes per day each year is defined as one “pack-year.” Subjects were recruited from the chest or surgical clinics at Michael E. DeBakey Houston Veterans Affairs Medical Center hospitals. Studies were approved by the Institutional Review Board at Baylor College of Medicine, and informed consents were obtained from all patients.
Antibodies and reagents
Antibodies used for western blotting and immunohistochemistry of C3 and monoclonal anti-human complement component C3a antibody were purchased from R&D Systems (Minneapolis, MN); mouse anti-human complement C3b-alpha monoclonal antibody for detection of C3b and C3c was purchased from Chemicon, Millipore (Billerica, MA); mouse anti-human C3d were purchased from AbD Serotec (Raleigh, NC). Rabbit polyclonal antibodies for immunofluorescent and flow cytometry staining of murine C3aR was generously provided by Dr. Scott Drouin from the University of Texas UT Houston Health Science Center as previously described 44. Alexa-Fluor 488 conjugated Goat anti-Rabbit IgG was purchased from Invitrogen (Grand Island, NY).
Complement protein cleavage assay and identification of its fragments
Purified human C3 (5μg; Complement Technology; Tyler, TX) was incubated for 4 hours (h) at 37°C with control (vehicle), or 0.5, 0.05, and 0.005μg of human neutrophil elastase (EMD, Calbiochem; Darmstadt, Germany), human MMP9, MMP12 (Anaspec; Fremont, CA), human cathepsin G (EMD Millipore; Billerica, MA) and human proteinase 3 (Novoprotein; Summit, NJ) in a total volume of 50μl diluted by substrate buffer (50mM Tris, 50mM CaCl2). Equal concentrations of cleaved (proteinase treated) and intact (control vehicle treated) C3 protein were loaded on 10% non-reducing tricine gel and resolved using electrophoresis. After separation, proteins on the gel were transferred to nitrocellulose membrane by iBlot® Gel Transfer Device (Life Technologies; Grand Island, NY). After blocking in 3% BSA for 1 hour at room temperature, western blotting was used to detect C3a, C3b-α, and C3d according to the manufacturer's instructions.
Mouse model of emphysema
Mice (8 weeks old) were exposed to active smoke from commercial cigarettes as described previously 10. Briefly mice were exposed to intermittent cycles of smoke (5 seconds) followed by 20 seconds of oxygen lasting 5 minutes per cigarette. Mice were given 4 cigarettes, 5 days a week for a total of six months. Intermittent cyclic delivery of smoke was designed to mimic puffing cycles in smokers and to prevent CO2-induced asphyxiation. Mice were given 10 min of rest in between cigarettes and total cigarettes were calculated to approximate greater than twenty pack year smoke exposure in humans10. Forty-eight hrs. following the last smoke exposure, mice were euthanized, and bronchoalveolar lavage (BAL) was collected by instilling and withdrawing 0.8 ml of sterile phosphate-buffered saline (PBS) twice through the trachea. Total and differential cell counts in the BAL fluid were determined with the standard hemocytometer and HEMA3 staining (Fisher Scientifics; Waltham, MA) of 200 microliter aliquots prepared with cytospin slides. Cytokine and chemokine concentrations in the BAL were measured by Milliplex kit according to manufacturer's instructions.
Micro-Computed Tomography (microCT) and mean linear intercept (MLI)
Quantification of lung emphysema in mice was determined by microCT volumetric and density measurements as previously described using the Animal Phenotyping Core in Baylor College of Medicine 10. Briefly, anesthetized mice were placed in an animal mCT scanner (Gamma Medica; Northridge, CA), and completed images of the chest were used for emphysema quantification with Amira 3.1.1 software according to manufacturer's instructions (Amira; San Diego, CA). In addition, mean linear intercept was used as a complementary method to quantify emphysema as previously described 25.
mRNA Isolation and qPCR
Total RNA was extracted from mouse BAL cells with TRIzol (Invitrogen; Grand Island, NY) following the manufacturer's instructions. cDNA was synthesized using RNase HRT (Invitrogen; Grand Island, NY) and analyzed by using iQ SYBR Green Supermix in an iCycler (both from Bio-Rad). All gene probes, Mmp9 (Mm00600164_g1), Mmp12 (Mm00500554_m1), C3ar1 (Mm02620006_s1), Il6 (Mm00446190_m1), Il1beta (Mm00434228_m1), Cd86 (Mm00444543_m1) and human C3AR1 (Hs00269693_s1) were purchased from Applied Biosystems (Carlsbad, CA). All data were normalized to 18S ribosomal RNA (Hs99999901_s1) expression.
Lung APCs immune phenotype
Red blood cell (RBC)–free single-cell suspensions of the lung tissue were blocked with 2ul/sample mouse Fc block (eBioscience; San Diego, CA) and labeled with fluorescent anti-B220, anti-CD11b, anti-CD11c and anti-Ly6C/G antibodies (BD Biosciences; San Jose, CA), followed by detection of fluorescent signal using LSRII (BD; Franklin Lakes, NJ). Alternatively, RBC-free single cell suspension of the lungs or BAL cells were blocked in 10% goat serum and 2ul Fc block for 30min on ice and then labeled with rabbit polyclonal antibody against mouse C3aR. Cells were washed with PBS three times and stained with Alexa Fluor-488 conjugated goat anti-rabbit IgG, along with primary antibodies; fluorescent signal was detected by LSRII.
In vitro T cell co-culture and cytokine measurements
Mouse lung or spleen single cell suspension was prepared by mincing whole organs through 40 μm cell strainer (BD Falcon; Franklin Lakes, NJ) followed by red blood cell lysis (ACK lysis buffer) (Sigma-Aldrich; St. Louis, MO) for 3 min. Lung antigen presenting cells were isolated from RBC-free whole lung cells labeled with paramagnetic bead-conjugated anti-CD11c (Miltenyi Biotec; San Diego, CA) and separated using autoMACS (Miltenyi Biotec; San Diego, CA) according to manufacturer's instructions. Mouse Splenic CD4+ T cells were isolated from RBC-free single cells suspension from wild type naïve mice as described above; and lung CD11c+ mDCs were isolated from RBC-free single cells suspension from WT, C3−/− and C3ar−/− mice exposed to air or cigarette smoke. Mouse Splenic CD4+ T cells were cultured in complete media for 3 days in vitro with congenic CD11c+ lung mDCs (10:1 ratio, 2×105 CD4+ T cells and 2×104 mDCs) in the presence of 1μg/ml soluble anti-mouse CD3 (BD; Franklin Lakes, NJ). Milliplex kit (EMD Millipore; Billerica, MA) was used to measure concentrations of a selected group of cytokines (IL-6, IL-17) according to manufacturer's instructions.
In vitro chemotaxis assay
Mouse bone marrow derived DCs (BMDCs) were differentiated using cells harvested from bone marrows of WT and C3ar−/− mice. Briefly, mouse femurs were flushed with RPMI supplemented with 10% heat inactivated fetal bovine serum (complete media) to extract bone marrow cells. Red blood cells in the crude cell suspension were lysed by ACK buffer and bone marrow cells were cultured in complete media with 10ng/ml IL-4 and 20ng/ml GM-CSF for 5-7 days to derive BMDCs 46. Human monocyte derived dendritic cells (MDDCs) were prepared from peripheral blood mononuclear cells (PBMCs) isolated from whole blood purchased from Gulf Coast Regional Blood Center. Briefly, PBMCs were purified using Ficoll-Paque Plus (GE Healthcare; Pittsburgh, PA) according to manufacturer's instruction. Red Blood Cell (RBC)-free PBMCs were seeded (9×106 cells in complete media) in 6-well plates for 3 hours at 37 °C and then non-adherent cells were removed by washing with sterile PBS. Adherent cells were cultured in complete media supplemented with 10ng/ml human IL-4 and 50ng/ml human GM-CSF for 5 to 6 days. Viable cells were suspended to a final working concentration of 1×106 cells/ml in RPMI and 5×104 cells were used in chemotaxis assays.
Chemotaxis was measured using 48-well chemotaxis chambers (Neuro Probe; Gaithersburg, MD) according to manufacturer's instruction. The chemotactic activity of C3a receptor agonist (CAS 944997-60-8 used at 100ng/ml; Sigma-Aldrich; St. Louis, MO), BAL fluid from WT and C3−/− mice exposed to air or cigarette smoke was assessed against BMDCs in triplicate as previously described 47. Briefly, BAL fluid supernatant were placed in lower chambers that were separated by 8-micron transfilters; BMDCs were placed on top chambers, and incubated at 37°C for 1 hr. In some experiments transfilters were harvested at 15-, 30- and 60-min time points. Transmigrating cells were enumerated at 20x magnification and cell numbers were reported as the average number of cells/field 47. Chemotactic activity of cleaved C3 fragments was examined using chemotaxis assays as described above. Briefly, NE- or MMP12-cleaved C3 products were purified in PBS using 3kD columns (EMD Millipore; Billerica, MA) and were used in chemotaxis assay at 5 μg/ml as described above.
Immunohistochemical detection of complement protein
Human lung tissue specimens from smokers with and without emphysema collected by surgical resection, were embedded into cryo-conserve media (Tissue-Tek; Torrance, CA), and 5 μm lung sections were used for immune staining. Briefly, lung sections were fixed in ice-cold acetone, blocked in 5% non-fat milk, and incubated with primary antibody for 1 hr. Lung sections were washed three times in PBST and signal was detected using fluorescent conjugated secondary antibodies. Nuclei were counter-stained with DAPI by HardSet kit (Vector Laboratories; Burlingame CA). Perfused mouse lung sections isolated from air control or smoke exposed mice were paraffin embedded. Briefly, following rehydration, and antigen retrieval 48, lung sections were subjected to immunohistochemical analyses as described above. Images were detected by a Nikon ECLIPSE TE2000 microscope using NIS-Elements software version 2.30 and Leica DFC300 FX.
Characterization of complement regulatory receptors
Red blood cell (RBC)–free single-cell suspensions of the human lung tissue were labeled with fluorescent anti-CD4, anti-CD19, anti-CD11c and anti-CD1a antibodies (BD Biosciences; San Jose, CA) to identify respectively, T cells, B cells, Macrophages and mDCs. Complement regulatory proteins, were detected using fluorescent anti-CD46, anti-CD55, anti-CD93 and anti-97 (BD Biosciences; San Jose, CA), and signal was detected using LSRII (BD; Franklin Lakes, NJ).
Quantification of complement 3 components in human plasma
C3 in heparinized human plasma was measured after samples were diluted (PBST with1% BSA), detected using a sandwich ELISA (GenWay Biotech; San Diego, CA), according to the manufacturer's instructions. Briefly, Immulon ELISA plates were coated with 500ng/ml chicken IgY anti-human C3 in PBS overnight at 4°C. Plates were washed with PBST three times and blocked in 1% BSA in PBS for one hour at room temperature. Plasma samples and standards were diluted and added to the plates. After 2 hours of incubation at room temperature, plates were washed with PBST for three times. 20ng/ml HRP-conjugated chicken IgY anti- human C3 was added to detect C3. After one-hour incubation at room temperature, plates were washed five times with PBST and developed by BD OptEIA TMB substrate (BD; Franklin Lakes, NJ). Final readout was detected by absorbance at 450nm. Concentration of C3a in plasma samples was detected using human C3a ELISA kit (BD; Franklin Lakes, NJ) according to the manufacturer's instruction.
In vitro culture and stimulation of human lung mDCs
Red blood cell (RBC)–free single-cell suspensions of the human lung tissue were first labeled with CD1a antibody conjugated beads and then CD1a positive cells were isolated by AutoMACS according to manufacturer's instruction (Miltenyi Biotec; San Diego, CA). After isolation, cells were counted and adjusted to a concentration of 1×106/ml. 2×105 cells were plated in each well of 96 well plates in triplicate and stimulated with purified human C3a or media alone for 24 hour. Cells were then collected and lysed in TRIzol (Invitrogen; Grand Island, NY) for further RNA isolation and analysis of C3AR1 expression.
Statistics
All statistical analyses were performed with the Prism software (GraphPad Software). Data points in the figures show the mean and error bars represent standard error of mean (SEM). For the comparison of BAL cellularity and gene expression from air- and smoke-exposed mice, we used the Student's t test or one-way analysis of variance (ANOVA) test. Complement 3 components concentration in human studies was analyzed by the Mann–Whitney nonparametric test. Linear regression evaluated the relationship between plasma Complement 3 or C3a concentration and the FEV1/FVC Ratio. For the comparison of CT quantifications of air- and smoke exposed mice, one-way ANOVA test with Bonferroni's multiple comparison was used. We considered differences significant when p<0.05.
Supplementary Material
Acknowledgements
The funding was provided by the National Institute of Health (HL117181) to FK and DC, and a VA merit award (CX000104-05) to FK. The project was supported in part by fellowship administered by the NIOSH to MS and RY. This project was also supported by Mouse Phenotyping Core, and the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (AI036211, CA125123, and RR024574) and the expert assistance of Joel M. Sederstrom. We thank Ms. Han-Fang Cheng for the technical assistance.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. The New England journal of medicine. 1974;291:755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 2.Wright JL, Churg A. Animal models of cigarette smoke-induced chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine. 2010;4:723–734. doi: 10.1586/ers.10.68. [DOI] [PubMed] [Google Scholar]
- 3.Houghton AM. Mechanistic links between COPD and lung cancer. Nature reviews. Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 4.Kheradmand F, Shan M, Xu C, Corry D. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence Expert review of clinical immunology. 2012;8:285–292. doi: 10.1586/eci.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maes T, et al. Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. International Archives of Allergy & Immunology. 2006;141:354–368. doi: 10.1159/000095462. [DOI] [PubMed] [Google Scholar]
- 6.Doz E, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 7.Phipps JC, et al. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infection and immunity. 2010;78:1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nature Reviews Immunology. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 9.Lee S-H, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat. Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 10.Shan M, et al. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;4:117ra119. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurimoto E, et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu C, et al. Autoreactive T Cells in Human Smokers is Predictive of Clinical Outcome. Frontiers in immunology. 2012;3:267. doi: 10.3389/fimmu.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppert BL, Wortham BW, Flury JL, Borchers MT. Functional characterization of T cell populations in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2013;190:1331–1340. doi: 10.4049/jimmunol.1202442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y, et al. Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. American Journal of Physiology Lung Cellular & Molecular Physiology. 2014;306:L132–143. doi: 10.1152/ajplung.00111.2013. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, et al. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. International Immunopharmacology. 2013;15:58–66. doi: 10.1016/j.intimp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Shan M, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 17.Holers VM. Complement and Its Receptors: New Insights Into Human Disease. Annual review of immunology. 2014 doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 18.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nature reviews. Immunology. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 19.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nature reviews. Immunology. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 20.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 21.Walters DM, Breysse PN, Schofield B, Wills-Karp M. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. American Journal of Respiratory Cell & Molecular Biology. 2002;27:413–418. doi: 10.1165/rcmb.4844. [DOI] [PubMed] [Google Scholar]
- 22.Li K, et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology. 2012;217:65–73. doi: 10.1016/j.imbio.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Molecular immunology. 2004;41:123–131. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI. Complement activation by cigarette smoke. The American journal of physiology. 1991;260:L254–259. doi: 10.1152/ajplung.1991.260.4.L254. [DOI] [PubMed] [Google Scholar]
- 25.Shan M, et al. Agonistic induction of PPARgamma reverses cigarette smoke-induced emphysema. The Journal of clinical investigation. 2014 doi: 10.1172/JCI70587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghannam A, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008;181:5158–5166. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- 27.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. The American journal of pathology. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 28.Claesson R, Kanasi E, Johansson A, Kalfas S. A new cleavage site for elastase within the complement component 3. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2010;118:765–768. doi: 10.1111/j.1600-0463.2010.02655.x. [DOI] [PubMed] [Google Scholar]
- 29.Del Nagro CJ, Kolla RV, Rickert RC. A critical role for complement C3d and the B cell coreceptor (CD19/CD21) complex in the initiation of inflammatory arthritis. J Immunol. 2005;175:5379–5389. doi: 10.4049/jimmunol.175.8.5379. [DOI] [PubMed] [Google Scholar]
- 30.Miller RD, Kueppers F, Offord KP. Serum concentrations of C3 and C4 of the complement system in patients with chronic obstructive pulmonary disease. The Journal of laboratory and clinical medicine. 1980;95:266–271. [PubMed] [Google Scholar]
- 31.Kosmas EN, Zorpidou D, Vassilareas V, Roussou T, Michaelides S. Decreased C4 complement component serum levels correlate with the degree of emphysema in patients with chronic bronchitis. Chest. 1997;112:341–347. doi: 10.1378/chest.112.2.341. [DOI] [PubMed] [Google Scholar]
- 32.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert review of cardiovascular therapy. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. American journal of respiratory and critical care medicine. 2012;186:11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 34.Vlahos R, Bozinovski S. Recent advances in pre-clinical mouse models of COPD. Clinical science. 2014;126:253–265. doi: 10.1042/CS20130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhami R, et al. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. American Journal of Respiratory Cell & Molecular Biology. 2000;22:244–252. doi: 10.1165/ajrcmb.22.2.3809. [DOI] [PubMed] [Google Scholar]
- 36.Tichaczek-Goska D. Deficiencies and excessive human complement system activation in disorders of multifarious etiology. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2012;21:105–114. [PubMed] [Google Scholar]
- 37.Matsuyama W, et al. Molecular analysis of hereditary deficiency of the third component of complement (C3) in two sisters. Internal medicine. 2001;40:1254–1258. doi: 10.2169/internalmedicine.40.1254. [DOI] [PubMed] [Google Scholar]
- 38.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 39.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song H, Qiao F, Atkinson C, Holers VM, Tomlinson S. A complement C3 inhibitor specifically targeted to sites of complement activation effectively ameliorates collagen-induced arthritis in DBA/1J mice. Journal of immunology. 2007;179:7860–7867. doi: 10.4049/jimmunol.179.11.7860. [DOI] [PubMed] [Google Scholar]
- 41.Ioannou A, Lieberman LA, Dalle Lucca JJ, Tsokos GC. Complement depletion protects lupus-prone mice from ischemia-reperfusion-initiated organ injury. American journal of physiology. Gastrointestinal and liver physiology. 2013;304:G283–292. doi: 10.1152/ajpgi.00371.2012. [DOI] [PubMed] [Google Scholar]
- 42.Kandasamy M, et al. Complement mediated signaling on pulmonary CD103(+) dendritic cells is critical for their migratory function in response to influenza infection. PLoS pathogens. 2013;9:e1003115. doi: 10.1371/journal.ppat.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nature reviews. Immunology. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 44.Dillard P, Wetsel RA, Drouin SM. Complement C3a regulates Muc5ac expression by airway Clara cells independently of Th2 responses. American journal of respiratory and critical care medicine. 2007;175:1250–1258. doi: 10.1164/rccm.200701-049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesselbacher S, et al. Cross-sectional analysis of the utility of pulmonary function tests in predicting emphysema in ever-smokers. Int J Environ Research Public Health. 2011;8(5):1324–1340. doi: 10.3390/ijerph8051324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G. Isolation of dendritic cells. Current protocols in immunology / edited by John E. Coligan. 2001 doi: 10.1002/0471142735.im0307s25. Chapter 3, Unit 3 7. [DOI] [PubMed] [Google Scholar]
- 47.Corry DB, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goswami S, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nature Immunology. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.