Abstract
The long-chain polyunsaturated fatty acids are considered to be of major health importance, and recent studies indicate that their endogenous metabolism is influenced by B-vitamin status and smoking habits. We investigated the associations of circulating B-vitamins and smoking habits with serum polyunsaturated fatty acids among 1,366 patients who underwent coronary angiography due to suspected coronary heart disease at Haukeland University Hospital, Norway. Of these, 52% provided information on dietary habits by a food frequency questionnaire. Associations were assessed using partial correlation (Spearman’s rho). In the total population, the concentrations of most circulating B-vitamins were positively associated with serum n-3 polyunsaturated fatty acids, but negatively with serum n-6 polyunsaturated fatty acids. However, the associations between B-vitamins and polyunsaturated fatty acids tended to be weaker in smokers. This could not be solely explained by differences in dietary intake. Furthermore, plasma cotinine, a marker of recent nicotine exposure, showed a negative relationship with serum n-3 polyunsaturated fatty acids, but a positive relationship with serum n-6 polyunsaturated fatty acids. In conclusion, circulating B-vitamins are, in contrast to plasma cotinine, generally positively associated with serum n-3 polyunsaturated fatty acids and negatively with serum n-6 polyunsaturated fatty acids in patients with suspected coronary heart disease. Further studies should investigate whether B-vitamin status and smoking habits may modify the clinical effects of polyunsaturated fatty acid intake.
Introduction
The n-3 long-chain polyunsaturated fatty acids [LCPUFAs5: EPA, docosapentaenoic acid (DPA) and DHA] are proposed to have several beneficial health effects [1]. The human body is able to synthesize most fatty acids, except for the n-3 alpha-linolenic acid (ALA) and the n-6 linoleic acid (LA), which must be obtained from foods or supplements [1]. These essential polyunsaturated fatty acids (PUFAs) can be further metabolized into n-3 and n-6 LCPUFAs, but in limited amounts, partly due to the competition for the same enzymes (elongases and desaturases) in their conversion [1]. Because of this internal competition and the importance of generating sufficient amounts of n-3 LCPUFAs, the Nordic recommendations specify that at least one percent of the energy intake should be from n-3 PUFAs [2]. Interestingly, recent research indicates that the endogenous metabolism of LCPUFAs might be influenced by other lifestyle and dietary factors in addition to dietary intake of the essential fatty acids. A recent study showed that an induced marginal vitamin B6 deficiency in humans leads to a reduction in plasma concentrations of both n-3 and n-6 LCPUFAs [3]. Moreover, studies in animals and cell cultures have shown that certain B-vitamins, including folate, vitamin B6 and cobalamin (vitamin B12), affect the circulating PUFA profile [4–6].
Interestingly, smoking is associated with lower concentrations of both circulating B-vitamins [7,8] and LCPUFAs [9–11], where dietary inequalities alone do not explain this. N-3 LCPUFAs are considered to be particularly beneficial in relation to cardiovascular disease (CVD) [12], and more knowledge on the determinants of PUFA status should be generated in patients at high risk of CVD. Thus, we investigated the association between circulating concentrations of B-vitamins [folate, riboflavin (vitamin B2), pyridoxal 5’-phosphate (vitamin B6) and vitamin B12], and smoking habits with serum PUFAs in a cross-sectional study among 1,366 patients with suspected coronary heart disease (CHD).
Materials and Methods
Study population
The Bergen Coronary Angiography Cohort (BECAC) includes an unselected cohort of 4,241 adult patients (> 98% white) who underwent coronary angiography for suspected CHD during the period 2000–2004 at Haukeland University Hospital (Bergen, Norway). The present substudy includes the initial 1,366 BECAC patients recruited during 2000–2001, of whom 709 (52%) also participated in the Western Norway B Vitamin Intervention Trial (WENBIT) [13].
A signed consent form was obtained from all participants. The study was approved by the Regional Committee for Medical and Health Research Ethics and the Norwegian Data Inspectorate.
Assessment of clinical and dietary data
A self-administered questionnaire completed by each patient was used to collect information about medical history, risk factors and medications, and was checked against medical records as previously reported [13]. Fasting was defined as not having ingested any food or beverages at least 6 hours prior to blood sampling, and hypercholesterolemia was defined as serum total cholesterol ≥ 6.5 mmol/L. Smokers included self-reported current smokers, those who had quit smoking within less than one month prior to baseline, and patients with plasma cotinine ≥ 85 nmol/L [14]. The majority of participants recruited in WENBIT were asked to complete a semiquantitative food-frequency questionnaire (FFQ) with 169 food items at trial enrollment, providing information on dietary habits and use of supplements during the last year [15].
Analysis of biochemical data
Blood samples were collected prior to coronary angiography, serum/plasma was separated and stored at -80°C until analysis. Serum fatty acid methyl esters were analyzed by gas-liquid chromatography (GC 8000 TOP, Finnigan, USA) on DB1-ms capillary column (j & W Scientific, USA) and quantified as previously described [16]. Fatty acids are given as percentage of total fatty acids in serum. The delta-5-desaturase (D5D) and delta-6-desaturase (D6D) activity indexes were calculated as the concentration of products divided by precursors: n-3 D5D, EPA/eicosatetraeonic acid (ETA); n-3 D6D, stearidonic acid (SDA)/ALA; n-6 D5D, arachidonic acid (AA)/dihomo-γ-linolenic acid (DGLA); n-6 D6D, γ-linolenic acid (GLA)/LA. The omega-3 index [17] was modified to represent the sum of serum EPA and DHA as a fraction of total fatty acids, and we used the EPA/AA-ratio [18] to present a n-3/n-6 PUFA ratio. Plasma concentrations of vitamin B2, vitamin B6 and cotinine were analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) [19] and serum concentrations of folate [20] and vitamin B12 [21] were determined by microbiological methods. Plasma concentration of total homocysteine and methylmalonic acid (MMA), a marker of vitamin B12 status, were measured using gas chromatography-tandem mass spectrometry (GC-MS/MS) [22]. B-vitamins, homocysteine, MMA and cotinine were analyzed by BEVITAL AS, Bergen, Norway (http://www.bevital.no/).
Statistical methods
Analyses were conducted in the total population and in non-smokers and smokers separately. Summary measures for continuous variables are reported as medians (25th, 75th percentiles) and categorical variables are reported as counts (percentages). The Kolmogorov-Smirnov test was used to assess normality of continuous variables. Mann-Whitney U and Chi-square tests were used to compare characteristics in smokers versus non-smokers as appropriate. Associations between continuous variables were assessed using partial non-parametric correlation (Spearman). Statins are suspected to modify the beneficial CVD effect of n-3 PUFA supplement [23]. Thus ranked values of effective statin dose [expected percent LDL-cholesterol reduction, based on type and dose of specified statin use [24,25] (ordinal)] was included together with age (continuous), gender, dietary intake of n-3 or n-6 PUFA (g/day), plasma vitamin B2, vitamin B6 and MMA (continuous), and serum folate and vitamin B12 (continuous) in the multivariate-adjusted correlation analysis. Further adjustments for BMI and blood glucose did not have any impact on the associations and were thus not included in the final model. Generalized additive models (GAM) were used to describe non-linear associations and were either adjusted for age and gender (simple) or the same covariates as those included in the multivariate correlation analyses. Unadjusted interaction analyses were used to assess differences in associations between non-smokers and smokers.
All probability values were 2-tailed and p-values < 0.05 were considered as statistically significant when comparing nonsmokers and smokers, whereas p-values < 0.01 were considered statistically significant in the multivariate correlations analysis. SPSS software version 21.0 was used for most statistical analysis (SPSS Inc., Chicago, IL, USA). R version 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria) with package “mgcv” was used to generate GAM curves and to conduct interaction analyses.
Results
Characteristics of participants
General characteristics of the total population (n = 1,366) and subgroups of non-smokers (n = 907) and smokers (n = 459) are shown in Table 1. Overall, 74.7% were men and the median (25th, 75th percentile) age was 61 (54, 69) years. The majority of the participants had stable angina pectoris (93.3%) and used statins (67.0%). As compared to non-smokers, smokers were younger, had lower BMI, a lower prevalence of diabetes mellitus (both type 1 and 2), lower Troponin T, but more frequently impaired left ventricular ejection fraction.
Table 1. Baseline characteristics of participants regarding medical history, risk factors and medications 1 .
| Total | Non-smokers | Smokers | ||
|---|---|---|---|---|
| Characteristics | (n = 1366) | (n = 907) | (n = 459) | P 2 |
| Demographic and clinical | ||||
| Men 3 | 1021 (74.7) | 661 (72.9) | 360 (78.4) | 0.03 |
| Age (years) 4 | 61.0 (54.0, 69.0) | 64.0 (56.0, 71.0) | 57.0 (50.0, 63.0) | < 0.001 |
| BMI (kg/m2) 4 | 26.2 (24.1, 28.7) | 26.4 (24.3, 29.0) | 26.0 (23.7, 28.4) | 0.01 |
| Fasting 3 | 187 (13.7) | 115 (12.7) | 71 (15.7) | 0.15 |
| Plasma cotinine (nmol/L) 4 | 1.6 (0.0, 510) | 0.0 (0.0, 1.6) | 1054 (508, 1583) | < 0.001 |
| Cardiovascular risk factors | ||||
| Hypercholesterolemia 3 , 5 | 755 (55.3) | 503 (55.5) | 252 (54.9) | 0.71 |
| Diabetes 3 , 6 | 140 (10.2) | 106 (11.7) | 34 (7.41) | 0.02 |
| Stable angina pectoris 3 | 1274 (93.3) | 857 (94.5) | 417 (90.8) | 0.02 |
| Homocysteine (μmol/L) 4 | 10.3 (8.6, 12.3) | 10.2 (8.5, 12.3) | 10.6 (8.8, 12.4) | 0.12 |
| Functional markers for cardiac function | ||||
| Left ventricular ejection fraction < 50% 3 | 114 (8.3) | 65 (7.2) | 49 (10.7) | 0.027 |
| Troponin T ≥14 ng/L 3 | 201 (14.7) | 151 (16.6) | 50 (10.9) | 0.021 |
| Medication 3 , 7 | ||||
| Statin | 915 (67.0) | 610 (67.3) | 305 (66.4) | 0.68 |
| Acetylsalicylic acid | 1075 (78.7) | 719 (79.3) | 356 (77.6) | 0.47 |
| β-blocker | 987 (72.3) | 679 (74.9) | 308 (67.1) | 0.002 |
| Calcium channel blocker | 305 (22.3) | 206 (22.7) | 99 (21.6) | 0.63 |
| ACE inhibitors | 255 (18.7) | 190 (20.9) | 65 (14.2) | 0.002 |
| Angiotensin II receptor antagonist | 129 (9.4) | 81 (8.9) | 48 (10.5) | 0.36 |
| Loop diuretics | 135 (9.9) | 100 (11.0) | 35 (7.6) | 0.047 |
1 Missing values: Cotinine: n = 3 (0.2%), β-blocker: n = 1 (0.1%); Fasting: n = 74 (5.4%); Hypercholesterolemia: n = 81 (5.9%); Troponin T: n = 170 (12.4%). ACE, angiotensin converting enzyme.
2 P values for differences between smokers and non-smokers were calculated by using Mann-Whitney U test for continuous variables and chi-square tests for categorical variables.
3 n (%).
4 Median (25th, 75th percentile).
5 Serum total cholesterol ≥ 6.5mmol/L.
6 Includes diabetes types 1 and 2.
7 Medication prior to coronary angiography.
Dietary intake and circulating concentrations of B-vitamins and PUFAs
Fifty-two percent of the participants completed a FFQ at trial enrollment. A short summary of dietary intake and circulating concentrations of B-vitamins and PUFAs are shown in Table 2 and Table 3, respectively. Even though the dietary intake of B-vitamins did not significantly differ between non-smokers and smokers, there were lower circulating concentrations of folate, vitamin B2 and vitamin B6 among smokers. Further, smokers had a higher dietary intake of ALA, but lower dietary intake of n-3 LCPUFAs and total serum n-3 PUFAs than non-smokers. Dietary intake of AA did not significantly differ between non-smokers and smokers, but participants who smoked had higher dietary intake of LA and total serum n-6 PUFAs.
Table 2. Dietary intake of B-vitamins and fatty acids according to smoking status 1 .
| Total | Non-smokers | Smokers | ||
|---|---|---|---|---|
| Nutrient 2 | (n = 709) | (n = 486) | (n = 223) | P 3 |
| B-vitamins | ||||
| Folate (μg/d) | 228 (181, 281) | 223 (179, 279) | 236 (183, 289) | 0.21 |
| B2 (mg/d) | 1.51 (1.15, 1.93) | 1.52 (1.16, 1.90) | 1.50 (1.10, 2.01) | 0.68 |
| B6 (mg/d) | 1.55 (1.19, 1.93) | 1.52 (1.18, 1.89) | 1.58 (1.98, 1.20) | 0.39 |
| B12 (μg/d) | 7.50 (5.40, 10.3) | 7.50 (5.35, 10.1) | 7.90 (5.50, 10.8) | 0.21 |
| Saturated fatty acids (g/d) | 26.5 (19.4, 34.2) | 25.4 (18.7, 32.8) | 29.4 (21.1, 37.6) | < 0.001 |
| MUFAs (g/d) | 23.1 (17.5, 29.8) | 22.6 (16.8, 29.1) | 24.8 (18.8, 34.2) | 0.001 |
| PUFAs (g/d) | ||||
| Total n-3 4 | 3.00 (2.21, 4.17) | 2.96 (2.20, 4.2) | 3.09 (2.30, 4.29) | 0.28 |
| ALA | 1.85 (1.36, 2.43) | 1.76 (1.30, 2.26) | 2.05 (1.49, 2.69) | < 0.001 |
| EPA, DPA and DHA | 1.05 (0.58, 1.76) | 1.13 (0.59, 1.91) | 0.92 (0.52, 1.59) | 0.03 |
| Total n-6 5 | 12.6 (8.91, 16.9) | 12.0 (8.71, 15.7) | 14.0 (10.3, 19.3) | 0.001 |
| LA | 12.5 (8.80, 16.7) | 11.93 (8.61, 15.6) | 13.8 (10.1, 19.2) | < 0.001 |
| AA | 0.11 (0.08, 0.14) | 0.11 (0.07, 0.14) | 0.11 (0.08, 0.15) | 0.15 |
1 Of participants who completed the food frequency questionnaire, missing values are: B6: n = 3 (0.4%), B12: n = 3 (0.4%). AA, arachidonic acid; ALA, alpha-linolenic acid; B2, riboflavin; B6, pyridoxal 5’-phosphate; B12, cobalamin; DPA, docosapentaenoic acid; LA, linoleic acid.
2 Median (25th, 75th percentile).
3 Difference between smokers and non-smokers were calculated by Mann-Whitney U test.
4 Sum of ALA, EPA, DPA and DHA.
5 Sum of LA and AA.
Table 3. Serum/plasma concentrations of vitamins, subgroups of fatty acids, fatty acid indexes and activity index of desaturases according to smoking status 1 .
| Total | Non-smokers | Smokers | ||
|---|---|---|---|---|
| Characteristics | (n = 1366) | (n = 907) | (n = 459) | P 2 |
| B-vitamins and vitamin-marker | ||||
| Folate (nmol/L) | 10.0 (7.3, 14.6) | 10.7 (7.9, 15.2) | 8.90 (6.5, 12.5) | < 0.001 |
| B2 (nmol/L) | 11.0 (7.4, 17.5) | 11.8 (7.8, 18.3) | 9.91 (6.8, 15.9) | < 0.001 |
| B6 (nmol/L) | 42.1 (29.9, 59.9) | 44.1 (32.0, 61.6) | 37.5 (25.9, 54.8) | < 0.001 |
| B12 (pmol/L) | 351 (269, 443) | 353 (270, 444) | 346 (266, 436) | 0.80 |
| MMA (μmol/L) | 0.16 (0.13, 0.20) | 0.16 (0.14, 0.21) | 0.16 (0.13, 0.20) | 0.11 |
| Fatty acids (% of total fatty acids) | ||||
| SFA | 33.0 (31.9, 34.8) | 33.0 (31.9, 34.7) | 33.0 (31.6, 34.9) | 0.99 |
| MUFA | 23.1 (20.7, 25.5) | 22.9 (20.8, 25.1) | 23.5 (20.5, 26.3) | 0.01 |
| n-3 PUFA | 7.27 (5.61, 9.35) | 7.83 (6.12, 10.0) | 6.18 (4.8, 8.09) | < 0.001 |
| n-6 PUFA | 35.0 (31.7, 38.7) | 34.6 (31.6, 37.9) | 35.6 (32.1, 39.6) | 0.001 |
| Fatty acid indexes | ||||
| Omega-3 index | 5.68 (4.14, 7.60) | 6.19 (4.59, 8.28) | 4.67 (3.40, 6.44) | < 0.001 |
| EPA/AA-ratio 3 | 34.5 (20.3, 58.8) | 40.2 (23.1, 65.6) | 26.1 (15.9, 47.9) | < 0.001 |
| Activity index of desaturases | ||||
| n-3 D5D | 13.2 (8.97, 19.4) | 14.4 (9.88, 20.7) | 10.8 (7.29, 16.3) | < 0.001 |
| n-6 D5D | 4.33 (3.60, 5.40) | 4.39 (3.61, 5.54) | 4.30 (3.55, 5.20) | 0.10 |
| n-3 D6D | 0.05 (0.03, 0.08) | 0.05 (0.05, 0.08) | 0.04 (0.03, 0.07) | 0.001 |
| n-6 D6D | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.10 |
1 Given as median (25th, 75th percentile). Missing values: Folate: n = 2 (0.1%); B2: n = 2 (0.1%); B6: n = 2 (0.1%); B12: n = 494 (36.2%); MMA: n = 2 (0.1%). AA, arachidonic acid; B2, riboflavin; B6, pyridoxal 5’-phosphatase; B12, cobalamin; D5D, delta 5 desaturase; D6D, delta 6 desaturase; MMA, methylmalonic acid; omega-3 index, (EPA + DHA) of total fatty acids; n-3 D5D, EPA/eicosatetraeonic acid; n-3 D6D, stearidonic acid/alpha linolenic acid; n-6 D5D, arachidonic acid/dihomo-γ-linolenic acid; n-6 D6D, γ-linolenic acid/ linoleic acid; SFA, saturated fatty acids.
2 Difference between smokers and non-smokers were calculated by Mann-Whitney U test.
3 EPA/AA-ratio * 100
Associations between circulating B-vitamins and PUFAs
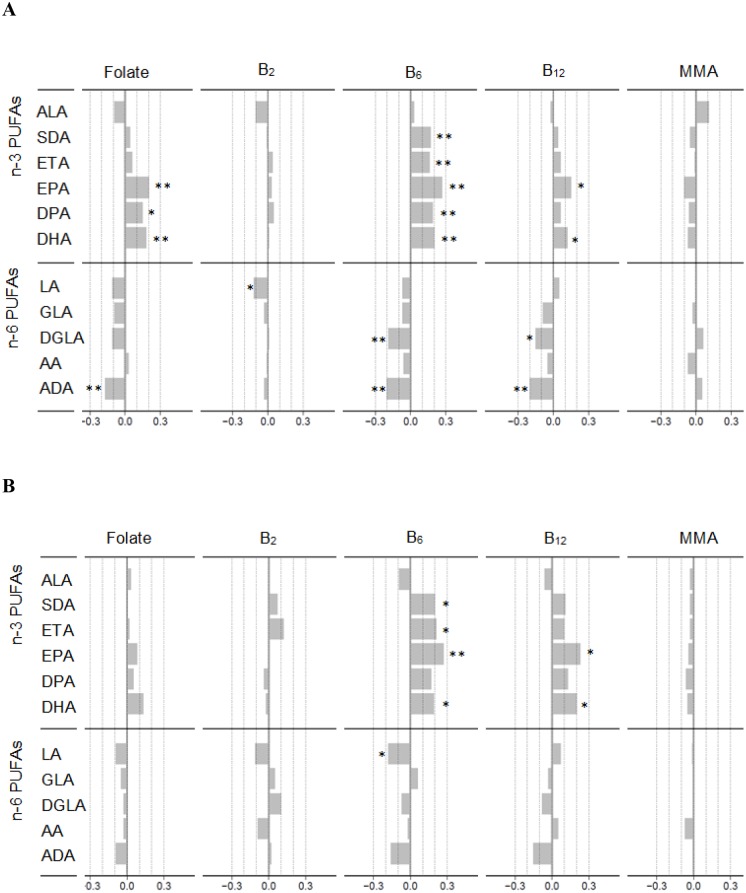
Among non-smokers, simple correlation analysis demonstrated positive associations of circulating folate, vitamins B6 and B12 with n-3 PUFAs, the omega-3 index, EPA/AA-ratio and n-3 D5D (S1 Table). Furthermore, circulating folate and vitamin B6 were negatively related with serum n-6 PUFAs and positively with n-3 D6D and n-6 D5D. None of the associations of serum vitamin B12 with fatty acids, fatty acid indexes or activity indexes of desaturases were reflected by a similar inverse relationship of the metabolic vitamin B12 marker, MMA. The strongest observed relation was between plasma B6 and the EPA/AA-ratio (S1 Table). These associations were essentially confirmed by multivariate analyses, demonstrating circulating B-vitamins to be positively related to serum n-3 PUFAs, and negatively related to serum n-6 PUFAs. The strongest associations were seen for plasma vitamin B6, which was directly associated with serum n-3 LCPUFAs and inversely with serum n-6 LCPUFAs, and declined in the order EPA, DHA/ADA and DGLA (Fig 1A).
Fig 1. The relationship between circulating B-vitamins and serum n-3 and n-6 PUFAs in non-smokers and smokers.
Spearman’s rho (r) of ranked values of circulating B-vitamins folate, B2, B6, B12 and MMA with serum n-3 and n-6 PUFAs in non-smokers (n = 480) (A) and smokers (n = 215) (B) who completed the food frequency questionnaire. The models for n-3 PUFAs were adjusted for gender, age, effective statin dose and dietary intake of n-3 PUFAs (ALA, EPA, DPA and DHA) (g/d); The models for n-6 PUFAs were adjusted for gender, age, effective statin dose and dietary intake of n-6 PUFAs (LA and AA) (g/d). AA, arachidonic acid; ADA, Adrenic acid; ALA, alpha linolenic acid; B2, riboflavin; B6, pyridoxal 5’-phosphate; B12, cobalamin; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; ETA, eicosatetraeonic acid; GLA, γ-linolenic acid; LA, linoleic acid; MMA, methylmalonic acid; SDA, Stearidonic acid. * p <0.01, ** p < 0.001.
Further, GAM was used to investigate the dose-response relationship for the strongest associations found by the multivariate correlation analyses among non-smokers for serum n-3 PUFAs (S1 Fig) and n-6 PUFAs (S2 Fig). The positive associations with most serum n-3 PUFAs leveled off at higher vitamin concentrations, whereas the inverse associations with the serum n-6 PUFAs were essentially linear across the whole distribution of circulating folate, vitamin B6 and vitamin B12 in non-smokers.
In smokers, crude associations were similar to those observed among non-smokers, except for serum folate that did not show any significant association with serum fatty acids, fatty acid indexes or activity indexes of desaturases in this group (S1 Table). Serum folate showed no significant correlation with serum n-3 or n-6 PUFAs in the multivariate analysis (Fig 1B). Most associations of plasma vitamin B6 and serum vitamin B12 with serum n-3 PUFAs were similar in smokers as compared to non-smokers in the multivariate analysis, whereas the associations with serum n-6 PUFAs tended to be weaker in smokers.
Even though the correlations between circulating B-vitamins and PUFAs tended to be generally weaker in smokers as compared to non-smokers (Fig 1A and 1B), interaction analyses did not detect any significant differences between these subgroups.
Associations between circulating cotinine and PUFAs
Crude correlation analyses showed strong inverse associations between plasma cotinine and variables containing serum n-3 PUFAs, and a positive, although weaker, association with serum n-6 PUFAs (S1 Table). The multivariate analyses demonstrated plasma cotinine to be inversely associated with all serum n-3 PUFAs [SDA (r = -.11, p = 0.003), ETA (r = -.11, p = 0.004), EPA (r = -.18, p < 0.001), DPA (r = -.25, p < 0.001) and DHA (r = -.24, p < 0.001)], with the exception of ALA. There were no significant associations between plasma cotinine and serum n-6 PUFAs in these analyses.
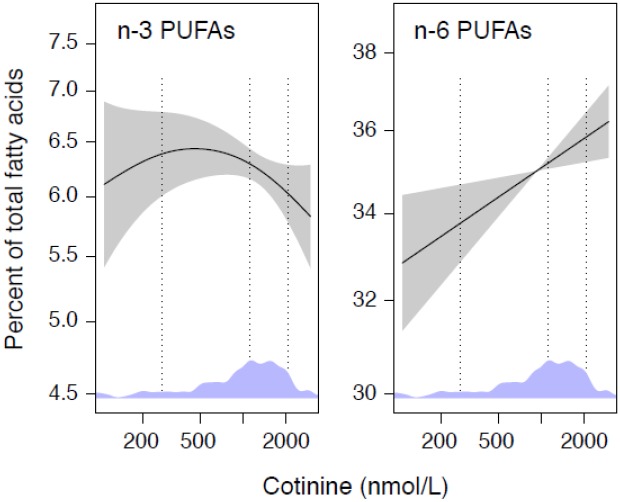
Among smokers, the multivariate analyses did not show any significant associations between plasma cotinine and serum n-3 PUFAs, but demonstrated a positive association between plasma cotinine and the essential n-6 PUFA, LA in serum (r = .20, p = 0.004). These relationships were further investigated in GAM-curves which demonstrated plasma cotinine to be inversely associated with total serum n-3 PUFAs at cotinine concentrations above ~500 nmol/L, and directly associated with serum n-6 PUFAs throughout the cotinine range (Fig 2).
Fig 2. The relationship between cotinine and serum n-3 and n-6 PUFAs.
The dose-response relationships between concentrations of plasma cotinine levels (nmol/L) and serum PUFAs (% of total fatty acids) in smokers (n = 459). Associations were modeled by GAM and adjusted for gender and age. Shaded areas indicate 95% confidence intervals. The y-axis spans 2 standard derivations of each outcome. Density plot for the distribution of cotinine are included in diagrams with 10th, 50th and 90th percentile marked by dotted, vertical lines.
Discussion
Principal findings
In this large cross-sectional study among patients with suspected CHD, we demonstrated that circulating B-vitamins are generally positively related with serum n-3 PUFAs, and negatively related with serum n-6 PUFAs, independent of smoking status. Overall, the strongest association was between plasma vitamin B6 and serum EPA. Circulating cotinine, a marker of recent nicotine exposure, tended to be inversely related to serum n-3 PUFAs, and directly related to serum n-6 PUFAs.
Clinical relevance
Our results indicate that there may be an interaction between B-vitamin status, smoking and circulating PUFA status, which are factors associated with risk of CVD [17,18,26–29]. No beneficial effect of treatment with folic acid/B12 or B6 was found in WENBIT [13]. Notably, we recently observed that a high dietary intake of n-3 LCPUFAs was associated with an increased risk of fatal myocardial infarction in patients without impairment of glucose metabolism, but with a reduced risk in patients with diabetes [30]. Furthermore, in contrast to results from older studies, recent randomized controlled trials do not find a protective effect of n-3 PUFA supplementation on CVD risk in the general population [31] or in individuals at high risk of cardiovascular events [32]. Moreover, the prescription of statins has increased during the last decade and such treatment may modify the effect of n-3 PUFAs on incident major cardiovascular events [23]. Adjustment for statin treatment did not influence the current results. In addition, a lower prevalence of smokers during the last decades may have contributed to an increase in the circulating concentration of n-3 PUFAs in the general population. This may further limit the possibility to detect beneficial effects of n-3 PUFA supplementation in relation to CVD. Our data should motivate subgroup analysis of B-vitamin status and/or smoking habits when exploring results from studies with PUFA or statin treatment.
Possible mechanisms
Circulating concentrations of PUFAs are related to the dietary intake of corresponding fatty acids [30]. Moreover, a study in healthy men demonstrated that an increased dietary intake of LA resulted in decreased plasma EPA and increased plasma n-6 eicosadienoic acid (a conversion product of LA), but no changes of plasma AA [33]. The authors suggested that an increased conversion of LA inhibited the ALA conversion to EPA [33]. This may be seen in context with smokers in our study who had a higher dietary intake of LA and a lower circulating EPA/AA-ratio than non-smokers. A low EPA/AA-ratio may also be associated with an increased production of AA generated pro-inflammatory eicosanoids, as opposed to anti-inflammatory eicosanoids which can be derived from EPA [34].
Fatty acids, including PUFAs, are incorporated into cellular phospholipids where important biological functions are exerted. Phosphatidylcholine (PC) is the most abundant group of phospholipids and can be synthesized from two different pathways. The most common pathway is the CDP-choline pathway, while the alternative route goes through the phosphatidylethanolamine (PE) methylation pathway. The latter includes the activity of phosphatidylethanolamine-N-methyltransferase (PEMT) to convert PE into PC via sequential methylation reactions [35]. This pathway has been reported to affect the circulating concentration of LCPUFAs in serum, plasma or erythrocytes [4,36,37]. Since PEMT seems to prefer LCPUFA PE as substrate, PC synthesized through this pathway contain more LCPUFA as compared to PC synthesized through the alternative CPD-choline pathway, which is composed of more saturated fatty acids [36]. An increased generation of PC through the PEMT pathway may thus increase the availability of LCPUFA to the peripheral tissue [37]. A study in mice showed that low PEMT activity led to reduced DHA in plasma PC, but an accumulation of DHA in hepatic PE, possibly demonstrating the role of PEMT in mobilization of DHA from liver into plasma [37]. Interestingly, homocysteine, an established risk marker for CVD [38] is formed from S-adenosylhomocysteine which inhibits most methyltransferases, including PEMT. Circulating concentrations of folate, vitamin B6 and vitamin B12 are inversely associated with that of homocysteine [38]. Thus, these homocysteine-lowering vitamins are suspected to be positively correlated with PEMT activity, and therefore also with the circulating concentrations of LCPUFA [4]. Accordingly, a reduced PEMT activity has been demonstrated in liver microsomes of vitamin B6-deficient rats [39]. In further support of this hypothesis, an inverse relationship between circulating homocysteine and DHA is observed in both animal and human studies [4,40,41]. Furthermore, serum AA was positively associated while hepatic AA was negatively associated to vitamin B supplementation in rats on an energy restricted diet [42]. Thus, B-vitamins may indirectly affect the circulating concentration of PUFAs through the hepatic PEMT activity. In the current study we did not measure hepatic PEMT activity, but we observed a positive relationship between the circulating concentrations of folate, vitamin B6 and vitamin B12 with serum n-3 LCPUFAs.
PUFA status may also be modified through desaturase activity, which introduces new double-bonds to the PUFAs during their conversion into LCPUFAs [1]. Low serum concentrations of vitamin B6 are associated with lower estimated D5D and D6D activity indexes in healthy people [43], and vitamin B6-deficiency has shown to decrease the activity index of D6D in rat liver microsomes [44]. Moreover, high levels of vitamin B6 have been reported to increase both D5D- and D6D mRNA levels in HepG2 cells [5]. These results may suggest that folate and vitamins B6 and B12 influence genes and enzymes involved in the metabolism of LCPUFA. In agreement with these experimental data, we demonstrated that the association between circulating B-vitamins and LCPUFAs was stronger than with the essential PUFAs [ALA and LA] in non-smokers. Furthermore, smoking has been reported to decrease the activity indexes of D5D and D6D in previous cell studies [10,11], and further reduce the LA conversion [11]. Our results are in line with these experimental findings in that plasma cotinine is inversely associated with the estimated activity indexes of D5D and D6D, but positively related to serum LA in smokers. Of note, previous studies present the activity indexes of desaturases calculated from n-6 PUFAs [10,11]. In the current study plasma cotinine was only significantly associated with the activity indexes of desaturases when calculated from n-3 PUFAs. However, a decreased activity of D5D and D6D due to smoking may explain the tendency of weaker associations of circulating B vitamins with serum n-3 and n-6 LCPUFA in smokers as compared to non-smokers. Moreover, different single-nucleotide polymorphisms of genes regulating the desaturase enzymes may also affect the PUFA profile [45]. Unfortunately, the present study does not include such information.
Since smoking has both short- and long-term effects on circulating B-vitamins [8], it may also indirectly affect the PUFA profile. Smoking induces systemic oxidative stress in humans [46], whereas folate, vitamin B2 and vitamin B6 have antioxidant properties [47–49]. Furthermore, smoking is associated with low muscle mass [50]. Muscles are a major depot of vitamin B6 [51], and a depletion of these depots may reduce circulating vitamin B6. In accordance to a tendency of higher oxidative stress and reduced muscle mass among smokers, we observed them to have lower concentrations of circulating folate, B2 and B6 as compared to non-smokers.
Taken together, both the current study and results from prior experimental studies suggest a complex metabolic interplay between B-vitamins, PUFAs and smoking status, which may further interfere with the progression of CHD.
Strengths and limitations
Strengths of the current study include its large, well-characterized population. Data from the FFQ made it possible to adjust for dietary PUFAs when investigating the associations of B-vitamins and cotinine with circulating PUFAs. Further, plasma cotinine was used to investigate the dose-response relationship between nicotine exposure and PUFAs.
There were some limitations of the data collection. Most patients were not fasting, which may influence some of the measurements, including the fatty acid composition. Of note, the circulating concentrations of n-3 PUFAs, n-6 PUFAs, folate, B2, B12 and cotinine did not differ between fasted and non-fasted participants, whereas plasma B6 was lower in those who fasted. However, no effect of prandial status on plasma vitamin B6 is reported (www.bevital.no). Furthermore, there were no differences in the prevalence of fasting between non-smokers and smokers. Since both cigarette smoke [14] and smokeless tobacco [52] affects the circulating cotinine concentration, subjects using smokeless tobacco may have been misclassified as smokers. However, most of the subjects (approximately 85%) confirmed through self-report that they were current smokers or had been smokers within the past month before included in the study. Moreover, only subjects enrolled in WENBIT were asked to fill in the FFQ, introducing a potential for selection bias. Respondents of the FFQ were more often non-smokers and had a higher frequency of stable angina pectoris. Since patients with known CHD may be more aware of their dietary intake, this may influence the results. However, the median dietary intake of n-3 PUFAs and B-vitamins in our study population was comparable with intakes observed in the general population from the same region using the same questionnaire [53]. Furthermore, our data are based on single measurements at baseline, which may lead to underestimation of the true strength of the associations, due to regression dilution bias [54].
Conclusions
In conclusion, we demonstrate that circulating B-vitamins and smoking habits are associated with serum PUFAs in patients with suspected CHD. These associations should be evaluated in general populations, and motivate further studies on interactions between B-vitamins, smoking status and lipid metabolism in relation to lifestyle diseases.
Supporting Information
The dose-response relationships between concentrations of serum folate, plasma vitamin B6 and serum vitamin B12 with concentrations of serum EPA and DHA in non-smokers (n = 480). Associations were modeled by GAM adjusted for age, gender, effective statin dose and dietary intake of n-3 PUFAs. Shaded areas indicate 95% confidence intervals. The y-axis spans 2 standard derivations of each outcome. Density plots for the distribution of B-vitamins are included in diagrams with 10th, 50th and 90th percentiles marked by dotted, vertical lines. B6, pyridoxal 5’-phosphate; B12, cobalamin.
(TIF)
The dose-response relationships between concentrations of serum folate, plasma vitamin B6 and serum vitamin B12 with concentrations of serum DGLA and ADA in non-smokers (n = 480). Associations were modeled by GAM adjusted for age, gender, effective statin dose and dietary intake of n-6 PUFAs. Shaded areas indicate 95% confidence intervals. The y-axis spans 2 standard derivations of each outcome. Density plot for the distribution of B-vitamins are included in diagrams with 10th, 50th and 90th percentiles marked by dotted, vertical lines. DGLA, dihomo-γ-linolenic acid; ADA, Adrenic acid; B6, pyridoxal 5’-phosphate; B12, cobalamin.
(TIF)
1 Values are given as Spearman’s rho. B2, riboflavin; B6, pyridoxal 5’- phosphate; B12, cobalamin; MMA, methylmalonic acid; omega-3 Index, (EPA + DHA) of total fatty acids; AA, arachidonic acid; D5D, delta 5 desaturase; n-3 D5D, EPA/eicosatetraenoic acid; n-6 D5D, arachidonic acid/dihomo-γ-linolenic acid; D6D, delta 6 desaturase; n-3 D6D, stearidonic acid/alpha linolenic acid; n-6 D6D, γ-linolenic acid/ linoleic acid. * p < 0.01, ** p < 0.001.
(TIF)
Acknowledgments
We are grateful to Liv Kristine Øysæd, Kari Helland Mortensen and Randi Sandvik for excellent technical assistance during FA composition analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Norwegian Foundation for Health and Rehabilitation, the Norwegian Heart and Lung Patient Organization, the Norwegian Ministry of Health and Care Services, the Western Norway Regional Health Authority, the Department of Heart Disease, Haukeland University Hospital, Johan Throne Holst Foundation for Nutrition Research and Freia Medical Research Fund, Norway. Bevital AS provided support in the form of salaries for authors OM and AU, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Das UN (2006) Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 1: 420–439. [DOI] [PubMed] [Google Scholar]
- 2. The Nordic Council (2013) New Nordic Nutrition Recommendations 2012. In: 2013. NCoM, editor. 3 October 2013. ed. pp. 85. [Google Scholar]
- 3. Zhao M, Lamers Y, Ralat MA, Coats BS, Chi YY, Muller KE,et al. (2012) Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and women. J Nutr 142: 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Wijk N, Watkins CJ, Hageman RJ, Sijben JC, Kamphuis PG, Wurtman RJ, et al. (2012) Combined dietary folate, vitamin B-12, and vitamin B-6 intake influences plasma docosahexaenoic acid concentration in rats. Nutr Metab (Lond) 9: 49 10.1186/1743-7075-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao M, Ralat MA, da Silva V, Garrett TJ, Melnyk S, James SJ, et al. (2013) Vitamin B-6 restriction impairs fatty acid synthesis in cultured human hepatoma (HepG2) cells. Am J Physiol Endocrinol Metab 304: E342–351. 10.1152/ajpendo.00359.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bordoni A, Hrelia S, Lorenzini A, Bergami R, Cabrini L, Biagi PL, et al. (1998) Dual influence of aging and vitamin B6 deficiency on delta-6-desaturation of essential fatty acids in rat liver microsomes. Prostaglandins Leukot Essent Fatty Acids 58: 417–420. [DOI] [PubMed] [Google Scholar]
- 7. Giraud DW, Martin HD, Driskell JA (1995) Erythrocyte and plasma B-6 vitamer concentrations of long-term tobacco smokers, chewers, and nonusers. Am J Clin Nutr 62: 104–109. [DOI] [PubMed] [Google Scholar]
- 8. Ulvik A, Ebbing M, Hustad S, Midttun O, Nygard O, Vollset SE, et al. (2010) Long- and short-term effects of tobacco smoking on circulating concentrations of B vitamins. Clin Chem 56: 755–763. 10.1373/clinchem.2009.137513 [DOI] [PubMed] [Google Scholar]
- 9. Block RC, Harris WS, Pottala JV (2008) Determinants of Blood Cell Omega-3 Fatty Acid Content. Open Biomark J 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghezzi S, Rise P, Ceruti S, Galli C (2007) Effects of cigarette smoke on cell viability, linoleic acid metabolism and cholesterol synthesis, in THP-1 cells. Lipids 42: 629–636. [DOI] [PubMed] [Google Scholar]
- 11. Rise P, Ghezzi S, Manzoni C, Colombo C, Galli C (2009) The in vitro effects of cigarette smoke on fatty acid metabolism are partially counteracted by simvastatin. Prostaglandins Leukot Essent Fatty Acids 80: 71–75. 10.1016/j.plefa.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 12. Abeywardena MY, Head RJ (2001) Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res 52: 361–371. [DOI] [PubMed] [Google Scholar]
- 13. Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. (2008) Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 300: 795–804. 10.1001/jama.300.7.795 [DOI] [PubMed] [Google Scholar]
- 14. Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y (1987) Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77: 1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manger MS, Strand E, Ebbing M, Seifert R, Refsum H, Nordrehaug JE, et al. (2010) Dietary intake of n-3 long-chain polyunsaturated fatty acids and coronary events in Norwegian patients with coronary artery disease. Am J Clin Nutr 92: 244–251. 10.3945/ajcn.2010.29175 [DOI] [PubMed] [Google Scholar]
- 16. Strand E, Bjorndal B, Nygard O, Burri L, Berge C, Bohov P, et al. (2012) Long-term treatment with the pan-PPAR agonist tetradecylthioacetic acid or fish oil is associated with increased cardiac content of n-3 fatty acids in rat. Lipids Health Dis 11: 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris WS (2008) The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 87: 1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 18. Domei T, Yokoi H, Kuramitsu S, Soga Y, Arita T, Ando K, et al. (2012) Ratio of serum n-3 to n-6 polyunsaturated fatty acids and the incidence of major adverse cardiac events in patients undergoing percutaneous coronary intervention. Circ J 76: 423–429. [DOI] [PubMed] [Google Scholar]
- 19. Midttun O, Hustad S, Ueland PM (2009) Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 23: 1371–1379. 10.1002/rcm.4013 [DOI] [PubMed] [Google Scholar]
- 20. Molloy AM, Scott JM (1997) Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 281: 43–53. [DOI] [PubMed] [Google Scholar]
- 21. Kelleher BP, Broin SD (1991) Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol 44: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S (2007) Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med 45: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 23. Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. (2012) Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction. Eur Heart J 33: 1582–1588. 10.1093/eurheartj/ehr499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones P, Kafonek S, Laurora I, Hunninghake D (1998) Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 81: 582–587. [DOI] [PubMed] [Google Scholar]
- 25. Rosenson RS (2003) Rosuvastatin: a new inhibitor of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev Cardiovasc Ther 1: 495–505. [DOI] [PubMed] [Google Scholar]
- 26. Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, et al. (1998) Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 97: 437–443. [DOI] [PubMed] [Google Scholar]
- 27. Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, et al. (1996) Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol 143: 845–859. [DOI] [PubMed] [Google Scholar]
- 28. Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, et al. (1998) Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 279: 359–364. [DOI] [PubMed] [Google Scholar]
- 29. Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, et al. (2003) Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 34: e51–54. [DOI] [PubMed] [Google Scholar]
- 30. Strand E, Pedersen ER, Svingen GF, Schartum-Hansen H, Rebnord EW, Bjorndal B, et al. (2013) Dietary intake of n-3 long-chain polyunsaturated fatty acids and risk of myocardial infarction in coronary artery disease patients with or without diabetes mellitus: a prospective cohort study. BMC Med 11: 216 10.1186/1741-7015-11-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS (2012) Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308: 1024–1033. 10.1001/2012.jama.11374 [DOI] [PubMed] [Google Scholar]
- 32. Risk, Prevention Study Collaborative Group, Roncaglioni MC, Tombesi M, Avanzini F, et al. (2013) n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 368: 1800–1808. 10.1056/NEJMoa1205409 [DOI] [PubMed] [Google Scholar]
- 33. Angela Liou Y, Innis SM (2009) Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-gamma-linolenic and eicosapentaenoic acid in plasma of adult men. Prostaglandins Leukot Essent Fatty Acids 80: 201–206. 10.1016/j.plefa.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 34. Tapiero H, Ba GN, Couvreur P, Tew KD (2002) Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 56: 215–222. [DOI] [PubMed] [Google Scholar]
- 35. Vance DE (2013) Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim Biophys Acta 1831: 626–632. 10.1016/j.bbalip.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 36. DeLong CJ, Shen YJ, Thomas MJ, Cui Z (1999) Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 274: 29683–29688. [DOI] [PubMed] [Google Scholar]
- 37. Watkins SM, Zhu X, Zeisel SH (2003) Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr 133: 3386–3391. [DOI] [PubMed] [Google Scholar]
- 38. Marti-Carvajal AJ, Sola I, Lathyris D, Karakitsiou DE, Simancas-Racines D (2013) Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 1: CD006612 10.1002/14651858.CD006612.pub3 [DOI] [PubMed] [Google Scholar]
- 39. She QB, Hayakawa T, Tsuge H (1995) Alteration in the phosphatidylcholine biosynthesis of rat liver microsomes caused by vitamin B6 deficiency. Biosci Biotechnol Biochem 59: 163–167. [DOI] [PubMed] [Google Scholar]
- 40. Li D, Mann NJ, Sinclair AJ (2006) A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids 41: 85–89. [DOI] [PubMed] [Google Scholar]
- 41. Kume A, Kurotani K, Sato M, Ejima Y, Pham NM, Nanri A, et al. (2013) Polyunsaturated fatty acids in serum and homocysteine concentrations in Japanese men and women: a cross-sectional study. Nutr Metab (Lond) 10: 41 10.1186/1743-7075-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bertrandt J, Klos A, Debski B (2004) Content of polyunsaturated fatty acids (PUFAs) in serum and liver of rats fed restricted diets supplemented with vitamins B2, B6 and folic acid. Biofactors 22: 189–192. [DOI] [PubMed] [Google Scholar]
- 43. Krajcovicova-Kudlackova M, Klvanova J, Dusinska M (2004) Polyunsaturated Fatty Acid Plasma Content in Groups of General Population with lowvitamin B6 or low iron serum levels. Ann Nutr Metab 48: 118–121. [DOI] [PubMed] [Google Scholar]
- 44. She QB HT, Tsuge H (1994) Effect of vitamin B6 deficiency on linoleic acid desaturation in the arachidonic acid biosynthesis of rat liver microsomes. Biosci Biotech Biochem 58: 459–463. [Google Scholar]
- 45. Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, et al. (2010) Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res 51: 2325–2333. 10.1194/jlr.M006205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF (2007) Systemic effects of smoking. Chest 131: 1557–1566. [DOI] [PubMed] [Google Scholar]
- 47. Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T (2001) Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med 30: 1390–1399. [DOI] [PubMed] [Google Scholar]
- 48. Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF (2000) Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol 71: 129–134. [DOI] [PubMed] [Google Scholar]
- 49. Toyosaki T (1992) Antioxidant effect of riboflavin in enzymatic lipid peroxidation. Journal of Agricultural and Food Chemistry 40: 1727–1730. [Google Scholar]
- 50. Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Douglas KM, Nevill AM, Jamurtas AZ, et al. (2008) Cigarette smoking associates with body weight and muscle mass of patients with rheumatoid arthritis: a cross-sectional, observational study. Arthritis Res Ther 10: R59 10.1186/ar2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coburn SP (1990) Location and turnover of vitamin B6 pools and vitamin B6 requirements of humans. Ann N Y Acad Sci 585: 76–85. [DOI] [PubMed] [Google Scholar]
- 52. Agaku IT, King BA (2014) Validation of self-reported smokeless tobacco use by measurement of serum cotinine concentration among US adults. Am J Epidemiol 180: 749–754. 10.1093/aje/kwu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Berstad P, Konstantinova SV, Refsum H, Nurk E, Vollset SE, Tell GS, et al. (2007) Dietary fat and plasma total homocysteine concentrations in 2 adult age groups: the Hordaland Homocysteine Study. Am J Clin Nutr 85: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 54. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. (1990) Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The dose-response relationships between concentrations of serum folate, plasma vitamin B6 and serum vitamin B12 with concentrations of serum EPA and DHA in non-smokers (n = 480). Associations were modeled by GAM adjusted for age, gender, effective statin dose and dietary intake of n-3 PUFAs. Shaded areas indicate 95% confidence intervals. The y-axis spans 2 standard derivations of each outcome. Density plots for the distribution of B-vitamins are included in diagrams with 10th, 50th and 90th percentiles marked by dotted, vertical lines. B6, pyridoxal 5’-phosphate; B12, cobalamin.
(TIF)
The dose-response relationships between concentrations of serum folate, plasma vitamin B6 and serum vitamin B12 with concentrations of serum DGLA and ADA in non-smokers (n = 480). Associations were modeled by GAM adjusted for age, gender, effective statin dose and dietary intake of n-6 PUFAs. Shaded areas indicate 95% confidence intervals. The y-axis spans 2 standard derivations of each outcome. Density plot for the distribution of B-vitamins are included in diagrams with 10th, 50th and 90th percentiles marked by dotted, vertical lines. DGLA, dihomo-γ-linolenic acid; ADA, Adrenic acid; B6, pyridoxal 5’-phosphate; B12, cobalamin.
(TIF)
1 Values are given as Spearman’s rho. B2, riboflavin; B6, pyridoxal 5’- phosphate; B12, cobalamin; MMA, methylmalonic acid; omega-3 Index, (EPA + DHA) of total fatty acids; AA, arachidonic acid; D5D, delta 5 desaturase; n-3 D5D, EPA/eicosatetraenoic acid; n-6 D5D, arachidonic acid/dihomo-γ-linolenic acid; D6D, delta 6 desaturase; n-3 D6D, stearidonic acid/alpha linolenic acid; n-6 D6D, γ-linolenic acid/ linoleic acid. * p < 0.01, ** p < 0.001.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.