Abstract
Thyroid cancer and thyroid nodules are more prevalent in women than men, so female sex hormones may have an etiological role in these conditions. There are no data about direct effects of progesterone on thyroid cells, so the aim of the present study was to evaluate progesterone effects in the sodium-iodide symporter NIS, thyroglobulin TG, thyroperoxidase TPO, and KI-67 genes expression, in normal thyroid follicular cells, derived from human tissue. NIS, TG, TPO, and KI-67 mRNA expression increased significantly after TSH 20 μUI/mL, respectively: 2.08 times, P < 0.0001; 2.39 times, P = 0.01; 1.58 times, P = 0.0003; and 1.87 times, P < 0.0001. In thyroid cells treated with 20 μUI/mL TSH plus 10 nM progesterone, RNA expression of NIS, TG, and KI-67 genes increased, respectively: 1.78 times, P < 0.0001; 1.75 times, P = 0.037; and 1.95 times, P < 0.0001, and TPO mRNA expression also increased, though not significantly (1.77 times, P = 0.069). These effects were abolished by mifepristone, an antagonist of progesterone receptor, suggesting that genes involved in thyroid cell function and proliferation are upregulated by progesterone. This work provides evidence that progesterone has a direct effect on thyroid cells, upregulating genes involved in thyroid function and growth.
1. Introduction
Thyroid nodules and thyroid cancer are more common in women [1, 2]. The incidence rate of thyroid cancer (TC) is increasing, which could be due to better surveillance techniques [3]. Nevertheless, other factors are probably involved because the incidence rates of tumors of all sizes increased [4]. As the increase in incidence of TC appears to be higher in women, sex hormones might have an etiological role [5]. Reproductive factors, as history of uterine fibroids, greater number of live births, greater number of reproductive years, and greater estimated number of ovulatory cycles, were associated with an increased risk of TC [6]. Also, during pregnancy, thyroid nodules were shown to increase in number and size [7, 8], and TC has been shown to affect one in a thousand pregnant women, being the second most common type of cancer in pregnancy [9].
Several studies have shown thyroid cells growth in response to estrogen [10–13], as reviewed recently [14], and some data suggest a decrease in differentiated function by this hormone in thyroid cells [11, 15].
There are no studies of progesterone effects on thyroid cells. Progesterone might have a protective effect on the thyroid because the use of oral contraceptives and menopausal hormone therapy, with the combination of estrogen and progesterone, were not associated with thyroid cancer risk [6], but irregular menstrual bleeding, usually due to anovulatory cycles, has been associated with it [16].
Although progesterone could exert its effects through genomic or nongenomic mechanisms [17, 18], its primary action is to regulate the expression of target genes through binding directly to its cognate sequence in the genes, called progesterone responsive elements [19]. As PR have been variably described in thyroid follicular cells [20–23], the aim of the present study was to evaluate the effects of progesterone on gene expression in a model of normal human thyroid cells in primary culture.
2. Methods
2.1. Reagents
All reagents were purchased from Sigma-Aldrich unless otherwise specified.
2.2. Thyroid Tissue Acquisition
Tissue samples were obtained from patients submitted to thyroidectomy as part of treatment for differentiated TC. After evaluation by two pathologists, normal tissue that would have been discharged was kept in Hank's solution at about 4°C until processing. The project was submitted to, and approved by, the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil (CEP: 09-454), which waived a written informed consent. In accordance with the Resolution HCPA 02/97, based on the Resolution CNS 196/96 of the National Health Council, Brazil, and the Guideline 9 of the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS, WHO, Geneva, 1993), there was no need to obtain informed consent of the patients, because only after the surgical procedure the researchers would know if a tissue sample would be available, they would not know the identity nor had access to the files of the patients, and the tissue samples included would have been discharged by the pathologists. The authors signed the “Statement of commitment to use of data,” which states that no data that could identify patients would be used and also that their anonymity would be preserved in any publication of the results.
2.2.1. Primary Culture of Normal Thyroid Cells
Thyroid follicular cells isolation and culture were performed as described previously [24]. Briefly, thyroid tissue was cut in fragments of about 1 mm3 and digested by 3 mg/mL type I collagenase (GIBCO, Grand Island, NY, USA). The suspension of cells was sequentially filtered through nylon meshes (250, 150, and 60 μm pore size) and the filtered fraction, containing epithelial thyroid cells, was resuspended and seeded in a 35 mm Petri dish at a density of 1 × 106 cells/cm2. Thyroid cells were cultured initially in Ham's F-12 Coon's modification medium supplemented with 10% fetal bovine serum, 10 μg/mL insulin, 5 μg/mL transferrin, 1 mU/mL TSH, and 100 U/mL kanamycin at 37°C with 5% CO2; after two changes, the concentration of serum was decreased to 5% (3H medium).
2.2.2. Treatments
Five or 7 days later, cells were deprived of TSH for 48 hours (3H medium minus TSH: 2H medium); subsequently, they were treated with 2H medium and progesterone, mifepristone, or vehicle ethanol, as follows: Group 1: 2H medium; Group 2: 2H medium + 20 μU/mL TSH; Group 3: 2H medium + 20 μU/mL TSH + 10 nM progesterone, and Group 4: 2H medium + 20 μU/mL TSH + 10 nM progesterone + 100 nM mifepristone, for 4 h or 48 h, before total RNA isolation. Ethanol was added so the final concentration was 0.1% for all groups.
2.2.3. Gene Expression Analysis
Total RNA was isolated using the Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). Concentration and purity were assessed by the Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Rockland, DE, USA) and stored at −80°C. 1 μg total RNA was transcribed into cDNA by Superscript III Reverse Transcriptase (Invitrogen Life Technologies) and stored at −20°C.
Expression of KI-67, thyroglobulin TG, thyroperoxidase TPO, and sodium-iodide symporter NIS genes was assessed by quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) on Applied Biosystems StepOne Real-Time PCR System using Kit Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen Life Technologies). β-actin was used as reference gene [24]. The primers used for the specific amplification of TG, TPO, and NIS were described previously [25]. The sequences of the primers for progesterone receptor were 5′-ACCCGCCCTATCTCAACTACC-3′ and 5′-AGGACACCATAATGACAGCCT-3′. Annealing temperature of 60°C was used for amplification; dissociation curves were performed by running a gradient of 60–95°C to confirm the specificity of the PCR amplification. All samples were amplified in triplicate, and cDNA standard curves were constructed using the threshold cycles with five successive 10-fold dilution points of a pool of cDNA samples.
2.3. Statistical Analysis
RT-qPCR experiments were performed in duplicate and repeated in three (NIS gene) or four (KI-67, TG, and TPO genes) independent experiments, with cells isolated from different patients. mRNA values were normalized by β-actin, before statistical analysis. Differences in the means of the treatment groups were evaluated with the generalized estimating equation (GEE). After GEE, post hoc Bonferroni test was used to identify which means were different. All statistical analyses were performed with SPSS software, version 18.0 (SPSS, Chicago, IL), and considered significant when P was less than 0.05.
3. Results
3.1. Thyroid Cells in Primary Cultures Were Responsive to Stimulation by TSH
Thyroid follicular cells viability and differentiation were assessed by characteristic morphology and by staining TPO protein in immunocytochemistry. As showed in Figure 1(a), the thyroid follicular cells isolated in our model present a cuboid shape, with the presence of many vacuoles around the nucleus as observed in previous studies of primary culture of follicular cells thyroid [26]. Also, as other epithelioid cells, these cells grow as islands and form a monolayer with extensive cell-to-cell contact. In Figure 1(b), TPO protein staining, characteristic of thyroid follicular cells, is shown. The specific thyroid genes expression (TG, TPO, and NIS) was also positive in our primary culture of thyroid follicular cells.
Figure 1.
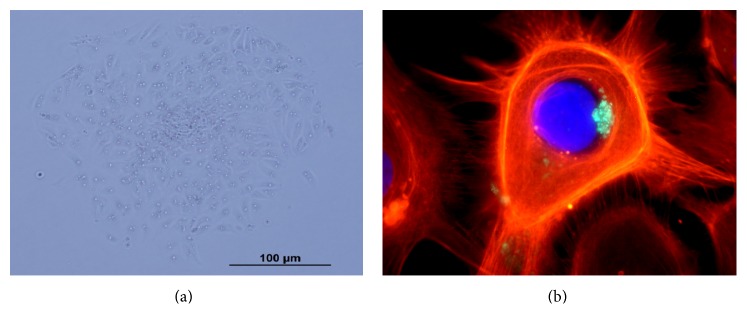
Normal human thyroid follicular cells in primary culture. (a) Thyrocytes in monolayers showing characteristic morphology by phase contrast microscopy and (b) immunocytochemistry of actin with phalloidin-fluorescein isothiocyanate (red), nuclei stained with DAPI (blue), and thyroperoxidase protein (green).
TSH increased significantly the NIS gene expression (2.08 times) after 4 h; it also increased significantly the TG (2.39 times), TPO (1.58 times), and KI-67 (1.87 times) genes expression, after 48 h, as shown in Figure 2.
Figure 2.
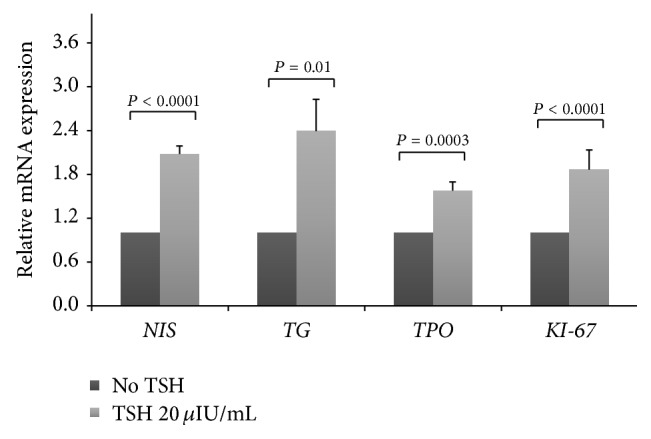
TSH increased mean mRNA expression of human normal thyroid follicular cells genes in primary culture. The sodium-iodide symporter NIS gene was evaluated after 4 h, and the thyroglobulin TG, thyroperoxidase TPO, and KI-67 genes were evaluated after 48 h. Data were obtained by RT-qPCR and normalized with β-actin gene expression and are shown as the mean ratio of TSH/no TSH ± standard deviation, considering no TSH = 1. The experiments were performed in duplicate and repeated in three independent cultures for NIS and four independent cultures for TG, TPO, and KI-67.
3.2. Progesterone Upregulated NIS, TG, TPO, and KI-67 mRNA Expression in Normal Human Thyroid Follicular Cells
To evaluate the effect of progesterone treatment on the expression of NIS, TG, TPO, and KI-67 genes, RT-qPCR was applied. Gene expression of progesterone receptor was present in all thyroid cells cultures used in this study (data not shown). When thyroid cells were treated with 20 μUI/mL TSH plus 10 nM progesterone, the mean expression of NIS mRNA after 4 h, and the mean expression of TG, TPO, and KI-67 mRNA after 48 h, increased, respectively, by 1.75, 1.77, 1.68, and 1.95 times, when compared to the group treated with TSH (Figure 3).
Figure 3.
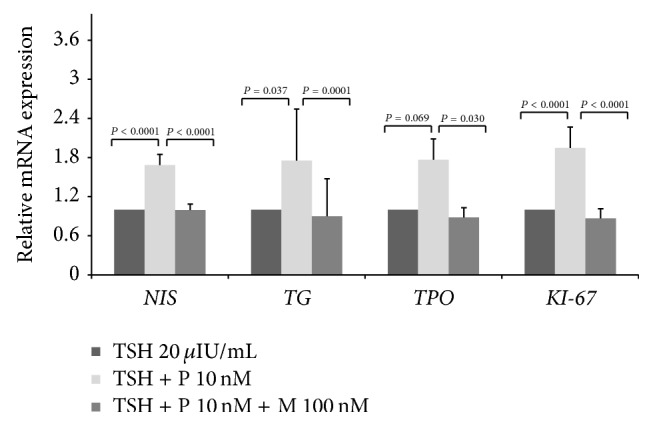
Progesterone (P) increased mean mRNA expression of human normal thyroid follicular cells genes in primary culture. The sodium-iodide symporter NIS gene was evaluated after 4 h, and the thyroglobulin TG, thyroperoxidase TPO, and KI-67 genes were evaluated after 48 h. These effects were abolished by mifepristone (M). Data were obtained by RT-qPCR and normalized with β-actin gene expression and are shown as the mean ratio of treatment/TSH ± standard deviation, considering TSH = 1. The experiments were performed in duplicate and repeated in three independent cultures for NIS and four independent cultures for TG, TPO, and KI-67.
4. Discussion
In the present study, for the first time, an effect of progesterone on thyroid cells was demonstrated. Genes involved in the differentiated protein expression of thyroid cells were upregulated by progesterone, as well as KI-67, a marker for growth; these effects were abolished by mifepristone.
Isolated human follicular cells derived from normal thyroid tissue have been studied previously [27, 28], with high functional correspondence with the original follicular cells. In our model, thyroid cells were grown in monolayer and exhibited the expected morphology. TG, TPO, and NIS mRNA were detected even when the cells were deprived of TSH for 48 h, which could be due to other components of the medium and its supplements, such as insulin, or constitutive activation of thyroid cells [29].
It is known that thyroid cell regulation varies in different species, and, sometimes, the cells can have different phenotypes and responses to TSH [29, 30]. In our model, normal human thyroid follicular cells in primary culture were shown to be differentiated and responsive to TSH. The response of gene expression to TSH has been shown to vary with the gene and time after treatment. Our cells responded to TSH, as expected [11, 31–37].
As no direct effect of progesterone on thyroid cells has been described until now, we have no other studies to compare our results with. As progesterone increased the expression of genes involved in growth and differentiated function of thyroid cells, its effect on the thyroid gland could be protective. Recently Sathi et al. observed an increase in free T4 levels in progesterone-treated women, and a tendency towards lower TSH levels during progesterone treatment, in a randomized controlled progesterone trial [38].
Our results suggest that progesterone effects on human thyroid cell occur via a transcriptional level through its nuclear receptor, since a specific antagonist, mifepristone, abolished it. Mifepristone exerts its antiprogesterone effects by binding PR with higher affinity than progesterone [39]. The ability of mifepristone to abolish progesterone effect in thyroid cells suggests that these receptors are functional in these cells, although its concentration in normal thyroid has been described as low or undetectable [22, 23]. In a recent study PR expression was seen in 75.8% of patients with papillary thyroid cancer [40]. In our study, PR gene expression was identified in all cell cultures.
As mifepristone blocks also the action of glucocorticoids and some synthetic progestogens, as medroxyprogesterone, are ligands for the glucocorticoids receptor (GR), the effects described in the present study could have been mediated by these receptors. Nevertheless, the ability of progesterone to bind to GR is disputable [41].
Although the model used in the present study has contributed to the understanding of thyroid cell growth regulation [42], the increase in gene expression does not always result in a proportional increase in functional proteins. Besides, our monolayer model does not reproduce the complex organization of thyrocytes in the follicle, so functional and growth studies are necessary to better understand a possible role of progesterone in thyroid cells.
5. Conclusion
This work provides evidences that progesterone has a direct effect on thyroid cells, through its receptor, upregulating genes involved in thyroid function and growth.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Vanderpump M. P. J. The epidemiology of thyroid disease. British Medical Bulletin. 2011;99(1):39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell I., Livingston E. H., Chang A. Y., et al. Trends in thyroid cancer demographics and surgical therapy in the United States. Surgery. 2007;142(6):823.e1–828.e1. doi: 10.1016/j.surg.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Davies L., Welch H. G. Current thyroid cancer trends in the United States. JAMA Otolaryngology—Head & Neck Surgery. 2014;140(4):317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 4.Horn-Ross P. L., Lichtensztajn D. Y., Clarke C. A., et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiology Biomarkers & Prevention. 2014;23(6):1067–1079. doi: 10.1158/1055-9965.epi-13-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enewold L., Zhu K., Ron E., et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiology Biomarkers and Prevention. 2009;18(3):784–791. doi: 10.1158/1055-9965.epi-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braganza M. Z., de González A. B., Schonfeld S. J., Wentzensen N., Brenner A. V., Kitahara C. M. Benign breast and gynecologic conditions, reproductive and hormonal factors, and risk of thyroid cancer. Cancer Prevention Research. 2014;7(4):418–425. doi: 10.1158/1940-6207.capr-13-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struve C. W., Haupt S., Ohlen S. Influence of frequency of previous pregnancies on the prevalence of thyroid nodules in women without clinical evidence of thyroid disease. Thyroid. 1993;3(1):7–9. doi: 10.1089/thy.1993.3.7. [DOI] [PubMed] [Google Scholar]
- 8.Kung A. W. C., Chau M. T., Lao T. T., Tam S. C. F., Low L. C. K. The effect of pregnancy on thyroid nodule formation. Journal of Clinical Endocrinology and Metabolism. 2002;87(3):1010–1014. doi: 10.1210/jc.87.3.1010. [DOI] [PubMed] [Google Scholar]
- 9.Imran S. A., Rajaraman M. Management of differentiated thyroid cancer in pregnancy. Journal of Thyroid Research. 2011;2011:5. doi: 10.4061/2011/549609.549609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filardo E. J., Quinn J. A., Bland K. I., Frackelton A. R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Molecular Endocrinology. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 11.Furlanetto T. W., Nguyen L. Q., Jameson J. L. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology. 1999;140(12):5705–5711. doi: 10.1210/endo.140.12.7197. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A., Klinge C. M., Goldstein R. E. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. International Journal of Oncology. 2010;36(5):1067–1080. doi: 10.3892/ijo-00000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manole D., Schildknecht B., Gosnell B., Adams E., Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. Journal of Clinical Endocrinology and Metabolism. 2001;86(3):1072–1077. doi: 10.1210/jc.86.3.1072. [DOI] [PubMed] [Google Scholar]
- 14.Santin A. P., Furlanetto T. W. Role of estrogen in thyroid function and growth regulation. Journal of Thyroid Research. 2011;2011:7. doi: 10.4061/2011/875125.875125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlanetto T. W., Nunes R. B., Jr., Sopelsa A. M. I., Maciel R. M. B. Estradiol decreases iodide uptake by rat thyroid follicular FRTL-5 cells. Brazilian Journal of Medical and Biological Research. 2001;34(2):259–263. doi: 10.1590/S0100-879X2001000200015. [DOI] [PubMed] [Google Scholar]
- 16.Truong T., Orsi L., Dubourdieu D., Rougier Y., Hémon D., Guénel P. Role of goiter and of menstrual and reproductive factors in thyroid cancer: a population-based case-control study in New Caledonia (South Pacific), a very high incidence area. American Journal of Epidemiology. 2005;161(11):1056–1065. doi: 10.1093/aje/kwi136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonhardt S. A., Edwards D. P. Mechanism of action of progesterone antagonists. Experimental Biology and Medicine. 2002;227(11):969–980. doi: 10.1177/153537020222701104. [DOI] [PubMed] [Google Scholar]
- 18.Weigel N. L., Moore N. L. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Molecular Endocrinology. 2007;21(10):2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 19.Leonhardt S. A., Boonyaratanakornkit V., Edwards D. P. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68(10–13):761–770. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 20.Bonacci R., Pinchera A., Fierabracci P., Gigliotti A., Grasso L., Giani C. Relevance of estrogen and progesterone receptors enzyme immunoassay in malignant, benign and surrounding normal thyroid tissue. Journal of Endocrinological Investigation. 1996;19(3):159–164. doi: 10.1007/BF03349859. [DOI] [PubMed] [Google Scholar]
- 21.Marugo M., Torre G., Bernasconi D., Fazzuoli L., Berta S., Giordano G. Thyroid and steroid receptors. Journal of Endocrinological Investigation. 1989;12(8):565–570. doi: 10.1007/bf03350762. [DOI] [PubMed] [Google Scholar]
- 22.Miki H., Oshimo K., Inoue H., Morimoto T., Monden Y. Sex hormone receptors in human thyroid tissues. Cancer. 1990;66(8):1759–1762. doi: 10.1002/1097-0142(19901015)66:8<1759::AID-CNCR2820660820>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Memon G. R., Arain S. A., Jamal Q., Ansari T. Immunohistochemical study of progesterone receptors in thyroid gland. Journal of the Pakistan Medical Association. 2005;55(8):321–324. [PubMed] [Google Scholar]
- 24.Santin A. P., Souza A. F. D., Brum L. S., Furlanetto T. W. Validation of reference genes for normalizing gene expression in real-time quantitative reverse transcription pcr in human thyroid cells in primary culture treated with progesterone and estradiol. Molecular Biotechnology. 2013;54(2):278–282. doi: 10.1007/s12033-012-9565-0. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K., Otsuki T., Sonoo H., et al. Semi-quantitative comparison of the differentiation markers and sodium iodide symporter messenger ribonucleic acids in papillary thyroid carcinomas using RT-PCR. European Journal of Endocrinology. 2000;142(4):340–346. doi: 10.1530/eje.0.1420340. [DOI] [PubMed] [Google Scholar]
- 26.Roger P. P., Dumont J. E. Factors controlling proliferation and differentiation of canine thyroid cells cultured in reduced serum conditions: effects of thyrotropin, cyclic AMP and growth factors. Molecular and Cellular Endocrinology. 1984;36(1-2):79–93. doi: 10.1016/0303-7207(84)90087-x. [DOI] [PubMed] [Google Scholar]
- 27.Chambard M., Verrier B., Gabrion J., Mauchamp J. Polarization of thyroid cells in culture: evidence for the basolateral localization of the iodide ‘pump’ and of the thyroid-stimulating hormone receptor-adenyl cyclase complex. Journal of Cell Biology. 1983;96(4):1172–1177. doi: 10.1083/jcb.96.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsch L., Wollman S. H. Suspension culture of separated follicles consisting of differentiated thyroid epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(1):472–476. doi: 10.1073/pnas.77.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura T., van Keymeulen A., Golstein J., Fusco A., Dumont J. E., Roger P. P. Regulation of thyroid cell proliferation by tsh and other factors: a critical evaluation of in vitro models. Endocrine Reviews. 2001;22(5):631–656. doi: 10.1210/er.22.5.631. [DOI] [PubMed] [Google Scholar]
- 30.Deleu S., Pirson I., Coulonval K., et al. IGF-1 or insulin, and the TSH cyclic AMP cascade separately control dog and human thyroid cell growth and DNA synthesis, and complement each other in inducing mitogenesis. Molecular and Cellular Endocrinology. 1999;149(1-2):41–51. doi: 10.1016/s0303-7207(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 31.Vélez M. L., Costamagna E., Kimura E. T., et al. Bacterial lipopolysaccharide stimulates the thyrotropin-dependent thyroglobulin gene expression at the transcriptional level by involving the transcription factors thyroid transcription factor-1 and paired box domain transcription factor 8. Endocrinology. 2006;147(7):3260–3275. doi: 10.1210/en.2005-0789. [DOI] [PubMed] [Google Scholar]
- 32.Furuya F., Shimura H., Suzuki H., et al. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 2004;145(6):2865–2875. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]
- 33.Akeno N., Smith E. P., Stefan M., et al. IFN-alpha mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. The Journal of Immunology. 2011;186(8):4693–4706. doi: 10.4049/jimmunol.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratt M. A. C., Eggo M. C., Bachrach L. K., Carayon P., Burrow G. N. Regulation of thyroperoxidase, thyroglobulin and iodide levels in sheep thyroid cells by TSH, tumor promoters and epidermal growth factor. Biochimie. 1989;71(2):227–235. doi: 10.1016/0300-9084(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 35.Eszlinger M., Holzapfel H.-P., Voigt C., Arkenau C., Paschke R. RGS 2 expression is regulated by TSH and inhibits TSH receptor signaling. European Journal of Endocrinology. 2004;151(3):383–390. doi: 10.1530/eje.0.1510383. [DOI] [PubMed] [Google Scholar]
- 36.Saito T., Endo T., Kawaguchi A., et al. Increased expression of the Na+/I− symporter in cultured human thyroid cells exposed to thyrotropin and in Graves' thyroid tissue. Journal of Clinical Endocrinology and Metabolism. 1997;82(10):3331–3336. doi: 10.1210/jc.82.10.3331. [DOI] [PubMed] [Google Scholar]
- 37.Ajjan R. A., Watson P. F., Findlay C., et al. The sodium iodide symporter gene and its regulation by cytokines found in autoimmmunity. Journal of Endocrinology. 1998;158(3):351–358. doi: 10.1677/joe.0.1580351. [DOI] [PubMed] [Google Scholar]
- 38.Sathi P., Kalyan S., Hitchcock C. L., Pudek M., Prior J. C. Progesterone therapy increases free thyroxine levels—data from a randomized placebo-controlled 12-week hot flush trial. Clinical Endocrinology. 2013;79(2):282–287. doi: 10.1111/cen.12128. [DOI] [PubMed] [Google Scholar]
- 39.Edwards D. P., Altmann M., DeMarzo A., Zhang Y., Weigel N. L., Beck C. A. Progesterone receptor and the mechanism of action of progesterone antagonists. Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1–6):449–458. doi: 10.1016/0960-0760(95)00091-d. [DOI] [PubMed] [Google Scholar]
- 40.Vannucchi G., de Leo S., Perrino M., et al. Impact of estrogen and progesterone receptors expression on the clinical and molecular features of papillary thyroid cancer. European Journal of Endocrinology. 2015 doi: 10.1530/eje-15-0054. [DOI] [PubMed] [Google Scholar]
- 41.Stanczyk F. Z., Hapgood J. P., Winer S., Mishell D. R., Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocrine Reviews. 2013;34(2):171–208. doi: 10.1210/er.2012-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ericson L. E., Nilsson M. Deactivation of TSH receptor signaling in filter-cultured pig thyroid epithelial cells. American Journal of Physiology: Endocrinology and Metabolism. 2000;278(4):E611–E619. doi: 10.1152/ajpendo.2000.278.4.E611. [DOI] [PubMed] [Google Scholar]


