Abstract
Human liver fluke, Opisthorchis viverrini, a food-borne trematode is a significant public health problem in Southeast Asia, particularly in Thailand. Despite a long history of control programs in Thailand and a nationwide reduction, O. viverrini infection prevalence remains high in the Northeastern Provinces. Therefore, a new strategy for controlling the liver fluke infection using the EcoHealth/One Health approach was introduced into the Lawa Lake area in Khon Kaen province where the liver fluke is endemic. A program has been carried using anthelminthic treatment, novel intensive health education methods both in the communities and in schools, ecosystem monitoring and active community participation. As a result, the infection rate in the more than 10 villages surrounding the Lake has declined to approximate one third of the average of 50% as estimated by a baseline survey. Strikingly, the Cyprinoid fish species in the Lake, which are the intermediate host, now showed less than 1% prevalence compared to a maximum of 70% at baseline. This liver fluke control program, named “Lawa model,” is now recognized nationally and internationally, and being expanding to other parts of Thailand and neighboring Mekong countries. Challenges to O. viverrini disease control, and lessons learned in developing an integrative control program using a community-based, ecosystem approach, and scaling-up regionally based on Lawa as a model are described.

Keywords: Opisthorchis viverrini, integrated control, ecosystems health, Lawa model, Thailand
1. Introduction
The human liver fluke, Opisthorchis viverrini remains an important public health problem in many parts of Southeast Asia, particularly in the Lower Mekong Basin, including Thailand, the Lao People’s Democratic Republic (Lao PDR), Cambodia and central/south Vietnam (Sripa et al., 2010; Sithithaworn et al., 2012). O. viverrini is acquired by eating traditional fish preparations in which the infective stage often remains viable.
Infection by this food-borne trematode can lead to hepatobiliary disease including hepatomegaly, cholangitis, cholecystitis, fibrosis of the periportal system, and gallstone (Sripa et al., 2010). Moreover, O. viverrini has been classified as a Group 1 carcinogens (metazoan parasites that are carcinogenic to humans) by the International Agency for Research on Cancer, World Health Organization (WHO) (Bouvard et al., 2009; IARC, 2012) since it is the major aetiological agent of bile duct cancer, cholangiocarcinoma (CCA) (Sripa et al., 2007; Sripa et al., 2012). As a result of this liver fluke, Khon Kaen Province of north-eastern Thailand has the highest incidence of this type of primary liver cancer in the world (Vatanasapt et al., 1990; Shin et al., 2010).
The first report of high prevalence of O. viverrini infection, which reaches 100% in certain villages of north-eastern Thailand, was done by Sadun (1955). Almost 30 years later, a similar near 100% prevalence and high intensity infection of O. viverrini was reported in the Chonnabot District of Khon Kaen Province, confirming Khon Kaen to be one of the hotspots of liver fluke infection (Upatham et al., 1982, 1984). The first nationwide survey (1980-1981) revealed an overall prevalence of O. viverrini infection of 14%; with the highest prevalence in the Northeast (34.6%) followed by the central part of the country (6.3%), then the North (5.6%) and the South (0.01%) (Jongsuksuntigul and Imsomboon, 2003).
Following a period of intensive and continuous control programmes and public health service activities, along with a significant demographic shift from traditional agriculture to modern urban-industrial life, the average national prevalence of this infection declined to 9.4% in the year 2000 (Jongsuksuntigul and Imsomboon, 2003) reaching 8.7% by the end of the decade (Sithithaworn et al., 2012). Based on national survey data in 2009, O. viverrini infection prevalence in Thailand still high, particularly in the North and north-eastern regions, with the total number of Opisthorchis-infected cases estimated to exceed 6 million, an assessment highly likely to be a gross underestimate (Andrews et al., 2008).
In spite of the recent apparent overall reduction in prevalence data indicate a reversal in some areas as well as numerous pockets, particularly in the north-eastern provinces, where prevalence remains as high as 50% (Sithithaworn et al., 2012). Clearly, the positive outcomes from previous control programmes were not sustainable. It seems that they failed to infuse a long-term affect in many communities, or other unidentified social or ecological influences on O. viverrini transmission dynamics are at work. As this became realized closer examination including more detailed studies began to suggest factors that might responsible for the intransigence of the O. viverrini infection rates.
What has emerged is the need for new ways of thinking about this disease and its control. For example, like that suggested for other neglected tropical diseases (NTDs) elsewhere, a multi-sectoral, multidisciplinary control efforts is required (e.g., Ehrenberg and Ault, 2005), and helminths generally (Grazzinelli et al., 2012). This is found to reflect zoonotic NTDs in particular, which are especially problematic in light of their complex life cycles, multiple determinants and limited funding (IOM, 2011). The recent designation including of persistent helminth diseases as simultaneously “diseases of environmental, agriculture and poverty” included pointing to the need new, systems oriented approaches such as “one health” or “ecohealth” (WHO, 2013).
What all these approaches have in common is integrated intervention methods developed based in integrative, transdisciplinary research using an “ecosystem approach to health (Charon, 2012; Forget and Lebel, 2001; Parkes et al 2005; Wilcox et al., 2012). The resulting integrated control methods necessitate the combining of technologies and expertise from different fields beyond biomedicine or conventional public health, including that from social, environmental and ecological sciences.
Unfortunately, in our experience, neither explicit guidelines nor examples exist of procedurally how to develop such an integrated control strategy. Among other challenges this requires overcoming barriers to collaboration between academic scientists from multiple disciplines, not to mention with and local communities and other stakeholders; i.e., expanding a project beyond interdisciplinary to transdisciplinary. In this paper we trace the history of the efforts to control O. viverrini infection in Thailand, identify the likely barriers to sustainable control, describe an evolving integrative programme for O. viverrini research and control and the lessons learned.
2. Opisthorchiasis control in Thailand: a success story?
2.1 History
Opisthorchiasis control in Thailand spans more than 50 years and can be roughly divided into three phases.
First phase
During the period from 1950 to 1958 the Department of Health, Ministry of Public Health, Thailand established Helminthiasis Control Units in five provinces with the support of USAID. These provinces were Nakorn Ratchasima, Udon Thani, Skol Nakorn, Ubon Ratchathani (in north-eastern Thailand) and Songkhla (in the South) (Jongsuksuntigul and Imsomboon, 2003). Following a decline in USAID support in 1958, the helminthiasis control activities were integrated into the national Rural Health Development Project (1967 to 1974). An Opisthorchiasis Control Unit was established in Sakol Nakorn Province in the Northeast to implement these control activities. The Unit focused mainly on health education at the community level, utilizing a variety of strategies such as demonstrations of how to prepare cooked fish, distribution of low-cost cooking pots, etc. Following the termination of the Rural Health Development Project in 1974, health education on liver fluke infection and “safe cooking” was the only approach used for opisthorchiasis control.
Second phase
After the introduction of the anthelmintic praziquantel in Thailand in the early 1980s, a joint field trial organized by Mahidol University and the Helminthiasis Section of the Department of Communicable Disease Control, Ministry of Public Health, was conducted in north-eastern Thailand (Jongsuksuntigul and Imsomboon, 2003). During the 1984-1987 period, the Department of Communicable Disease Control organized opisthorchiasis treatment units in four provinces in the Northeast: Khon Kaen, Roi-Et, Sakol Nakorn and Ubon Ratchathani. A region-wide line of attack against the disease was started in 1987 with the inclusion of an opisthorchiasis control programme included as part of the Sixth Five-year National Public Health Development Plan (1987-1991), under the auspices of the Department of Communicable Disease Control. In addition, the German Society for International Cooperation (GTZ) provided technical and partial operational support for the Project for Promotion of Community Health through Parasite Control in seven north-eastern provinces (from 1989 to 1992). During this period, a total of 7,077,875 individuals were examined and 2,306,104 positive cases treated. The Seventh National Health Development Plan (1992-1996) extended the Opisthorchiasis Control Programme to an additional 17 provinces in northern Thailand and six provinces in the centre of the country. With the Eighth National Health Plan (1997-2001), the opisthorchiasis control programme was integrated into the Nationwide Disease Control Aims, with the objective of reducing the prevalence of O. viverrini infection to below 10 %. Many campaigns against the consumption of “raw fish” were organized by different governmental and non-government organizations. During this period (1981-2001) the level of O. viverrini infection prevalence fell from 34.6% to 15.7% in the Northeast to an overall national prevalence of 9.4% (Jongsuksuntigul and Imsomboon, 2003).
Third phase
After 2000, the National Opisthorchiasis Control Programme activities subsided after the Asian Economic crisis, due to a reduction in government funding and a diversion of resources to other health priorities. The most recent data from the National Helminthic Survey showed an overall O. viverrini infection prevalence of 8.7%, with the highest level in the Northeast (16.6%), followed by the North (10.0%), the Centre (1.3%) and the South (0.1%) (Sithithaworn et al., 2012). Indeed, the prevalence in 2009 in the northeastern provinces was similar or even higher than that of the previous survey 10 years before in 2001 (15.7%).
Even more alarming, although the average prevalence nationally shows an overall declining trend, reliably estimated Opisthorchis infection rates as high as 85% were still being reported for some villages in the Northeast in the 2009 National Survey (Sithithaworn et al., 2012) - similar to that reported 60 years ago! Moreover, the age-standardized incidence rate (ASR) of CCA is still high with average of 44.3 per 100,000 for male in Khon Kaen province, Northeast Thailand (Kamsa-ard et al., 2011).
2.2 What happened to the control programme in Northeast Thailand?
First, an historical distinction between the Northeast and the rest of Thailand should be noted. The above historically reported nationwide reductions of Opisthorchis infection prevalence occurred during the period of Thailand’s most dramatic economic transition, from an almost entirely rural-agricultural to a largely urban-industrial economy. Thus, these control programs were implemented when much of the national population was shifting away from deeply traditional lifestyle, including a softening of adherence to food preferences associated with rural life. Yet this transition has taken longer to reach the Northeastern provinces, where the majority of the population is still rural-agricultural and adherence to traditions remains strongly rooted in many places. Nonetheless, infrastructure development has been extensive during this period, particularly aimed at water resources development. Substantial alteration of river and wetland ecosystems, thus habitat of O. viverrini’s intermediate hosts, has occurred as result, apparently dramatically increasing host population density in some places.
Thus, the control programs employed in Thailand over the past 30 years - involving conventional “top-down” medical and public health interventions including parasitic treatment, sanitation improvement and health education - neglected to consider the distinct socio-culture and environmental context of O. viverrini transmission in the Northeast.
Within this contextual challenge, particularly but not totally exclusive to the rural Northeast, we have identified six categories of obstacles to be overcome in crafting a new control strategy. These include: (1) lack of continuity in government policy and control activities including geographic coverage; (2) culturally determined behaviors associated with fishing, food preparation, and eating uncooked fish deeply embedded as part of the indigenous rice-fish culture of this region; (3) wetland ecosystem-dependent livelihood and an equally ancient co-evolutionary relationship of the O. viverrini and humans in this region; (4) a parasite highly efficient in its transmission though this coupled human-natural system; (5) a complex set of pathological consequences associated with infection and treatment community awareness; and, (6) historically unprecedented environmental change, including those caused by regional and local water resources management and flood control projects.
2.3 Research gaps and needs
A seventh, overarching obstacle is the lack of understanding of O. viverrini transmission dynamics, and a clear strategy for filling research gaps aimed specifically at interrupting transmission by combining top-down with bottom-up methods aligned with socio-culture and environmental circumstances unique not only to different regions, but often to neighboring districts or even villages.
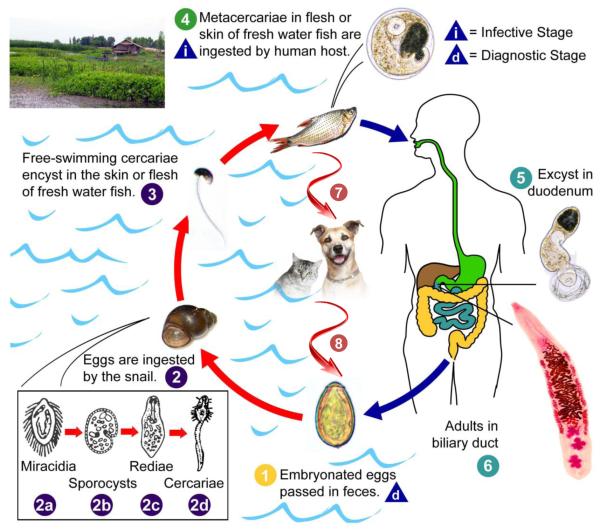
The general life cycle of liver flukes is well-known with that of O. viverrini shown in Fig. 1. However, much less is known about the underlying dynamics O. viverrini and liver flukes in general than is the case for other helminths of medical significance. Yet analysis of transmission dynamics using quantitative modelling is essential to the design effective and effective integrated control programs (Basanez et al., 2012). This includes determining the relative importance of life cycle/transmission components and of intrinsic and extrinsic influences in determining infection prevalence. Variation in parasite survival and infectivity in different transmission stages and snail density and influences of environmental factors such as water quality parameters, for example, have not been adequately studied. Moreover, the contribution of other fish-eating vertebrate host populations found in the human-wetland ecosystem complexes has not been adequately determined.
Figure 1.
Life cycle of Opisthorchis viverrini. Briefly, the parasite eggs are shed in the biliary tree, enter the intestine are passed with the faeces. They need to reach freshwater bodies and be ingested by Bithynia snails, the first intermediate host, to develop into sporocysts which eventually develop into free-swimming cercariae, the stage infective for the second intermediate fish host. Humans as well as cats, dogs and other fish eating mammals may serve as reservoir hosts acquire the infection by ingesting raw or inadequately cooked fish. After infection, the metacercariae humans acquire from eating the fish mature into adult worms, the sexual stage, which establish themselves in the bile ducts of the liver over a period of about a month. In humans, the adult fluke may have a life span of over 20 years (Attwood and Chou, 1978), which along with a high rate of continued re-infection explains the persistency of the infection (Upatham et al., 1988) in endemic areas. (Figure modified from http://www.cdc.gov/dpdx/opisthorchiasis/ and Sripa et al., 2010)
Finally, human behaviour and the social ecological aspects of food processing, preparation and eating fish preparations are similarly only superficially understood, and require systematic investigation (WHO, 2012; Sithithaworn and Haswell-Elkins, 2003).
The above points to the need for a more strategic research effort, linking clinical, laboratory, ecological and community-based research and intervention should be studied. A helminth research agenda was recently produced by a WHO expert panel (WHO, 2012), including mention of “ecohealth approaches”, though without specific attention to the need for better understanding of integrative research and stakeholder participation processes, including community participation. Nonetheless, sufficient understanding of food-borne trematode transmission exists indicating basic control measures, as outlined some time ago by another WHO expert panel (WHO, 1995). Public education with an emphasis on school children (also see WHO, 2011) with the aim of informing entire communities about the parasite’s life cycle and mode of transmission, especially food-related behavior.
Informed by this, the need for better understanding of transmission dynamics and how to develop an integrated control programme, ultimately leading to a more bottom-up, community participatory approach, the Lawa Project was developed.
3. The Lawa Project: An ecosystems health approach
3.1 Background
Opisthorchiasis primarily afflicts people of rural rice farming communities, whose staple food besides rice traditionally has been the Cyprinoid fish that inhabit the paddies, irrigation ditches and adjacent reservoirs. This fish, along with Bithyniid snail hosts, invade these manmade habitats from the streams, lakes and wetlands, nearly all of which have been displaced by a human-cultivated landscape. Understanding this ecology, particularly how people in these communities procure, prepare and consume fish, is clearly important.
The first milestone in our understanding of the recalcitrance of high opisthorchiasis rates was the finding of a geographic gradient of endemicity in which tends to decline with distance from major water bodies and wetlands, such as the Lawa area (Sripa, unpublished). Northeast Thailand supports an extensive network including three major river basins and their tributaries winding across the Isaan Plateau to the Mekong River. These produce numerous water bodies and wetlands, mostly substantially altered by water management and construction projects. As a seasonally dry region prone to droughts these are the source of irrigated agriculture and rice paddy production, as well as habitat for an array of fish and wildlife including the primary and secondary intermediate hosts of O. viverrini. These include the species of the Bythniid spp complex and those of Cyprinoid fish, the main ingredient of the region’s traditional staple food, along with “sticky rice.” Rural livelihoods of many villagers still are intimately tied to these ecosystems that, besides these staple foods, provide numerous other plants and animals traditionally used as food, fibre and fodder for livestock, along with critical environmental services including flood control, waste recycling and water purification.
Our understanding of how people and these ecosystems are entwined making interventions to interrupt the O.viverrini transmission cycle especially challenging, has only been possible by beginning to study on of these human ecosystem complexes.
Rural, natural resource-based communities present a particular set of challenges, and opportunities, for linking ecosystem and human health issues, described generally as “ecosystem approaches to health” (Charon, 2012; Forget and Lebel, 2001). Parkes and Panelli (2001) provide a comprehensive description of community-based participatory action approach, including principles and practical steps. Subsequently, Parkes et al. (2005) described similar transdisciplinary programmes based on a social ecological framework for addressing zoonotic diseases.
A historically parallel development has been integrated vector management (IVM) promoted by WHO (2004) as framework to sustainably control vector-borne diseases. Summed-up as control in “sustainable and ecologically-sensitive ways relies on packages of evidence-based interventions, tailor-made for local settings, and provides a way to coordinate and refocus resources for vector control, while at the same time reducing reliance on insecticides. This approach aims to control, manage and monitor vector-borne diseases at all relevant points in the life-cycle and transmission-cycle (Townson et al., 2005). While strictly speaking IVM may be inapplicable to non-vector-borne helminthes, we reason the same general principles should apply to zoonotic diseases and especially O. viverrini whose Bithynia snail hosts have proven uncontrollable by molluscicides.
3.2 Building an integrated control programme for O. viverrini
The situation with intransigence of O. viverrini infection prevalence and record high CCA incidence in Khon Kaen, motivated us to gradually add a community-based component to our clinical and laboratory-based research, beginning approximately 10 years ago, initially consisting of just collecting fish samples for parasite surveillance, followed several years later (2007) by a disease-screening initiative in five villages in the Lawa District of Khon Kaen Province. The screening, involved stool examination with respect to Opisthorchis eggs, ultrasound for the detection of potential CCA lesions, and basic health information component.
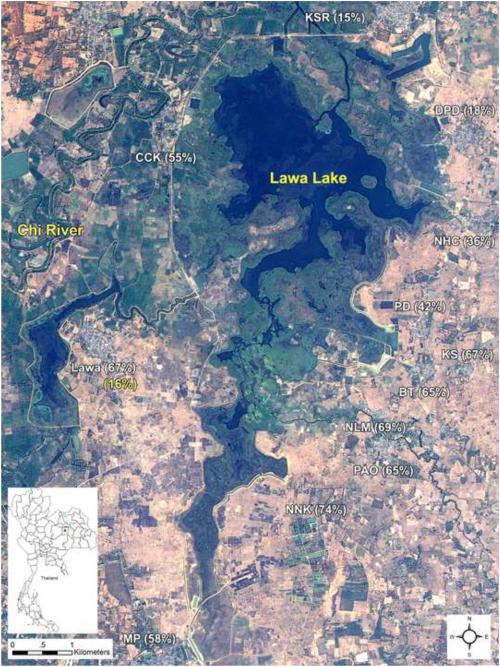
In 2008 we initiated a pilot demonstration project in the Lawa Lake region of Khon Kaen as a first step toward developing an integrated, community-based control model. After collecting prevalence data from the Lawa Lake region villages (see map in Fig. 2) we targeted one, the village of Lawa, with praziqantel treatment and an intensive community education curriculum. Other villages received praziqantel and the basic education component. Lawa village was selected as the initial program development test site because of its very high infection prevalence and proximity to a community hospital for assessing feasibility for incorporating the community education strategies into routine work practices. The prevalence of liver fluke infection in Lawa village declined from 67% to 16% over three years with minimal re-infection. After discovering the school baseline infection prevalence was 9.2% (of 1,136 children), an intensive “Liver Fluke-free School” multi-component program was also developed and introduced in 2009 in 9 schools around the Lawa Lake. The program consisted of stool examination, praziqantel treatment, health education, exhibitions and curriculum focusing on liver fluke infection and liver cancer (for student grades 4-6). Prevalence has now been reduced to an undetectable level (no positive stool samples among the entire population). In 2012 all nine schools were certified as liver fluke free schools.
Figure 2.
Satellite map of the Lawa Lake area and baseline prevalence of Opisthorchis viverrini infection in villages around. The prevalence of the liver fluke infection in the Lawa village after 3 years of implementation is shown in yellow. (THEOS Satellite image, GISTDA, Thailand, photo taken in January 2013)
Encouraged by the largely positive reception from our target communities, including community members voluntarily assisting with these activities, we decided to scale-up surveillance and to incorporate a more comprehensive education component than previously offered, targeting schools as well as the general public. By 2012, this program had developed into a district-wide initiative covering 11 villages consisting of a range of activities (Table 1). Also, by this time we had clearly recognized the parallels of O. viverrini control needs and challenges with that of other vector borne and zoonotic diseases for which ecosystem approaches and integrated management were being advocated. The same year Lawa project joined several other helminth control projects funded in Asia by the Canada International Development Research Centre (IDRC) to develop its ecosystem approach.
Table 1.
Lawa project components.
| Component | Description | Intended Impact/Benefit |
|---|---|---|
|
Treatment and
Intensive Education |
Tailored treatment program with intensive education in different age groups including adults, employing a range of media and information technologies including video, placed in key locations (village markets, shops, community centers, etc.). |
People in community both infected and non-infected have knowledge and are aware of the cause of liver fluke infection. |
|
School-based IEC
and Science Curriculum |
Integrating of liver fluke disease ecology in science curriculum using primary school students, and junior high school. |
Students who live in the community with their parents have knowledge and aware of the causes of liver fluke infection. They also have knowledge are a competent to remind parents not to eat raw fish. |
|
Technical Training
for Liver Fluke Control |
Community hospital staff and community volunteer training programs. |
Community hospital staff and public health volunteers are capable to oversee liver fluke control program suitable to their villages, are competent to make recommendations and monitor peoples’ eating behavior in the village. |
|
Manual of Liver
Fluke Control Strategy |
Production and distribution of a manual for establishing community- based liver fluke control programmes aimed at district and village-level administrators, school administrators, community health volunteers and other key groups. |
As a media for distribution, as well as the manual for community hospital staffs and public health volunteers to study for their references. |
|
Disease Surveillance
and Environmental Monitoring |
Continual measurement of liver fluke prevalence in treated human populations, and host wildlife populations (snails and fish). Investigate and monitor environmental conditions that promote infection. |
Detect environmental trends in relation to snail and fish host ecology potentially associated with liver fluke transmission dynamics. |
In summary, the Lawa project has been carried out for over five years using chemotherapy, intensive health education methods both in the communities and in schools, ecosystem monitoring and active community participation as mentioned above. During this time liver fluke infection rate in the more than 10 villages surrounding the Lake has declined to less than one third of the estimated average of 50% of the baseline survey. People living around the lake gained more knowledge and awareness of liver fluke infection and its link with liver cancer. Strikingly, the Cyprinoid fish species, which are the second intermediate host, now show less than 1% prevalence compared to a maximum of 70% during the baseline survey (B. Sripa, unpublished data). The project is now entering a new phase, more deliberately attempting to incorporate elements aligned with an integrated, ecologically informed disease management principle as well as a more formal impact evaluation. A major focus is on collaborative research initiatives to fill gaps necessary to understand how refine the project’s intervention strategy to sustain the initial encouraging results.
3.3 Some key lessons learned
The project’s primary challenge has been learning how to build an effective disease prevention and control program from the “bottom-up”, including creating an understanding of liver fluke infection disease risk with the community. Our preliminary results suggest treatment and education campaigns are only effective to the extent we engage with the community in a reciprocal learning process. This includes understanding the local culture and broader health and well-being concerns held by a community and ensuring these are aligned with intervention programme approaches and activities.
In particular, we found that information-gathering and, when feasible, intervention actions are best carried out by community public health volunteers and teachers as intermediaries (from the same communities). Awareness-building and education efforts designed and implemented independently by outside “experts” may be neither as culturally appropriate nor effective.
Our preliminary research on the details of sharing and eating fish dishes has confirmed how deeply they can be embedded in the local rice-fish culture and the social connections within a village. Thus, interventions aimed at behavioral change insensitive to the traditional values and practices potentially could negatively impact the social, cultural and ecological integrity essential to community health and well-being overall and in the long term.
To address these and other issues that undoubtedly arise we find it necessary to continually review and revise protocols for community engagement at all levels; treatment and education, school science curriculum, etc., drawing on a community participatory approach in which the community, and local knowledge is at least as equally valued to outside experts.
3.4 Scaling up the Lawa model?
The Lawa project and its integrated liver fluke control model, now known as “The Lawa Model” has gained national recognition among policy makers, government agencies and NGOs for its potential to control opisthorchiasis regionally. Lawa village and its community hospital is being used as a demonstration and training site for liver fluke control for health personnel from other districts and provinces. Thus, at least in part the Lawa model is now being expanding to other districts of Northeast Thailand and neighbouring Mekong countries.
Yet, scaling up presents a number of challenges. Besides overcoming the obstacles listed earlier addressing the lessons immediately two that appear to us to most significant are as follows: the time and resource limitations and the absence of a detailed understanding of transmission dynamics. As is apparent from the Lawa project experience an extraordinary amount of time and a wide range of expertise is required to ensure integration with a community at adequate level to sustain disease control. Guidelines for managing this kind of a project including written protocols for community engagement should take into account. Along with such guidance, cost-effective and sustainable intervention designs are needed.
4. Discussion and Conclusion
When the Lawa project began there were no detailed models or guidelines for community-based surveillance and intervention targeting opisthorchiasis or, more generally, foodborne trematodiases. Until recently, large-scale control initiatives like those of the major helminithiases have been non-existent (Lustigman et al., 2012). Yet valuable guidance has been provided by several WHO expert groups over two decades who identify as research priorities such as on diagnosis and treatment, and education focused especially on school children. The results of the Lawa project thus far demonstrate the validity of these interventions.
During the past decade “integrated” approaches to disease control have been frequently mentioned. Integrated methods have referred to combining various programmatic dimensions including multiple diseases in the case of NDTs in general. Simultaneously, holistic approaches complimentary to conventional biomedical and epidemiological approaches to disease control and prevention have been suggested. Often identified as ecosystem approaches to health, this is characterized as transdisciplinary, participatory, and including consideration of gender and social equity, as well as incorporating ecological principles. These are typically associated with so-called one health and ecohealth approaches.
There are few reports and case studies of the successful application of community-based approaches including participatory principles. Those that exist and are applicable to tropical, zoonotic diseases in rural settings including Southeast Asia (Espina et al., 2004; Preston et al., 2010; Hafton et al., 2013) emphasize the difficulties and the lack of success stories. At the same time the critical importance of participatory principles is uncontested (including by these authors).
In the case of O. viverrini infection and associated hepatobiliary disease such approaches should help us isolate the “variables” primarily responsible for transmission and develop an integrated intervention programme that can be sustained, ultimately without intensive “top-down” involvement. Lessons can be learned from the Lawa project and other similar attempts, both in terms of advancing the methodologies for integrative research and integrated disease control, as well as that of our understanding of the parasite and disease. Yet, significant challenges remain in the development of integrated control model that demonstrably sustains reduced infection rates.
Research highlights.
Integrated liver fluke control using ecohealth approach in Lawa Lake, Thailand
Key interventions include treatment, IEC, fish, snail and ecological monitoring
5-years implementation revealed human infection rate declined to less than one third
Cyprinoid fish showed < 1% prevalence compared to a maximum of 70% at baseline
The “Lawa model” has now applied to other endemic areas of Thailand
Acknowledgements
This cumulative works were supported by Khon Kaen University; the Ecohealth Emerging Infectious Diseases Initiative (EcoEID) [funded by Canada’s International Development Research Centre; Foreign Affairs, Trade and Development Canada (through the Global Health Research Initiative); and the Australian Agency for International Development]; the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS); the Grand Challenges Canada (grant no. 0221-01); the Thailand Research Fund under the TRF Senior Scholar; the National Research Council of Thailand; and the National Health Security Office, Thailand. Initial works of the Lawa project was partially supported by the National Institute of Allergy and Infectious Diseases (NIAID), award number P50AI098639. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH or the funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood HD, Chou ST. The longevity of Clonorchis sinensis. Pathology. 1978;10:153–156. doi: 10.3109/00313027809063494. [DOI] [PubMed] [Google Scholar]
- Basáñez MG, McCarthy JS, French MD, Yang GJ, Walker M, Gambhir M, Churcher TS. A research agenda for helminth diseases of humans: modelling for control and elimination. PLoS Negl. Trop. Dis. 2012;6:e1548. doi: 10.1371/journal.pntd.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. WHO International Agency for Research on Cancer Monograph Working Group. [DOI] [PubMed] [Google Scholar]
- Charron DF. In Ecohealth Research in Practice. Springer; New York: 2012. Ecohealth: Origins and approach; pp. 1–30. [Google Scholar]
- Choffnes ER, Relman DA. National Academies Press; 2011. The causes and impacts of neglected tropical and zoonotic diseases: opportunities for integrated intervention strategies: Workshop summary. [PubMed] [Google Scholar]
- Ehrenberg JP, Ault SK. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health. 2005;5:119. doi: 10.1186/1471-2458-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino FE, Koops V, Manderson L. World Health Organization: 2004. Community participation and tropical disease control in resource-poor settings. Special Topics in Social, Economic and Behavioural (SEB) Research, No. 2. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) [Google Scholar]
- Forget G, Lebel J. An ecosystem approach to human health. Int. J. Occup. Environ. Health. 2001;7(2 Suppl):S3–38. [PubMed] [Google Scholar]
- Gazzinelli A, Correa-Oliveira R, Yang GJ, Boatin BA, Kloos H. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl. Trop. Dis. 2012;6:e1603. doi: 10.1371/journal.pntd.0001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halton K, Sama M, Barnett A, Leonardo L, Graves N. A systematic review of community-based interventions for emerging zoonotic infectious diseases in Southeast Asia. JBI Database Syst. Rev. Impl. Rep. 2013;11:1–235. doi: 10.11124/jbisrir-2012-252. [DOI] [PubMed] [Google Scholar]
- IARC IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- IOM . Institute of Medicine. The National Academic Press; Washington D.C.: 2011. The causes and impacts of neglected tropical and zoonotic diseases. [PubMed] [Google Scholar]
- Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- Kamsa-ard S, Wiangnon S, Suwanrungruang K, Promthet S, Khuntikeo N, Kamsa-ard S, Mahaweerawat S. Trends in liver cancer incidence between 1985 and 2009, Khon Kaen, Thailand: cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2011;12:2209–13. [PubMed] [Google Scholar]
- Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl. Trop. Dis. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes MW, Bienen L, Breilh J, Hsu LN, McDonald M, Patz JA, Rosenthal JP, Sahani M, Sleigh A, Waltner-Toews D, Yassi A. All hands on deck: transdisciplinary approaches to emerging infectious disease. EcoHealth. 2005;2:258–272. [Google Scholar]
- Parkes M, Panelli R. Integrating catchment ecosystems and community health: the value of participatory action research. Ecosystem Health. 2001;7:85–106. [Google Scholar]
- Preston R, Waugh H, Larkins S, Taylor J. Community participation in rural primary health care: intervention or approach? Aust. J. Prim. Health. 2010;16:4–16. doi: 10.1071/py09053. [DOI] [PubMed] [Google Scholar]
- Sadun EH. Studies on Opisthorchis viverrini in Thailand. Am. J. Hyg. 1955;62:81–115. doi: 10.1093/oxfordjournals.aje.a119772. [DOI] [PubMed] [Google Scholar]
- Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, Wiangnon S, Sripa B, Hong ST. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma-focus on East and South-Eastern Asia. Asian Pac. J. Cancer Prev. 2010;11:1159–1166. [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Sripa B. Concerted action is needed to tackle liver fluke infections in Asia. PLoS Negl. Trop. Dis. 2008;2:e232. doi: 10.1371/journal.pntd.0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr. Opin. Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson H, Nathan MB, Zaim M, Guillet P, Manga L, Bos R, Kindhauser M. Exploiting the potential of vector control for disease prevention. Bull. World Health Organ. 2005;83:942–947. [PMC free article] [PubMed] [Google Scholar]
- Upatham ES, Viyanant V, Brockelman WY, Kurathong S, Lee P, Kraengraeng R. Rate of re-infection by Opisthorchis viverrini in an endemic northeast Thai community after chemotherapy. Int. J. Parasitol. 1988;18:643–649. doi: 10.1016/0020-7519(88)90099-9. [DOI] [PubMed] [Google Scholar]
- Upatham ES, Viyanant V, Kurathong S, Brockelman WY, Menaruchi A, Saowakontha S, Intarakhao C, Vajrasthira S, Warren KS. Morbidity in relation to intensity of infection in Opisthorchiasis viverrini: study of a community in Khon Kaen, Thailand. Am. J. Trop. Med. Hyg. 1982;31:1156–1163. doi: 10.4269/ajtmh.1982.31.1156. [DOI] [PubMed] [Google Scholar]
- Upatham ES, Viyanant V, Kurathong S, Rojborwonwitaya J, Brockelman WY, Ardsungnoen S, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull. World Health Organ. 1984;62:451–461. [PMC free article] [PubMed] [Google Scholar]
- Vatanasapt V, Uttaravichien T, Mairiang EO, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- WHO . WHO Technical Report Series. Vol. 849. World Health Organization: 1995. Control of foodborne trematode infections: report of a WHO study group. [PubMed] [Google Scholar]
- WHO . World Health Organization: 2004. Global strategic framework for integrated vector management. [Google Scholar]
- WHO . 2nd World Health Organization: 2011. Helminth control in school age children: a guide for managers of control programmes. [Google Scholar]
- WHO . World Health Organization Technical Report Series. 972. xv. World Health Organization: 2012. Research priorities for helminth infections. [PubMed] [Google Scholar]
- WHO . World Health Organization Technical Report Series. 976. i. World Health Organization: 2013. Research priorities for the environment, agriculture and infectious diseases of poverty. [PubMed] [Google Scholar]
- Wilcox BA, Aguirre A, Horwitz P. 2nd Oxford University Press: 2012. Ecohealth: Connecting Ecology, Health and Sustainability. Conservation Medicine; pp. 17–32. [Google Scholar]