Abstract
Background/purpose
Current treatment options for HCC≥10 cm (huge HCC) are limited. Otherwise, the margin status is known as a prognostic factor. Our aim was to determine the safety, effectiveness, and risk factors for overall survival and disease-free survival for these patients.
Methods
A total of 211 consecutive patients from 2000/08 to 2010/12 were enrolled. Characteristics of patients, tumors, and treatment were compared between the huge group (HCCs; ≥10 cm, n = 23; 11%) and those with smaller group (HCC; <10 cm n = 188; 89%). Disease-free survival (DFS), overall survival (OS), and risk factors were analyzed.
Results
Median follow up was 37 months. Patients with huge HCC were more likely to be symptomatic, positive for preoperative portal vein thrombosis, longer surgical time, more blood loss and transfusions, and significantly shorter median OS and DFS. Both groups had similar postoperative mortality and morbidity rates. In the huge HCC, multivariate analysis identified two significant determinants of DFS (preoperative portal vein thrombosis on imaging and tumor-free margin less than 1 mm) and two significant determinants of OS (age over 80 and preoperative portal vein thrombosis). Even with positive margins, it still had no impact on OS. For DFS, 1 mm free margins appeared to be adequate.
Conclusion
Tumor-free margin is an independent risk factor for recurrence but has no impact on OS. Surgical margin >1 mm is adequate in patients with tumors ≥10 cm. Postoperative close follow up, especially of distant metastasis, and appropriate treatment of recurrence by a multidisciplinary approach may improve prognosis.
Keywords: Huge HCC, Smaller HCC, Surgical margin, Disease-free survival, Overall survival
Highlights
-
•
No such comparative study before.
-
•
Surgery for huge HCCs is safe as small ones.
-
•
Even 1 mm surgical margin is good enough.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common liver tumor [1]. In Taiwan, it is commonly due to chronic hepatitis B infection. Surgery including liver resection and transplantation is the first choice of treatment [2]. However, huge HCC tumors (≥10 cm in diameter) are often treated non-surgically. The reported 5-year survival rate after trans-arterial chemo-embolization (TACE) for huge HCC tumors is only 7–10% and the prognosis is worse in patients with poor liver reserve. Nevertheless, surgically treated groups seem to have had a better prognosis [3–5] and their five-year survival rate reached 35% [5,6]. However, increased risk of morbidity and mortality after surgery was also reported [7]. In this study, we aimed to determine the safety and effectiveness of resection as well as survival rates and risk factors for overall survival (OS) and disease-free survival (DFS) after resection of HCC ≥10 cm in diameter. Additionally, we explored the impact of free margins on the prognosis.
2. Materials and methods
2.1. Patients
Data from 287 patients who underwent liver resection for pathologically confirmed HCC at our Hospital from September 2000 to December 2010 were retrospectively analyzed. The data from 7 patients who received hepatectomy previously at other hospitals, 40 patients who received prior non-surgical treatment including radiotherapy (RT), TACE, and percutaneous alcohol injection (PEI), 9 patients who received only palliative resection, and 20 who had incomplete pathological or laboratory data were excluded from the analysis. The 211 cases finally included in the study were divided into two groups: those with huge HCCs, defined as tumors over 10 cm in diameter (n = 23) and those with smaller HCCs, defined as tumors under 10 cm in diameter (n = 188). These patients were followed up till 2012/07. Surgical mortality was defined as death occurring within 90 days of surgery. Morbidity was defined as any complication occurring during the perioperative period. The severity of complications was scored using the Dindo Classification system [7]. This study was approved by our Institutional Research Board (IRB).
2.2. Surgical technique and follow up
Before operation, all patients were evaluated by surgeons. The resectable lesions were identified as adequate liver reserve after necessary liver resection for tumor clearance. All patients received traditional laparotomy with intra-operative sonography to determine the extent of disease and to map the line of parenchymal transaction. Excision was performed using a Cavitational Ultrasonic Surgical Aspirator (CUSA) and, in select patients, the Pringle maneuver (portal triad clamping for 15 min and release for 5 min). Postoperatively, the wound was routinely checked for bile leakage with a dry white gauze pad and repaired by sutures if leakage was found. Closed drainage tubes including Jackson–Pratt (J-P) or J-VAC drains were routinely placed in the subphrenic space, subhepatic space, or resection surface.
After being discharged, patients were followed up at the outpatient clinic with a physical examination, ultrasonography, and assessment of alpha-fetoprotein (AFP) level every 3 months for first 2 years and thereafter every 6 months, and received annual computed tomography. Recurrence of HCC was identified on images showing new or growing lesions with or without rising AFP. HCC lesions with atypical appearance were confirmed by biopsy. The treatment choice for recurrent HCC including local treatment, transcatheter arterial embolization (TAE), chemoembolization (TACE), or repeat hepatectomy based on the patient's condition and the severity of disease.
2.3. Statistical analysis
Patient demographics, and tumor, operative, and treatment characteristics were evaluated. The variables analyzed included age, gender, comorbidities, hepatitis serology, AFP, other common laboratory data, Child-Pugh class, and Model for End-stage Liver Disease (MELD) score. The analyzed characteristics included tumor laterality, patient with abdominal symptoms or not, pre-operative portal vein thrombosis, preoperative tumor rupture or not, operative variables, mortality, morbidity, severity of complications, and post-operative tumor characteristics. Patients were staged according to the sixth edition of the American Joint Commission on Cancer (AJCC) Manual. Major hepatic resection was defined as right or left lobectomy or resection of more than 3 Couinaud's segments. The recurrence patterns and treatment were also analyzed. As the intrahepatic recurrence site, the “marginal” means that the any recurrence located just on the resection margin. The “Adjacent Section” means the recurrence located at the nearby section. For example, the primary tumor located at Segment 4a, the location of recurrence tumor classified as “ Adjacent Section ” if the lesion locates at Segment 3, 4b, or 8. If the recurrence located at other section it was classified as “distal sections”. If several recurrent tumors located in different segments, it defined as “Multiple”.
Comparisons between groups were performed using the Chi-square test for categorical variables, the Student t test for continuous variables with normal distribution and Mann Whitney U test for continuous variables without normal distribution. Time to recurrence (disease-free survival) and time to death were determined by the Kaplan–Meier method and differences were compared by the log-rank test. Several variables including age, sex, hepatitis virus infection, symptoms, pre-operative portal vein thrombosis, liver reserve, operative outcomes, tumor stage, and pathology risk factors including vascular invasion, venous thrombi and satellite nodules were inserted into a backward stepwise Cox proportional hazards model to calculate the hazard ration and identify significant factors. All risk factors which were significantly associated with disease-free survival or overall survival in univariate analysis were entered into a backward stepwise Cox proportional hazards model again as multivariate analysis. p-values of <0.05 was considered to indicate statistical significance.
3. Results
The clinical features and tumor characteristics in the 211 patients with hepatic resection from the huge group (tumor ≥10 cm, n = 23) and the smaller group (tumor <10 cm, n = 188) are summarized in Tables 1 and 2. Totally 40 patients got lost followed up including 38 patients in smaller group (20.2%) and 2 patients in huge group (8.6%). There was no between-group difference in age, sex, and co-morbidities. Hepatitis B infection occurred more frequently in patients with huge tumors than those with smaller tumors. Moreover, patients with huge tumors had a significantly higher rate of symptoms, significantly higher preoperative platelet count, and poorer liver function (i.e., lower albumin and more Child B cases, but similar MELD scores). Although the laterality, frequency of solitary or multiple tumors and rate of rupture are similar in the two groups, the rate of preoperative portal vein thrombosis seen in imaging studies is significantly higher in the huge group. Patients with larger tumor size also had more advanced stage disease and higher rate of vascular invasion, vascular thrombi, satellite nodules, positive surgical margin, and narrow surgical margin (<1 mm).
Table 1.
The clinical features of all 211 patients with hepatic resection.
| Variables | HCC < 10 cm (n = 188) | HCC ≧ 10 cm (n = 23) | p-value |
|---|---|---|---|
| Clinical characteristics | |||
| Age | 59.77 ± 10.25 | 61.52 ± 12.18 | 0.449# |
| Gender | 0.14 | ||
| Male | 135 | 20 | |
| Female | 53 | 3 | |
| Symptoms (+) | 33 (17.6%) | 17 (73.9%) | <0.001 |
| Virus type | 0.022 | ||
| Non-B Non-C | 18 (9.6%) | 5(21.7%) | |
| HBV | 70(37.2%) | 13(56.6%) | |
| HCV | 83(44.2%) | 3(13%) | |
| HBV+HCV | 17(9%) | 2(8.7%) | |
| PLT (103/uL) | 155.75±61.42 | 258.48±134.08 | <0.001 |
| PT (INR) | 1.12±0.71 | 1.07±0.75 | 0.939 |
| Albumin(g/dL) | 3.96±0.55 | 3.51±0.60 | <0.001 |
| ALB ≧3.5 | 157 | 12 | 0.001 |
| ALB <3.5 | 31 | 11 | |
| Child-Pugh class | 0.044 | ||
| A | 183 | 20 | |
| B | 5(2.8%) | 3 (13%) | |
| MELD score | 8.75±3.99 | 7.83±1.51 | 0.408 |
| Median AFP level (ng/ml) | 18.225 | 30.15 | 0.256 |
| (2.87–65433) | (1.25– 677930) | ||
| AFP ≧1000 | 28 | 8 | 0.034 |
| AFP <1000 | 160 | 15 | |
#: Student t test, Other continuous variables: Mann Whitney U test.
Abbreviations: HBV: hepatitis B virus, HCV: hepatitis C virus, PLT: platelet, Cre: Creatinine, PT: Prothrombin time, INR: International normalized ratio, AFP: Alpha-fetoprotein.
Table 2.
The tumor related factors of all 211 patients with hepatic resection.
| Variables | HCC < 10 cm (n = 188) | HCC ≧ 10 cm (n = 23) | p-value |
|---|---|---|---|
| Tumor-related factors | |||
| Number of tumors | 0.741 | ||
| Solitary | 166 | 20 | |
| Multiple | 22 (11.7%) | 3 (13%) | |
| Preoperative portal vein thrombosis diagnosed by imaging | 5 (2.7%) | 4(17.4%) | 0.009 |
| Preoperative tumor rupture | 9 (4.8%) | 1 (4.3%) | 1 |
| Tumor size (mm) | 37.58±19.17 | 131.22±26.09 | <0.001 |
| (12-97mm) | (100-180mm) | ||
| T1/2/3/4 | 84/90/6/8 | 1/13/6/3 | <0.001 |
| Positive vascular invasion | 71 (37.8%) | 11 (47.8%) | 0.002 |
| Positive venous thrombi | 26 (13.8%) | 8 (34.8%) | 0.016 |
| Positive satellite nodules | 21 (11.2%) | 8 (34.8%) | 0.006 |
| Free margin | 8.08±9.56 | 3.83±5.51 | 0.011 |
| Free Margin <1mm | 29 (15.4%) | 9 (39.1%) | 0.01 |
Surgical outcomes are compared between the groups in Tables 3 and 4. Patients with large tumors had higher operative stress (i.e., significantly longer surgical time, more major hepatic resections, more estimated blood loss, more intra-operative blood transfusions, and longer intensive care unit (ICU) and postoperative hospital stays) yet similar mortality, morbidity, morbidity severity, and rates of complications and their grades.
Table 3.
Surgical outcomes of patients with HCC resection.
| Variables | HCC < 10 cm (n = 188) | HCC ≧ 10 cm (n = 23) | p-value |
|---|---|---|---|
| Operative outcome | |||
| Surgical time (minutes) | 220.59±66.28 | 315.65±166.89 | <0.001 |
| ≧220 minutes | 87 | 21 | <0.001 |
| <220 minutes | 101 | 2 | |
| Major hepatic resection | 22(11.7%) | 14 (60.9%) | <0.001 |
| Surgical blood loss (ml) | 700.98±833.57 | 1639.13±1198.0 | <0.001 |
| ≧1000 ml | 42 | 14 | <0.001 |
| <1000 ml | 146 | 9 | |
| PRBC transfusion (%) | 84(44.7%) | 19 (82.6%) | 0.001 |
| Morbidity and mortality | |||
| Mortality | 3 (1.6%) | 1 (4.3%) | 0.372 |
| Morbidity | 56(29.8%) | 9 (39.1%) | 0.35 |
| Major morbidity (Dindo grade ≧3) | 29(51.8%) | 4(44.4%) | 0.41 |
Abbreviation: PRBC: Packed red blood cell.
Table 4.
Surgical complications and their severity in patients with HCC resection.
| Variables | HCC < 10 cm (n = 188) | HCC ≧ 10 cm (n = 23) |
|---|---|---|
| Complications and severity | ||
| Total complications | 56 (29.8%) | 9 (39.1%) |
| Grade I | ||
| CNS-seizures | 1 | 0 |
| Bile leakage | 1 | 2 |
| Ascites | 16 | 2 |
| Pleural effusion | 2 | 0 |
| Transient hepatic insufficiency | 1 | 0 |
| Wound hematoma | 1 | 0 |
| Others | 1 | 0 |
| Grade II | ||
| Newly onset atrial fibrillation | 2 | 0 |
| Postoperative bleeding | 1 | 0 |
| Pneumonia | 0 | 1 |
| Acute renal impairment | 1 | 0 |
| Grade III | ||
| Bile leakage | 1 | 0 |
| Pleural effusion needing drainage | 14 | 0 |
| Grader IV | ||
| Pneumonia, pulmonary edema, ARDS | 9 | 2 |
| Acute renal impairment | 1 | 0 |
| Consciousness changes of unknown cause | 0 | 1 |
| Adrenal insufficiency | 1 | 0 |
| Grade V | ||
| Liver failure | 2 | 1 |
| Acute renal impairment | 1 | 0 |
Abbreviation: ARDS: Acute respiratory distress syndrome.
The median follow-up period was 37 months. Patients with huge tumors had significantly shorter overall median survival (30.23 vs. 119.53 months, p < 0.001; Fig. 1), significantly lower 1-, 3-, and 5-year survival rates (67.6%, 41.5%, and 35.6% vs. 90.4%, 75.8%, and 62.7%, respectively), significantly shorter median DFS (7.4 vs. 31.2 months; p = 0.002; Fig. 2), and significantly lower 1-, 3-, 5-year disease-free survival rates (29.2%, 14.6%, and 9.7% vs. 74.3%, 44.3%, and 25.8%, respectively).
Fig. 1.
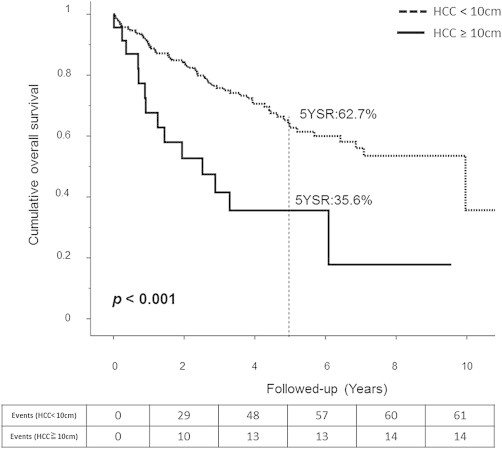
Overall survival for Huge HCC is lower but still have a fair prognosis.
Fig. 2.
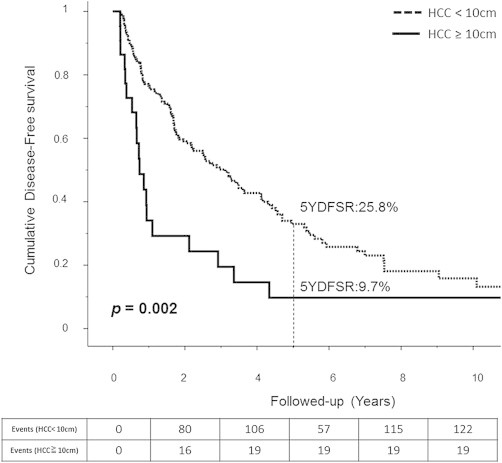
Disease free survival for both groups.
We also analyzed the prognostic factors for patients from the huge group, as Table 5. Univariate analysis identified four negative prognostic factors for disease-free survival including preoperative portal vein thrombosis diagnosed by imaging, advanced tumor stage (T3 and T4), positive satellite nodules, and disease-free margin less than 1 mm and two risk factors for overall survival (age over 80 and preoperative portal vein thrombosis). Multivariate analysis identified two independent risk factors of DFS (preoperative portal vein thrombosis [HR: 7.744, 95%CI 1.880-31.897] and disease-free margin less than 1 mm [HR: 3.423, 95%CI 1.197-9.790]. To OS, both age over 80 [HR: 4.037, 95%CI 1.018-16.003] and preoperative portal vein thrombosis [HR: 6.785, 95%CI 1.670-27.576] were independent risk factors.
Table 5.
The prognostic factor analysis for the huge group patients.
| Disease-free survival factors | Univariant analysis |
Multivariant analysis |
|---|---|---|
| HR ( 95% CI) | HR ( 95% CI) | |
| Portal vein thrombosis | 7.533 (1.971–28.788) | 7.774 (1.880–31.897) |
| Free margin < 1 mm | 3.403 (1.231–9.411) | 3.423 (1.197–9.790) |
| Advanced T stage (T3+T4) | 2.940 (1.154–7.411) | N.S. |
| Satellite nodules | 3.132 (1.100–8.918) | N.S. |
| Overall survival factors | Univariant analysis |
Multivariant analysis |
|---|---|---|
| HR ( 95% CI) | HR ( 95% CI) | |
| Age over 80 | 4.037 (1.018–16.003) | 12.993 (2.535–66.599) |
| Portal vein thrombosis | 6.785 (1.670–27.576) | 8.953 (1.772–45.223) |
Abbreviation: N.S.: Non-significance.
We also analyzed the impact of different values of the free margin on overall survival and disease-free survival, and these are listed in Table 6. We identified similar results. The margin status showed no benefit on the overall survival rate, but showed benefits for the overall disease-free survival rate. Moreover, a 1 mm free margin is adequate to get benefits with regard to the recurrence rates [HR: 3.403, 95%CI 1.231-9.411]. Free margins over 2 mm showed no benefits on the recurrence rate.
Table 6.
The impact of different free margin distances on overall survival and disease-free survival (Analyzed by the Univariate Cox regression).
| Disease-free survival |
Overall survival |
|
|---|---|---|
| HR ( 95% CI) | HR ( 95% CI) | |
| Free margin <10 mm | 1.353 (0.381–4.810) | 1.805 (0.495–6.581) |
| Free margin <5 mm | 0.764 (0.252–2.310) | 0.977 (0.302–3.158) |
| Free margin <2 mm | 0.624 (0.248–1.571) | 0.768 (0.263–2.237) |
| Free margin <1 mm | 3.403 (1.231–9.411) | 1.624 (0.523–5.040) |
The first recurrence site after liver resection is compared between groups in Table 7. Overall, at the end of this study, 19 patients (82.6%) with huge HCCs and 122 patients (64.9%) with smaller HCCs got a recurrence. The rate of extra-hepatic recurrence (lung, bone, brain, or other site) or combined intra-hepatic and extra-hepatic recurrence and the frequency of multiple intrahepatic site, distal section area, and resection margin recurrence were higher in patients with huge tumors. After the first recurrence, all the patients with huge HCCs and most patients with smaller HCCs received non-surgical treatment like TACE, hepatic arterial infusion chemotherapy (HAIC), or PEI and had significantly shorter overall survival.
Table 7.
Site and pattern of recurrences after hepatic resection.
| Valuables | HCC < 10 cm | HCC ≧ 10cm | p-value |
|---|---|---|---|
| Recurrence numbers | 122 | 19 | |
| Type of recurrence | <0.001 | ||
| Intra-hepatic | 107 (87.6%) | 11 (62.5%) | |
| Extra-hepatic | 2 (1.9%) | 3 (12.5%) | |
| Intra and extra-hepatic | 13 (10.5%) | 5 (25%) | |
| Site of intra-hepatic recurrence | 0.008 | ||
| No intra-hepatic recurrence | 2 (1.6%) | 3 (15.8%) | |
| Marginal | 6 (4.9%) | 1(5.3%) | |
| Adjacent Section | 68 (55.7%) | 5 (26.3%) | |
| Distal Section | 20 (16.4%) | 3 (15.8%) | |
| Multiple | 26 (21.3%) | 7 (36.8%) | |
| 1st recurrence treatment | 0.332 | ||
| Non-curative | 100(82%) | 18(94.7%) | |
| RFA | 9 (7.4%) | 0 | |
| Surgery | 13 (10.7%) | 1(5.3%) | |
|
Overall Survival After 1st recurrence (m/o) |
44.23±10.15 | 11.60±7.16 | 0.002 |
4. Discussion
In previous studies, the 5-year survival rate was 16.7–54.0% in patients with HCC ≥10 cm who received surgical intervention [5–7,9–13] and less than 10% in patients with HCC ≥10 cm who received TACE [3,4], suggesting that surgical intervention is the better treatment for HCC ≥10 cm. In the study by Yamashita et al. [5], comparing surgical and non-surgical intervention for HCC ≥10 cm, 35% in the surgical intervention group survived 5 years, while no one in the non-surgical treatment group survived 2 years. Mok et al. [14] reported that the 5-year survival rate in the resection group was significantly better than in the non-resection group (24.5% vs. 8.2%, p < 0.001) for huge HCC. The huge group in our study showed 1-, 3-, and 5-year overall survival rates of 67.6%, 41.5%, and 35.6% and DFS of 29.2%, 14.6%, and 9.7%, respectively. Moreover, Min YW et al. [15] reported directly compared the prognosis between surgery and TACE. Surgery group showed higher 1-, 2-, and 3-year overall survival rates than TACE group (69.7%, 58.6%, and 51.7% vs 40.2%, 33.9%, and 18.5%, respectively, p < 0.001) during median follow up of 14.5 months (range: 0–103). Although we did not directly compare TACE or other non-surgical treatments to surgical intervention, all data in our literature review agreed that surgical intervention provides a better prognosis.
Another concern is perioperative morbidity and mortality. The reported mortality and complication rates for resection of HCCs ≥10 cm are 2%∼15% [5–7,9–13,15] in mortality and about 24.5%∼50% [5,6,8,9] in morbidity respectively. In our study, the mortality was 4.3% and the morbidity was 39.1%. It is compatible to previous studies. Also, the mortality and morbidity of operation in huge group showed no statically difference to smaller group. It means the surgical treatment is safe for selected huge HCC patients.
Previously reported factors indicative of poor prognosis include advanced stage [5], capsule involvement [10,13], tumor rupture [16], satellite nodules [10,13,16], portal vein thrombosis [5,10,13,17], pathological vascular invasion [7], high AFP value over 200, 400, or 1000 ng/ml [14,16–18], impaired liver function including liver cirrhosis [9,19], and pro-thrombin time elongation [4]. However, liver function and the biologic behavior of the tumor cannot be changed by liver resection with curative intent. The pre-operative general condition and liver tissue reserve in surgical patients should be analyzed very carefully before deciding on surgery.
Another factor that the surgeon can control is the surgical margin. Theoretically, wider margin of excision facilitates more adequate resection. However, this is difficult to achieve for patients with huge HCCs, because more extensive normal liver tissue has to be removed, and this may elevate the operative risk due to inadequate liver preservation. In addition, larger free margins do not appear to benefit overall survival. Liau et al. [11] reported that the margin status showed no impact on patient survival. Yamashita et al. [5] found that a free margin over 5 mm did not affect overall survival rates and tumor recurrence rates.
In our study, we analyzed the impact of different values of the free margin on overall survival and disease-free survival. The margin status showed no benefit on the overall survival rate, even the margin is not free. But it showed benefits for the overall disease-free survival rate. An 1 mm free margin is adequate to get benefits with regard to the recurrence rates. Free margins over 2 mm showed no benefits on the recurrence rate.
The reported extra-hepatic recurrence rates vary in patients with resected huge HCCs. The rate was 34% in a study of 98 patients by Lee et al. [18] and higher in cases of huge HCCs than in cases of smaller HCCs (refer to the studies by Mok et al. [14]. [31.9% vs. 12.8%] and Yamashita et al. [5] [38% vs. 10%]). The lungs, followed by brain and bone, were the most frequent metastatic sites. Similarly, in our study, the rates of extra-hepatic recurrence, combined intra- and extra-hepatic recurrence as well as intrahepatic multi-site, distal, and marginal recurrence were significantly higher in those patients with huge tumors. Moreover, in our subgroup analysis, the extra-hepatic metastases seem to be associated with poorer prognosis than those without non-extra-hepatic metastases by shorter overall survival, disease-free survival, and survival after diagnosis of the first recurrence. Although none of these results showed a significant difference from what we obtained in our research, they reveal that extra-hepatic recurrences are one of the reasons for poorer prognosis in patients with huge HCC. Therefore, the postoperative follow-up abdominal CT and bone scintigraphy for the huge HCC patients should paid more attention due to the high rate of extra-hepatic metastasis, particularly important in the presence of a ruptured tumor (T4), venous thrombi, or a left lobe tumor. Once the recurrence is noted, appropriate treatment should be applied for these patients. For extra-hepatic recurrences, aggressive management with surgery for the isolated recurrences has been reported to offer better prognosis [20].
Our study has limitations. First, it is a retrospective single institutional non-randomized study and therefore there is a possibility of selection bias. Moreover, the single-institution nature of this study may limit the generalizability to whole population. Second, the heterogeneity of group is great and some of the clinical features between groups were not compatible. Third, sample size of huge is relatively small. We may not detect a difference in OS or DFS with different margins because of lack power.
In conclusion, although it is technically difficult and involves the prospect of an extended recovery period, surgical resection is still a relatively safe and effective treatment for selected patients with huge HCCs. The tumor-free margin width is an independent risk factor for recurrence but has no impact on overall survival. Moreover, 1 mm free margins are adequate to get the benefit of decreased recurrence rates. During follow-up, more attention should be directed to extra-hepatic recurrences especially in patients with huge HCCs. Once a recurrence is noted, appropriate treatment, including aggressive surgical intervention, may improve the prognosis.
Conflicts of interest
We don't have any conflicts of interest.
Funding
No funding for our research.
Ethical approval
Our research was approved by hospital Ethic Committee and IRB.
Author contribution
Study Design: Chen Jian-Han, Lee Cheng-Hung, Yin Wen-Yao.
Acquisition of data: Chen Jian-Han, Lee Cheng-Hung, Wei Chang-Kuo, Chang Chun-Ming, Hsu Ta-Wen.
Analysis and interpretation: Chen Jian-Han, Lee Cheng-Hung.
Manuscript drafted by: Chen Jian-Han
Revision: Lee Cheng-Hung, Yin Wen-Yao.
Acknowledgments
We would like to express our gratitude to the cancer tumor center in our hospital for their support and assistance during the data collection period.
Contributor Information
Jian-Han Chen, Email: JAMIHAN1981@gmail.com.
Chang-Kuo Wei, Email: wck@tzuchi.com.tw.
Cheng-Hung Lee, Email: 76543@tzuchi.com.tw.
Chun-Ming Chang, Email: dm335280@tzuchi.com.tw.
Ta-Wen Hsu, Email: B120018@tzuchi.com.tw.
Wen-Yao Yin, Email: wenyao4748@gmail.com.
References
- 1.Salhab M., Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J. Cancer Res. Ther. 2011;7(4):463–475. doi: 10.4103/0973-1482.92023. [DOI] [PubMed] [Google Scholar]
- 2.de Lope C.R., Tremosini S., Forner A., Reig M., Bruix J. Management of HCC. J. Hepatol. 2012;56(Suppl. 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 3.Poon R.T., Ngan H., Lo C.M., Liu C.L., Fan S.T., Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J. Surg. Oncol. 2000;73(2):109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y.H., Wu J.C., Chen S.C., Chen C.H., Chiang J.H., Huo T.I. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Alimentary Pharmacol. Ther. 2006;23(1):129–135. doi: 10.1111/j.1365-2036.2006.02704.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y., Taketomi A., Shirabe K., Aishima S., Tsuijita E., Morita K. Outcomes of hepatic resection for huge hepatocellular carcinoma (>/= 10 cm in diameter) J. Surg. Oncol. 2011;104(3):292–298. doi: 10.1002/jso.21931. [DOI] [PubMed] [Google Scholar]
- 6.Tsoulfas G., Mekras A., Agorastou P., Kiskinis D. Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J. Surg. 2012;82(7–8):510–517. doi: 10.1111/j.1445-2197.2012.06079.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah S.A., Wei A.C., Cleary S.P., Yang I., McGilvray I.D., Gallinger S. Prognosis and results after resection of very large (>or=10 cm) hepatocellular carcinoma. J. Gastrointest. Surg. Off. J. Soc. Surg. Alimentary Tract. 2007;11(5):589–595. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanazaki K., Kajikawa S., Shimozawa N., Shimada K., Hiraguri M., Koide N. Hepatic resection for hepatocellular carcinoma in diameter of > or = 10 cm. Hepato-gastroenterology. 2002;49(44):518–523. [PubMed] [Google Scholar]
- 10.Chen X.P., Qiu F.Z., Wu Z.D., Zhang B.X. Chinese experience with hepatectomy for huge hepatocellular carcinoma. Br. J. Surg. 2004;91(3):322–326. doi: 10.1002/bjs.4413. [DOI] [PubMed] [Google Scholar]
- 11.Liau K.H., Ruo L., Shia J., Padela A., Gonen M., Jarnagin W.R. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104(9):1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 12.Nagano Y., Tanaka K., Togo S., Matsuo K., Kunisaki C., Sugita M. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J. Surg. 2005;29(1):66–71. doi: 10.1007/s00268-004-7509-y. [DOI] [PubMed] [Google Scholar]
- 13.Chen X.P., Qiu F.Z., Wu Z.D., Zhang B.X. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J. Gastroenterol. 2006;12(29):4652–4655. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok K.T., Wang B.W., Lo G.H., Liang H.L., Liu S.I., Chou N.H. Multimodality management of hepatocellular carcinoma larger than 10 cm. J. Am. Coll. Surg. 2003;197(5):730–738. doi: 10.1016/j.jamcollsurg.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Min Y.W., Lee J.H., Gwak G.Y., Paik Y.H., Rhee P.L., Koh K.C. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J. Gastroenterol. Hepatol. 2014;29(5):1043–1048. doi: 10.1111/jgh.12504. [DOI] [PubMed] [Google Scholar]
- 16.Yeh C.N., Lee W.C., Chen M.F. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann. Surg. Oncol. 2003;10(9):1070–1076. doi: 10.1245/aso.2003.03.072. [DOI] [PubMed] [Google Scholar]
- 17.Huang X., Wei W., Ya N., Zeng J., Zeng Y., Ma C. A Mathematical model to Predict Short-term recurrence and metastasis of primary hepatocellular carcinoma larger than 10cm in diameter. Hepato-gastroenterology. 2012;60(122) doi: 10.5754/hge. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.G., Hwang S., Jung J.P., Lee Y.J., Kim K.H., Ahn C.S. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br. J. Surg. 2007;94(3):320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 19.Taniai N., Yoshida H., Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J. Hepato-Biliary-Pancreatic Surg. 2008;15(4):410–416. doi: 10.1007/s00534-007-1317-3. [DOI] [PubMed] [Google Scholar]
- 20.Poon R.T., Fan S.T., O'Suilleabhain C.B., Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J. Am. Coll. Surg. 2002;195(3):311–318. doi: 10.1016/s1072-7515(02)01226-7. [DOI] [PubMed] [Google Scholar]


