Abstract
Context:
Osteoporosis is often a presenting sign of celiac disease (CD). Whether skeletal fragility in CD is associated with microarchitectural abnormalities is not known.
Objective:
The objective of the study was to evaluate microarchitecture and biomechanical properties of bone in CD.
Design:
This was a case-control study.
Setting:
The study was conducted at a university hospital outpatient facility.
Patients:
Patients included premenopausal women with newly diagnosed CD (n = 33) and healthy controls (n = 33).
Main Outcome Measures:
Areal bone mineral density by dual-energy x-ray absorptiometry was measured as was trabecular and cortical volumetric bone mineral density (vBMD) and microarchitecture by high-resolution peripheral computed tomography of the distal radius and tibia. Whole-bone stiffness estimated by finite element analysis. PTH, 25-hydroxyvitamin D, and bone turnover markers were also measured.
Results:
Groups had similar age, race, and body mass index. Both groups had sufficient 25-hydroxyvitamin D and normal calcium and PTH. Areal bone mineral density was lower in CD. By high-resolution peripheral computed tomography, CD had lower trabecular vBMD, fewer, more widely, and irregularly spaced trabeculae at both the radius and tibia (8%–33%). At the tibia, they also had lower total density (8%) and thinner cortices (10%). Whole-bone stiffness and failure load were lower (11%–21%) in CD at both sites. Biomechanical deficits were associated with trabecular abnormalities.
Conclusions:
Women with CD had abnormal vBMD and microarchitecture at both the radius and tibia. Trabecular bone was preferentially affected. These deficits were associated with lower estimates of skeletal strength. These findings suggest a potential structural mechanism for skeletal fragility in CD and support further research into the pathogenesis of fracture in this population.
Celiac disease (CD) is a chronic intestinal disorder affecting approximately 1% of the population of Europe and the United States (1). Patients with CD have an immune reaction to the gliadin fraction of gluten, a protein found in barley, wheat, and rye, the ingestion of which leads to villous atrophy and chronic inflammation of the small bowel mucosa (2, 3). CD can have many extraintestinal manifestations, including osteomalacia, and osteoporosis. Fractures are often a presenting sign of CD. Low bone mineral density (BMD) has been reported in many studies of patients with CD, with estimates as high as 70%, depending on the age, sex, menopausal status, and the general health of the population studied (4–8). Although many studies have not been designed to look at fracture rates, some epidemiological studies (9–17) and a recent meta-analysis (18) have demonstrated an increased risk of fractures in patients with CD. More recently a population-based study demonstrated that persistent villous atrophy was associated with an increased risk of hip fracture, despite a gluten-free diet (19).
Abnormal bone metabolism in patients with CD is multifactorial. Low BMD in celiac patients has been directly related to severity of histological disease by intestinal biopsy, providing further support for the role of malabsorption and inflammation as factors in the development of bone disease (20). Malabsorption of calcium, magnesium, and vitamin D resulting from villous atrophy can result in secondary hyperparathyroidism (21–23). Patients often have concurrent lactose intolerance, which can also contribute to inadequate calcium and vitamin D intake. Increased inflammatory cytokines, IL-1, IL-6, TNFα, and receptor activator of nuclear factor-κB ligand in CD patients may contribute to increased osteoclast activity, decreased osteoblast activity, and uncoupled bone turnover (24–26). Other factors that may contribute to fragility and fracture risk in CD patients include zinc deficiency and low IGF-1, low body mass index (BMI), malnutrition and hypogonadism, and autoantibodies (3, 27). These factors have the potential to increase fragility in various ways and may have differential effects on the cortical and trabecular compartments of bone. These disparate effects cannot be captured by dual-energy x-ray absorptiometry (DXA), the standard tool for assessment of BMD. Furthermore, DXA is affected by bone size and may be artifactually low in CD patients who were ill during adolescence and thus may not have attained peak bone mass or predicted adult height.
The availability of high-resolution peripheral quantitative computed tomography (HR-pQCT) has provided us with the ability to measure true volumetric BMD (vBMD) in patients with CD and to study the differential effects of CD on cortical and trabecular bone. This technology, with an isotropic voxel size of 82 μm permits noninvasive measurement of vBMD and trabecular and cortical microarchitecture. In addition, data sets from individual HR-pQCT scans can be computationally modeled by microstructural finite element analysis to assess bone stiffness and failure load, surrogate measures of strength. In recent years, this technology has become an important tool for investigation of microstructural and biomechanical mechanisms of fragility in many populations (28–35). In this study, we compared premenopausal women with CD with premenopausal controls using standard DXA and HR-pQCT. We hypothesized that women with CD would have lower vBMD and worse microarchitecture compared with controls. We further hypothesized that cortical bone would be preferentially affected due to the effects of secondary hyperparathyroidism.
Materials and Methods
Participants
Premenopausal women with CD (n = 33), between the ages of 20 and 50 years, were recruited at Columbia University Medical Center by physician referral. The CD diagnosis was made on the basis of serology and small bowel biopsy. Histology was not available in four of the CD subjects whose biopsies had been performed at outside institutions. Women were included if they were newly diagnosed with celiac disease and were untreated. Subjects who had been following a gluten-free diet for more than a month were excluded. Control subjects (n = 33) were healthy premenopausal women who had negative serological antibody tests for CD. Controls were recruited as part of another ongoing study that mandated they have normal bone density (Z-score ≥ −1) and no medical conditions associated with osteoporosis including endocrinopathies (eg, untreated hypothyroidism, Cushing's syndrome, or prolactin-secreting pituitary adenoma), renal insufficiency, liver disease, or medication exposures that could affect bone metabolism (eg, glucocorticoids, anticonvulsants, anticoagulants, methotrexate, and aromatase inhibitors).
All subjects provided written informed consent, and the Institutional Review Board of Columbia University Medical Center approved this study. At the study visit, past medical history and medication use were assessed with questionnaires. A physical examination was performed including height by Harpenden stadiometer and weight, and BMI was calculated. Fasting blood samples were obtained.
Areal bone mineral density (aBMD)
aBMD was measured by DXA (QDR-4500; Hologic Inc) at the lumbar spine L1-L4 (LS), total hip (TH), femoral neck (FN), ultradistal radius (UDR), and the one third radius (1/3R).
High-resolution peripheral quantitative computed tomography
HR-pQCT (XtremeCT) was performed by immobilizing the nondominant forearm and the ipsilateral tibia in a carbon fiber shell and scanning as previously described (28–30). The machine provided measures of volumetric bone density, bone size, and three-dimensional quantitative in vivo measurements of the microarchitectural structures. The European Forearm Phantom was scanned daily for quality control. All scans were acquired by the same highly experienced technician. The HR-pQCT analysis methods used in this study have described and validated in several of our previously published studies (28–30). In addition to standard cortical and trabecular measurements of vBMD, cortical thickness, and trabecular microarchitecture, HR-pQCT data were computationally modeled using microfinite element analysis to calculate whole-bone stiffness and failure load, measures of bone's resistance to force. To evaluate the cortical bone structure, a validated autosegmentation method was applied to separate the cortical and trabecular compartments and measure cortical porosity (percentage). Cortical porosity was calculated as the percentage of void space in the cortex. This method, distributed by the manufacturer (Scanco Medical), has been validated for accuracy and reproducibility (36).
Biochemical measurements
Serum 25-hydroxyvitamin D (25OHD) was measured by ultraperformance liquid chromatography combined with tandem mass spectrometry using a 1290 UPLC and a 6410 tandem mass spectrometer (Agilent). Interassay coefficient of variation (CV) is 2.9% for 25OHD2 and 5.4% for 25OHD3. Serum 1,25-dihydroxyvitamin D was measured by RIA (Diasorin; CV 12%). Intact PTH was measured by immunoradiometric assay (Scantibodies Laboratories; CV 6.8%). Serum C-telopeptide (CTX) was measured by an ELISA (Immunodiagnostics Systems; CV < 10%). Osteocalcin was measured by ELISA (Immunodiagnostic Systems; CV 2.7%). Type 1 procollagen amino-terminal-propeptide (P1NP) was measured by a RIA (Orion Diagnostica; CV 7.0%). Bone-specific alkaline phosphatase (BSAP) was measured by an ELISA (Quidel; CV 8%). Serum was archived at −80ºC and analyzed in one batch after all visits were completed.
Statistical methods
All analyses were performed using SAS version 9.3 (SAS Institute). Group means were compared by independent Student's t test for continuous measures or a Fisher's exact test for categorical indices. The percentage difference in measures of celiac patients relative to controls was calculated as a difference between group means with the SE of the difference (see Figures 2 and 4). Two-sided values of P < .05 were considered to indicate statistical significance. No penalty for multiple comparisons was applied.
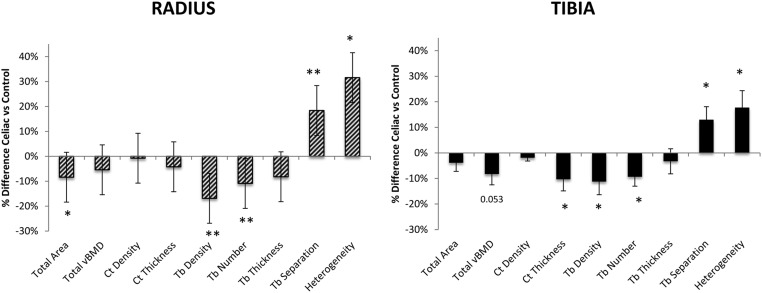
Figure 2.
Comparison of the percentage difference ± SEM in vBMD and microarchitecture by HR-pQCT in celiac and control subjects at the radius (above) and tibia (below). *, P < .05, **, P < .01. Ct Density (cortical density); Ct Thickness (cortical thickness); Heterogeneity (trabecular network heterogeneity); Tb. Density (trabecular density); Tb Number (trabecular number); Tb Separation (trabecular separation); Tb Thickness (trabecular thickness); Total vBMD (total density).
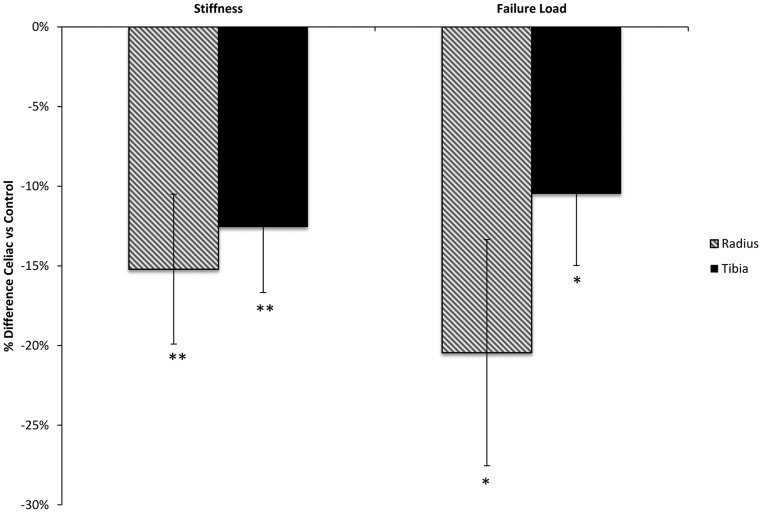
Figure 4.
Comparison of percentage difference ± SEM in whole-bone stiffness and failure load in celiac and control subjects at the radius (striped bars) and tibia (black bars), presented as percentage difference. *, P < .05; **, P < .01.
Results
Sixty-six premenopausal women were enrolled (mean age 33 ± 8 y), 33 with CD and 33 controls. Group characteristics are detailed in Table 1. Women with CD and controls were of similar age and BMI. Use of calcium supplements was greater among celiac subjects, accounting for a daily calcium intake twice that of controls (1278 ± 664 vs 595 ± 120 mg/d; P < .01). Similarly, use of vitamin D supplements was twice as great among patients with CD compared with controls. Vitamin D intake varied widely, with five celiac subjects using high-dose vitamin D supplementation (>1600 IU daily). Average daily dose of vitamin D was 1400 ± 1977 IU among CD patients and 475 ± 96 IU among controls (P = .37). Duration of supplementation among CD patients was also quite variable, between 2 weeks and 5 years (average 17 ± 23 mo). There were no fractures in the control group. Among the women with celiac disease, 5 of 33 had a history of fracture. Fracture sites included the tibia, wrist, and clavicle. Three subjects had metatarsal stress fractures.
Table 1.
Characteristics of the Study Population (Mean ± SD)
| Celiac (n = 33) | Control (n = 33) | P Value | |
|---|---|---|---|
| Age, y | 33 ± 8 | 33 ± 9 | .88 |
| Race, % white | 100% | 100% | n/a |
| Height, cm | 164 ± 7 | 165 ± 7 | .03 |
| Weight, kg | 65 ± 14 | 68 ± 15 | .56 |
| BMI, kg/m2 | 24 ± 5 | 25 ± 5 | .58 |
| Use of calcium supplements, % | 36% | 21% | .29 |
| Calcium supplements, total daily dose, mg | 1278 ± 664 | 595 ± 120 | .01 |
| Use of vitamin D supplements, % | 67% | 33% | .02 |
| Vitamin D supplements, total daily dose, IUa | 1399 ± 1977 | 475 ± 96 | .37 |
Abbreviation: n/a, not available.
Includes five patients on high-dose vitamin D supplementation.
Serum 25-hydroxyvitamin D was in the sufficient range in both groups but substantially higher in celiac subjects than in controls (51%; P < .01), likely reflecting the increased use of supplements. Serum 1,25-dihydroxyvitamin D was also higher among subjects than controls (37%; P < .01). PTH, in contrast, was well within the normal range and did not significantly differ. Bone turnover, measured both by bone resorption (CTX) and bone formation markers (osteocalcin, BSAP, and P1NP) was similar between groups (Table 2).
Table 2.
Biochemistries in Celiac and Control Subjects (Mean ± SD)
| Celiac | Control | P Value | |
|---|---|---|---|
| Calcium (8.6–10.2 mg/dL) | 9.0 ± 0.1 | 9.3 ± 0.1 | .02 |
| 25OHD (30–80 ng/mL) | 42.7 ± 16.1 | 32.9 ± 13.9 | <.01 |
| 1,25(OH)2D (25–66 pg/mL) | 74.9 ± 35.1 | 48.8 ± 10.6 | <.001 |
| PTH (14–66 pg/mL) | 29.5 ± 12.5 | 27.4 ± 9.8 | .92 |
| BSAP (11.6–42.7 U/L) | 24.3 ± 10.4 | 21.6 ± 7.0 | .30 |
| OC (8.4–33.9 ng/mL) | 17.0 ± 6.8 | 19.4 ± 8.2 | .26 |
| P1NP (16–96 μg/L) | 48.9 ± 15.0 | 55.0 ± 22.9 | .26 |
| CTX (0.11–0.74 ng/mL) | 0.38 ± 0.18 | 0.40 ± 0.25 | .80 |
Abbreviations: OC, osteocalcin; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
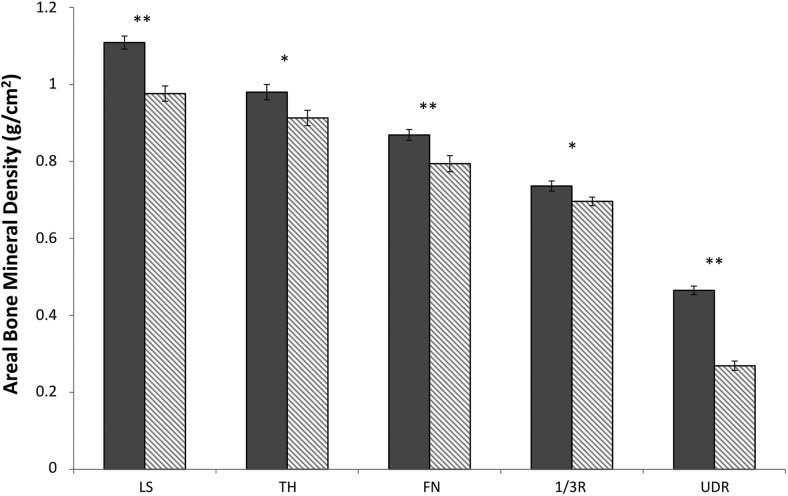
aBMD measurements by DXA were significantly lower in celiac subjects compared with controls at all sites (Figure 1). Z-scores were normal at all sites but on average were 0.6–1.3 SD lower in celiac subjects (LS: celiac, −0.5 ± 1.3 vs control, 0.8 ± 1.0, P < .001; TH: celiac, −0.1 ± 0.9 vs control, 0.5 ± 0.8, P < .01; FN: celiac, −0.3 ± 1.1 vs control, 0.3 ± 0.8, P < .01; 1/3R: celiac, 0.2 ± 1.0 vs control, 0.8 ± 1.0, P < .03; UDR: celiac, −0.4 ± 1.2 vs control, 0.4 ± 0.9, P < .01).
Figure 1.
Comparison of areal BMD by DXA in premenopausal women with celiac disease (gray bars) and matched controls (striped bars). *, P < .05; **, P < .01.
Bone size, vBMD, and cortical and trabecular microarchitecture were assessed by HR-pQCT (Figure 2). At the radius, the total cross-sectional area was smaller in celiac subjects (−8.4%; P < .02). Both cortical (−8.9%; P = .07) and trabecular (−8.3%; P = .07) compartments tended to be smaller in celiac subjects. Trabecular indices were worse in celiac subjects, who had lower trabecular density (−16.9%; P < .009) and number (−10.9%; P < .007) and greater trabecular separation (18.4%; P < .007) and network heterogeneity (32.6%; P < .02). Trabecular thickness tended to be lower in celiac subjects (−8.2%; P = .07). In contrast, total and cortical density and cortical thickness did not significantly differ.
At the tibia, total cross-sectional and trabecular areas were similar between groups, whereas the cortical area was lower in CD patients (−12.7%; P < .002). Total density tended to be lower in celiac subjects (−8.3%; P = .053). Cortical density was similar, but cortical thickness was lower in celiac subjects (−10.4%; P < .03). As at the radius, trabecular abnormalities were observed in celiac subjects, with lower trabecular density (−11.3%; P < .04) and number (−9.4; P < .02) and greater separation and network heterogeneity (13.0%; P < .02, and 17.8%; P < .02, respectively). Trabecular thickness did not differ. Cortical porosity was significantly higher in celiac subjects compared with the controls at the radius (celiac: 1.6% ± 1.0% vs control: 0.9% ± 0.5%; P < .001) but did not differ at the tibia (celiac: 2.9% ± 1.5% vs control 2.9% ± 1.6%; P = .83). Representative HR-pQCT scans of the radius and tibia in a CD subject and a control of similar age and BMI are shown in Figure 3.
Figure 3.
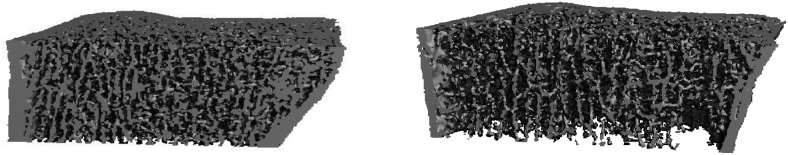
Representative HR-pQCT scans of a control (left panel) and celiac subject (right panel) of similar age and BMI, which illustrate the trabecular abnormalities observed in the celiac group.
Biomechanical properties of bone were assessed by microstructural finite element analysis (Figure 4). Celiac subjects had lower whole-bone stiffness at both the radius (−15.2%; P < .002) and tibia (−12.6%; P < .007). Similarly, failure load was lower in celiac patients at both the radius (−20.5%; P < .01) and tibia (−10.5%; P < .03). Lower stiffness and failure load at the radius were directly associated with worse microarchitectural parameters. The strongest associations were with trabecular density (r = 0.69; P < .0001 for both stiffness and failure load) and trabecular separation (r = −0.58; P < .001 for association with stiffness; r = −0.60; P < .0006 for association with failure load).
To determine whether vitamin D supplementation by the CD subjects impacted the structural results, we compared CD subjects who were (n = 22) and were not (n = 11) using vitamin D. There was no difference in aBMD at the spine, hip, or FN, although aBMD at the 1/3R was lower in the group on vitamin D supplementation (P < .05). There was no difference between patients who were and were not using vitamin D supplements in vBMD or any microarchitectural variable.
Discussion
This study demonstrated for the first time that premenopausal women with celiac disease have lower vBMD, abnormal microarchitecture, and lower strength, measured as stiffness and failure load compared with normal premenopausal women of similar age, BMI, gender, and race/ethnicity. In contrast to our hypothesis that cortical abnormalities would predominate, we found pronounced deficits in trabecular bone, which were associated with impaired biomechanical properties. In accordance with prior literature, we found that aBMD by DXA was also lower in CD patients.
Although we hypothesized that cortical bone would be preferentially affected as a result of secondary hyperparathyroidism, we did not find this to be the case. Many other groups have reported that vitamin D deficiency and secondary hyperparathyroidism are common in CD (23, 37, 38). Patients with secondary hyperparathyroidism have lower BMD than those with normal PTH and less improvement in BMD after initiation of gluten-free diet (39). In contrast, our patients with CD did not have secondary hyperparathyroidism. They had normal serum calcium and sufficient 25OHD levels, possibly because most of our CD patients were taking high doses of calcium and vitamin D. Some of these patients had been diagnosed with vitamin D deficiency in the past and started on treatment before the diagnosis of CD was established, which likely influenced our results.
It is noteworthy that despite substantially higher calcium intake, serum calcium was lower in CD patients compared with controls, suggesting that these patients did have calcium malabsorption. Measurements of 24-hour urine samples, which we do not have, would have been helpful in making that assessment. Lower serum calcium may also have reflected active skeletal remineralization in CD patients. Serum 25OHD was greater among CD patients and was likely the primary influence governing PTH in this group. Several of the CD patients were using high doses of vitamin D at the time of the study evaluation. Mean vitamin D intake was substantially higher among CD patients compared with controls, although this difference was not significant due to the large variability in vitamin D dose. The cross-sectional nature of this study precludes our assessment of past abnormalities in calciotropic hormones, which may have significantly influenced skeletal structure and strength, particularly if the abnormal calciotropic hormones were longstanding.
We found substantial abnormalities in trabecular bone and associated biomechanical compromise despite normal PTH and preserved cortical bone in CD patients. Although recent evidence has demonstrated abnormalities in both cortical and trabecular microarchitecture in patients with primary hyperparathyroidism (40), the preferential effect on trabecular bone suggests that other factors may play a role in fragility in this population. Other potential mechanisms that could preferentially affect trabecular bone because of its increased metabolic activity include uncoupled bone turnover related to increased inflammatory cytokines and low IGF-1 (3, 24). Autoimmune factors may also play a role, including direct effects of tissue transglutaminase antibodies on bone (27). The role of all of these factors warrants further investigation.
Compared with controls, premenopausal women with CD had lower trabecular density and fewer, more widely and irregularly spaced trabeculae. These abnormalities were directly associated with lower stiffness and failure load. In contrast, cortical abnormalities were more variable and did not appear to play as significant a role in biomechanical compromise in this group. Cortical porosity was higher at the radius in CD patients but did not differ at the tibia. Cortical area was smaller and thickness was lower compared with controls at the tibia, but we found no difference in cortical density at either site. A study using an older, lower resolution peripheral computed tomography scanner (unable to assess bone microarchitecture) found that women with CD had lower total and cortical vBMD and smaller cortical cross-sectional area as well as lower trabecular vBMD compared with controls (37). Bone strength estimated by cross-sectional moment of inertia and bending strength index was lower. Most subjects in that study had vitamin D deficiency and secondary hyperparathyroidism, which may have accounted for the observed abnormalities in cortical parameters (37). After treatment with a gluten-free diet, calcium and 25OHD increased and PTH decreased; vBMD and estimates of strength increased as well. Men in this study did not have the same abnormalities or response to treatment, suggesting that there may be differences in the extent of skeletal disease in CD according to sex and providing support for studying the groups separately.
We found that aBMD by DXA was lower in women with CD compared with controls. This finding was universal at all skeletal sites. Our results are consistent with other studies finding lower aBMD in newly diagnosed celiac patients compared with healthy controls (4–8, 21). Patients in many of these studies had vitamin D and calcium deficiency and secondary hyperparathyroidism, whereas we detected lower aBMD in the absence of these derangements. It is conceivable that the abnormalities we observed in BMD reflect past derangements in PTH, calcium, and vitamin D. In addition, CD patients did have lower cross-sectional area at the radius by HR-pQCT, and smaller bone size may have contributed to lower aBMD values by DXA.
This study had both unique strengths and limitations. To our knowledge this is the first study to evaluate the relationship between vBMD, microarchitecture, and strength in patients with CD. Our sample was restricted to premenopausal women, which allowed us to investigate the relationship between CD and bone without the influence of differences in sex or menopausal status. Furthermore, groups were matched by race and of similar age and BMI. Limitations of the study include the small size and cross-sectional design. Although our subjects had normal calcium, vitamin D, and PTH at the time of evaluation, it is not possible to ascertain whether prior derangements in calciotropic hormones resulted in the observed densitometric and microstructural abnormalities. It is conceivable that CD patients had abnormalities in skeletal mineralization, not detected by our techniques. Increased osteoid may have contributed to lower areal and volumetric BMD. Threshold-based measurements, such as estimated bone stiffness, would likely not account for increased osteoid. Another drawback is that our controls had been recruited for another study that required normal BMD for inclusion. We might not have detected as robust differences if the selection of controls had been population based and there were no BMD criterion for entry. However, this cutoff also ensured that we selected healthy controls and not women with an undiagnosed cause of secondary osteoporosis. The controls were also slightly but significantly taller than CD women, which may have contributed to larger bone size, and may have masked potential differences in cortical thickness. As mentioned earlier, there was some selection bias in our CD group because these were women primarily referred from the celiac practice at Columbia University Medical Center. Those who were willing to participate may have had more concerns about their bone health than the general population, as evidenced by their high intakes of calcium and vitamin D.
In conclusion, we found premenopausal women with CD had lower bone strength than controls matched for race and of similar age and BMI. Biomechanical deficits were directly related to abnormalities in trabecular bone vBMD and microarchitecture. These deficits existed despite sufficient calcium and 25OHD and normal PTH at the time of evaluation. These findings suggest a potential structural mechanism for skeletal fragility in CD and lay the foundation for further research into the pathogenesis of fracture in this population.
Acknowledgments
This work was supported by National Institutes of Health grant 2K24 AR052665 and the Thomas L. Kempner and Katheryn C. Patterson Foundation.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- aBMD
- areal BMD
- BMD
- bone mineral density
- BMI
- body mass index
- BSAP
- bone-specific alkaline phosphatase
- CD
- celiac disease
- CTX
- C-telopeptide
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- FN
- femoral neck
- HR-pQCT
- high-resolution peripheral quantitative computed tomography
- LS
- lumbar spine L1-L4
- 25OHD
- 25-hydroxyvitamin D
- P1NP
- type 1 procollagen amino-terminal-propeptide
- 1/3R
- one third radius
- TH
- total hip
- UDR
- ultradistal radius
- vBMD
- volumetric BMD.
References
- 1. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. [DOI] [PubMed] [Google Scholar]
- 2. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419–2426. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle AM, Apalset E, Mielnik P, Bollerslev J, Lundin KE, Tell GS. Celiac disease and risk of fracture in adults—a review. Osteoporos Int. 2014;25:1667–1676. [DOI] [PubMed] [Google Scholar]
- 4. Meyer D, Stavropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol. 2001;96:112–119. [DOI] [PubMed] [Google Scholar]
- 5. Casella S, Zanini B, Lanzarotto F, Villanacci V, Ricci C, Lanzini A. Celiac disease in elderly adults: clinical, serological, and histological characteristics and the effect of a gluten-free diet. J Am Geriatr Soc. 2012;60:1064–1069. [DOI] [PubMed] [Google Scholar]
- 6. Duerksen DR, Leslie WD. Positive celiac disease serology and reduced bone mineral density in adult women. Can J Gastroenterol. 2010;24:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bianchi ML, Bardella MT. Bone in celiac disease. Osteoporos Int. 2008;19:1705–1716. [DOI] [PubMed] [Google Scholar]
- 8. Szymczak J, Bohdanowicz-Pawlak A, Waszczuk E, Jakubowska J. Low bone mineral density in adult patients with coeliac disease. Endokrynol Pol. 2012;63:270–276. [PubMed] [Google Scholar]
- 9. Heikkila K, Heliovaara M, Impivaara O, et al. Coeliac disease autoimmunity and hip fracture risk: findings from a prospective cohort study. J Bone Miner Res. 2015;30(4):630–636. [DOI] [PubMed] [Google Scholar]
- 10. Moreno ML, Vazquez H, Mazure R, et al. Stratification of bone fracture risk in patients with celiac disease. Clin Gastroenterol Hepatol. 2004;2:127–134. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez MI, Mohaidle A, Baistrocchi A, et al. Risk of fracture in celiac disease: gender, dietary compliance, or both? World J Gastroenterol. 2011;17:3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. West J, Logan RF, Card TR, Smith C, Hubbard R. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003;125:429–436. [DOI] [PubMed] [Google Scholar]
- 13. Jafri MR, Nordstrom CW, Murray JA, et al. 2008. Long-term fracture risk in patients with celiac disease: a population-based study in Olmsted County, MN. Dig Dis Sci. 53:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures—a general population-based cohort study. Aliment Pharmacol Ther. 2007;25:273–285. [DOI] [PubMed] [Google Scholar]
- 15. Vasquez H, Mazure R, Gonzalez D, et al. Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol. 2000;95:183–189. [DOI] [PubMed] [Google Scholar]
- 16. Davie MW, Gaywood I, George E, et al. Excess non-spine fractures in women over 50 years with celiac disease: a cross-sectional, questionnaire-based study. Osteoporos Int. 2005;16:1150–1155. [DOI] [PubMed] [Google Scholar]
- 17. Olmos M, Antelo M, Vazquez H, Smecuol E, Maurino E, Bai JC. Systematic review and meta-analysis of observational studies on the prevalence of fractures in coeliac disease. Dig Liver Dis. 2008;40:46–53. [DOI] [PubMed] [Google Scholar]
- 18. Heikkila K, Pearce J, Maki M, Kaukinen K. Coeliac disease and bone fractures: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(1):25–34. [DOI] [PubMed] [Google Scholar]
- 19. Lebwohl B, Michaelsson K, Green PH, Ludvigsson JF. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab. 2014;99:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia-Manzanares A, Tenias JM, Lucendo AJ. Bone mineral density directly correlates with duodenal Marsh stage in newly diagnosed adult celiac patients. Scand J Gastroenterol. 2012;47:927–936. [DOI] [PubMed] [Google Scholar]
- 21. Di Stefano M, Mengoli C, Bergonzi M, Corazza GR. Bone mass and mineral metabolism alterations in adult celiac disease: pathophysiology and clinical approach. Nutrients. 2013;5:4786–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corazza GR, Di Sario A, Cecchetti L, et al. Bone mass and metabolism in patients with celiac disease. Gastroenterology. 1995;109:122–128. [DOI] [PubMed] [Google Scholar]
- 23. Selby PL, Davies M, Adams JE, Mawer EB. Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res. 1999;14:652–657. [DOI] [PubMed] [Google Scholar]
- 24. Moreno ML, Crusius JB, Chernavsky A, et al. The IL-1 gene family and bone involvement in celiac disease. Immunogenetics. 2005;57:618–620. [DOI] [PubMed] [Google Scholar]
- 25. Taranta A, Fortunati D, Longo M, et al. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J Bone Miner Res. 2004;19:1112–1121. [DOI] [PubMed] [Google Scholar]
- 26. Bardella MT, Bianchi ML, Teti A. Chronic inflammatory intestinal diseases and bone loss. Gut. 2005;54:1508. [PMC free article] [PubMed] [Google Scholar]
- 27. Sugai E, Chernavsky A, Pedreira S, et al. Bone-specific antibodies in sera from patients with celiac disease: characterization and implications in osteoporosis. J Clin Immunol. 2002;22:353–362. [DOI] [PubMed] [Google Scholar]
- 28. Stein EM, Liu XS, Nickolas TL, et al. Microarchitectural abnormalities are more severe in postmenopausal women with vertebral compared to nonvertebral fractures. J Clin Endocrinol Metab. 2012;97:E1918–E1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab. 2011;96:2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25:2572–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen A, Liu XS, Stein EM, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94:4351–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. [DOI] [PubMed] [Google Scholar]
- 33. Melton LJ, 3rd, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22:1442–1448. [DOI] [PubMed] [Google Scholar]
- 34. Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res. 2009;24:737–743. [DOI] [PubMed] [Google Scholar]
- 35. Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int. 2013;24:1733–1740. [DOI] [PubMed] [Google Scholar]
- 36. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–890. [DOI] [PubMed] [Google Scholar]
- 37. Ferretti J, Mazure R, Tanoue P, et al. Analysis of the structure and strength of bones in celiac disease patients. Am J Gastroenterol. 2003;98:382–390. [DOI] [PubMed] [Google Scholar]
- 38. Armagan O, Uz T, Tascioglu F, Colak O, Oner C, Akgun Y. Serological screening for celiac disease in premenopausal women with idiopathic osteoporosis. Clin Rheumatol. 2005;24:239–243. [DOI] [PubMed] [Google Scholar]
- 39. Valdimarsson T, Toss G, Lofman O, Strom M. Three years' follow-up of bone density in adult coeliac disease: significance of secondary hyperparathyroidism. Scand J Gastroenterol. 2000;35:274–280. [DOI] [PubMed] [Google Scholar]
- 40. Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013;28:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]