Abstract
Objective:
To determine dose-dependent effects of T administration on voice changes in women with low T levels.
Methods:
Seventy-one women who have undergone a hysterectomy with or without oophorectomy with total T < 31 ng/dL and/or free T < 3.5 pg/mL received a standardized transdermal estradiol regimen during the 12-week run-in period and were then randomized to receive weekly im injections of placebo or 3, 6.25, 12.5, or 25 mg T enanthate for 24 weeks. Total and free T levels were measured by liquid chromatography-tandem mass spectrometry and equilibrium dialysis, respectively. Voice handicap was measured by self-report using a validated voice handicap index questionnaire at baseline and 24 weeks after intervention. Functional voice testing was performed using the Kay Elemetrics-Computer Speech Lab to determine voice frequency, volume, and harmonics.
Results:
Forty-six women with evaluable voice data at baseline and after intervention were included in the analysis. The five groups were similar at baseline. Mean on-treatment nadir total T concentrations were 13, 83, 106, 122, and 250 ng/dL in the placebo, 3-, 6.25-, 12.5-, and 25-mg groups, respectively. Analyses of acoustic voice parameters revealed significant lowering of average pitch in the 12.5- and 25-mg dose groups compared to placebo (P < .05); these changes in pitch were significantly related to increases in T concentrations. No significant dose- or concentration-dependent changes in self-reported voice handicap index scores were observed.
Conclusion:
Testosterone administration in women with low T levels over 24 weeks was associated with dose- and concentration-dependent decreases in average pitch in the higher dose groups. These changes were seen despite the lack of self-reported changes in voice.
There has been an increasing interest in the use of T therapy to improve sexual function, body composition, and bone mass in postmenopausal women. Potential safety concerns of androgen supplementation include hirsutism, acne, clitoromegaly, and changes in voice. We recently demonstrated that 24 weeks of T administration over a wide range of doses to women with low T levels who have undergone a hysterectomy improved several domains of sexual function, body composition, and muscle performance with few androgenic adverse effects (1). In particular, none of the participants reported any intervention-related changes in voice. However, there are relatively few quantitative data on the magnitude of functional voice changes as determined by sophisticated acoustic testing in androgen-deficient women exposed to exogenous T.
The vocal cord is an important target of gonadal hormones and expresses both androgen and estrogen receptors (2). Androgens are known to exert a profound effect on the development, structure, and function of the human larynx by causing hypertrophy of thyroarytenoid muscles, which results in lowering of the voice pitch (3). Increased levels of androgens during puberty in boys results in an increased mass and thickening of the vocal folds, which is responsible for the characteristic lower frequency of the male voice (4). Indeed, boys who undergo orchiectomy before achieving puberty do not achieve these changes in the vocal folds and retain the female voice (5). Women with congenital adrenal hyperplasia and polycystic ovary syndrome, both associated with higher serum androgen levels, have been reported to have lower voice frequency than healthy controls (6, 7). Preliminary studies in women treated with androgens have reported voice changes such as pitch fluctuations and hoarseness (8–10).
In female-to-male transsexuals, supraphysiological im T injections result in the desired voice change to an acceptable male voice within 6 months of therapy (11). These findings are in contrast to those observed among postmenopausal women treated with a physiological dose of transdermal T for the same period where no adverse effects on voice were reported (12). Despite a lack of subjective voice changes reported by participants, it is plausible that subclinical changes in functional voice parameters, which can only be determined by sophisticated acoustic testing, may occur. Hence, it remains unclear whether the virilizing effects of T replacement are limited to pharmacological doses and whether androgen replacement at physiological doses can be safely administered in postmenopausal women without alterations in voice. To date, randomized controlled trials evaluating the dose-dependent effects of exogenous T on objective and subjective voice parameters in women with low T levels have not been conducted. Accordingly, we investigated the dose-response relationships of T administration on both subjective and objective voice parameters in women with low serum T concentrations who have undergone a hysterectomy.
Subjects and Methods
Study design
The Testosterone Dose Response in Surgically Menopausal Women (TDSM) trial was a two-center, parallel group, placebo-controlled, double-blind randomized trial designed to determine the dose-response effects of T on a range of androgen-dependent outcomes. The eligibility criteria and design of the TDSM trial have been previously reported (1) and are described here briefly. The trial consisted of a 12-week run-in period of transdermal estradiol administration, a 24-week treatment period, and a 16-week recovery period. The study was approved by the institutional review boards of Boston University Medical Center and the Charles Drew University of Medicine and Science (Los Angeles, California). All participants provided written informed consent.
Participants
The participants were healthy women, 41–62 years of age, who had undergone hysterectomy with or without partial or total oophorectomy. The participants had serum total T concentrations < 31 ng/dL or free T concentrations < 3.5 pg/mL (less than the median for healthy young women) (13). We included women who had hysterectomy alone or partial oophorectomy if their FSH levels were ≥ 30 U/L or if they were already receiving estrogen therapy. Inclusion required a documented normal Papanicolaou test and mammogram within the last 12 months. We excluded women with major psychiatric illness, recent hospitalization, active cancers, poorly controlled diabetes mellitus (Hemoglobin A1c > 8.5%), uncontrolled hypertension, severe obesity (body mass index [BMI] > 40 kg/m2), illicit drug use, alcohol dependence, and abnormal liver function. Women with a history of breast, ovarian, endometrial, or cervical cancer; hyperandrogenic disorders; cardiac disease; or thromboembolic disorders; and those taking glucocorticoids, androgens, spironolactone, and GnRH agonists were also excluded.
Randomization and study interventions
All eligible women were administered a regimen of transdermal estradiol patch applied twice a week and designed to achieve nominal delivery of 50-μg estradiol daily (Alora; Watson Pharmaceuticals) for a 12-week run-in phase. After the run-in, the subjects were randomized in a double-blinded fashion to one of five groups to receive weekly im injections of placebo, 3, 6.25, 12.5, or 25 mg T enanthate (ENDO Pharmaceuticals) for 24 weeks.
Hormone assays
Serum total T levels were measured by liquid chromatography-tandem mass spectrometry with sensitivity of 2 ng/dL, as described elsewhere (14). The cross-reactivity of dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, androstenedione, and estradiol in the T assay was negligible at 10 times the circulating concentrations of these hormones. The interassay coefficient of variation (CV) was 15.8% at 12.0 ng/dL, 10.6% at 23.5 ng/dL, 7.9% at 48.6 ng/dL, 7.7% at 241 ng/dL, 4.4% at 532 ng/dL, and 3.3% at 1016 ng/dL, respectively. As part of the Centers for Disease Control's (CDC) Testosterone Assay Harmonization Initiative, quality control samples provided by the CDC were run every 3 months; the bias in quality control samples in the 3.47-to-34.7 nmol/L (100-to-1000 ng/dL) range was < 6.2%. Free T was measured using equilibrium dialysis with an interassay CV of 12.3% (13, 15). SHBG levels were measured using an immunofluorometric assay with a sensitivity of 0.5 nmol/L (16). The interassay CVs were 8.3, 7.9, and 10.9%, and intra-assay CVs were 7.3, 7.1, and 8.7%, respectively, in the low, medium, and high pools.
Voice analysis
Functional voice assessments
Functional voice analyses were performed by a single clinical phonetician who was blinded to treatment assignment. Digital audio recordings of the subjects' voices were made in a soundproof room with a head-mounted electret microphone placed at a distance of 15 cm from the mouth. The first test was a sustained “Ah.” After practicing a couple of times in their regular voice, subjects were then instructed to sustain an “Ah” for at least 4–6 seconds on a single breath. In the second test, subjects were instructed to read aloud the whole sentence of the following passage within an 8-second window (“When the sunlight strikes raindrops in the air, they act as a prism and form a rainbow”). The audio recordings were saved as sound files for acoustic analysis. Using the Kay Elemetrics-Computer Speech Lab program, the voice recordings were analyzed for average fundamental frequency (hertz), mean volume (decibels), mean percentage vocal jitter and shimmer, and harmonics-to-noise ratio (decibels). Fundamental frequency (or pitch) measures the number of vibrations produced by the vocal fold per second. Jitter and shimmer measures cycle-to-cycle variability in pitch and amplitude, respectively. Harmonics-to-noise ratio compares the relative amplitude of harmonics compared to relative amplitude of additional noise coming from the vocal folds.
Voice handicap index
Self-assessment of voice handicap was measured using a validated voice handicap index (VHI-10) questionnaire before and after 24 weeks of intervention (17). This questionnaire consists of 30 items asking individuals to describe their voice and the effects of their voice on their daily activities. Three subscales assess the functional, physical, and emotional aspects of voice impairment. Points from the questions are combined to assign a total score and combined by subscale.
Statistical analysis
Analyses were performed on all subjects who had data on VHI scores at baseline and 24 weeks. Outcome questionnaires were imputed at the level of domains (subscores) such that partially complete records were retained and used. Analyses were also performed on a subset of women who underwent objective functional voice testing at baseline and again at either 12 or 24 weeks. Functional voice analyses included all participants with baseline and at least one postrandomization measurement of acoustic data. Tabular and graphical displays were used to compare baseline characteristics of groups. Mean change in outcomes was compared across treatment doses using analysis of covariance models for VHI data and linear mixed models for functional voice data incorporating adjustment for baseline outcome measurements. Linear regression models, adjusted for baseline values, were used to test the association between change in each voice outcome measure with changes in total and free T levels. All tests were performed at α = 0.05 level of significance. No adjustments for multiple comparisons were made because of the exploratory nature of these analyses. Statistical analyses were conducted using SAS 9.3 software (SAS Institute, Inc).
Results
Flow of participants through the study
Of the 850 women who underwent telephone screening, 218 met eligibility criteria, 85 entered the estrogen run-in period, 71 were randomized, 59 completed the study, and 46 who had baseline and postintervention voice outcome data constituted the analytic sample (placebo, n = 10; 3 mg, n = 8; 6.25 mg, n = 9; 12.5 mg, n = 11; or 25 mg, n = 8.
Baseline characteristics
Baseline characteristics across the five treatment groups are displayed in Table 1. Mean age was 53 years, and average BMI was 29.8 kg/m2. Participants across the dose groups were similar in terms of age, BMI, smoking status, T concentrations, baseline voice handicap scores, and baseline acoustic voice parameters; 74% of the women had undergone bilateral oophorectomy.
Table 1.
Baseline Demographic and Voice Characteristics by Treatment Group of the Analytic Sample (n = 46)
| Dose of Testostrone Enanthate, mg/wk (n = 46) | Placebo (n = 10) | 3 (n = 8) | 6.25 (n = 9) | 12.5 (n = 11) | 25 (n = 8) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 54 (6) | 53 (6) | 52 (6) | 52 (5) | 54 (4) |
| BMI, kg/m2 | 32 (5) | 32 (7) | 30 (5) | 29 (5) | 26 (6) |
| Current smoker, n | 1 | 1 | 2 | 1 | 1 |
| Hysterectomy alone, n (%) | 3 (30) | 2 (25) | 1 (11) | 1 (9) | 3 (38) |
| Partial oophorectomy, n (%) | 1 (10) | 1 (13) | 0 (0) | 0 (0) | 0 (0) |
| Bilateral oophorectomy, n (%) | 6 (60) | 5 (63) | 8 (89) | 10 (91) | 5 (63) |
| Baseline hormone levels | |||||
| Total T, ng/dL | |||||
| Screening | 11 (9) | 17 (14) | 20 (18) | 16 (17) | 16 (11) |
| Post-estrogen run-in | 15 (9) | 16 (5) | 14 (14) | 11 (6) | 17 (10) |
| Free T, pg/mL | |||||
| Screening | 1 (1) | 1 (0) | 1 (1) | 1 (1) | 1 (1) |
| Post-estrogen run-in | 3 (2) | 3 (1) | 2 (3) | 2 (1) | 2 (1) |
| SHBG, nmol/L | 64 (24) | 64 (29) | 53 (18) | 63 (38) | 82 (14) |
| VHI, n = 41 | n = 9 | n = 7 | n = 8 | n = 9 | n = 8 |
|---|---|---|---|---|---|
| Functional score | 4 (3) | 5 (6) | 11 (15) | 7 (5) | 5 (5) |
| Physical score | 9 (8) | 4 (6) | 10 (14) | 9 (7) | 4 (4) |
| Emotional score | 7 (7) | 4 (7) | 10 (16) | 4 (5) | 2 (4) |
| Overall VHI score | 21 (14) | 13 (17) | 30 (44) | 21 (14) | 11 (10) |
| Acoustic Values, n = 36 | n = 9 | n = 6 | n = 6 | n = 9 | n = 6 |
|---|---|---|---|---|---|
| Sustained “Ah” test | |||||
| Average pitch, Hz | 182 (28) | 187 (26) | 189 (49) | 173 (63) | 184 (38) |
| Pitch range, Hz | 47 (36) | 50 (42) | 43 (27) | 45 (22) | 38 (33) |
| Average volume, dB | 71 (6) | 73 (7) | 74 (7) | 69 (4) | 72 (4) |
| Volume range, dB | 11 (7) | 12 (5) | 9 (2) | 19 (11) | 8 (2) |
| Jitter, % | 0.5 (0.2) | 0.5 (0.3) | 0.4 (0.2) | 0.8 (0.9) | 0.4 (0.1) |
| Shimmer, dB | 0.3 (0.2) | 0.4 (0.1) | 0.4 (0.3) | 0.5 (0.2) | 0.4 (0.2) |
| Harmonics/noise | 19 (2) | 19 (2) | 22 (4) | 17 (7) | 17 (8) |
| Sentence test | |||||
| Average pitch, Hz | 164 (34) | 174 (15) | 160 (30) | 166 (23) | 163 (32) |
| Pitch range, Hz | 147 (61) | 137 (33) | 129 (47) | 145 (38) | 149 (52) |
| Average volume, dB | 66 (6) | 70 (6) | 68 (4) | 67 (5) | 68 (6) |
| Volume range, dB | 35 (7) | 31 (4) | 28 (4) | 31 (7) | 31 (5) |
Data represent mean (SD) or n (%). Overall VHI and domain baseline scores are out of a maximum possible score of 120 and 40, respectively. Jitter is cycle-cycle variability in pitch; normal range for jitter is 0.2–1.0%. Shimmer is cycle-cycle variability in amplitude; normal values are < 0.5 dB. Harmonics-to-noise ratio measures relative amplitude of harmonics compared to relative amplitude of noise. Higher values indicate that harmonics is louder than noise. Lower values indicate that noise is louder relative to the harmonics.
Hormone levels
Baseline mean total and free T concentrations were 16.0 ng/dL and 1.0 pg/mL, respectively, well below the range for healthy, menstruating women. Serum nadir total and free T levels, measured during week 24, one week after the previous injection, increased from baseline in a dose-dependent fashion. Mean on-treatment nadir total T concentrations were 13, 83, 106, 122, and 250 ng/dL, and free T concentrations were 2.4, 14, 15, 22, and 48 pg/mL at the 0, 3-, 6.25-, 12.5-, and 25-mg doses, respectively.
Voice outcomes
Sustained “Ah” test
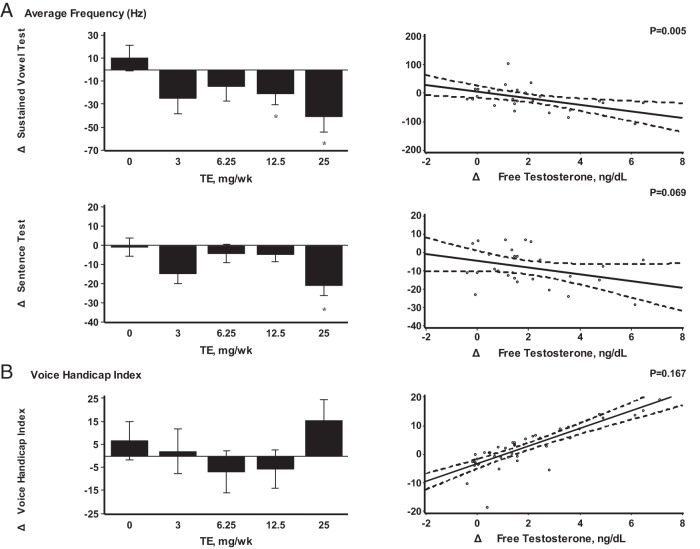
A significant decrease in average pitch was observed in the 12.5- and 25-mg dose groups when compared to placebo (Δ-21 and Δ-40 Hz, respectively; P < .05). These changes in pitch were related significantly to increases in serum free T concentrations (Figure 1A). Changes in the harmonics-to-noise ratio were not related to changes in T concentrations. There was a small increase in shimmer in the 12.5-mg dose group, but overall, the changes in shimmer were not significantly related to T concentrations. No dose or concentration-dependent changes were seen in average volume or percentage jitter (Supplemental Tables 1 and 3).
Figure 1.
A, Average frequency (hertz). B, VHI. A and B, In the bar graphs on the left, data represent absolute mean changes ± SE from baseline to end of the study for each treatment group. *, A significant difference between mean on-treatment change in dose group vs placebo at the 0.05 α levels. Scatterplots on the right show linear regression line with 95% confidence intervals as a function of change in free T levels adjusted to baseline value of outcome. The P values displayed are from significance test of no association. Hz, Hertz; TE, T enanthate.
Sentence test
There was a significant decrease in average pitch at the highest dose when compared to placebo (Δ-21 Hz; P < .05) (Figure 1A). A trend toward reduction in pitch was observed with increasing T concentrations; however, in contrast to the sustained “Ah” test, it was not statistically significant (P = .069) (Supplemental Tables 1 and 3). Average volume increased by 3.58 dB at the highest dose group relative to placebo, but the changes were not statistically significant or related to T concentrations (P > .05).
Voice handicap index
Overall, VHI scores did not change significantly at any dose group compared to placebo and were not related to increases in T concentrations (Figure 1B). Similarly, there were no significant dose- or concentration-dependent changes in functional, physical, or emotional subscores of the VHI (Supplemental Tables 2 and 3).
Discussion
Exogenous T administration for 6 months in women who have undergone hysterectomy was associated with dose- and concentration-dependent changes in average pitch. These findings were further corroborated by the significant relation between the changes in pitch and the increases in serum free T concentrations. The changes in pitch were noted during acoustic voice analysis despite the lack of significant self-reported changes in voice. Moreover, the changes in pitch were observed more consistently in the sustained vowel phonation (“Ah” test) than during the sentence test, consistent with the observations that changes in voice quality are more accurately measured in the sustained vowel phonation than during articulation of running speech (18). We conclude that supraphysiological doses of T in hysterectomized women may induce changes in voice frequency that are detectable during acoustic voice analysis even before the participants are able to perceive changes in their voice quality.
Previous studies have shown that the human larynx is a highly sensitive target of androgen action (2). The sex differences in voice quality have been well recognized, although there are cultural and ethnic variations in these sex differences. However, across various cultural and ethnic groups that have been studied, the most consistent difference between men and women is the higher pitch in women than in men. Historically, the deliberate induction of male hypogonadism by prepubertal castration in choir boys was widely practiced to preserve the high-pitched feminine voice of the castrati that dominated opera in western Europe in the 18th century (5). Increased levels of androgens are known to increase mass of the laryngeal tissues, resulting in lowering of the pitch of the voice in both sexes (3). Women with congenital adrenal hyperplasia have significantly lower fundamental frequency of the voice compared to healthy controls (6). Similarly, women with polycystic ovary syndrome report more vocal complaints (deepening of voice) compared to healthy controls (19); however, elevated serum androgen levels in women with polycystic ovary syndrome have not been consistently correlated with subjective and objective voice parameters (7). Similarly, in female-to-male transsexuals, long-term administration of supraphysiological doses of T results in a significant lowering of pitch into the male range (11). Thus, it is possible that a certain threshold of T dose and concentration and duration of exposure has to be achieved before virilizing voice changes are detectable. Furthermore, subclinical changes in functional voice parameters may precede clinical complaints.
Women treated with supraphysiological doses of androgenic drugs have reported dysfunction in their speaking and singing after starting treatment, with some subjects suffering permanent vocal dysfunction (8, 9). In our trial, a standardized self-assessment questionnaire with the widely used VHI showed no significant dose- or concentration-dependent changes in total score or in subscores for emotional, functional, and psychological aspects of voice impairment, even at doses that achieved supraphysiological T concentrations. Based on our findings, it appears that T doses given over a wide range for 24 weeks had no significant effect on their voice-related quality of life. However, none of our study participants were professional speakers or singers; it is conceivable that voice professionals would differ in their self-perceived voice quality. Although there were no adverse changes in subjective vocal parameters, this 24-week dose-response interventional trial showed a significant lowering of vocal pitch at the two highest T-dose groups (12.5 and 25 mg) when compared to placebo, and these changes were significantly associated with increases in T concentrations. The early changes in acoustic parameters before clinical manifestation have also been reported in patients with Parkinson's disease, in whom changes in vocal frequency can be detected a decade before clinical diagnosis (20), supporting the notion that early changes detected during functional acoustic testing can be a useful marker for subsequent clinical voice changes from T administration.
Our study has notable strengths and some limitations. The trial had many features of a good trial design: concealed randomization, placebo control, blinding and oversight by an independent Data Safety Monitoring Board. Total and free T levels were measured using liquid chromatography-tandem mass spectrometry and equilibrium dialysis, respectively, both of which are widely considered the reference methods with the highest sensitivity and specificity. Testosterone injections were effective in raising T concentrations in a dose-dependent fashion over a wide range. The dose-response effects of T administration on acoustic parameters in postmenopausal women have not been previously studied in the setting of clinical trials. However, measurement of voice outcomes was not the primary outcome of the trial, and the trial was not designed to detect changes in these voice parameters. Our analysis was therefore limited by small sample size, which may have reduced our power to detect small effects. Furthermore, because some small studies have reported better subjective voice quality in postmenopausal women on estrogen replacement (21), it is possible that virilizing effects of T on some voice parameters may have been attenuated because of concurrent estrogen therapy.
In conclusion, short-term T administration over a wide range of doses for 24 weeks in hysterectomized women was associated with dose- and concentration-dependent changes in lowering of vocal pitch, even in the absence of subjective voice changes. Based on the findings of this trial, referral for functional voice assessment may be useful for early detection of any potential changes in voice characteristics in women receiving similar doses of T.
Acknowledgments
We thank the staff of the General Clinical Research Unit of Boston University's Clinical and Translational Science Institute and the Clinical Research Center of Charles Drew University of Medicine and Science for their help with these studies and the study participants for their commitment and generosity.
Data Safety Monitoring Board: Dr Jan Shifren, Massachusetts General Hospital, Boston, Massachusetts (Chair); Dr Raja Sayegh, Boston Medical Center; and Dr Anita Nelson, Harbor-UCLA Medical Center.
This study was supported by Grants 5U54HD041748–04 (to Charles Drew University of Medicine and Science) and 2008 TF D2274G (sub award to Boston University) from the National Institute of Child Health and Human Development and the Boston Claude D. Pepper Older Americans Independence Center Grant 5P30AG031679 from the National Institute of Aging. Watson Pharmaceuticals provided the transdermal estradiol patch for this trial.
Clinical Trials Registration No. NCT00494208.
Disclosure Summary: S.B. has received grant support from Abbott Pharmaceuticals for investigator-initiated studies. S.B. has previously consulted for Eli Lilly, Inc. S.B. has received research grant support from Abbvie Pharmaceuticals and Eli Lilly and Co. for investigator-initiated research; these research grants are managed by the Brigham and Women's Hospital and are unrelated to this study. No other potential conflict of interest relevant to this article was reported.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- VHI
- voice handicap index.
References
- 1. Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21(6):612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voelter Ch, Kleinsasser N, Joa P, et al. Detection of hormone receptors in the human vocal fold. Eur Arch Otorhinolaryngol. 2008;265:1239–1244. [DOI] [PubMed] [Google Scholar]
- 3. Damrose EJ. Quantifying the impact of androgen therapy on the female larynx. Auris Nasus Larynx. 2009;36:110–112. [DOI] [PubMed] [Google Scholar]
- 4. Abitbol J, Abitbol P, Abitbol B. Sex hormones and the female voice. J Voice. 1999;13:424–446. [DOI] [PubMed] [Google Scholar]
- 5. Jenkins JS. The voice of the castrato. Lancet. 1998;351:1877–1880. [DOI] [PubMed] [Google Scholar]
- 6. Nygren U, Södersten M, Falhammar H, Thorén M, Hagenfeldt K, Nordenskjöld A. Voice characteristics in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2009;70:18–25. [DOI] [PubMed] [Google Scholar]
- 7. Gugatschka M, Lichtenwagner S, Schwetz V, et al. Subjective and objective vocal parameters in women with polycystic ovary syndrome. J Voice. 2013;27:98–100. [DOI] [PubMed] [Google Scholar]
- 8. Baker J. A report on alterations to the speaking and singing voices of four women following hormonal therapy with virilizing agents. J Voice. 1999;13:496–507. [DOI] [PubMed] [Google Scholar]
- 9. Damsté PH. Voice change in adult women caused by virilizing agents. J Speech Hear Disord. 1967;32:126–132. [DOI] [PubMed] [Google Scholar]
- 10. Talaat M, Talaat AM, Kelada I, Angelo A, Elwany S, Thabet H. Histologic and histochemical study of effects of anabolic steroids on the female larynx. Ann Otol Rhinol Laryngol. 1987;96:468–471. [DOI] [PubMed] [Google Scholar]
- 11. Cosyns M, Van Borsel J, Wierckx K, et al. Voice in female-to-male transsexual persons after long-term androgen therapy. The Laryngoscope. 2014;124:1409–1414. [DOI] [PubMed] [Google Scholar]
- 12. Braunstein GD, Sundwall DA, Katz M, et al. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. [DOI] [PubMed] [Google Scholar]
- 13. Sinha-Hikim I, Arver S, Beall G, et al. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1998;83:1312–1318. [DOI] [PubMed] [Google Scholar]
- 14. Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi HH, Gray PB, Storer TW, et al. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab. 2005;90:1531–1541. [DOI] [PubMed] [Google Scholar]
- 16. Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. [DOI] [PubMed] [Google Scholar]
- 17. Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. The Laryngoscope. 2004;114:1549–1556. [DOI] [PubMed] [Google Scholar]
- 18. Parsa V, Jamieson DG. Acoustic discrimination of pathological voice: sustained vowels versus continuous speech. J Speech Lang Hear Res. 2001;44:327–339. [DOI] [PubMed] [Google Scholar]
- 19. Hannoun A, Zreik T, Husseini ST, Mahfoud L, Sibai A, Hamdan AL. Vocal changes in patients with polycystic ovary syndrome. J Voice. 2011;25:501–504. [DOI] [PubMed] [Google Scholar]
- 20. Harel BT, Cannizzaro MS, Cohen H, Reilly N, Synder PJ. Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment. J Neurolinguist. 2004;17:439–453. [Google Scholar]
- 21. Caruso S, Roccasalva L, Sapienza G, Zappalá M, Nuciforo G, Biondi S. Laryngeal cytological aspects in women with surgically induced menopause who were treated with transdermal estrogen replacement therapy. Fertil Steril. 2000;74:1073–1079. [DOI] [PubMed] [Google Scholar]