Abstract
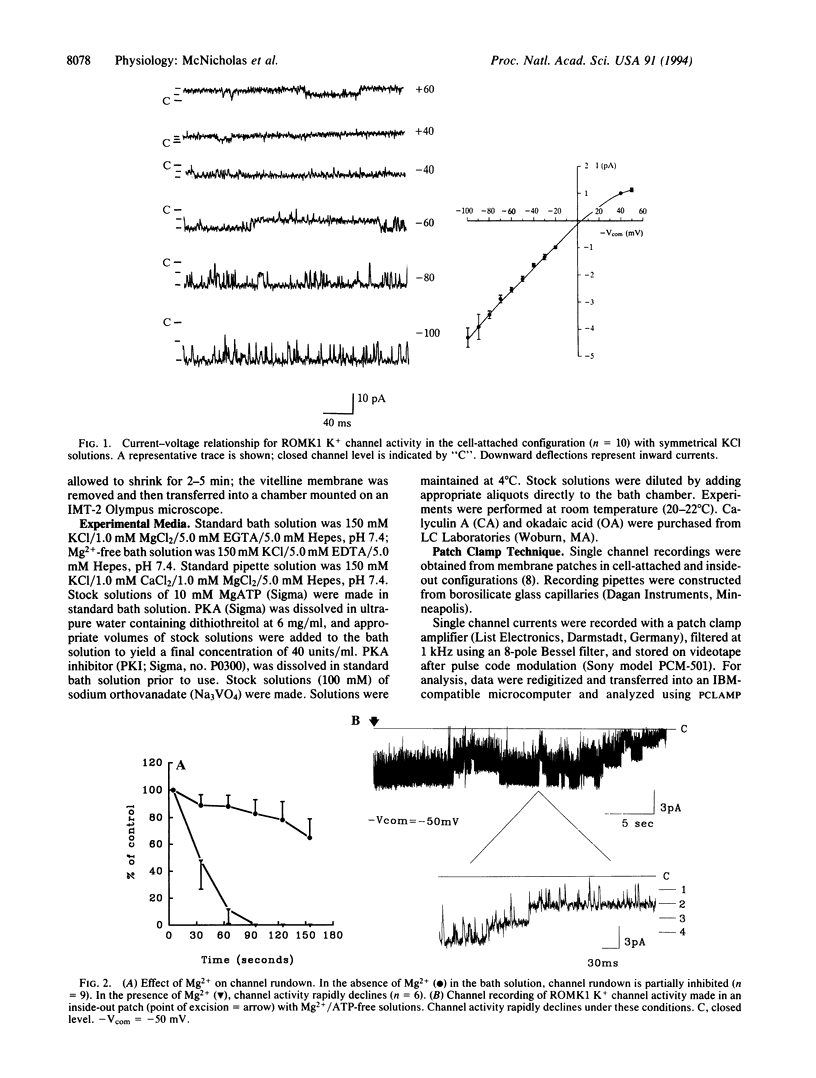
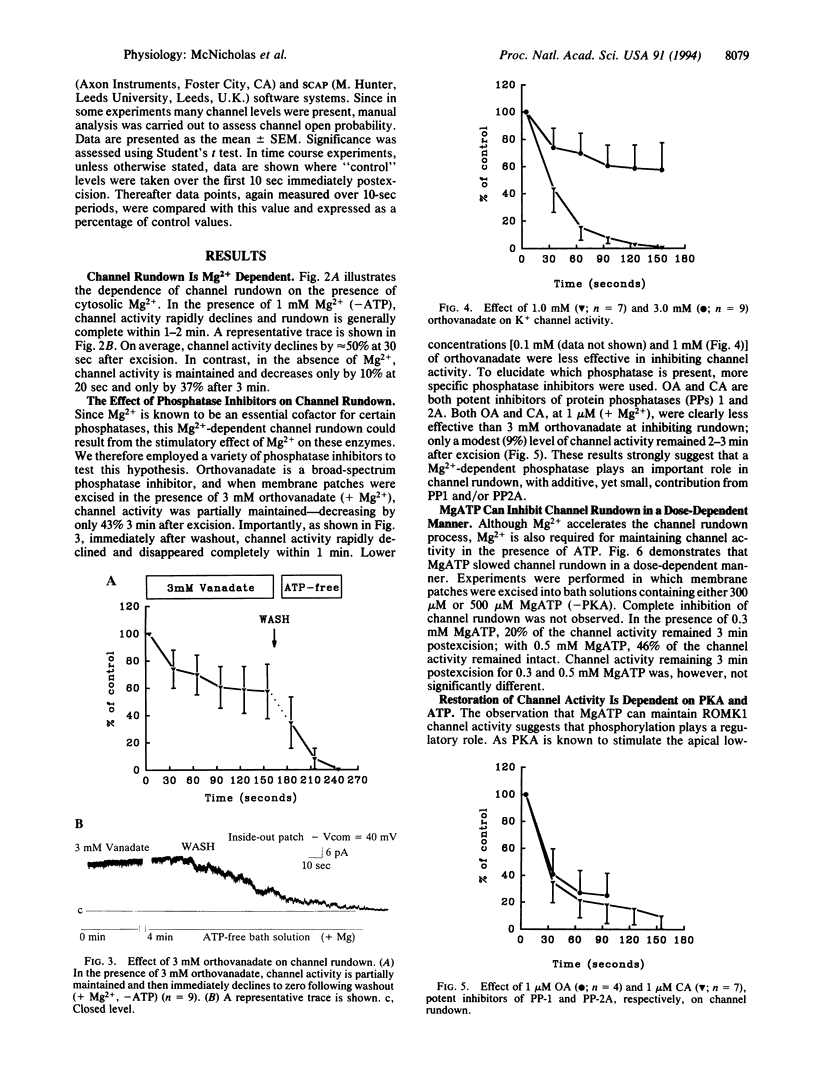
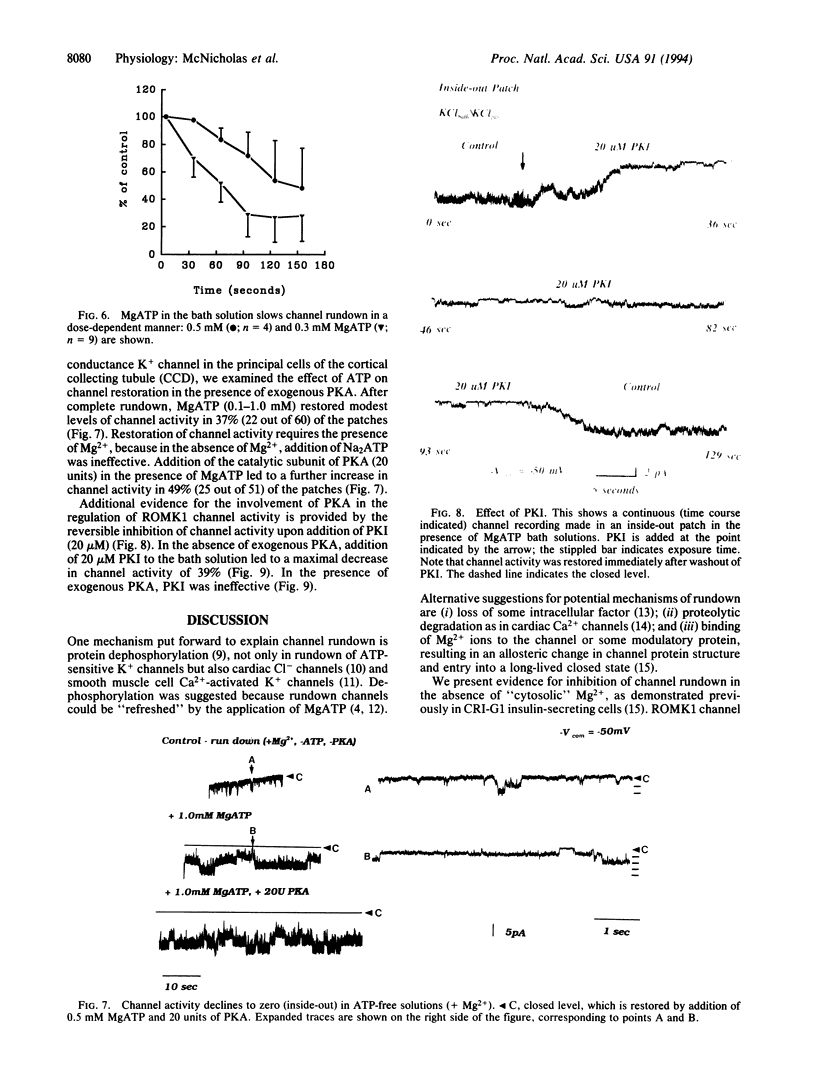
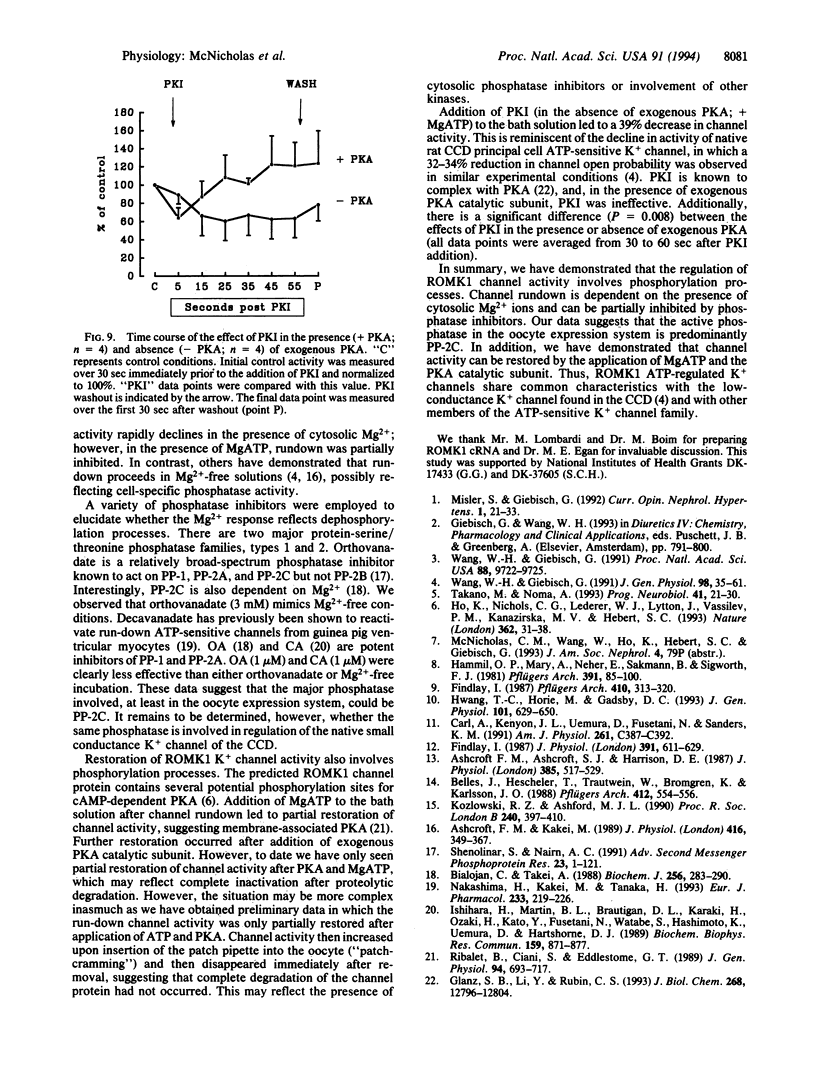
An inwardly rectifying, ATP-regulated K+ channel with a distinctive molecular architecture, ROMK1, was recently cloned from rat kidney. Using patch clamp techniques, we have investigated the regulation of ROMK1 with particular emphasis on phosphorylation/dephosphorylation processes. Spontaneous channel rundown occurred after excision of membrane patches into ATP-free bath solutions in the presence of Mg2+. Channel rundown was almost completely abolished after excision of patches into either Mg(2+)-free bathing solutions or after preincubation with the broad-spectrum phosphatase inhibitor, orthovanadate, in the presence of Mg2+. MgATP preincubation also inhibited channel rundown in a dose-dependent manner. In addition, the effect of the specific phosphatase inhibitors okadaic acid (1 microM) and calyculin A (1 microM) was also investigated. The presence of either okadaic acid or calyculin A failed to inhibit channel rundown. Taken together, these data suggest that rundown of ROMK1 involves a Mg(2+)-dependent dephosphorylation process. Channel activity was also partially restored after the addition of MgATP to the bath solution. Addition of exogenous cAMP-dependent protein kinase A (PKA) catalytic subunit led to a further increase in channel open probability. Addition of Na2ATP, in the absence of Mg2+, was ineffective, suggesting that restoration of channel activity is a Mg(2+)-dependent process. Addition of the specific PKA inhibitor, PKI, to the bath solution led to a partial, reversible inhibition in channel activity. Thus, PKA-dependent phosphorylation processes are involved in the modulation of channel activity. This observation is consistent with the presence of potential PKA phosphorylation sites on ROMK1.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol. 1987 Apr;385:517–529. doi: 10.1113/jphysiol.1987.sp016505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989 Sep;416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles B., Hescheler J., Trautwein W., Blomgren K., Karlsson J. O. A possible physiological role of the Ca-dependent protease calpain and its inhibitor calpastatin on the Ca current in guinea pig myocytes. Pflugers Arch. 1988 Oct;412(5):554–556. doi: 10.1007/BF00582548. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A., Kenyon J. L., Uemura D., Fusetani N., Sanders K. M. Regulation of Ca(2+)-activated K+ channels by protein kinase A and phosphatase inhibitors. Am J Physiol. 1991 Aug;261(2 Pt 1):C387–C392. doi: 10.1152/ajpcell.1991.261.2.C387. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol. 1987 Oct;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz S. B., Li Y., Rubin C. S. Characterization of distinct tethering and intracellular targeting domains in AKAP75, a protein that links cAMP-dependent protein kinase II beta to the cytoskeleton. J Biol Chem. 1993 Jun 15;268(17):12796–12804. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Gadsby D. C. Functionally distinct phospho-forms underlie incremental activation of protein kinase-regulated Cl- conductance in mammalian heart. J Gen Physiol. 1993 May;101(5):629–650. doi: 10.1085/jgp.101.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Misler S., Giebisch G. ATP-sensitive potassium channels in physiology, pathophysiology, and pharmacology. Curr Opin Nephrol Hypertens. 1992 Oct;1(1):21–33. doi: 10.1097/00041552-199210000-00005. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Kakei M., Tanaka H. Activation of the ATP-sensitive K+ channel by decavanadate in guinea-pig ventricular myocytes. Eur J Pharmacol. 1993 Mar 23;233(2-3):219–226. doi: 10.1016/0014-2999(93)90053-k. [DOI] [PubMed] [Google Scholar]
- Ribalet B., Ciani S., Eddlestone G. T. ATP mediates both activation and inhibition of K(ATP) channel activity via cAMP-dependent protein kinase in insulin-secreting cell lines. J Gen Physiol. 1989 Oct;94(4):693–717. doi: 10.1085/jgp.94.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Takano M., Noma A. The ATP-sensitive K+ channel. Prog Neurobiol. 1993 Jul;41(1):21–30. doi: 10.1016/0301-0082(93)90039-u. [DOI] [PubMed] [Google Scholar]
- Wang W. H., Giebisch G. Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9722–9725. doi: 10.1073/pnas.88.21.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Giebisch G. Dual effect of adenosine triphosphate on the apical small conductance K+ channel of the rat cortical collecting duct. J Gen Physiol. 1991 Jul;98(1):35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]




