Abstract
Context:
Like all hormones, GH has variable physiological effects across people. Many of these effects initiated by the binding of GH to its receptor (GHR) in target tissues are mediated by the expression of the IGF1 gene. Genetic as well as epigenetic variation is known to contribute to the individual diversity of GH-dependent phenotypes through two mechanisms. The first one is the genetic polymorphism of the GHR gene due to the common deletion of exon 3. The second, more recently reported, is the epigenetic variation in the methylation of a cluster of CGs dinucleotides located within the proximal part of the P2 promoter of the IGF-1 (IGF1) gene, notably CG-137.
Objective:
The current study evaluates the relative contribution of these two factors controlling individual GH sensitivity by measuring the response of serum IGF-1 to a GH injection (IGF-1 generation test) in a sample of 72 children with idiopathic short stature.
Results:
Although the d3 polymorphism of the GHR contributed 19% to the variance of the IGF-1 response, CG-137 methylation in the IGF-1 promoter contributed 30%, the combined contribution of the two factors totaling 43%.
Conclusion:
Our observation indicates that genetic and epigenetic variation at the GHR and IGF-1 loci play a major role as independent modulators of individual GH sensitivity.
Tens of thousands of children affected by various causes of short stature currently receive recombinant GH to improve their final height. There is, however, a highly variable individual GH response to this treatment, which does not allow pediatric endocrinologists to predict final height reliably, although clinical predictive models have been developed (1) as well as biological models based on GH/IGF-1 hormones measured before treatment (2). The multiple causes underlying the variability of the therapeutic response to GH include the etiology of short stature (3, 4), GH dose (5), child age (1), treatment regimens (6), and therapeutic compliance (7). Because IGF-1 is the mediator of GH effects, the individual variation in growth responses is in large part due to the variable IGF-1 production under GH treatment, reflected by circulating IGF-1 concentration (8–10). On the other hand, the personal capacity to respond to GH has prompted few studies in search of biological mechanisms able to vary across individuals (11, 12). The increment in IGF-1 induced by GH administration in healthy children with idiopathic short stature is normally distributed (8, 10, 13, 14) and can thus be modeled as a continuous quantitative trait. Among the molecular determinants involved in the variability of IGF-1 responses to GH, it has become clear that genetic variation plays a role (11). Notably, the deletion of exon 3 within the GH receptor (GHRd3) gene has been recognized as a significant predictor of GH growth-promoting effects in children with idiopathic short stature (15–18) or other etiologies of short stature (11, 12, 19).
Estimates of the proportion of variance in circulating IGF-1 that is genetically determined vary between 38% and 80% (20, 21). The association of serum IGF-1 concentration with several genetic variants is debated (22–24), but variants at the IGF-1 locus do not seem to influence circulating IGF-1 in Caucasian adults (22, 25) except, perhaps, the most common Z allele of the microsatellite located 1 kb upstream the IGF-1 gene (26, 27). Overall, the genetic basis for serum IGF-1 variability remains largely unknown in adults and has not been studied during the period of physiological growth in normal children. In children with idiopathic short stature, considered to be a variant category of normal children, the GHRd3 is associated with higher circulating IGF-1 in response to GH injection (28) .
Unlike pharmacogenetics, pharmacoepigenetics is a nascent field of clinical medicine (29), and the epigenetics of growth and IGF-1 responses have only recently started to be investigated (30). A recent study showed that the methylation of a cluster of dinucleotides (CGs) located within the P2 promoter of the IGF-1 (IGF-1) gene, notably CG-137, is inversely closely correlated with the response of growth and circulating IGF-1 to GH treatment (30).
In growing children, GH responsiveness is important to physiology and in some of them to therapeutics. The current study investigates the individual response of children to a GH injection with the objective of evaluating the respective role of genetic polymorphism of the GHR and the degree of methylation of the P2 promoter of the IGF-1 gene. We selected children who have not entered puberty to avoid the confounding effect of the variable tempo of sexual maturation, which adds to the variability of basal (31–33) and GH-stimulated (34) circulating IGF-1. We used the long-studied generation test (31, 34–37) to study the GH responsiveness of circulating IGF-1 in 72 children. We studied the GH receptor (GHR) d3 genotype and the methylation of the IGF-1 P2 promoter in these children to study how these factors contribute to the individual variability of the GH response to the test.
Materials and Methods
Participants
Seventy-two children with various degrees of short stature (−1.1 SD to −3.2 SD) belonging to the Epigrowth cohort (30) had venous blood sampling at 8:00 pm before dinner, 10 minutes before receiving an injection of 100 μg/kg body weight of recombinant human growth hormone (rhGH) im in the left thigh, had a normocaloric standard dinner at 8:10 pm, fasted overnight, and had a second blood sampling at 8:00 am. Their main characteristics are depicted in Table 1. All children were healthy and had normal clinical examination. Thirty children had criteria of idiopathic short stature, as defined in (11). In these children, GH deficiency was excluded with a stimulated GH peak greater than 15 ng/mL, TSH levels were normal. Subtle chondrodysplasia were excluded by forearm, pelvis, and spine radiographs. Pubertal stages were estimated using the Tanner definition.
Table 1.
Main characteristics of the studied children, mean ± SD
| Acute rhGH Injection test | |
|---|---|
| n | 72 |
| Sex, M/F | 45/27 |
| Age, y | 11 ± 2.5 |
| Height (SDS) | −1.8 ± 0.7 |
| Tanner stages, n | |
| 1 | 41 (57%) |
| 2 | 20 (28%) |
| 3 | 10 (14%) |
| 4 | 1 (1%) |
| Serum IGF-1 before test | |
| Nanograms per milliliter | 236 ± 149 |
| SDS | −1.16 ± 0.8 |
| Serum IGF-1 12 h after rhGH | |
| Nanograms per milliliter | 326 ± 150 |
| SDS | −0.55 ± 0.84 |
| GHR genotype | |
| fl/fl | 33 (46%) |
| fl/d3 | 32 (44%) |
| d3/d3 | 7 (10%) |
| IGF-1 P2 promoter methylation, % | |
| CG-232 | 62 ± 6 |
| CG-224 | 74 ± 7 |
| CG-218 | 72 ± 6 |
| CG-207 | 45 ± 7 |
| CG-137 | 47 ± 4 |
| CG-108 | 60 ± 6 |
| Averagea | 58 ± 4 |
Abbreviations: F, female; M, male.
Mean value for the six studied CGs.
Parents of all studied children gave their written informed consent for the study according to the French rules of bioethics in biomedical research checked by our institutional review board.
Serum IGF-1 and IGF binding protein-3 (IGFBP3) concentrations
Serum IGF-1 concentration was measured at approximately 7:00–8:00 am before breakfast in 136 children using an immune-radiometric assay after ethanol-acid extraction using Cisbio reagents. Intra- and interseries coefficients of variation were 1.5% and 3.7% at 260 ng/mL and 3.9% at 760 ng/mL. The sensitivity was 4 ng/mL. IGF-1 SD score (SDS) was calculated using the norms of Alberti et al (33) in French children. Serum IGFBP3 was measured using an immune-radiometric assay after ethanol-acid extraction with Diagnostic Systems Laboratory reagents (Beckman-Coulter). Intra- and interseries coefficient of variation was 10.4 and 14% and the sensitivity limit was 6.2 ng/mL.
GHR genotype
Analysis of the GHR exon 3 polymorphism was carried out in patients by quantitative PCR performed on ABI 7500 fast (Applied Biosystems). Genotyping protocol was adapted from Bernabeu et al (38). Primer/probe sets were targeted to exon 3 and exon 10 of GHR, which was used as an internal positive control. We used oligonucleotide primers and probes previously described in (38).
For each analysis, 50 ng genomic DNA was quantitative PCR amplified in 96-well plates in a volume of 12 μL using Gotaq probe quantitative PCR master mix (Promega), 1.25 μL GHR exon 3 primer/probe set (3 pm/μL each), 1.25 μL GHR exon 10 primer/probe set (9 pm/μL each), and 2.75 μL sterile water. Cycle conditions were 50ºC for 2 minutes and 95ºC for 10 minutes, followed by 40 cycles of 95ºC for 15 seconds and 60ºC for 1 minute. Differences in cycle threshold (Ct) between the exon 3 and exon 10 amplicons were used to determine the exon 3 copy number for each sample. A δCt value of 1 indicates two exon 3 copies (genotype fl/fl), a δCt value of 2 indicates one exon 3 copy (genotype fl/d3), and no signal for exon 3 in the presence of a normal exon 10 signal indicates an absent exon 3 (genotype d3/d3).
DNA methylation
For methylation measurements, 6 mL peripheral blood samples were obtained, from which peripheral blood mononuclear cells were purified immediately.
For promoter P2, we studied the six CGs located upstream from the major transcription start site within the proximal part of the P2 promoter. CGs are denominated according to position vs each promoter transcription start site. Nucleic acids were extracted from peripheral blood mononuclear cells using Gentra Puregene blood kit (QIAGEN). A bisulfite-PCR-pyrosequencing technique (39) was used to measure the methylation of the CGs. We improved the resolution of this method from a handful of bases to up to 100 nucleotides, with the ability to quantify methylation in the same sample of blood with a coefficient of variation (SD/mean) as little as 1%–5%. Briefly, 400 ng of genomic DNA was treated with an EZ DNA Methylation-Gold kit, according to manufacturer's protocol (Zymo Research Corp). The bisulfite-treated genomic DNA was PCR amplified using unbiased IGF-1 primers (30) and performed quantitative pyrosequencing using a PyroMark Q96 ID Pyrosequencing instrument (QIAGEN). Pyrosequencing assays were designed using MethPrimer (http://www.urogene.org/methprimer/index1.html). Biotin-labeled single-stranded amplicons were isolated according to protocol using the QIAGEN Pyromark Q96 Work Station and underwent pyrosequencing with 0.5 μM primer. The percentage methylation for each of the CGs within the target sequence was calculated using PyroQ CpG Software (QIAGEN).
Calculations and statistics
The IGF-1 response to GH administration was expressed as increment in serum IGF-1 concentration, ie, the difference between serum IGF-1 12 hours after injecting GH and serum IGF-1 concentration before GH injection. Pearson correlations were calculated as adjusted R square that measures the proportion of the variation in the dependent variable accounted for by the explanatory variables. The fraction of explained variance is calculated under the linear regression model, using the usual definition: r2 × 100. We fitted a multivariate linear model to the data to estimate the proper effect of GHR genotype and CG-137 methylation on response to GH, adjusted for the effect of the other covariates contributing to the IGF-1 response, such as age and sex. We carried out tests of independence of each covariate one at a time, keeping the others in the model. Statistics and estimations of effect given in the tables are thus adjusted for the others whenever appropriate and are not subject to marginal association. We checked the normality of the residuals, and the residuals vs the fitted values did not show any trend, indicating that there was no noticeable deviation from the assumption of the linear model. All statistics and linear model were computed using R 2.10.1. Results are expressed as mean ± SD.
Results
The response of serum IGF-1 concentration to the GH test averaged 90 ± 54 ng/mL, an increase of 38 ± 36% from the basal IGF-1 value. There was no correlation between basal IGF-1 and the increase of IGF-1 during the test. Within the studied group of children, the IGF-1 response to GH showed a nonsignificant trend of positive association with age (P = .11) and Tanner pubertal stages (P = .45).
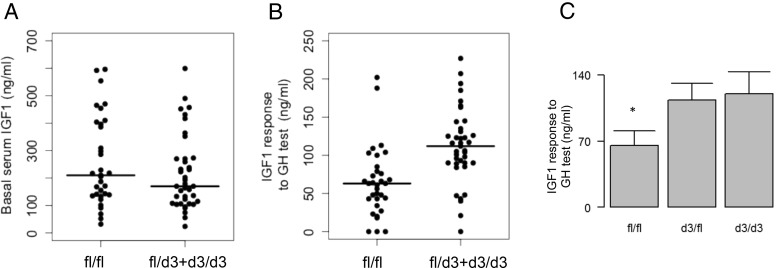
Basal IGF-1 concentration was comparable in children of various GHR genotypes (Figure 1A), but a significant difference appeared after the GH injection (Figure 1B). Children carrying the dominant GHR-allele d3 (15) had a 56% higher IGF-1 response to GH than the fl/fl homozygotes. Because d3/3 children and fl/d3 showed comparable IGF-1 responses to GH, they were merged in a single group for analysis.
Figure 1.
Relationship between GHR-d3 genotype and serum IGF-1 concentration in the studied children. A, Basal IGF-1 shows no difference across the genotypic groups. B, The response of serum IGF-1 to GH test is greater in children carrying a d3 allele (P = 2.10−5). C, Mean values of IGF-1 response in the three GHR genotypic groups show that d3/fl and d3/d3 classes have a comparable response to GH.
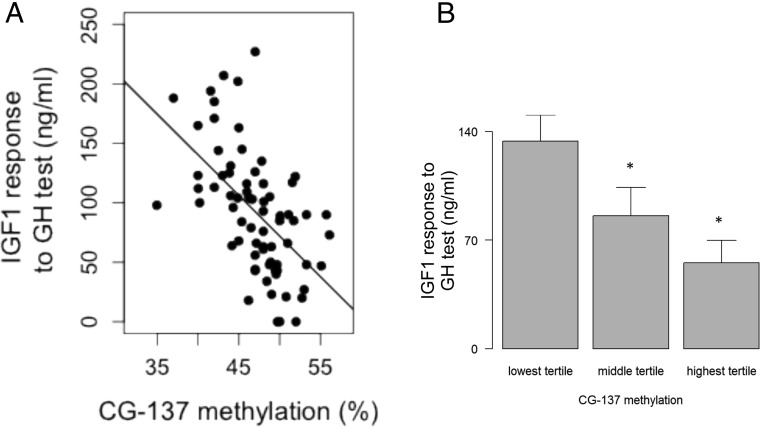
The serum IGF-1 response to the GH test showed a negative correlation with the methylation of three of six CGs of the IGF-1 P2 promoter (Supplemental Table 1), notably with CG-137 methylation (R = −0.54, P = 4.10−7) as shown in Figure 2A. Children in the highest tertile for CG-137 methylation showed a 142% increase in IGF-1 response compared with those in the lowest tertile (Figure 2B). CG methylation was comparable in the GHR genotype groups.
Figure 2.
Relationship between CG-137 methylation and IGF-1 response to the GH test. A, Correlation between serum IGF-1 and CG-137 methylation (R = −0.54, P = 4.10−7, y = −6.8X + 413). B, IGF-1 response across the three tertiles of CG-137 methylation (P = 10−6).
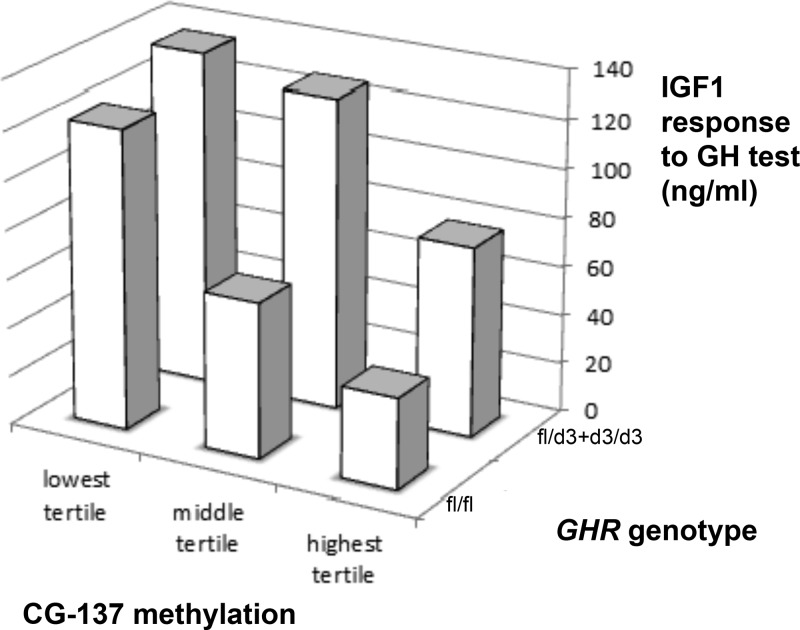
Once combined in a multivariate analysis with age, pubertal stages, and sex, both GHR d3 genotype and the methylation of CG-137 showed a significant association with the serum IGF-1 response to the GH test (Table 2). The combined effect of GHR genotype and IGF-1 epigenotype is illustrated by the three-dimensional histograms of Figure 3. Overall, the GHR d3 genotype contributed 19% to the variance of the IGF-1 response and the IGF-1 P2 methylation contributed 30%, resulting in a total contribution of 43% to the individual GH sensitivity evaluated by the IGF-1 generation test. Strikingly, the children who had both a GHR fl/fl genotype and a CG-137 methylation in the highest tertile (n = 18) had an IGF-1 response to the GH test 3.4 times smaller than children carrying one or two GHR d3 alleles combined with CG-137 methylation in the lowest tertile (n = 11) (Figure 3).
Table 2.
General linear model for regression of age, sex, Tanner stage, GHR d3 polymorphism, and methylation of CG-137 on IGF-1 response to the GH test
| Estimates | SE | t Value | Pr (> t ) | |
|---|---|---|---|---|
| Intercept | 275 | 58 | 4.7 | 10−5 |
| Age, y | 6.3 | 2.74 | 2.30 | 0.025 |
| Sex | 15.03 | 10.3 | 1.46 | 0.15 |
| Tanner stage (1–4) | −12.84 | 9.3 | −1.40 | 0.17 |
| GHR d3 polymorphism | 35.7 | 9.20 | 3.87 | 2.10−4 |
| CG-137 methylation, % | −5.60 | 1.13 | −4.95 | 5.6.10−6 |
Pr (> t), probability of a value > t.
Figure 3.
3-D relationship between IGF-1 response to GH test and both GHR-d3 genotype and CG-137 methylation showing higher responses in children carrying a d3 allele and having low levels of CG-137 methylation (P = 10−9).
We found no correlation of serum IGFBP3 with the GHR genotype or with the IGF-1 P2 promoter methylation, in the basal state as well as in response to the GH test.
Discussion
Our study supports that both the GHR genotype and the CG methylation of the P2 promoter of the IGF-1 gene are major determinants of the individual IGF-1 response to GH in childhood.
IGF-1 generation tests were developed more than 20 years ago (31, 34–37) and are currently used in evaluating GH sensitivity in children with unexplained short stature, notably when characterized by low serum IGF-I. Major limitations have included variability in protocols for administration of GH, timing of samples, differences in IGF assay methodologies, and lack of adequate normative data (40). The latter is particularly problematic, given the well documented age-related variability in IGF-I concentrations (12, 32) as well as gender-related differences in responsiveness to exogenous GH (41, 42). The results of the generation test in children with idiopathic short stature are of particular interest, as previously reported (36), to have serum IGF-I concentrations in the lower portion of the normal range or below the lower limits of normal. Interestingly, these subjects also failed, in general, to raise their serum IGF-1 concentrations in response to GH; many did not even attain levels within the baseline normal range. According to another report (34), this was previously considered to result from mild mutations of the GHR gene or from subtle postreceptor mechanisms (36, 43, 44). The current results establish the GHR d3 genotype and the IGF-1 P2 epigenotype as major sources of individual variation of the IGF-1 generation test. This can be of importance for understanding the phenotypic diversity in GH sensitivity at the individual level.
The vast majority of the increase in serum IGF-1 concentration occurring in response to GH is considered to result from GH effects on IGF-1 expression in child liver, mediated by GH binding to its hepatic receptor. A weakness of our study is that we could not study the association of liver P2 methylation with GH responsiveness. Such lack of availability and analysis of epigenomes in specific cells of physiological tissues is a common but major limitation of epigenetic epidemiology (45, 46), which most often has to rely on blood cells (47, 48) .
The expression of the IGF-1 gene in liver is controlled by GH at a transcriptional level. In mammals including humans, the IGF-1 gene is composed of six exons and five introns that span greater than 80 kb of chromosomal DNA (49, 50). Tandem promoters direct IGF-1 gene transcription through unique leader exons. Promoter 1, which uses heterogeneous transcription initiation sites, is active in multiple animal tissues (51), whereas the smaller and simpler promoter 2 is primarily but not exclusively active in the liver of cattle (52), unlike in rodents in which promoter 2 activity seems exclusively hepatic (14). GH exerts its effects through the Janus kinase/signal transducer and activator of transcription pathway with translocation of activated signal transducer and activator of transcription-5b transcription factor to the nucleus in which it regulates IGF-1 transcription (53). In the liver of hypophysectomized rats, GH induces dramatic changes in chromatin at the IGF-1 locus and activates IGF-1 transcription by distinct promoter-specific epigenetic mechanisms (54, 55). Yet not only CG location and composition are different in human and rat IGF-1 promoters, but also the pattern of methylation and its transcriptional effects on rat promoters are still unknown.
Although the d3 polymorphism at the GHR locus and the variation of methylation at the IGF-1 P2 promoter account for a total 43% of the individual variability of GH effects on serum IGF-1, the remaining 57% of variance are yet to be explained by other factors. Age, puberty, or sex could be important but do not play a significant role in the current group of children recruited within a relatively narrow age range. Because serum IGF-1 concentration mostly reflects IGF-1 production by the liver in response to GH, it is possible that some of the variance comes from the factors controlling GH pharmacokinetics or from the molecular pathway involved in the hepatic signaling of GH upstream IGF-1 production. This pathway encompasses the GHR, JAK2, and STAT5b gene products whose expression can vary, depending on individual genotypes and epigenotypes. We are not aware that genome-wide association studies or methylome-wide association studies for serum IGF-1 in children (basal or in response to GH) have yet been carried out. The challenge of understanding the remaining part of individual GH sensitivity thus remains entire.
Our observation indicates that genetic and epigenetic variation at the GHR and IGF-1 loci play a major role as independent modulators of individual GH sensitivity.
Acknowledgments
We thank Pfizer France for supporting the GH PharmacoEpigenomics (“GH-EPiGx”) program.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ct
- cycle threshold
- GHR
- GH receptor
- IGFBP3
- IGF binding protein-3
- rhGH
- recombinant human growth hormone
- SDS
- SD score.
References
- 1. Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2. Dahlgren J, Kriström B, Niklasson A, Nierop AFM, Rosberg S, Albertsson-Wikland K. Models predicting the growth response to growth hormone treatment in short children independent of GH status, birth size and gestational age. BMC Med Inform Decis Mak. 2007;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee PA, Sävendahl L, Oliver I, et al. Comparison of response to 2-years' growth hormone treatment in children with isolated growth hormone deficiency, born small for gestational age, idiopathic short stature, or multiple pituitary hormone deficiency: combined results from two large observational studies. Int J Pediatr Endocrinol. 2012;2012(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collett-Solberg PF. Update in growth hormone therapy of children. J Clin Endocrinol Metab. 2011;96(3):573–579. [DOI] [PubMed] [Google Scholar]
- 5. Albertsson-Wikland K, Aronson AS, Gustafsson J, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008;93(11):4342–4350. [DOI] [PubMed] [Google Scholar]
- 6. Sotos JF, Tokar NJ. Growth hormone significantly increases the adult height of children with idiopathic short stature: comparison of subgroups and benefit. Int J Pediatr Endocrinol. 2014;2014(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–154. [DOI] [PubMed] [Google Scholar]
- 8. Cohen P, Germak J, Rogol AD, et al. Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J Clin Endocrinol Metab. 2010;95(5):2089–2098. [DOI] [PubMed] [Google Scholar]
- 9. Cohen P, Rogol AD, Weng W, et al. Efficacy of IGF-based growth hormone (GH) dosing in nonGH-deficient (nonGHD) short stature children with low IGF-I is not related to basal IGF-I levels. Clin Endocrinol (Oxf). 2013;78(3):405–414. [DOI] [PubMed] [Google Scholar]
- 10. Cohen P, Rogol AD, Howard CP, et al. Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. 2007;92(7):2480–2486. [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld RG. The Pharmacogenomics of Human Growth. J Clin Endocrinol Metab. 2006;91(3):795–796. [DOI] [PubMed] [Google Scholar]
- 12. Ranke MB. Treatment of children and adolescents with idiopathic short stature. Nat Rev Endocrinol. 2013;9(6):325–334. [DOI] [PubMed] [Google Scholar]
- 13. Park P, Cohen P. Insulin-like growth factor I (IGF-I) measurements in growth hormone (GH) therapy of idiopathic short stature (ISS). Growth Horm IGF Res. 2005;15(suppl A):S13–S20. [DOI] [PubMed] [Google Scholar]
- 14. Kriström B, Lundberg E, Jonsson B, Albertsson-Wikland K, Study Group. IGF-1 and growth response to adult height in a randomized GH treatment trial in short non-GH-deficient children. J Clin Endocrinol Metab. 2014;99(8):2917–2924. [DOI] [PubMed] [Google Scholar]
- 15. Dos Santos C, Essioux L, Teinturier C, Tauber M, Goffin V, Bougnères P. A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat Genet. 2004;36(7):720–724. [DOI] [PubMed] [Google Scholar]
- 16. Bougnères P. The exon-3 deletion of the growth hormone receptor (GHR) gene still has a limited impact in clinical endocrinology. J Clin Endocrinol Metab. 2010;95(1):56–59. [DOI] [PubMed] [Google Scholar]
- 17. Renehan AG, Solomon M, Zwahlen M, et al. Growth hormone receptor polymorphism and growth hormone therapy response in children: a Bayesian meta-analysis. Am J Epidemiol. 2012;175(9):867–875. [DOI] [PubMed] [Google Scholar]
- 18. Wassenaar MJE, Dekkers OM, Pereira AM, et al. Impact of the exon 3-deleted growth hormone (GH) receptor polymorphism on baseline height and the growth response to recombinant human GH therapy in GH-deficient (GHD) and non-GHD children with short stature: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2009;94(10):3721–3730. [DOI] [PubMed] [Google Scholar]
- 19. Rosenfeld RG. Pharmacogenomics and pharmacoproteomics in the evaluation and management of short stature. Eur J Endocrinol. 2007;157(suppl 1):S27–S31. [DOI] [PubMed] [Google Scholar]
- 20. Kao PC, Matheny AP, Lang CA. Insulin-like growth factor-I comparisons in healthy twin children. J Clin Endocrinol Metab. 1994;78(2):310–321. [DOI] [PubMed] [Google Scholar]
- 21. Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98(11):2612–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palles C, Johnson N, Coupland B, et al. Identification of genetic variants that influence circulating IGF-1 levels: a targeted search strategy. Hum Mol Genet. 2008;17(10):1457–1415. [DOI] [PubMed] [Google Scholar]
- 23. Al-Zahrani A, Sandhu MS, Luben RN, et al. IGF-1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF-1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15(1):1–101. [DOI] [PubMed] [Google Scholar]
- 24. Wong H-L, DeLellis K, Probst-Hensch N, et al. A new single nucleotide polymorphism in the insulin-like growth factor I regulatory region associates with colorectal cancer risk in Singapore Chinese. Cancer Epidemiol Biomarkers Prev. 2005;14(1):144–151. [PubMed] [Google Scholar]
- 25. Ester WA, Hokken-Koelega ACS. Polymorphisms in the IGF-1 and IGF-1R genes and children born small for gestational age: results of large population studies. Best Pract Res Clin Endocrinol Metab. 2008;22(3):415–431. [DOI] [PubMed] [Google Scholar]
- 26. Vaessen N, Janssen JA, Heutink P, et al. Association between genetic variation in the gene for insulin-like growth factor-I and low birthweight. Lancet. 2002;359(9311):1036–1037. [DOI] [PubMed] [Google Scholar]
- 27. Frayling TM, Hattersley AT, McCarthy A, et al. A putative functional polymorphism in the IGF-I gene association studies with type 2 diabetes, adult height, glucose tolerance, and fetal growth in UK populations. Diabetes. 2002;51(7):2313–2316. [DOI] [PubMed] [Google Scholar]
- 28. Toyoshima MTK, Castroneves LA, Costalonga EF, Mendonca BB, Arnhold IJP, Jorge AAL. Exon 3-deleted genotype of growth hormone receptor (GHRd3) positively influences IGF-1 increase at generation test in children with idiopathic short stature. Clin Endocrinol (Oxf). 2007;67(4):500–504. [DOI] [PubMed] [Google Scholar]
- 29. Ivanov M, Kacevska M, Ingelman-Sundberg M. Epigenomics and interindividual differences in drug response. Clin Pharmacol Ther. 2012;92(6):727–736. [DOI] [PubMed] [Google Scholar]
- 30. Ouni M, Belot MP, Castell AL, Fradin D, Bougnères P. The IGF-1 P2 promoter is a major epigenetic locus for GH responsiveness. Pharmacogenomics. Published online ahead of print March 13, 2015. doi:10.1038/tpj.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coutant R, Dörr H-G, Gleeson H, Argente J. Diagnosis of endocrine disease: limitations of the IGF-1 generation test in children with short stature. Eur J Endocrinol. 2012;166(3):351–373. [DOI] [PubMed] [Google Scholar]
- 32. Cole T j., Ahmed M l., Preece M a., Hindmarsh P, Dunger D b. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol (Oxf). Published online ahead of print November 23, 2014. doi: 10.1111/cen.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alberti C, Chevenne D, Mercat I, et al. Serum concentrations of insulin-like growth factor (IGF)-1 and IGF binding protein-3 (IGFBP-3), IGF-1/IGFBP-3 ratio, and markers of bone turnover: reference values for French children and adolescents and z-score comparability with other references. Clin Chem. 2011;57(10):1424–1435. [DOI] [PubMed] [Google Scholar]
- 34. Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86(11):5176–5183. [DOI] [PubMed] [Google Scholar]
- 35. Copeland KC, Underwood LE, Van Wyk JJ. Induction of immunoreactive somatomedin C human serum by growth hormone: dose-response relationships and effect on chromatographic profiles. J Clin Endocrinol Metab. 1980;50(4):690–697. [DOI] [PubMed] [Google Scholar]
- 36. Attie KM, Carlsson LMS, Rundle AC, Sherman BM. Evidence for partial growth hormone insensitivity among patients with idiopathic short stature. J Pediatr. 1995;127(2):244–250. [DOI] [PubMed] [Google Scholar]
- 37. Rudman D, Kutner MH, Blackston RD, Cushman RA, Bain RP, Patterson JH. Children with normal-variant short stature: treatment with human growth hormone for six months. N Engl J Med. 1981;305(3):123–131. [DOI] [PubMed] [Google Scholar]
- 38. Bernabeu I, Alvarez-Escolá C, Quinteiro C, et al. The exon 3-deleted growth hormone receptor is associated with better response to pegvisomant therapy in acromegaly. J Clin Endocrinol Metab. 2010;95(1):222–291. [DOI] [PubMed] [Google Scholar]
- 39. Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2(9):2265–2275. [DOI] [PubMed] [Google Scholar]
- 40. Schwarze CP, Wollmann HA, Binder G, Ranke MB. Short-term increments of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 predict the growth response to growth hormone (GH) therapy in GH-sensitive children. Acta Paediatr Suppl (Oslo Nor). 1999;88(428):200–208. [DOI] [PubMed] [Google Scholar]
- 41. Cook DM, Ludlam WH, Cook MB. Route of estrogen administration helps to determine growth hormone (GH) replacement dose in GH-deficient adults. J Clin Endocrinol Metab. 1999;84(11):3956–3601. [DOI] [PubMed] [Google Scholar]
- 42. Bouhours-Nouet N, Gatelais F, Boux de Casson F, Rouleau S, Coutant R. The insulin-like growth factor-I response to growth hormone is increased in prepubertal children with obesity and tall stature. J Clin Endocrinol Metab. 2007;92(2):629–635. [DOI] [PubMed] [Google Scholar]
- 43. Clayton PE, Freeth JS, Whatmore AJ, Ayling RM, Norman MR, Silva CM. Signal transduction defects in growth hormone insensitivity. Acta Paediatr Suppl (Oslo Nor). 1999;88(428):174–178; discussion 179. [DOI] [PubMed] [Google Scholar]
- 44. López-Bermejo A, Buckway CK, Rosenfeld RG. Genetic defects of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab TEM. 2000;11(2):39–49. [DOI] [PubMed] [Google Scholar]
- 45. Mill J, Heijmans BT. From promises to practical strategies in epigenetic epidemiology. Nat Rev Genet. 2013;14(8):585–594. [DOI] [PubMed] [Google Scholar]
- 46. Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12(8):529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feinberg AP, Irizarry RA, Fradin D, et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med. 2010;2(49):49ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Talens RP, Boomsma DI, Tobi EW, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24(9):3135–3144. [DOI] [PubMed] [Google Scholar]
- 49. Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261(11):4828–4832. [PubMed] [Google Scholar]
- 50. Rotwein P. Structure, evolution, expression and regulation of insulin-like growth factors I and II. Growth Factors. 1991;5(1):3–181. [DOI] [PubMed] [Google Scholar]
- 51. Hall LJ, Kajimoto Y, Bichell D, et al. Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol. 1992;11(4):301–313. [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Price SE, Jiang H. Cloning and characterization of the bovine class 1 and class 2 insulin-like growth factor-I mRNAs. Domest Anim Endocrinol. 2003;25(4):315–328. [DOI] [PubMed] [Google Scholar]
- 53. Heim MH. The Jak-Stat pathway: cytokine signalling from the receptor to the nucleus. J Recept Signal Transduct. 1999;19(1–4):75–120. [DOI] [PubMed] [Google Scholar]
- 54. Chia DJ, Young JJ, Mertens AR, Rotwein P. Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol. 2010;24(4):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chia DJ, Rotwein P. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol. 2010;24(10):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]