Abstract
Context:
Marked elevations of 17-hydroxyprogesterone (17OHP) are characteristic of classic 21-hydroxylase deficiency (21OHD). Testing of 17OHP provides the basis for 21OHD diagnosis, although it suffers from several pitfalls. False-positive or false-negative results and poor discrimination of nonclassic 21OHD from carriers limit the utility of serum 17OHP and necessitate dynamic testing after cosyntropin stimulation when values are indeterminate.
Objective:
The objective was to provide a detailed characterization of 21-carbon (C21) steroids in classic 21OHD, which might identify other candidate steroids that could be employed for the diagnosis of 21OHD.
Setting and Participants:
Patients (11 women, 10 men) with classic 21OHD and 21 sex- and age-matched controls seen in a tertiary referral center were studied.
Methods:
C21 steroids in the peripheral sera from all subjects, as well as in media from cultured testicular adrenal rest tumor (TART) cells and normal adrenal (NA) cells, were analyzed using liquid chromatography/tandem mass spectrometry (10 steroids). Additionally, the dynamics of C21 steroid metabolism in TART and NA cells were assessed with radiotracer studies.
Results:
Five C21 steroids were significantly higher in 21OHD patients: 17OHP (67-fold; P < .01), 21-deoxycortisol (21dF; 35-fold; P < .01), 16α-hydroxyprogesterone (16OHP; 28-fold; P < .01), progesterone (2-fold; P < .01), and 11β-hydroxyprogesterone (11OHP; not detected in controls; P < .01). The same steroids were the highest in media from TART cells relative to the NA cells: 11OHP, 58- to 65-fold; 21dF, 30- to 41-fold; 17OHP, 9-fold; progesterone, 9- to 12-fold; and 16OHP, 7-fold.
Conclusion:
Measurement of 16OHP and 11OHP along with 17OHP and 21dF by liquid chromatography/tandem mass spectrometry might comprise a biomarker panel to accurately diagnose all forms of 21OHD.
Congenital adrenal hyperplasia (CAH) encompasses a group of autosomal recessive enzymatic defects in cortisol biosynthesis. Worldwide, 21-hydroxylase deficiency (21OHD) accounts for over 90% of all CAH cases (1). The spectrum of disease severity is a continuum dependent on the underlying CYP21A2 mutation and on the resulting residual activity of the encoded enzyme. The most severe or classic form of 21OHD leads to complete or nearly complete loss of 21-hydroxylase activity and affects approximately one in 15 000 individuals worldwide (2, 3). Nonclassic or “late onset” 21OHD is one of the most common genetic disorders, occurring in approximately one in 1000 Caucasians and up to one in 27 in certain ethnic groups, such as Hispanics, Yugoslavs, Yupik Eskimos, and Ashkenazi Jews (4–8).
Clinically, adrenal androgen excess is a central feature of 21OHD and prompts the diagnosis more commonly in females. Girls with classic 21OHD are born with masculinized, ambiguous genitalia, whereas adult women with nonclassic 21OHD might present with hirsutism, irregular menses, acne, and subfertility (1, 9), features indistinguishable from those of polycystic ovary syndrome (10). Males with nonclassic 21OHD are sometimes identified due to premature pubarche and accelerated bone age (1), but many remain undiagnosed unless evaluated along with an affected female family member.
Although most patients with classic 21OHD are diagnosed in infancy, often by newborn screening (2), nonclassic cases are typically identified in adolescence or later in life. In both children and adults, 17-hydroxyprogesterone (17OHP), the principal substrate of the defective enzyme, is the initial test in screening for 21OHD (1). Marked elevations of 17OHP, along with cortisol deficiency, are characteristic for classic 21OHD. Intermediate values of 17OHP cannot distinguish nonclassic forms of 21OHD from carriers (11, 12) and mandate dynamic testing with cosyntropin stimulation (13).
Beyond 17OHP, the pathophysiology of 21OHD promotes the accumulation of other 21-carbon (C21) steroids, such as 21-deoxycortisol (21dF) (14–16), which might be formed by the 11β-hydroxylation of 17OHP. The dynamics of cortisol precursor production and metabolism, as well as the potential for generation of additional characteristic C21 steroids in 21OHD, however, has been only minimally investigated. The goal of the present study was to provide a detailed characterization of the C21 steroid profiles in 21OHD.
Patients and Methods
Patient sera
Peripheral serum was obtained from 21 adult patients (11 women) with classic 21OHD, ages 19–59 years (Supplemental Table 1). The diagnosis was based on traditional clinical and biochemical criteria, and in all nine patients for whom genotyping was obtained, classic 21OHD was confirmed. Four samples were obtained at 8 am before the first morning dose of hydrocortisone from women with a serum androstenedione greater than 345 ng/dL (>12 nmol/L) from another study (17). In the other 17 patients, random serum samples were obtained during routine clinical visits. Additionally, we analyzed the sera of 21 sex- and age-matched controls, selected from patients undergoing measurement of a lipid profile, who were not taking corticosteroids. All samples were collected under protocols approved by the Institutional Review Board (IRB) at the University of Michigan. Written informed consent was obtained from 17 21OHD patients. A waiver of consent was granted by the IRB for using any leftover serum collected as part of standard clinical care for the control group and 4 of the 21OHD patients.
Tissue collection
A 7-cm testicular adrenal rest tumor (TART) was surgically removed from a 46-year-old man with classic 21OHD (one copy of CYP21A2 deleted; p.R356W mutation in the remaining copy). Written informed consent was obtained from the patient to use excess tissue for research purposes under a separate IRB-approved protocol. Normal adrenal glands were obtained from cadaveric kidney donors and stored in our biorepository. All studies were conducted after IRB approval.
Cell culture and steroid production experiments
Adrenal and TART cells were isolated as previously described (18). Briefly, tissue was minced and dissociated into a single cell suspension by repeated exposure of the tissue fragments to DMEM/F12 medium (Invitrogen) containing 1 mg/mL collagenase dispase and 0.25 mg/mL DNase-1 (Hoffmann-La Roche Ltd). Four 1-hour cycles of digestion at 37°C and mechanical dispersion were performed. Cells were collected between each digestion and combined before storage at −150°C.
For steroid production experiments, cells were rapidly thawed and plated in 48-well plates at a density of 3600 TART cells/well and 15 000 primary adrenal cells/well, respectively. Cells were grown to 60% confluence in 10% cosmic calf serum with antibiotics. Before treatment, cells were starved with 1% cosmic calf serum for 18 hours. Cells were then treated with ACTH (10 nmol/L), LH (25 ng/mL), or experimental medium. Each experimental condition was run in three biological replicates. Medium was collected after 48 hours for steroid quantitation by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Radiotracer steroid metabolism experiments and analysis
Immediately after media collection from the steroid production experiments (above), the same cells were incubated with 1 mL medium containing pregnenolone, progesterone, or 17OHP (1 μmol/L) and the corresponding [3H]labeled steroid (106 cpm/mL; PerkinElmer). This substrate concentration was chosen to assure enzymatic Vmax conditions for metabolism. Aliquots (0.3 mL) of medium were collected at 2, 4, and 8 hours, transferred to 2-mL microfuge tubes, and extracted with 1 mL dichloromethane. After centrifugation at 8000 rpm for 1 minute, the organic phase was transferred into glass tubes and concentrated under nitrogen. Samples were reconstituted with 20 μL methanol, and 5-μL samples were injected into an Agilent1 260 Infinity HPLC system equipped with UV detector and β-RAM4 in-line scintillation counter (LabLogic). Steroids were resolved on a Kinetex 50 × 2.1 mm, 2.6 μm particle size C8 column (Phenomenex) and methanol-water gradients as described (19), mixed with Bio-SafeII scintillation cocktail (Research Products International), and quantitated by integration of radioactivity peaks using Laura4 software (LabLogic).
Steroid quantitation by LC-MS/MS
A 50- or 100-μL aliquot of serum or medium was deproteinated with 225 μL acetonitrile containing 200 μL internal standard deuterated steroids (C/D/N Isotopes and Sigma-Aldrich) (Supplemental Table 2) at known concentrations and 150 μL methanol. The suspension was mixed and centrifuged for 5 minutes at 15 000 rpm. The supernatant was extracted with 1 mL of methyl-t-butyl ether, and the organic phase was concentrated under nitrogen. The dried extract was reconstituted with 100–200 μL of methanol/deionized water (1:1) and transferred to a 0.25-mL vial insert. Samples (10 μL) were injected via autosampler and resolved with an Agilent 1290 binary pump HPLC on a Kinetex 50 × 2.1 mm, 3 μm particle size biphenyl column (Phenomenex) using gradient elution with 0.25 mmol/L ammonium fluoride and methanol. The column effluent was directed into the source of an Agilent 6490 triple quadrupole mass spectrometer using electrospray ionization in positive ionization mode and analyzed using multiple reaction monitoring mode. Quantitation was accomplished by comparing ion currents for the monitored ions with 13-point quadratic external calibration curves (r2 was minimum 0.995) and corrected for specimen dilution and recovery of internal standards using ChemStation and MassHunter software (Agilent). Intra-assay coefficients of variability ranged from 2 to 8% for steroid concentrations > 100 ng/dL and from 5 to 18% for steroid concentrations < 100 ng/dL. The lower limit of detection for each steroid, defined as the minimum concentration achieving an extrapolated signal-to-noise ratio of 3, ranged from 0.7 to 30 ng/dL (Supplemental Table 2). Linearity of response was assessed by measuring four separate dilutions per sample (n = 3 samples), which rendered r2 values consistently > 0.95.
Protein extraction and protein assay
Cells were lysed in 0.1 mL Mammalian Protein Extraction Reagent (Pierce Chemical Co), and the protein content was estimated by the bicinchoninic acid protein assay using the micro bicinchoninic acid protocol (Thermo Scientific).
Statistical analyses
Nonparametric Mann-Whitney U test was employed to compare the 21OHD patients and controls, using GraphPad Prism 6 (GraphPad Software). Comparison between TART and normal adrenal cells was assessed using the mean steroid concentrations per mass of protein from three biological replicates. Correlation between pairs of steroids was assessed using the nonparametric Spearman correlation test. Statistical significance was accepted for P < .05.
Results
C21 steroids profile in sera of 21OHD patients
Using LC-MS/MS, we compared the abundance of 10 C21 steroids in the sera of both 21OHD patients and controls (Table 1). Five C21 steroids were significantly higher in 21OHD patients: 17OHP (67-fold; P < .0001), 21dF (35-fold; P < .0001), 16α-hydroxyprogesterone (16OHP; 28-fold; P < .0001), progesterone (2-fold; P < .01), and 11β-hydroxyprogesterone (11OHP). All controls had undetectable 11OHP, whereas nine of 21 (43%) 21OHD patients had detectable levels (33–387 ng/dL). Within the 21OHD group, there was a strong correlation between serum 17OHP and 16OHP concentrations (r = 0.96; P < .0001), and a weaker correlation between serum 17OHP and 21dF (r = 0.6; P = .003) or 11OHP concentrations (r = 0.7; P = .0002), respectively (Supplemental Figure 1). No direct correlation was observed between serum 21dF and 11OHP concentrations (r = 0.4; P = .1).
Table 1.
Concentrations of C21 Steroids (ng/dL) in Serum
| 21OHD Patients (n = 21) | Controls (n = 21) | Fold | P | |
|---|---|---|---|---|
| Progesterone | 540 [251.5–1899] | 245 [137–358.5] | 2.2 | <.01 |
| 17OHP | 7875 [830–20 202] | 118 [36–178] | 66.7 | <.0001 |
| 16OHP | 111 [15.5–551.5] | 4 [0.5–7.5] | 27.8 | <.0001 |
| 21dF | 1130 [322–6355] | 32 [22–68.5] | 35.3 | <.0001 |
| 11OHP | 0 [0–49] | 0 [0–0] | a | <.01 |
| 11-Deoxycorticosterone | 7 [3.5–11] | 6 [3–5] | 1.2 | .7 |
| 11-Deoxycortisol | 11 [7–53.5] | 58 [24–151] | 0.2 | <.01 |
| Corticosterone | 24 [6.5–133] | 295 [110.5–558] | 0.1 | <.0001 |
| Cortisol | 2295 [429–4632] | 10 395 [6660–12 450] | 0.2 | <.0001 |
| Cortisone | 459 [110–1163] | 2195 [1492–2666] | 0.2 | <.0001 |
Data are expressed as median [interquartile range]. Folds represent the 21OHD/controls ratio and were calculated using the medians for each steroid. To convert ng/dL to nmol/L, multiply by 0.0318 for progesterone; 0.0303 for 11OHP, 16OHP, 17OHP, and 11-deoxycorticosterone; 0.0289 for 11-deoxycortisol, 21dF, and corticosterone; 0.0276 for cortisol; and 0.0275 for cortisone.
Nine of 21 (43%) 21OHD patients and no controls had detectable levels of 11OHP.
Of the 11 female participants in this study, two were in the luteal phase, three had amenorrhea (one was 3 months postpartum, not nursing), two were postmenopausal, and four were taking continuous oral contraceptive pills. No significant difference was observed between males and females for 21dF (P = .45), 16OHP (P = .17), or 11OHP (P = .4); 17OHP was higher in women (P = .04).
Steroid profile of 21OHD TART cells: LC-MS/MS C21 steroid quantitation
To estimate steroid production directly from 21OHD adrenals compared to controls, we measured the same 10 C21 steroids in media from TART cells obtained from a 21OHD patient and normal adrenal cells, under basal conditions and after ACTH or LH stimulation. For comparison between TART and normal adrenal cells, steroid concentrations were corrected for protein mass. The five most abundant C21 steroids produced by the TART cells at baseline were 17OHP (64% of measured steroids), 21dF (16%), 16OHP (8%), progesterone (6%), and 11OHP (4%), proportions similar to that found in this patient's serum (Table 2). After ACTH stimulation, 17OHP, 21dF, 16OHP, and progesterone remained the predominant steroids (75, 7, 8, and 5% of measured steroids, respectively). In contrast, cortisol and 11-deoxycortisol dominated the steroid profiles of normal adrenal cells, both at baseline (41 and 25% of measured steroids, respectively) and after ACTH stimulation (65 and 14% of measured steroids, respectively). For the normal adrenal cells, 17OHP was the fourth most abundant steroid at baseline (9%) and the third most abundant after ACTH stimulation (10%), whereas 21dF, 16OHP, and 11OHP were produced in only negligible amounts. LH treatment resulted in steroid profiles similar to those seen in untreated TART cells or normal adrenal cells, respectively (data not shown).
Table 2.
Percentages of C21 Steroids in Patient Serum and TART Cell-Conditioned Medium
| Steroid | % of Steroids |
TART/NAa |
|||||
|---|---|---|---|---|---|---|---|
| Patient Serum | TART Basal | TART ACTHb | NA Basal | NA ACTH | Basal | ACTH | |
| Progesterone | 1.4 | 6 | 4.8 | 0.8 | 0.5 | 8.7 | 11.7 |
| 17OHP | 78 | 64 | 75 | 8.7 | 9.8 | 8.8 | 9.2 |
| 16OHP | 6.6 | 7.7 | 8.25 | 1.4 | 1.5 | 6.6 | 6.6 |
| 11OHP | 0.2 | 4 | 1 | 0.08 | 0.02 | 58.5 | 65 |
| 21dF | 7 | 15.5 | 7 | 0.46 | 0.3 | 41 | 30 |
| 11-Deoxycorticosterone | 0.2 | 0.14 | 0.05 | 2.4 | 1.3 | 0.07 | 0.04 |
| 11-Deoxycortisol | 0.45 | 0.45 | 0.24 | 25 | 14.1 | 0.02 | 0.02 |
| Corticosterone | 0.6 | 0.6 | 0.8 | 3.4 | 5.65 | 0.2 | 0.17 |
| Cortisol | 4.6 | 1.75 | 2.6 | 41.5 | 64.5 | 0.05 | 0.05 |
| Cortisone | 1.9 | 0.15 | 0.06 | 16 | 2.3 | 0.01 | 0.03 |
Abbreviation: NA, normal adrenal. Medium was collected under basal conditions and after ACTH (10 nmol/L) stimulation. Ratios were calculated by dividing the means of steroid concentrations from three biological replicates for each condition.
TART cells from a patient with 21OHD/NA cells. Patient serum is from the same patient before TART excision surgery.
Under these conditions, we estimate a total steroid production rate of approximately 100 ng/10 000 cells/24 h.
The ratio of individual steroids produced between TART and normal adrenal cells was highest for 11OHP (58- and 65-fold) and 21dF (41- and 30-fold) at baseline and after ACTH stimulation, respectively (Table 2). In comparison, 17OHP production was approximately 9-fold higher at baseline and after ACTH stimulation in TART cells vs normal adrenal cells; progesterone production was 9-fold higher at baseline and 12-fold higher after ACTH stimulation; and 16OHP production was 7-fold higher by TART cells relative to the normal adrenal cells, both at baseline and after ACTH stimulation.
Steroid metabolism in 21OHD TART cells
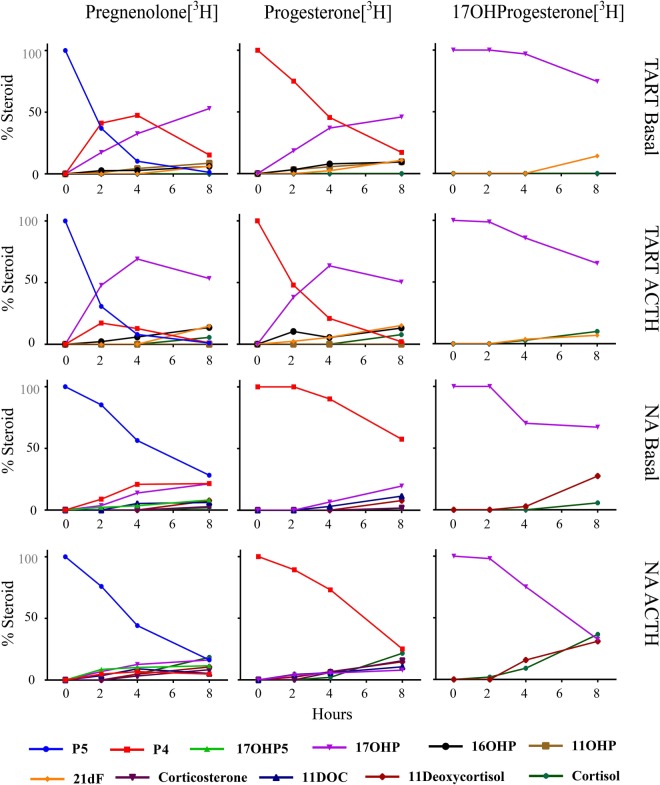
To elucidate the pathways of C21 steroid formation in the 21OHD adrenal, we performed a time course of steroid metabolism using cultured TART cells and radiotracer steroids (Figure 1). In agreement with our LC-MS/MS data, we found predominant accumulation of the radiolabeled substrates as 17OHP, 21dF, 16OHP, and 11OHP in the TART cells and minimal cortisol production. Conversely, the normal adrenal cells produced a balanced panel of steroids late in the traditional glucocorticoid and mineralocorticoid pathways, with no measurable 21dF, 16OHP, or 11OHP.
Figure 1.
Steroid metabolism in TART cells and normal adrenal (NA) cells. Cells were first treated with ACTH (10 nmol/L) or experimental medium for 48 hours. Immediately after media collection, cells were incubated with medium containing pregnenolone (P5), progesterone (P4), or 17OHP (1 μmol/L) and the corresponding [3H]labeled steroid (106 cpm/mL). Aliquots of medium were collected at 2, 4, and 8 hours. Samples were extracted, and radioactivity was quantified using HPLC. Data shown represent the percentage of total detected radiolabeled steroids (% steroid). 17OHP5, 17α-hydroxypregnenolone; 11DOC, 11-deoxycorticosterone.
Discussion
In addition to the 17OHP and 21dF as established biomarkers of 21OHD, we demonstrate elevated production of 16OHP and 11OHP in 21OHD patients compared to matched unaffected subjects. These steroids are direct products of the adrenal because cultured TART cells from a 21OHD patient metabolized pregnenolone and progesterone primarily to these C21 steroids.
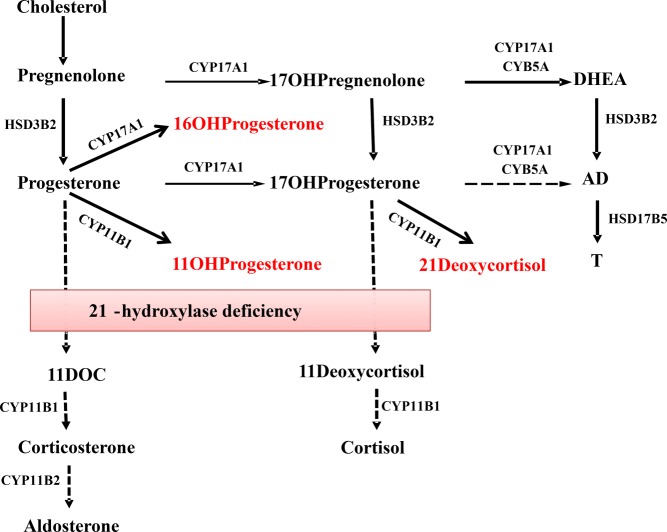
Deficiency of 21-hydroxylase promotes the accumulation of progesterone and its conversion to 17OHP, but also to other unconventional steroids, including 16OHP, 21dF, and 11OHP via the enzymes CYP17A1 and CYP11B1 (Figure 2). Human CYP17A1 17-hydroxylates pregnenolone and progesterone with equal efficiencies (20, 21) but also 16α-hydroxylates up to 30% of progesterone (22). In 1969, using radiolabeled substrates and chromatography, Janoski et al (23) showed that excretion of 16OHP was substantially higher in the urine of two patients with classic 21OHD as compared to controls, particularly when the glucocorticoid replacement was discontinued. This study should be interpreted with caution because CYP3A4 in the liver also metabolizes progesterone to 16OHP (24), and hepatic rather than adrenal metabolism dominates the disposition of peripherally administered steroids. We could find no further studies that investigated 16OHP production in 21OHD since this first report. Herein, we implemented accurate LC-MS/MS quantitation of 16OHP in the peripheral serum of 21OHD patients as well as in 21OHD TART cells. We documented a 28-fold elevation of 16OHP in the peripheral serum of patients with classic 21OHD compared to controls, despite concurrent glucocorticoid replacement therapy, which limits the accumulation of these biomarker steroids. Furthermore, 16OHP was the third most abundant C21 steroid produced by TART cells in culture.
Figure 2.
Pathways of C21 steroids synthesis in 21OHD. HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; CYP17A1, 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome b5 type A; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; HSD17B5, 17β-hydroxysteroid dehydrogenase type 5.
In the normal steroidogenic pathways to aldosterone and cortisol, progesterone and 17OHP are first hydroxylated at C-21 by CYP21A2 and subsequently metabolized further. In 21OHD, progesterone and 17OHP accumulate and are both substrates for 11-hydroxylase (CYP11B1), leading to11OHP and 21dF, respectively. Elevated levels of 21dF have been previously documented in patients with 21OHD, and both 21dF and the (21dF+17OHP)/cortisol ratio by LC-MS/MS have been employed to improve the positive predictive value of newborn screening, for which 17OHP alone affords many false-positives (25–27). Cosyntropin-stimulated levels of 21dF have been proven useful in distinguishing heterozygote carriers from nonclassic 21OHD (14, 15, 27). We confirm that 21dF is a good discriminator of 21OHD from control patients, even in random samples during treatment, with a 35-fold elevation in 21OHD.
In contrast, we found no reports that measured 11OHP in 21OHD patients. Although 11OHP is produced in only small amounts in comparison to 17OHP, 16OHP, or 21dF, its simultaneous presence might add diagnostic value when corroborated with the other three more abundant C21 steroids. Although our assay had low sensitivity for 11OHP relative to the other steroids measured, the absence of measurable 11OHP in all controls suggests that this steroid might increase the specificity of 21OHD testing when present. In our radiotracer studies, 11OHP was produced from progesterone only by the TART cells under basal conditions. After ACTH stimulation, 21dF further increased, whereas 11OHP was no longer measurable, suggesting that ACTH-mediated induction of CYP17A1 subsequently directs the steroid flux toward 21dF.
Marked elevations of 17OHP are a hallmark of 21OHD, and 17OHP has traditionally been used for both diagnosis and disease monitoring (28). Nonetheless, serum 17OHP testing has multiple pitfalls. False-positive results are common in premature and sick newborns (29, 30). Weight- and gestational age-adjusted cutoffs for 17OHP have been implemented by many centers to improve the positive predictive value of newborn screening protocols (31–33). False-negative rates of up to 22% have been reported in infant screening (34) and are more common for girls (35), particularly when mothers were exposed to glucocorticoids prenatally (36). Although 17OHP is generally adequate for diagnosing classic 21OHD, basal 17OHP alone often does not discriminate between nonclassic 21OHD and carriers (11, 12), and intermediate 17OHP values require dynamic confirmatory testing under cosyntropin stimulation (13).
The use of TART cells in culture as a model of the 21OHD adrenal is a limitation of our study. Other groups, however, have found that the expression of steroidogenic enzymes and the production of steroids in TART tissue are similar to that of the 21OHD adrenal (37–40). In addition, treatment of these TART cells with LH did not demonstrably alter the steroid production profile compared to basal conditions. Furthermore, the relative proportions of the steroids secreted were similar in medium from cultured TART cells and serum from the same patient (Table 2). The 11OHP concentrations were too low to measure in sera from many patients, and future assay modifications might improve the limit of detection. Nevertheless, LC-MS/MS assays avoid the inherent cross-reactivity problems of immunoassays and permit comparison of multiple steroids using small samples. Of the steroids measured, 17OHP, which also derives from the ovary, was the only biomarker that was significantly higher in women. Our cohort, however, was heterogeneous in regard to disease control, treatment regimens, and compliance. Consequently, we cannot conclude that this difference can be attributed to sex. Because our 21OHD patients did not interrupt glucocorticoid treatment when providing samples, differences in biomarker steroids with control patients might be even greater in untreated and poorly controlled 21OHD patients.
In summary, in addition to 17OHP and 21dF, we demonstrate the elevation of two other upstream C21 steroids in 21OHD: 16OHP and 11OHP. Larger studies, including patients undergoing diagnostic testing for both classic and nonclassic 21OHD before initiation of glucocorticoids, are required to validate the utility of these steroids and to optimize parameters for diagnosis and treatment monitoring. Implementation of LC-MS/MS allows simultaneous measurement of multiple steroids from a single small volume sample. A panel of steroids upstream of the enzymatic blockage and their ratio to cortisol might improve the sensitivity and specificity of 21OHD diagnostic testing in the future.
Acknowledgments
We thank Dr Thomas Giordano and the Molecular Pathology Research Laboratory (Department of Pathology, University of Michigan) for TART tissue procurement. We thank Janssen Research and Development for returning leftover serum samples from study 212082HPL1002 under written informed consent for research purposes.
This work was supported by pilot grants from the University of Michigan Reproductive Sciences Program and MCubed 1.0 Pilot Cycle and by National Institutes of Health Grants R01GM086596 (to R.J.A.) and R01DK069950 (to W.E.R.). A.F.T. was supported by Grant F32DK103461 from the National Institutes of Health. Mass spectrometry used core services supported by Grant DK089503 from the National Institutes of Health to the University of Michigan under the Michigan Nutrition Obesity Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CAH
- congenital adrenal hyperplasia
- 21dF
- 21-deoxycortisol
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- 21OHD
- 21-hydroxylase deficiency
- 11OHP
- 11β-hydroxyprogesterone
- 16-OHP
- 16α-hydroxyprogesterone
- 17OHP
- 17-hydroxyprogesterone
- TART
- testicular adrenal rest tumor.
References
- 1. Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–788. [DOI] [PubMed] [Google Scholar]
- 2. Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. [DOI] [PubMed] [Google Scholar]
- 3. Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:15–30. [DOI] [PubMed] [Google Scholar]
- 4. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–291. [DOI] [PubMed] [Google Scholar]
- 5. Pang S, Murphey W, Levine LS, et al. A pilot newborn screening for congenital adrenal hyperplasia in Alaska. J Clin Endocrinol Metab. 1982;55:413–420. [DOI] [PubMed] [Google Scholar]
- 6. Ferenczi A, Garami M, Kiss E, et al. Screening for mutations of 21-hydroxylase gene in Hungarian patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1999;84:2369–2372. [DOI] [PubMed] [Google Scholar]
- 7. Pang SY, Wallace MA, Hofman L, et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81:866–874. [PubMed] [Google Scholar]
- 8. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650–667. [PMC free article] [PubMed] [Google Scholar]
- 9. Azziz R, Dewailly D, Owerbach D. Clinical review 56: nonclassic adrenal hyperplasia: current concepts. J Clin Endocrinol Metab. 1994;78:810–815. [DOI] [PubMed] [Google Scholar]
- 10. Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. [DOI] [PubMed] [Google Scholar]
- 11. New MI, Lorenzen F, Lerner AJ, et al. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983;57:320–326. [DOI] [PubMed] [Google Scholar]
- 12. Wilson RC, Mercado AB, Cheng KC, New MI. Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab. 1995;80:2322–2329. [DOI] [PubMed] [Google Scholar]
- 13. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa-Barbosa FA, Carvalho VM, Nakamura OH, Bachega TA, Vieira JG, Kater CE. Zona fasciculata 21-hydroxysteroids and precursor-to-product ratios in 21-hydroxylase deficiency: further characterization of classic and non-classic patients and heterozygote carriers. J Endocrinol Invest. 2011;34:587–592. [DOI] [PubMed] [Google Scholar]
- 15. Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, et al. Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2010;73:700–706. [DOI] [PubMed] [Google Scholar]
- 16. Milewicz A, Vecsei P, Gruszka S, Szymczak J, Bednarek-Tupikowska G, Grabiski M. Diagnosis of congenital adrenal hyperplasia based on plasma 21-deoxycortisol level determined with a specific radioimmunoassay. Mater Med Pol. 1984;16:95–98. [PubMed] [Google Scholar]
- 17. Auchus RJ, Buschur EO, Chang AY, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99:2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassett MH, Suzuki T, Sasano H, et al. The orphan nuclear receptor NGFIB regulates transcription of 3β-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630. [DOI] [PubMed] [Google Scholar]
- 19. Peng HM, Liu J, Forsberg SE, Tran HT, Anderson SM, Auchus RJ. Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5. J Biol Chem. 2014;289:33838–33849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. [DOI] [PubMed] [Google Scholar]
- 21. Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995;308:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swart AC, Storbeck KH, Swart P. A single amino acid residue, Ala 105, confers 16α-hydroxylase activity to human cytochrome P450 17α-hydroxylase/17,20 lyase. J Steroid Biochem Mol Biol. 2010;119:112–120. [DOI] [PubMed] [Google Scholar]
- 23. Janoski AH, Roginsky MS, Christy NP, Kelly WG. On the metabolism of 16-hydroxy-C21-steroids. III. Evidence for high rates of production of 16α-hydroxyprogesterone and 16α-hydroxypregnenolone in the salt-losing form of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1969;29:1301–1309. [DOI] [PubMed] [Google Scholar]
- 24. Gomes LG, Huang N, Agrawal V, Mendonça BB, Bachega TA, Miller WL. Extraadrenal 21-hydroxylation by CYP2C19 and CYP3A4: effect on 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2009;94:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fiet J, Gueux B, Raux-Demay MC, et al. 21-deoxycortisol. A new marker of virilizing adrenal hyperplasia caused by 21-hydroxylase deficiency [in French]. Presse Med. 1989;18:1965–1969. [PubMed] [Google Scholar]
- 26. Janzen N, Peter M, Sander S, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92:2581–2589. [DOI] [PubMed] [Google Scholar]
- 27. Bidet M, Bellanné-Chantelot C, Galand-Portier MB, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab. 2009;94:1570–1578. [DOI] [PubMed] [Google Scholar]
- 28. Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98:2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coulm B, Coste J, Tardy V, et al. Efficiency of neonatal screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency in children born in mainland France between 1996 and 2003. Arch Pediatr Adolesc Med. 2012;166:113–120. [DOI] [PubMed] [Google Scholar]
- 30. Cavarzere P, Samara-Boustani D, Flechtner I, et al. Transient hyper-17-hydroxyprogesteronemia: a clinical subgroup of patients diagnosed at neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol. 2009;161:285–292. [DOI] [PubMed] [Google Scholar]
- 31. Steigert M, Schoenle EJ, Biason-Lauber A, Torresani T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J Clin Endocrinol Metab. 2002;87:4106–4110. [DOI] [PubMed] [Google Scholar]
- 32. Olgemöller B, Roscher AA, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab. 2003;88:5790–5794. [DOI] [PubMed] [Google Scholar]
- 33. van der Kamp HJ, Oudshoorn CG, Elvers BH, et al. Cutoff levels of 17-α-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J Clin Endocrinol Metab. 2005;90:3904–3907. [DOI] [PubMed] [Google Scholar]
- 34. Sarafoglou K, Banks K, Kyllo J, Pittock S, Thomas W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA. 2012;307:2371–2374. [DOI] [PubMed] [Google Scholar]
- 35. Varness TS, Allen DB, Hoffman GL. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. J Pediatr. 2005;147:493–498. [DOI] [PubMed] [Google Scholar]
- 36. Gatelais F, Berthelot J, Beringue F, et al. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: implication for neonatal screening of congenital adrenal hyperplasia. Pediatr Res. 2004;56:701–705. [DOI] [PubMed] [Google Scholar]
- 37. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, et al. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92:3674–3680. [DOI] [PubMed] [Google Scholar]
- 38. Bercovici JP, Fiet J, Gibault L, et al. Testicular adrenal rest tumours in salt wasting congenital adrenal hyperplasia (in vivo and in vitro studies). J Steroid Biochem Mol Biol. 2005;93:67–72. [DOI] [PubMed] [Google Scholar]
- 39. Combes-Moukhovsky ME, Kottler ML, Valensi P, Boudou P, Sibony M, Attali JR. Gonadal and adrenal catheterization during adrenal suppression and gonadal stimulation in a patient with bilateral testicular tumors and congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1994;79:1390–1394. [DOI] [PubMed] [Google Scholar]
- 40. Blumberg-Tick J, Boudou P, Nahoul K, Schaison G. Testicular tumors in congenital adrenal hyperplasia: steroid measurements from adrenal and spermatic veins. J Clin Endocrinol Metab. 1991;73:1129–1133. [DOI] [PubMed] [Google Scholar]