Abstract
Context:
Estrogen levels and their metabolites are higher in obese vs lean postmenopausal women, and obesity increases breast cancer risk. Quinone derivatives of 4-hydroxylated estrogen metabolites, independently of the estrogen receptor, cause depurination and impaired DNA repair (genotoxic). 16α-Hydroxy (16α-OH)-estrone (E1), eg, promotes tumor proliferation and 2-methoxy-estradiol (E2) may be chemoprotective. Childhood obesity increases breast cancer death risk in women, but levels of estrogen derivatives had not been previously studied in young children.
Objective:
The objective of the study was to investigate whether total and genotoxic estrogens are increased in prepubertal obese girls compared with lean controls.
Design:
Stored sera from 12 lean and 23 obese prepubertal girls (Tanner stage I breast and pubic hair) studied previously were assayed for E1, E2, and their multiple metabolites (12 steroids total) using highly sensitive liquid chromatography and tandem mass spectrometry.
Results:
E2 concentrations were significantly higher in obese [3.45 (0.5, 4.65) pg/ml (median [quartile 1, quartile 3])] vs lean girls [0.5 (0.5, 2.37), P = .04], 57% of values upper quartile or greater (quartile 3) of controls. Concentrations of 16α-OH-E1 were higher in obese [7.17 (0.5, 9.64) pg/mL] vs lean girls [0.5 (0.5, 1.72, P = .007)], 65% of values quartile 3 or greater of controls. 2-Methoxy-E2 concentrations were lower in the obese group (P = .012). 16α-OH-E1 concentrations were positively correlated with body mass index, percentage fat mass, and IL-6 concentrations (P < .001).
Conclusions:
E2 and genotoxic metabolites were higher in obese vs lean prepubertal girls. These data suggest that obesity is associated with an increased extraglandular estrogen production and metabolism before the onset of puberty in girls. Long-term epidemiological studies are needed to assess any potential increase in breast cancer risk.
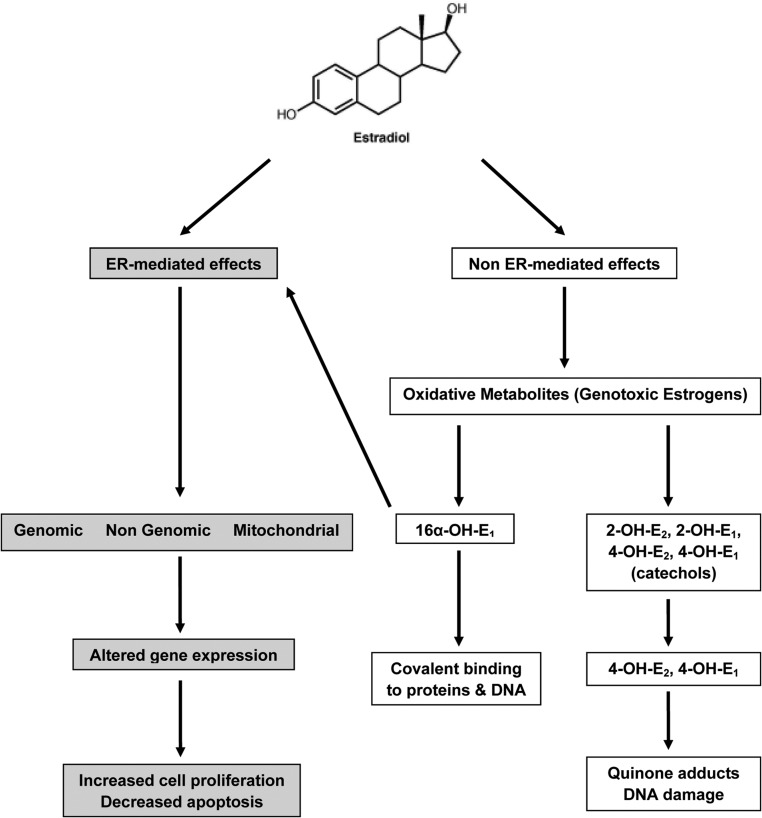
A large body of data suggests that there is a mechanistic link between estradiol production in women and the development of breast cancer. Epidemiological studies show a higher breast cancer risk is linked to early menarche, late menopause, use of postmenopausal hormone therapy and circulating estrogen levels (1–3). A current hypothesis suggests that this increased risk with estrogen exposure involves both estrogen receptor (ER)-mediated increased cell proliferation and ER-independent effects of estradiol metabolites (4). Estradiol (17β-E2) is extensively metabolized to catechol derivatives with hydroxylations at the 2 and 4 positions to form 2-hydroxyestradiol (2-OH-E2) and 4-hydroxyestradiol (4-OH-E2), respectively. The 4-OH-E2 is mutagenic in rat and human cells (5, 6) and is associated with endometrial cancer in vivo (7). Both these catechol estrogenic compounds are further metabolized to their detoxified derivatives 2-methoxy (MeO)-3-hydroxy-17β-estradiol (2-MeO-E2) and 4-MeO-E2. The 2-MeO-E2 in particular is considered chemopreventive (8, 9). The quinones formed from catechol estrogens are backconverted to 4-hydroxyestrogens via quinone reductase to create a redox cycle, resulting in the generation of reactive oxygen species, also forming unstable depurinating adducts (particularly the 3,4 quinones) with adenine and guanine. These adducts in turn cause depurination of DNA, resulting in error-prone DNA repair, ultimately increasing the risk for mutagenesis in breast tissue (10–12). Hence, the term, genotoxic estrogens, is used to describe these mutagenic and potentially carcinogenic estrogen metabolites, a subject thoroughly reviewed previously (4, 9). Estrone (E1) and E2 in addition to undergoing similar metabolism to the 2- and 4-OH-E1 metabolites also undergo metabolism to 16α-hydroxyestrone (16α-OH-E1), a compound thought to be involved in tumor proliferation due to its genotoxic effects and through activation of the ER (13–17) (Figure 1).
Figure 1.
Schematic representation of ER-mediated and non-ER-mediated metabolism. 16α-OH-E1 operates both ways through covalent binding to proteins and DNA and by activating the ER.
Estrogen levels and their metabolites are higher in obese vs lean postmenopausal women as a result of extraglandular steroid production through the aromatase enzyme. In these women, the risk of breast cancer is associated with the levels of parent estrogens, E1 and E2, and with their metabolites. A large body of epidemiological data show that obesity is associated with increased risk and mortality for breast cancer in postmenopausal women (18–21), and ever being overweight in childhood increases the risk of all-cause and breast cancer deaths in women, with 2.6 times higher risk if obese as a child than not obese (22). The mechanisms of these associations have not been well characterized.
The development of highly sensitive stable isotope dilution liquid chromatography-selected reaction monitoring/mass spectrometry assays for estrogen metabolites now allows for their quantification at much lower concentrations than previously possible (9, 23). The new high-sensitivity approach uses preionized N-methyl pyridinium sulfonyl derivatives of the estrogens coupled with positive nanospray ionization. This technique makes it possible to quantify very small estrogen concentrations in plasma of very young children. Using the new high sensitivity estrogen assays, we designed studies to examine whether genotoxic estrogens are increased in obese girls prior to the onset of puberty, another clinical situation in which ovarian function is inactive and extraglandular production can be analyzed, as compared with age-matched lean controls.
Materials and Methods
Study subjects
Studies were approved by the Institutional Review Board at the Wolfson Children's Hospital (Jacksonville, Florida) and informed written consent obtained from the parents and children's assent. Stored sera were used from 35 prepubertal girls recruited as part of a previously reported study in childhood obesity published earlier (24, 25). We intentionally selected only subjects who had no signs of puberty, ie, had both breast and pubic hair Tanner stage I and were either obese [body mass index (BMI) > 95th percentile] or lean (BMI 10 to < 85th percentile). All participants had normal lipid panels, glucose tolerance, and blood pressure. Stored sera were assayed for parent estrogens, E1, and E2, and their multiple metabolites (12 steroids total). All subjects had a physical examination with anthropometric parameters recorded.
Anthropometric and laboratory data
Concentrations of highly sensitive C-reactive protein (hsCRP), IL-6, fibrinogen, insulin, and glucose (to calculate the homeostatic model assessment for insulin sensitivity) were measured as previously described (24). Accurate height was measured by a Harpenden stadiometer and weight by a digital scale to calculate BMI. All subjects had a physical examination at the Pediatric Endocrine Clinic at the Nemours Children's Clinic (Jacksonville, Florida) and pubertal assessment made according to the standards of Tanner. All subjects had body composition assessed by dual X-ray absorptiometry (DEXA) using a Hologic DEXA.
Estrogen assays
E2, E1, and their 4-hydroxy (OH), 2-OH, 16α-OH, 4-methoxy (MeO), and 2-MeO metabolites (12 steroids in all) were quantified using new state-of-the-art liquid chromatography-mass spectrometry (LC-MS) methodology as described recently by the Blair laboratory (23). The marked improvement in sensitivity results from four factors: 1) the reduced background from microflow liquid chromatography techniques; 2) the latest generation mass spectrometer used, which has a 20-fold higher theoretical level of sensitivity; 3) the use of [13C]-labeled rather than deuterium-labeled steroids, which allows the precise coelution of specific analytes and their corresponding internal standards; and 4) the novel use of preionized Girard P hydrazone and N-methyl-pyridinium-sulfonate derivatives. This enabled a much higher sensitivity than with standard stable isotope dilution LC-MS-based methods. For E1 levels, the total of conjugated and unconjugated steroids was measured. Samples were incubated at 37°C in β-glucuronidase/arylsulfatase to cleave the conjugation bond and to release free steroid. The lower limits of quantitation for serum E2, E1, 16α-OH-E2, 16α-OH-E1, 4-MeO-E2, 4-Meo-E1, 2-MeOE2, and 2-MeO-E2 were 1 fg on the column and were 10 fg on the column for 4-OH-E2, 4-OH-E1, 2-OH-E2, and 2-OH-E1. All analytes demonstrated a linear response from 0.5 to 200 pg/mL (5–2000 pg/mL for 4-OH-E2, 4-OH-E1, 2-OH-E2, and 2-OH-E1). The coefficients of variation for analysis of estrogen standards extracted from charcoal-stripped plasma were all less than 10% and accuracies were between 85% and 115%.
Statistical analysis
Descriptive statistics were performed comparing differences in all anthropometric characteristics and estrogen metabolites between the lean and obese group using a Student's t test. Due to a lack of normality and failure to meet the assumptions of parametric models and tests, we report data as medians and interquartile [quartile (Q) 1, Q3)] range and perform a nonparametric Mann-Whitney U test to compare differences in median between the groups. Significance was established as P < .05. Ratios for statistically significant analytes were calculated dividing analyte concentration over parent compound concentration (eg, 16α-OH-E1/ E1). The ratio of 2-OH-E1 to 16α-OH-E1 was also calculated. Spearman rank correlation was calculated between the key variables found significantly different between the lean and obese groups and pertinent anthropometric measures of adiposity (BMI, percentage fat mass) and laboratory measures of inflammation (hsCRP, IL-6). The statistical analysis software (SAS Institute) was used for these calculations.
Results
Stored sera from lean (n = 12) and obese (n = 23) (BMI > 95th percentile) prepubertal girls who had participated in a previous study were used to measure the parent estrogens. Their clinical characteristics are summarized in Table 1.
Table 1.
Clinical Characteristics of Prepubertal Study Subjects
| Lean | Obese | P Value | |
|---|---|---|---|
| n | 12 | 23 | |
| Age, y | 9.5 ± 0.3 | 9.0 ± 0.2 | NS |
| Race | 83% C, 8% AA, 8% O | 70% C, 17% AA, 9% O, 4% H | |
| BMI, kg/m2 | 16.2 ± 0.3 | 27.8 ± 0.6 | <10−6 |
| BMI Z score | −0.2 ± 0.1 | 2.3 ± 0.1 | <10−6 |
| Waist circumference, cm | 59.4 ± 1.2 | 87.9 ± 1.3 | <10−6 |
| Fat mass, % | 23.2 ± 1.0 | 42.1 ± 0.8 | <10−6 |
| hsCRP, mg/L | 0.2 ± 0.0 | 2.4 ± 0.5 | 0.002 |
| Fibrinogen, mg/dL | 298 ± 19 | 358 ± 15 | 0.02 |
| IL-6, pg/mL | 0.4 ± 0.1 | 2.3 ± 0.4 | 0.001 |
| HOMA | 1.4 ± 0.3 | 3.8 ± 0.5 | <10−3 |
Abbreviation: AA, African American; H, Hispanic; HOMA, homeostatic model assessment; NS, not significant; O, Oriental.
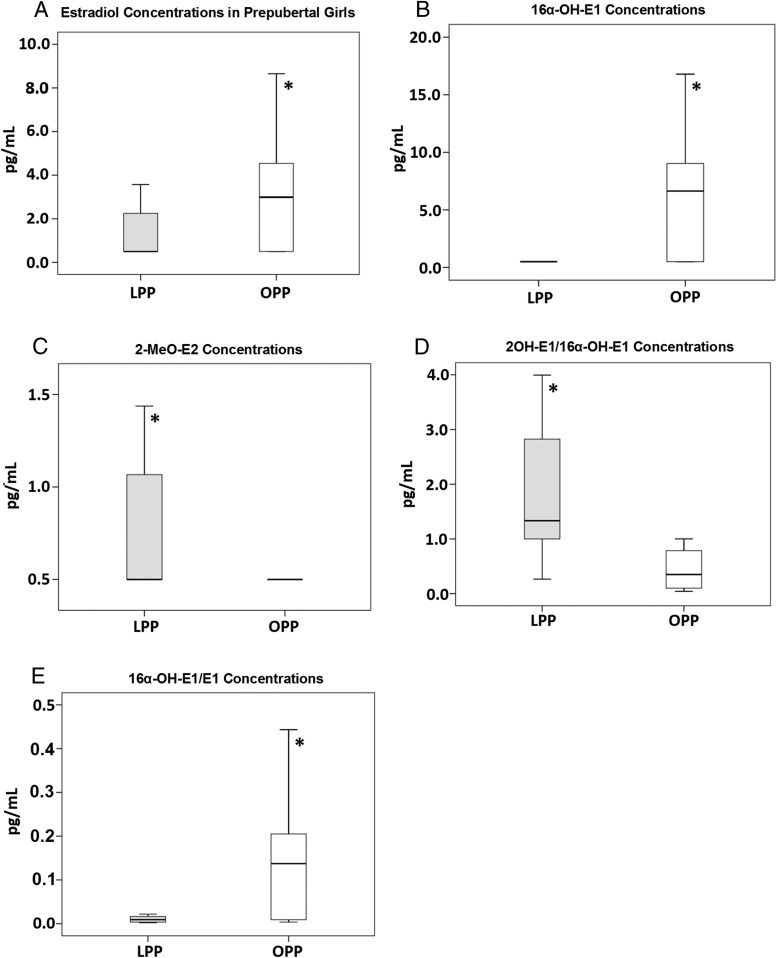
Using a highly sensitive liquid chromatography-tandem mass spectrometry assay, total E2 concentrations were significantly higher in the obese vs lean prepubertal group, with 57% of values of the upper quartile or greater (Q3) of controls, as was the concentration of 16α-OH-E1, a potentially carcinogenic estrogen, with 65% of values of Q3 or greater of controls (Table 2 and Figure 2). In contrast, 2MeO-E2 concentrations, thought to be chemopreventive, were higher in the lean vs the obese group (Figure 2). The ratios of these analytes to their parent compound are shown in Figure 2 as well as the ratio of 2-OH-E1 to 16α-OH-E1, thought to be reciprocally related to breast cancer risk (9). Estrogens circulate as conjugated metabolites (glucuronidated and sulfated) and as unconjugated (free) steroids. We measured the free E2 levels, but for E1 we measured the conjugated compounds.
Table 2.
Concentrations of Estrogens and Their Metabolites in Lean and Obese Prepubertal Girls Median (Interquartile Range) Levels in Picograms per Millilitera
| Lean | Obese | P Valueb | |
|---|---|---|---|
| E2 | 0.5 (.5, 2.37) | 3.45 (0.5, 4.65) | .04 |
| Estriol (16α-OH-E2) | 6.97 (2.77, 10.07) | 5.0 (0.5, 8.32) | .134 |
| 4-MeO-E2 | 1.85 (0.62, 2.12) | 2.12 (0.5, 5.75) | .462 |
| 2-Methoxyestradiol (2-MeO-E2) | 0.628 (0.5, 1.29) | 0.5 (0.5, 0.5) | .012 |
| E1c | 53.12 (31.7, 126.79) | 49.17 (23.79, 77.42) | .266 |
| 16α-OH-E1 | 0.5 (0.5, 0.91) | 7.17 (0.5, 9.64) | .007 |
| 4-Methoxyestrone (4-MeO-E1) | 0.5 (0.5, 1.72) | 0.5 (0.5, 0.5) | .426 |
| 2-Methoxyestrone (2-MeO-E1) | 0.72 (0.49, 1.97) | 0.5 (0.5, 2.68) | .859 |
| 4-OH-estradiol (4-OH-E2) | 0.5 (0.5, 0.5) | 0.5 (0.5, 0.5) | NS |
| 2-OH-estradiol (2-OH-E2) | 0.5 (0.5, 0.5) | 0.5 (0.5, 0.5) | NS |
| 4-OH-estrone (4-OH-E1) | 0.5 (0.5, 0.5) | 0.5 (0.5, 0.5) | NS |
| 2-OH-estrone (2-OH-E1) | 1.56 (0.5, 3.44) | 0.5 (0.5, 7.9) | 0.971 |
Bold denotes statistical significance.
SI unit conversion: for picomoles per liter, multiply E2 by 3.671; 16α-OH-E2, 4-OH-E2, and 2-OH-E2 by 3.467; 4-MeO-E2 and 2-MeO-E2 by 3.307; E1 by 3.698; 16α-OH-E1, 4-OH-E1, and 2-OH-E1 by 3.492; and 4-MeO-E1, and 2-MeO-E1 by 3.329.
Mann-Whitney U test.
The E1 values represent the total conjugated and unconjugated steroid after glucuronidase/sulfatase hydrolysis, whereas the levels of all other steroids represent the free fractions.
Figure 2.
Comparison of the median concentrations of estrogen and metabolites in lean (gray bars) and obese (white bars) prepubertal girls: E2 (A) estradiol (P = .04), 16α-OH-E1 (B) (P = .007), 2-MeO-E2 (C) (P = .012). D, Ratios of 2OH-E1/16α-OH-E1 [lean: 2.11 (1.9, 3.85); obese: 0.602 (0.13, 1.0)]. E, Ratio of 16α-OH-E1 to E1 in the same cohorts [lean: 0.014 (0.004, 0.02); obese: 0.139 (0.009, 0.27), P = .014].
Table 3 shows the Spearman correlation analysis performed for the three estrogen analytes observed to be significantly different between both obese and lean prepubertal girls and pertinent laboratory and anthropometric metrics including IL-6, hsCRP, and BMI.
Table 3.
Spearman Correlations
| n = 35 | BMI |
Fat Mass, % |
hsCRP |
IL-6 |
||||
|---|---|---|---|---|---|---|---|---|
| R | P Value | R | P Value | R | P Value | R | P Value | |
| E2 | 0.175 | .315 | 0.248 | .164 | 0.26 | .15 | 0.176 | .321 |
| 2-MeO-E2 | −0.430 | .01 | −0.376 | .03 | −0.309 | .085 | −0.444 | .008 |
| 16α-OH-E1 | 0.573 | <10−6 | 0.466 | .006 | 0.229 | .208 | 0.550 | .001 |
Bold denotes statistical significance.
Discussion
Using sensitive isotope dilution LC-MS assays (9), we have been able to detect very small concentrations of estrogen metabolites in the plasma of prepubertal girls. These subjects were chosen because they had no physical evidence of puberty, with no breast or pubic hair development. E2 concentrations were increased in the obese group and 16α-OH-E1 was also significantly increased as compared with lean controls. In addition, levels of 2-MeO-E2, which in postmenopausal women are reciprocally related to breast cancer risk, were substantially lower in the obese cohort. These results are remarkable because they represent the first line of evidence in humans that genotoxic concentrations of estrogen metabolites can be detected before the onset of puberty and that these concentrations are higher in obese girls.
Spearman correlation analysis showed a strong positive association between levels of genotoxic 16α-OH-E1 and BMI and percentage fat mass as measured by DEXA. These data are suggestive that the accumulation of these compounds is likely due to excess adiposity. Epidemiological studies in postmenopausal women, but not in premenopausal women, have shown that obesity increases the risk for breast cancer (18–21). In addition, increased BMI at the time of cancer diagnosis has a negative impact on the prognosis in both pre- and postmenopausal women. Although the data on this association between obesity and breast cancer risk are conflicting in premenopausal women (26), recent epidemiological study from the third Harvard Growth Study after more than 1800 participants showed that ever being overweight in childhood increased the risks of all-cause and breast cancer deaths (22). This study suggest that the underlying mechanism of that risk may be similar during the prepubertal years as during much later stages of life; this requires further study. Given that the children in our study had no signs of either gonadal or adrenal puberty, this made it easier to quantify the levels of these metabolites without the confounding effect of varying concentrations, depending on the time in the menstrual cycle.
We previously reported that these obese prepubertal girls have increased concentrations of prothrombotic and proinflammatory cytokines as compared with lean controls (24, 25). The correlation analysis here shows a significant association of genotoxic estrogens with IL-6 concentrations and reciprocal associations with the chemoprotective methoxyderivatives, suggesting a relationship between adipokines and genotoxic estrogen concentrations. This is congruent with experimental data demonstrating that cytokines TNFα and IL-6 are both secreted by adipocytes and can act either in autocrine or paracrine manners to increase production of aromatase, the latter directly related to increased synthesis of estrogen (27), also suggestive of an obesity-inflammation-aromatase axis in breast tissue (28).
The impact of the accumulation of genotoxic estrogens in obese prepubertal girls is ultimately unknown. However, studies in postmenopausal women show an increased accumulation of genotoxic metabolites in breast cancer tumors. The laboratory of Ziegler and colleagues at the National Institutes of Health (29) conducted a prospective case-control study of women with invasive breast cancer with the measurement of 2-OH and 4-OH estrogen metabolites along with methoxyderivatives of E2 and E1. Ziegler observed that nearly all estrogens, estrogen metabolites, and metabolic pathway groups were associated with an increased risk of breast cancer. In particular, more extensive 2-hydroxylation of parent estrogens was associated with a lower risk, and less extensive methylation of potentially genotoxic 4-hydroxylation pathway catechols was associated with higher risk of postmenopausal breast cancer (29). In our cohort we did not observe differences in the catechol metabolites of either E2 or E1; however, we detected a significant increase in the 16α-OH-E1 concentrations. It has been suggested that this compound might be involved in tumor initiation thorough its genotoxic effects (16, 17) and in tumor promotion and progression through its ability to induce cellular proliferation (9). Interestingly, we demonstrated an increase in the ratio of 2-OH-E1 to 16α-OH-E1 in the lean girls. It has been proposed that a shift toward 2-OH-E1 from the 16α-OH-E1 metabolic pathway, as indexed by the 2-OH-E1 to 16α-OH-E1 ratio, is inversely associated with breast cancer risk (9). In fact, several epidemiological studies have found that women with a high ratio of serum 2-OH-E1 metabolites to 16α-OH-E1 metabolites are at a decreased risk for breast cancer (30–32).
This study was made feasible by the development of new, highly sensitive methodology. This state-of-the-art technique exhibits much higher sensitivity than with standard LC-MS-based methods with enhanced specificity (23). The ability of this assay to detect minuscule quantities of these estrogen metabolites in girls prior to puberty establishes this as one of the more sensitive assays presently available to study estrogen biology.
Our study has limitations, including a relatively small sample size and the lack of a family history of breast cancer in the mothers of the participants. These data will need to be confirmed in larger data sets, and more specific studies in first-degree relatives of women with early-onset breast cancer will be needed.
In conclusion, we observed significant increases in estradiol concentrations and in genotoxic estrogen metabolites and decreases in the methoxyderivatives in obese prepubertal girls compared with lean age-matched controls. These data, made possible by new generation assay methodology, suggest that extraglandular estrogen production and metabolism occurs even before the onset of puberty in females. The changes observed in the prepubertal obese girls raise caution about the potential for increased breast cancer risk due to these effects. Long-term epidemiological studies will now be required to assess this potential risk.
Acknowledgments
We are grateful to Ligeia Damaso (Nemours Children's Clinic) for research assistance with the data organization and analysis and to Sylvia Kyle (librarian at Nemours Children's Clinic) and the children and families that participated in these studies.
This work was supported in part by grant support from the Thrasher Research Fund (to N.M.), Mayo Clinic Comprehensive Cancer Center NCIP50-CA01508 (G.C-O.), and National Institutes of Health grants R01CA158328 and P30ES013508 (to I.A.B.).
Disclosure Summary: J.H., Q.W., C.M., and I.A.B. have nothing to declare. N.M. received grant support (2010–2015) from AstraZeneca, Novartis, and Pfizer. G.C.-O. received grant support (2015) from Novartis. R.J.S. has served on an advisory board for Pfizer (2013).
Footnotes
- BMI
- body mass index
- DEXA
- dual X-ray absorptiometry
- E1
- estrone
- E2
- estradiol
- ER
- estrogen receptor
- hsCRP
- highly sensitive C-reactive protein
- LC-MS
- liquid chromatography-mass spectrometry
- MeO
- 2-methoxy
- 2-MeO-E2
- MeO-3-hydroxy-17β-estradiol
- 16α-OH-E1
- 16α-hydroxyestrone
- 2-OH-E2
- 2-hydroxyestradiol
- 4-OH-E2
- 4-hydroxyestradiol
- Q
- quartile.
References
- 1. Santen RJ, Boyd NF, Chlebowski RT, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. [DOI] [PubMed] [Google Scholar]
- 2. The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. [DOI] [PubMed] [Google Scholar]
- 3. Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. [DOI] [PubMed] [Google Scholar]
- 4. Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. Published online ahead of print August 26, 2014. pii: S0039-128X(14)00196-2. doi: 10.1016/j.steroids.2014.08.003. [Google Scholar]
- 5. Zhao Z, Kosinska W, Khmelnitsky M, et al. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem Res Toxicol. 2006;19:475–479. [DOI] [PubMed] [Google Scholar]
- 6. Gao N, Nester RA, Sarkar MA. 4-Hydroxy estradiol but not 2-hydroxy estradiol induces expression of hypoxia-inducible factor 1α and vascular endothelial growth factor A through phosphatidylinositol 3-kinase/Akt/FRAP pathway in OVCAR-3 and A2780-CP70 human ovarian carcinoma cells. Toxicol Appl Pharmacol. 2004;196:124–135. [DOI] [PubMed] [Google Scholar]
- 7. Bransfield LA, Rennie A, Visvanathan K, et al. Formation of two novel estrogen guanine adducts and HPLC/MS detection of 4-hydroxyestradiol-N7-guanine in human urine. Chem Res Toxicol. 2008;21:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol. 2003;15:425–430. [DOI] [PubMed] [Google Scholar]
- 9. Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids. 2010;75:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol. 2011;125:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavigne JA, Goodman JE, Fonong T, et al. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001;61:7488–7894. [PubMed] [Google Scholar]
- 12. Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. [DOI] [PubMed] [Google Scholar]
- 13. Castagnetta LA, Granata OM, Traina A, et al. Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin Cancer Res. 2002;8:3146–3155. [PubMed] [Google Scholar]
- 14. Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16α-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84:634–638. [DOI] [PubMed] [Google Scholar]
- 15. Lewis JS, Thomas TJ, Pestell RG, Albanese C, Gallo MA, Thomas T. Differential effects of 16α-hydroxyestrone and 2-methoxyestradiol on cyclin D1 involving the transcription factor ATF-2 in MCF-7 breast cancer cells. J Mol Endocrinol. 2005;34:91–105. [DOI] [PubMed] [Google Scholar]
- 16. Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;(27):75–93. [DOI] [PubMed] [Google Scholar]
- 17. Liehr JG. Role of DNA adducts in hormonal carcinogenesis. Regul Toxicol Pharmacol. 2000;32:276–282. [DOI] [PubMed] [Google Scholar]
- 18. Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92. [DOI] [PubMed] [Google Scholar]
- 20. Key TJ, Appleby PN, Reeves GK, et al. Endogenous Hormones Breast Cancer Collaborative Group. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. [DOI] [PubMed] [Google Scholar]
- 21. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study. BMJ. 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Must A, Phillips SM, Naumova EN. Occurrence and timing of childhood overweight and mortality: findings from the Third Harvard Growth Study. J Pediatr. 2012;160:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Q, Rangiah K, Mesaros C, et al. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids. 2015;96:140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauras N, Delgiorno C, Kollman C, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. 2010;95:1060–1068. [DOI] [PubMed] [Google Scholar]
- 25. Mauras N, DelGiorno C, Hossain J, et al. Metformin use in children with obesity and normal glucose tolerance—effects on cardiovascular markers and intrahepatic fat. J Pediatr Endocrinol Metab. 2012;25:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1121–1127. [PubMed] [Google Scholar]
- 27. Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanczyk FZ, Mathews BW, Sherman ME. Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: a critical appraisal of current science. Steroids. Published online ahead of print December 30, 2014. pii: S0039-128X(14)00304-3. doi: 10.1016/j.steroids.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 29. Fuhrman BJ, Schairer C, Ziegler RG, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Im A, Vogel VG, Ahrendt G, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30:1532–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16α-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jernstrom H, Klug TL, Sepkovic DW, Bradlow HL, Narod SA. Predictors of the plasma ratio of 2-hydroxyestrone to 16α-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24:991–1005. [DOI] [PubMed] [Google Scholar]