Abstract
Context:
Glucagon-like peptide-1 (GLP-1) is an insulinotropic factor made in the gastrointestinal tract that is essential for normal glucose tolerance. Infusion of GLP-1 increases insulin secretion in both diabetic and nondiabetic humans. However, the degree to which people vary in their β-cell sensitivity to GLP-1 and the factors contributing to this variability have not been reported.
Objective:
The objective was to measure the sensitivity of insulin secretion to GLP-1 in cohorts of lean and obese subjects across a broad range of insulin sensitivity.
Methods:
Insulin secretion was measured during clamped hyperglycemia (7.2 mmol/L) and graded GLP-1 infusion in young, healthy subjects, and GLP-1 sensitivity was computed from the insulin secretion rate (ISR) during progressive increases in plasma GLP-1.
Results:
All subjects had fasting glucose values <5.2 mm. The obese subjects were insulin resistant compared to the lean group (homeostasis model of assessment 2 for insulin resistance: obese, 2.6 ± 0.5; lean, 0.8 ± 0.1; P < .001). ISR increased linearly in both cohorts with escalating doses of GLP-1, but the slope of ISR in response to GLP-1 was greater in the obese than in the lean subjects (obese, 0.17 ± 0.03 nmol/min/pm; lean, 0.05 ± 0.01 nmol/min/pm; P < .001). There was a significant association of β-cell GLP-1 sensitivity and insulin resistance (r = 0.83; P < .001), and after correction for homeostasis model of assessment 2 for insulin resistance, the slopes of ISR vs GLP-1 concentration did not differ in the two cohorts (obese, 0.08 ± 0.01; lean, 0.08 ± 0.01; P = .98). However, within the entire study group, β-cell GLP-1 sensitivity corrected for insulin resistance varied nearly 10-fold.
Conclusions:
Insulin secretion in response to GLP-1 is proportional to insulin resistance in healthy subjects. However, there is considerable variability in the sensitivity of the β-cell to GLP-1 that is independent of insulin sensitivity.
Insulin secretion is greater when glucose is ingested than when an equal glycemic stimulus is given iv (1, 2). This phenomenon, termed the incretin effect, is mediated in great part by two hormones released from the gastrointestinal tract, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), which stimulate insulin secretion in proportion to blood glucose concentrations (3, 4). The incretins are secreted from enteroendocrine cells in the mucosa of the intestine after meal ingestion and are essential for normal glucose tolerance (5–8).
Numerous studies have demonstrated that administration of GLP-1 to healthy subjects causes a large increase of insulin secretion (3, 9, 10). However, previous studies have generally used amounts of peptide that caused supraphysiological plasma levels of bioactive GLP-1. Inhibition of endogenous GLP-1 action with the GLP-1 receptor (GLP-1r) antagonist exendin-(9–39) reduced the insulin response to oral glucose by 30–40% (11) but with a widely variable effect on insulin secretion among subjects, suggesting differences in the β-cell sensitivity to GLP-1. This supposition is supported by a study demonstrating that the magnitude of the incretin effect varies 1.5- to 2-fold among nondiabetic subjects and is reproducible day to day (4). The sensitivity of insulin secretion to GLP-1 has clinical importance because the incretin effect is reduced in persons with type 2 diabetes (12, 13), a finding recently proposed to extend to obese subjects (14–16). Moreover, with the recent development of GLP-1r agonists as therapy for type 2 diabetes, the possibility exists that differences in sensitivity to GLP-1 between people could explain variability in the responses to these agents.
In this study, the sensitivity of insulin secretion to GLP-1 was measured in nondiabetic lean and obese subjects who differed in insulin sensitivity but had unequivocally normal fasting glucose. We hypothesized that insulin secretion would be less sensitive to GLP-1 stimulation in obese compared to lean subjects (14, 15).
Subjects and Methods
Subjects
Ten lean (five male, five/female) and eight obese (five male, three female) adults aged 19–36 years were studied. All subjects were healthy, had no family history of diabetes, and did not use any regular medication, except for birth control pills among some of the women. Subjects had stable body weight over the previous 3 months and were asked to refrain from strenuous physical exercise and consume their usual diet for 3 days before the study. Anthropometric data and fasting biochemistries in the two groups are summarized in Table 1. The treatment and experimental protocols described below were approved by the Institutional Review Board of the University of Cincinnati, and all participants provided written informed consent before the studies.
Table 1.
Patient Characteristics and Fasting Plasma Glucose, Insulin, and C-Peptide
| Lean | Obese | P Value | |
|---|---|---|---|
| Age, y | 27.5 ± 1.5 | 25.8 ± 1.8 | .47 |
| Body weight, kg | 68.1 ± 3.0 | 127.5 ± 7.0 | <.001 |
| BMI, kg/m2 | 23.6 ± 0.9 | 40.5 ± 2.7 | <.001 |
| Sex (M/F) | 5/5 | 5/3 | |
| Fasting glucose, mm | 4.5 ± 0.1 | 4.8 ± 0.1 | <.05 |
| Fasting insulin, pm | 45 ± 4 | 148 ± 31 | <.01 |
| Fasting C-peptide, nm | 0.46 ± 0.04 | 1.12 ± 0.13 | <.01 |
| HOMA2-IR | 0.8 ± 0.1 | 2.6 ± 0.5 | <.01 |
Abbreviations: M, male; F, female. HOMA2-IR and modelled parameters for insulin sensitivity and β-cell function are based on fasting insulin and glucose using the HOMA-calculator (19). Characteristics are presented as mean ± SEM and were compared using unpaired t tests.
Experimental procedure
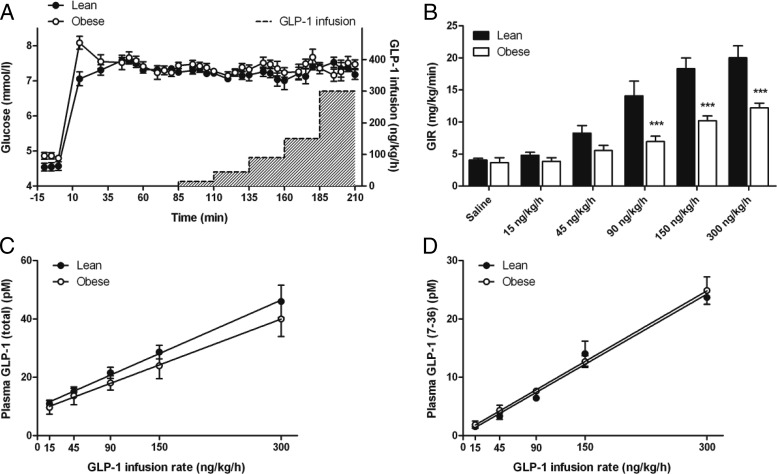
Subjects were admitted to the Clinical Translational Research Center (CTRC) at the Cincinnati Children's Hospital in the morning after an overnight fast of at least 10 hours. Body weight and height were measured, and pregnancy was excluded in female patients. A dual-energy x-ray absorptiometry scan was performed to determine lean body mass (LBM) in the obese subjects. Intravenous catheters were placed in each forearm for withdrawal of blood samples and infusion of glucose and GLP-1. The arm and hand used for blood sampling was wrapped in a heating pad to maintain stable blood flow. After fasting blood samples were taken, a primed infusion of 20% dextrose was started at time 0 to rapidly achieve a target blood glucose concentration of 7.2 mmol/L; this concentration was chosen because it approximates the typical peak of postprandial glycemia in normal glucose tolerant persons. After 85 minutes of stable hyperglycemia, step-wise administration of GLP-1 was begun, with increments changing every 25 minutes to progressively higher doses (15, 45, 90, 150, and 300 ng/kg LBM/h; Figure 1). Glucose infusion rate (GIR) was adjusted to maintain stable blood glucose levels throughout the study. In a pilot study of obese subjects, we noted that using body weight to calculate the GLP-1 infusion rate resulted in significant overdosing of obese compared to lean individuals. Therefore lean mass was used to calculate the GLP-1 infusion rate in the obese subjects, whereas the lean subjects were dosed by body weight. Blood samples were taken at scheduled intervals to measure plasma insulin, C-peptide, and GLP-1 concentrations.
Figure 1.
A, Blood glucose concentrations before and after a hyperglycemic clamp in lean (filled circles) and obese (open circles) subjects. At 85 minutes, a graded infusion of GLP-1 was started and increased every 25 minutes. The gray area shows the GLP-1 infusion rate indicated at the right Y-axis. B, GIR to maintain constant hyperglycemia during the glucose clamp with and without GLP-1. C and D, Plasma total (C) and intact GLP-1 (D) concentrations in response to the graded GLP-1 infusion. ***, Differences between lean and obese subjects. P < .001.
Peptide and analytical procedures
Purified GLP-1(7–36)amide ready for human use was purchased from Clinalfa (Merck Biosciences AG). Peptide infusions were prepared in the morning before each study by the CTRC research pharmacy by mixing lyophilized GLP-1 and 0.25% human serum albumin in 250 mL of saline. The use of synthetic GLP-1 in human research is approved under the US Food and Drug Administration IND 32,977.
Blood samples for glucose measurements were taken at 5-minute intervals and immediately analyzed using a bedside glucose monitor (YSI Life Sciences) to adjust the GIR. Samples for determination of insulin and C-peptide were collected in prechilled heparinized tubes and stored immediately on ice. Samples for determination of total GLP-1 were collected in a mix of heparin, EDTA, and aprotinin. In a subgroup of 11 subjects, the dipeptidyl peptidase-4 (DPP-4) inhibitor Diprotin A (Sigma-Aldrich) was added to allow measurement of active GLP-1(7–36). All samples were kept on ice, centrifuged within 1 hour of withdrawal, frozen, and stored at −80°C for later measurements of peptide hormone concentrations.
Insulin and C-peptide concentrations were assayed by RIA as previously described (17). Concentrations of total GLP-1 were measured by a commercially available RIA (Millipore). Active GLP-1 was measured by a commercially available ELISA for GLP-1(7–36) (Millipore) in the subgroup of subjects in both cohorts (four obese and seven lean) where Diprotin A was added to the sample upon withdrawal.
Calculations and statistical analysis
Data are presented as mean ± SEM unless otherwise noted. Anthropometrics and fasting biochemistries were compared in the lean and obese cohorts by t tests. Insulin secretion rate (ISR) was calculated by deconvolution of C-peptide concentrations, using population values for C-peptide kinetics (17, 18). Homeostasis model of assessment 2 for insulin resistance (HOMA2-IR) was determined with an online-calculator http://www.dtu.ox.ac.uk/homacalculator/index.php (19). Insulin sensitivity was also estimated using the mean GIR and plasma C-peptide concentrations from 75–85 minutes of the glucose clamp. Insulin sensitivity computed from GIR/C-peptide during the clamp correlated significantly with HOMA2-IR (r = −0.61; P = .0077) and was significantly reduced in the obese subjects (1.29 ± 0.27 and 4.21 ± 0.63 mg/kg/min × nM−1, for obese and lean subjects, respectively; P = .0001). Because HOMA2-IR correlated better with fasting C-peptide and glucose-stimulated ISR, this factor was used as the measure for insulin sensitivity in the primary analyses; secondary use of GIR/C-peptide from the clamp did not alter the results. The primary experimental variables GIR, insulin, C-peptide, GLP-1, and ISR were averaged over the final 10 minutes (three samples) of each GLP-1 infusion step.
Disposition index (DI) was computed using glucose-stimulated ISR (time, 60–85 min) during the clamp multiplied by 1/HOMA2-IR. Responses to the graded GLP-1 infusion were compared between lean and obese subjects using two-way ANOVA for repeated measures with Bonferroni post hoc analysis (GraphPad Prism; GraphPad Software). β-Cell sensitivity to GLP-1 was calculated for each subject as the slope of ISR and plasma GLP-1 levels, or ISR and GLP-1 infusion rate, and means were computed for the lean and obese groups. Variability in β-cell sensitivity to glucose was calculated as the maximum divided by the minimum of these slopes. Individual and group differences in ISR:GLP-1 slopes were analyzed by linear regression. Adjustment of β-cell function for insulin resistance was made by dividing ISR by HOMA2-IR.
Results
Fasting glucose, insulin, and insulin sensitivity
Mean fasting glucose was 4.5 ± 0.1 mmol/L in lean and 4.8 ± 0.1 mmol/L in obese subjects. Although the obese subjects had significantly higher fasting glucose (P = .048) they were well within the normal range of fasting with no subject exceeding a concentration of 5.2 mmol/L. Both fasting plasma insulin and C-peptide were significantly higher in the obese cohort (Table 1), corresponding to greater insulin resistance. HOMA2-IR was significantly higher in obese subjects compared to lean subjects (obese, 2.6 ± 0.5; lean, 0.8 ± 0.1; P = .001).
Hyperglycemic clamp and GIR
Constant hyperglycemia was induced by a primed glucose infusion and maintained by adjusting the GIR throughout 210 minutes. Between 61 and 210 minutes, the average glucose level was similar between the two groups (lean, 7.25 ± 0.04 mmol/L; obese, 7.36 ± 0.05 mmol/L; P = .110). There was no significant difference in the glucose increment over fasting between lean and obese subjects (lean, 2.70 ± 0.13 mmol/L; obese, 2.51 ± 0.07 mmol/L; P = .254), and the clamp was stable from 61 to 210 minutes with coefficients of variation of 5.7% (lean) and 5.4% (obese) (Figure 1A). The initial GIR needed to clamp blood glucose did not differ in the two groups (lean, 4.1 ± 0.38 mg/kg/min; obese, 3.7 ± 0.8 mg/kg/min; P = .635) and rose significantly in both groups with increasing GLP-1 dose (P < .001), although GIR appeared to reach a near maximal rate in the obese subjects with the latter two doses of GLP-1. The GIRs necessary to maintain constant hyperglycemia during the GLP-1 infusion were significantly higher in lean compared to obese subjects from 110 minutes through the completion of the experiments (P < .001; Figure 1B).
Plasma GLP-1 concentrations
Plasma levels of total GLP-1 during saline infusion were 7.5 ± 1.3 pmol/L in the lean and 10.1 ± 3.1 pmol/L in the obese cohorts (P = .665) and increased significantly with the graded infusion (P < .001; Figure 1C). Calculation of GLP-1 infusion rates based on LBM in the obese group resulted in average GLP-1 levels that were similar to the lean subjects during each infusion step (P = .492), and plasma GLP-1 was linearly related to infusion dose with slopes that did not differ between the two cohorts (P = .40; Figure 1C). In a subgroup of lean and obese subjects, plasma levels of active GLP-1(7–36) were measured to account for possible differences in clearance by DPP-4 between lean and obese subjects. The step-wise infusion resulted in a linear increase of intact GLP-1(7–36) that paralleled total GLP-1 and was almost identical for lean and obese subjects (Figure 1D). All subjects tolerated the infusion of GLP-1 across the dose range without nausea or other symptoms.
Insulin, C-peptide, and ISR in response to hyperglycemia and GLP-1
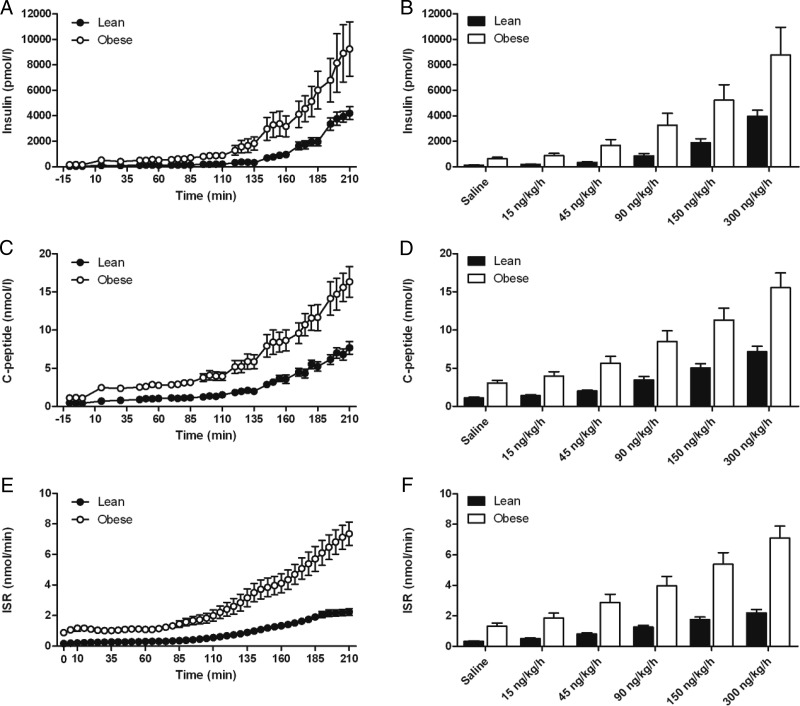
During hyperglycemia alone, before initiation of the GLP-1 infusion, the obese subjects had significantly higher concentrations of insulin (obese, 639 ± 114 pmol/L; lean, 140 ± 13 pmol/L; P = .003), C-peptide (obese, 3.06 ± 0.35 nmol/L; lean, 1.11 ± 0.12 nmol/L; P < .001), and ISR (obese, 1.33 ± 0.19 nmol/min; lean, 0.33 ± 0.03 nmol/min; P = .001). DI determined from the ISR response to hyperglycemia alone did not differ in the two groups (lean, 0.43 ± 0.04; and obese, 0.38 ± 0.06; P = .55). In both cohorts, there was a significant increase of insulin, C-peptide, and ISR with step-wise increments of GLP-1 infusion, and this was significantly greater in obese than in lean subjects (Figure 2; interaction of dose and group, P < .001). Plasma insulin and C-peptide concentrations increased at each step of the GLP-1 infusion in both groups such that no maximum β-cell response could be detected at the doses used in these experiments. Compared to the hyperglycemic baseline (60–85 min), insulin and C-peptide concentrations and ISR had doubled between the 45 and 60 ng/kg/h rates of GLP-1 infusion.
Figure 2.
Insulin (A, B), C-peptide (C, D), and ISR (E, F) in response to hyperglycemia and a graded infusion of GLP-1. Panels B, D, and F show average plasma concentrations and ISR during the last 10 minutes of saline and each of the GLP-1 infusion rates. *, Differences between the lean and obese subjects. P < .05.
β-Cell sensitivity to GLP-1 corrected for insulin resistance
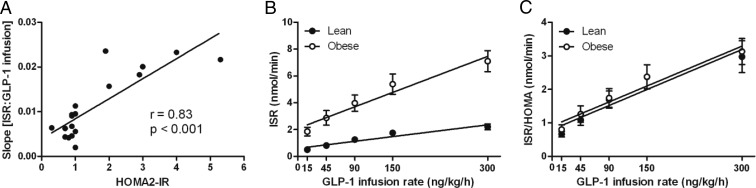
There was a significant relationship between ISR:GLP-1 and HOMR-IR (Figure 3A), indicating that β-cell sensitivity to GLP-1 increased in proportion to insulin resistance. Correction of ISR:GLP-1 for HOMA-IR resolved the different slopes in the lean and obese cohorts, resulting in a linear relationship that did not differ in the two groups (Figure 3B and C). Thus, when insulin resistance was accounted for, the mean slopes of GLP-1-stimulated ISR vs the amount of GLP-1 for the lean and obese groups no longer differed (lean, 0.008 ± 0.001; obese, 0.008 ± 0.001; P = .988).
Figure 3.
A, The relationship between β-cell sensitivity to GLP-1 and insulin resistance in lean and obese subjects; all 18 study participants are presented as filled circles. B, The relationship between ISR and the GLP-1 infusion rate in lean (filled circles) and obese (open circles) subjects. C, The relationship between ISR corrected for insulin resistance and the GLP-1 infusion rate in lean (filled circles) and obese (open circles) subjects.
To determine whether insulin secretion in response to GLP-1 was part of a generalized β-cell compensation for insulin sensitivity, ISR during hyperglycemia alone was compared to the slope of ISR:GLP-1. These factors correlated significantly across all subjects (r = 0.68; P < .05), suggesting that compensation for insulin resistance was an important determinant of the response to both glucose and incretin stimulation.
Variability in the β-cell response to GLP-1
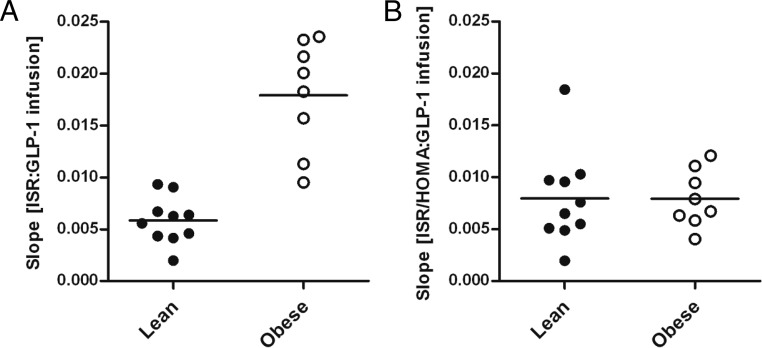
The slopes of ISR:GLP-1 infusion or ISR:plasma GLP-1 were used as indices of β-cell GLP-1 sensitivity in each subject (Figure 4A). Across all subjects, uncorrected β-cell GLP-1 sensitivity showed a roughly 20-fold difference between the maximum and minimum slope. A large portion of this variability was explained by the effect of insulin resistance on β-cell GLP-1 sensitivity. However, even after correction for HOMA2-IR, there remained a nearly 10-fold difference across the entire group of subjects (Figure 4B). Before adjustment for HOMA2-IR, there were 4.7- and 2.5-fold differences across the lean and obese groups, which increased to 9.3-fold in the lean cohort and 3.0-fold in the obese cohort after correction.
Figure 4.
β-Cell sensitivity to GLP-1 in lean and obese subjects uncorrected (A) and corrected (B) for insulin resistance.
Discussion
GLP-1 is a potent insulin secretagogue, and the GLP-1r system has now been harnessed for pharmaceutical use. Previous studies suggest that the insulinotropic response to GLP-1 (11) and the incretin effect (4) differ significantly within groups of healthy humans. In this study we have demonstrated directly that β-cell sensitivity to GLP-1 varies considerably among nondiabetic subjects. Among our cohort, GLP-1 enhanced insulin release in proportion to the degree of insulin resistance, a finding most apparent in obese subjects who had a wide range of insulin sensitivity. When the β-cell sensitivity to GLP-1 was corrected for insulin sensitivity, healthy lean and obese subjects had GLP-1-ISR relationships that were essentially identical, refuting our initial hypothesis. However, whereas the adjusted, mean responses of our lean and obese subjects to the graded GLP-1 infusion did not differ, there was considerable interindividual variation, demonstrating a wide range of GLP-1 sensitivity within healthy people. These results suggest that substantial differences exist in the contribution of GLP-1 action to glucose metabolism in nondiabetic individuals. Moreover, they raise the possibility that among patients treated with GLP-1r agonists, responses may vary in a predictable manner depending on their inherent sensitivity to GLP-1.
In this study we selected both lean and obese subjects who were young and otherwise healthy to allow the effects of β-cell GLP-1 sensitivity to be examined across a range of insulin sensitivity without other confounders. The two cohorts were well-matched for age but varied over a wide range of leanness and obesity. Although we did not perform oral glucose tolerance tests or determine glycated hemoglobin in these subjects, both cohorts had fasting glycemia consistent with normal glycemic regulation. Moreover, the obese subjects had appropriate compensatory insulin secretion for their degree of insulin resistance with DI that did not differ from the lean group. Glucose was clamped at 7–8 mm to study GLP-1 sensitivity at levels in the prandial range. We used a graded infusion of GLP-1 to determine the dose-response in a single study for subject convenience and to limit day-to-day variability. Given that the plasma half-life of active GLP-1 is 1–2 minutes in humans (20), each 25-minute step in dose is easily sufficient to give steady-state concentrations for assessment of insulin secretion. To achieve similar levels of GLP-1 in both cohorts, we performed dual-energy x-ray absorptiometry and dosed the GLP-1 infusion by lean mass in obese subjects. The resulting plasma concentrations of total and active GLP-1 were similar between the two groups, allowing direct comparison of the ISR responses. Total plasma GLP-1 concentrations increased linearly with GLP-1 infusion rate, suggesting constant clearance of the peptide at least within the dose-range of our study. Furthermore, analysis of the active plasma levels suggests no significant difference in DPP-4-mediated GLP-1 clearance between lean and obese individuals, confirming previous results (21).
Similar to studies of glucose-stimulated insulin secretion using a stepped glucose infusion protocol (22, 23), there was a linear relationship between GLP-1, either plasma concentration or infusion rate, and ISR in our study. As expected, the obese subjects had higher insulin and C-peptide in the fasting state and increased ISR during the initial glucose clamp, in keeping with their greater degree of insulin resistance. Interestingly, the difference in ISR between the lean and obese cohorts became even more pronounced at the higher doses of GLP-1, causing a left shift of the dose-response curve in obese subjects with significantly steeper slopes. Again, this finding is consistent with previous studies comparing ISR in lean and obese subjects in response to a graded glucose infusion or a hyperglycemic clamp (22, 24). Although these results suggest that obese individuals have relatively enhanced β-cell sensitivity to GLP-1, correction of their ISR for insulin resistance gave results nearly identical to the lean subjects. Thus, in nondiabetic subjects, β-cell sensitivity to GLP-1 holds to, and may help maintain, the fundamental relationship between insulin secretion and insulin action (25). In addition, the strong relationship between GLP-1 sensitivity and glucose-stimulated insulin secretion in this cohort is consistent with a major effect of insulin resistance to increase the effect of multiple β-cell stimuli. More refined studies are necessary to determine whether individual β-cell sensitivity to glucose and GLP-1 is independent.
A second notable finding of this study was the intersubject variability in β-cell responsiveness to GLP-1. After correction for insulin resistance, there remained a nearly 10-fold difference between the least, and the most, GLP-1-sensitive subjects. These findings suggest substantial differences inherent among healthy subjects to the insulinotropic properties of GLP-1, a finding recently suggested in a study of rats (26). A number of studies have connected the action of GLP-1 to potentiate glucose-stimulated insulin secretion to specific genetic variants (27–30), so it is reasonable to expect inherent variability among individuals. What is surprising is the magnitude of the difference between high and low GLP-1 responders in this group of normal subjects. Because we only studied each subject once, we cannot say for sure how much of this variation in β-cell GLP-1 sensitivity is a persistent characteristic of these individuals. However, our results, and the accumulation of genotype-phenotype interactions in GLP-1 responsiveness have implications for the use of GLP-1r agonists in diabetes, specifically the possibility that patients could be defined as more or less likely to respond to this class of drugs.
Although we did not design this study to determine the threshold level of plasma GLP-1 necessary to stimulate insulin secretion, our results provide some basis for comment. GLP-1 infusion rates of 45 and 90 ng/kg/h gave concentrations of total GLP-1 that are comparable to what has been reported after meals (31, 32). The mean ISR in both the lean and obese cohorts had doubled from the rates during glucose stimulation alone at the 45 ng/kg/h dose. This observation supports some endocrine action of secreted GLP-1 in postprandial insulin secretion, a mechanism that has recently been questioned by our group and others (33–35).
There are several limitations to our study that need to be considered. We did not directly measure glucose tolerance or the incretin effect in our subjects, and so cannot extend our findings on GLP-1 sensitivity to physiological regulation. Moreover, we did not measure other parameters of insulin secretion, and so we cannot comment on how these related to GLP-1 sensitivity. Secondly, our samples of lean and obese subjects were relatively small, limiting statistical power. However, the nearly superimposable ISR responses, when corrected for insulin resistance, support our conclusions by considering the group as a whole when evaluating the range of β-cell GLP-1 sensitivity. Third, it will ultimately be necessary to assess the insulin response to GLP-1 in repeated studies of individuals to determine the day-to-day variability, as we have done for the incretin effect (4). Finally, we did not include subjects with known diabetes or glucose intolerance in our study, groups where β-cell response to GLP-1 has the most relevance to pathophysiology and therapeutics; this too is an important consideration for future work.
In summary, we have described β-cell sensitivity to GLP-1 in groups of healthy lean and obese individuals. There was a high correspondence between glucose- and GLP-1-stimulated insulin secretion, emphasizing the need to consider the magnitude of the incretin effect in the context of overall β-cell function (4). The slope of the ISR:GLP-1 dose-response corrected for insulin sensitivity did not differ in the two groups, indicating that neither obesity nor insulin resistance impairs the insulinotropic effect of GLP-1. The positive relationship between the sensitivity to GLP-1 and insulin resistance raises the possibility that this is a mechanism whereby β-cells adapt to increasing demand for insulin. Lastly, the wide variation in β-cell GLP-1 sensitivity across our cohorts supports this parameter as one that could provide insights into the susceptibility for developing impaired glucose tolerance and diabetes, or for responding to GLP-1-based medications in the presence of these conditions.
Acknowledgments
The authors appreciate the careful assays provided by Kay Ellis, Brianne Reedy, Clinton T. Elfers, and Dr Radha Krishna. We also thank the staff of the Cincinnati Children's Hospital Clinical Translational Research Center for their expert technical assistance in the care of the subjects and performance of the studies.
This research was supported by a Merit Award from the Department of Veterans Affairs and US Public Health Service Grant R01 57900 (both to D.A.D.). This study was also supported by the National Institutes of Health Clinical and Translational Science Award program Grant 8 UL1 TR000077-05 (to the University of Cincinnati and the Cincinnati Children's Hospital).
B.A.A. contributed to study design, data collection, and analysis and wrote the manuscript. T.P.V. contributed to study design and data collection and reviewed/edited the manuscript. H.E.W.-P. contributed to the data analysis and discussion and reviewed/edited the manuscript. R.L.P. modeled the insulin secretion rate and contributed to data analysis. D.A.D. contributed to study design and data analysis, and reviewed/edited the manuscript. None of the authors have a conflict of interest to declare related to this work.
Disclosure Summary: B.A.A., T.P.V., H.E.W.-P., and R.L.P. have nothing to declare. D.A.D. has consulted for Boehringer-Ingelheim, Intarcia, Janssen, Lilly, Merck, Novo Nordisk and Roche.
Footnotes
- DI
- disposition index
- DPP-4
- dipeptidyl peptidase-4
- GIP
- glucose-dependent insulinotropic polypeptide
- GIR
- glucose infusion rate
- GLP-1
- glucagon-like peptide-1
- GLP-1r
- GLP-1 receptor
- HOMA2-IR
- homeostasis model of assessment 2 for insulin resistance
- ISR
- insulin secretion rate
- LBM
- lean body mass.
References
- 1. Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. [DOI] [PubMed] [Google Scholar]
- 2. Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. [DOI] [PubMed] [Google Scholar]
- 3. Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. [DOI] [PubMed] [Google Scholar]
- 4. Salehi M, Aulinger B, D'Alessio DA. Effect of glycemia on plasma incretins and the incretin effect during oral glucose tolerance test. Diabetes. 2012;61:2728–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scrocchi LA, Brown TJ, MaClusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2:1254–1258. [DOI] [PubMed] [Google Scholar]
- 6. D'Alessio DA, Vogel R, Prigeon R, et al. Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest. 1996;97:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes. 1999;48:86–93. [DOI] [PubMed] [Google Scholar]
- 8. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. [DOI] [PubMed] [Google Scholar]
- 9. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. [DOI] [PubMed] [Google Scholar]
- 11. Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93:4909–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. [DOI] [PubMed] [Google Scholar]
- 13. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. [DOI] [PubMed] [Google Scholar]
- 14. Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–1348. [DOI] [PubMed] [Google Scholar]
- 15. Knop FK, Aaboe K, Vilsbøll T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab. 2012;14:500–510. [DOI] [PubMed] [Google Scholar]
- 16. Hansen KB, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. Impaired incretin-induced amplification of insulin secretion after glucose homeostatic dysregulation in healthy subjects. J Clin Endocrinol Metab. 2012;97:1363–1370. [DOI] [PubMed] [Google Scholar]
- 17. Salehi M, Aulinger B, Prigeon RL, D'Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. [DOI] [PubMed] [Google Scholar]
- 19. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 20. Vahl TP, Paty BW, Fuller BD, Prigeon RL, D'Alessio DA. Effects of GLP-1-(7–36)NH2, GLP-1-(7–37), and GLP-1-(9–36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;88:1772–1779. [DOI] [PubMed] [Google Scholar]
- 21. Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878. [DOI] [PubMed] [Google Scholar]
- 22. Polonsky KS, Given BD, Hirsch L, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brandt A, Katschinski M, Arnold R, Polonsky KS, Göke B, Byrne MM. GLP-1-induced alterations in the glucose-stimulated insulin secretory dose-response curve. Am J Physiol Endocrinol Metab. 2001;281:E242–E247. [DOI] [PubMed] [Google Scholar]
- 24. Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–E508. [DOI] [PubMed] [Google Scholar]
- 25. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 26. Habegger KM, Heppner KM, Amburgy SE, et al. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2014;63:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müssig K, Staiger H, Machicao F, Häring HU, Fritsche A. Genetic variants affecting incretin sensitivity and incretin secretion. Diabetologia. 2010;53:2289–2297. [DOI] [PubMed] [Google Scholar]
- 28. Pilgaard K, Jensen CB, Schou JH, et al. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia. 2009;52:1298–1307. [DOI] [PubMed] [Google Scholar]
- 29. Schäfer SA, Tschritter O, Machicao F, et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schäfer SA, Müssig K, Staiger H, et al. A common genetic variant in WFS1 determines impaired glucagon-like peptide-1-induced insulin secretion. Diabetologia. 2009;52:1075–1082. [DOI] [PubMed] [Google Scholar]
- 31. Hansen KB, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab. 2010;95:3309–3317. [DOI] [PubMed] [Google Scholar]
- 32. Aulinger BA, Bedorf A, Kutscherauer G, et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63:1079–1092. [DOI] [PubMed] [Google Scholar]
- 33. Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. [DOI] [PubMed] [Google Scholar]
- 34. D'Alessio DA. What if gut hormones aren't really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152:2925–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donath MY, Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(suppl.2):S145–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]